Abstract
Objective
To investigate the relationship between brain structure and psychopathic traits in maximum-security incarcerated male adolescents: Do the associations between brain volumes in paralimbic and limbic regions and psychopathic traits observed in incarcerated adult men extend to an independent sample of incarcerated male adolescents?
Method
A structural magnetic resonance imaging (MRI) study of regional gray matter volumes (GMV) by using voxel-based morphometry (VBM) in maximum-security incarcerated male adolescents (N=218) assessed for psychopathic traits using the Hare Psychopathy Checklist–Youth Version (PCL-YV). All analyses controlled for effects of age, substance use, and brain size.
Results
Consistent with hypotheses and the adult literature, psychopathic traits were associated with decreased regional GMV in diffuse paralimbic regions, including orbitofrontal cortex, bilateral temporal poles, and posterior cingulate cortex.
Conclusions
These results strengthen the interpretation that paralimbic regions are central for understanding neural dysfunction associated with psychopathic traits and that psychopathy is best conceptualized as a neurodevelopmental disorder.
Keywords: paralimbic dysfunction, juvenile delinquency, voxel-based morphometry (VBM), psychopathy, antisocial
For most antisocial male adolescents, problematic behavior peaks in late adolescence or early adulthood and drops off rapidly thereafter, but some individuals remain on a life-course persistent trajectory of antisocial behavior throughout adulthood1. Some adults on the life-course persistent trajectory meet diagnostic criteria for psychopathy, a predictor of persistence in criminal activity and violent behavior2-4.
Psychopathy is a serious and enduring personality disorder marked by interpersonal, affective, and behavioral traits such as glibness, lack of moral emotions (e.g., remorse, empathy), irresponsibility, and impulsivity5. Clinical psychopathy is commonly assessed with the Hare Psychopathy Checklist– Revised (PCL-R)6, the most widely accepted diagnostic instrument for psychopathy in adults. The assessment of psychopathic traits in youth raises a number of important issues. In the DSM7, antisocial personality disorder (in adults) and conduct disorder (in youth) are most closely related to the construct of psychopathy; however, these diagnoses focus on more readily observable behavioral traits, rather than affective and interpersonal traits conceptualized to be at the core of the disorder5. Thus, others have emphasized the particular importance of callous and unemotional traits in conceptualizing psychopathy in adolescents8-9. Hare and colleagues constructed a modified version of the PCL-R, the Psychopathy Checklist–Youth Version (PCL-YV)10, designed for use with adolescents, identifying both interpersonal and affective traits (Factor 1) and lifestyle and antisocial traits (Factor 2). Although distinguishing psychopathic traits from normative adolescent development can be challenging11 and it is critical to avoid the assumption that adolescents with psychopathic traits are on a predetermined path of adult psychopathy12-13 or are untreatable14, the assessment of psychopathic traits in youth evidences reliability and construct validity11,15. Furthermore, male adolescents, like adults16, scoring high on these traits are quicker to reoffend, including violent offenses17-18.
The pervasive nature of emotional and behavioral symptoms of psychopathy suggests that a number of associated brain regions may contribute to the disorder. Among adults, converging evidence from electrophysiology19-20, functional neuroimaging21-22, and lesion studies23 implicates paralimbic cortex and limbic structures as dysfunctional in psychopathy24. These regions are linked based on cytoarchitectural similarities25, which suggests a partially shared neurodevelopmental trajectory. These regions include the temporal poles, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), orbitofrontal cortex (OFC), parahippocampal regions, amygdala, and hippocampus24.
The existing structural imaging literature supports the hypothesis that dysfunction in paralimbic and limbic regions is associated with psychopathy. Structural differences in the form of reduced regional gray matter volumes (GMV) are observed in adult men with psychopathic traits26-28. However, it is not known when these differences begin to appear and whether they are apparent in adolescence, a time when the brain is still undergoing significant development29 and psychopathic traits may begin to be observed11.
To date, no research has examined brain structure in incarcerated adolescents with psychopathic traits assessed by the PCL-YV. Some previous work has looked at structural differences in (primarily) male adolescents with psychopathic traits, using diverse assessment methods. Youths (M=16 years) with early onset conduct disorder (CD) had reduced temporal lobe volumes; prefrontal volumes were also reduced in these youths, but the difference did not reach statistical significance30. Reduced GMV in bilateral insula and left amygdala was reported in male adolescents (M=13 years) with CD compared to controls, but there were no significant differences in the ACC or OFC31. However, reduced GMV was reported in the temporal lobes bilaterally, left amygdala, left hippocampus, OFC, and ventromedial PFC, and greater GMV in the cerebellum, in male adolescents (M=14 years) with early onset CD compared to controls32. In contrast, one study of boys from the community (M=11 years) with callous and unemotional traits found increased GMV compared to controls in several regions, including the OFC, ACC, superior temporal gyrus, left hippocampus, insula, and PCC33.
These studies suggest that adolescents with early CD symptoms have aberrant paralimbic and limbic structure; however no studies have examined psychopathic traits assessed with the expert-rater based PCL-YV and thus may not be directly comparable to adult samples. Additionally, prior studies30-33 have relied on relatively small samples (Ns=20–48), drawn largely from community and outpatient populations; thus, these samples were likely only associated with low to moderate levels of psychopathic traits and had limited ability to control for potential confounds (e.g., substance abuse).
Here we begin to address these limitations by presenting results from a voxel-based morphometry (VBM) analyses on the relationship between brain structure and psychopathic traits in large sample of maximum-security incarcerated male adolescents. The aberrant structure and dysfunction observed in paralimbic cortex and limbic structures in adult psychopathy predicts that higher scores on psychopathic traits will be associated with reduced GMV in the parahippocampus, amygdala, hippocampus, temporal pole, ACC, PCC, and OFC.
Method
Participants
The data analyzed were drawn from the NIMH-funded SouthWest Advanced Neuroimaging Cohort, Youth sample (SWANC-Y), collected between June, 2007, and March, 2011, from ongoing research studies at a maximum-security youth detention facility in New Mexico. This research was approved by the University of New Mexico Health Sciences Center Human Research Review Committee and individuals volunteered to participate after providing written informed consent (if >=18 years of age) or after providing written informed assent and parent/guardian written informed consent (if <18 years of age). Participants were excluded from participation if they had a history of seizures, psychosis, traumatic brain injury, other major medical problems, or failed to show fluency in English at or above a grade four reading level. High-resolution structural magnetic resonance imaging (MRI) scans and PCL-YV scores were available from 218 male adolescents. Our final sample included N=191 individuals, after excluding n=18 for excessive motion or radiological findings and n=9 who were determined to meet the above exclusion criteria after scanning.
Participants were incarcerated for crimes that included murder, assault, rape, arson, weapons possession, burglary, fraud, drug possession/distribution, and criminal mischief (Table 1). Participants were predominantly Hispanic/Latino (56.6%), white/Caucasian (14.8%), or Native American (11.7%). From self-report, 89.0% of participants were right-handed, 9.4% left-handed, and 1.6% ambidextrous. Participants were paid a flat rate, yoked to the standard institutional hourly pay scale, for participation in the study.
Table 1.
Descriptive statistics for analyzed sample (N=191).
| Variable | Mean | SD | n |
|---|---|---|---|
| Age | 17.3 | 1.18 | 191 |
| IQ | 92.8 | 12.06 | 178 |
| Substance Dependence | 2.2 | 1.63 | 191 |
| Regular Substance Use | 5.5 | 2.70 | 184 |
| Criminal Convictions a | |||
| Total | 7.7 | 7.84 | 153 |
| Violent | 1.6 | 1.96 | 153 |
| Non-violent | 6.6 | 7.54 | 153 |
| Psychopathic Traits | |||
| Total Scores | 23.6 | 6.19 | 191 |
| Factor 1 ( interpersonal/affective) | 6.6 | 3.14 | 191 |
| Factor 2 (impulsive/antisocial) | 11.3 | 2.69 | 191 |
| KSADS Diagnoses, n (%) | Current | Past | Total |
| Anxiety disorders | 8 (4.2) | 8 (4.2) | 16 (8.4) |
| Depressive disorders | 6 (3.1) | 25 (13.1) | 31 (16.2) |
| Attention-Deficit/Hyperactivity Disorder (ADHD) |
5 (2.6) | 17 (8.9) | 22 (11.5) |
| Oppositional Defiant Disorder | 11 (5.7) | 46 (24.1) | 57 (29.8) |
| Conduct Disorder | 180 (94.3) | ||
| Childhood Onset | 96 (50.3) | ||
| Adolescent Onset | 84 (44.0) |
Note: From state records. KSADS = Kiddie Schedule for Affective Disorders and Schizophrenia.
Psychopathy
The PCL-YV10 assessment includes a review of institutional records and a semi-structured interview that reviews individuals’ school, family, work, and criminal histories, and their interpersonal and emotional skills. Individuals are scored on 20 items that measure personality traits and behaviors characteristic of psychopathy. Scores range from 0 to 40. For adults, the accepted diagnostic cutoff for psychopathy is 30 and above34. For comparison to adult samples, in addition to Total PCL-YV scores, we examined a two factor model of psychopathic traits6,35, with Factor 1 composed of interpersonal and affective traits and Factor 2 composed of lifestyle and antisocial traits.
This sample covered a wide range of PCL-YV scores, including a sufficient number of high scorers (PCL-YV>=30, n=35; Figure S1, available online) indicating a high level of psychopathic traits. The mean scores are comparable to those observed in adult male incarcerated populations6. Interviews were conducted by trained researchers and videotaped for reliability assessment (intra-class correlation coefficient (ICC 1,1)=.90 for PCL-YV Total scores; 12% of interviews (randomly selected) were double-rated).
Control Measures
Full-scale IQ (IQ) was estimated from the Vocabulary and Matrix Reasoning sub-tests of the Wechsler Adult Intelligence Scale36-37 for participants older than 16 years of age and from the Wechsler Intelligence Scale for Children–Fourth Edition38-39 for participants younger than 16 years of age (Table 1). The mean IQ estimate in this sample is consistent with other studies of juvenile offenders suggesting a negative relationship between delinquency and IQ40.
Trained researchers administered a post-head injury symptoms questionnaire41 to evaluate history of traumatic brain injury (TBI) and the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS)42. From the KSADS, we counted the total number of substances (alcohol and drug) for which an individual met the lifetime dependence diagnostic criteria (“substance dependence”). A modified version of the Addiction Severity Index43 was also administered. Following the method we used previously in adults28, years of regular use were summed for each substance (alcohol and drug) that the participant reported using regularly (3 or more times per week for a minimum period of one month), total scores were then divided by age (to control for opportunity to use), multiplied by 100, and a square root transformation was applied to correct for any skew (“regular substance use”; Table 1).
MRI Acquisition
High-resolution T1-weighted structural MRI scans were acquired using the Mind Research Network Mobile Siemens 1.5T Avanto MRI scanner, stationed at the detention facility. A multi-echo MPRAGE pulse sequence (repetition time=2530ms, echo times=1.64ms, 3.50ms, 5.36ms, 7.22ms, inversion time=1100ms, flip angle=7°, slice thickness=1.3mm, matrix size=256×256) was used, yielding 128 sagittal slices with an in-plane resolution of 1.0mm×1.0mm. Data were pre-processed and analyzed using Statistical Parametric Mapping software (SPM5). T1 images were manually inspected by an operator blind to subject identity and realigned to ensure proper spatial normalization. Images were spatially normalized to the SPM5 T1 Montreal Neurological Institute (MNI) template using non-linear registration, segmented into gray matter, white matter, and cerebrospinal fluid, and modulated to preserve total volume44-45. These segments were then averaged to create a study-specific template. Next, the original gray matter segments were normalized to the customized template. Finally, the images were resampled to 2×2×2mm and smoothed with a 10mm full-width at half-maximum (FWHM) Gaussian kernel. Voxels with a gray matter value of <.15 were excluded in order to remove possible edge effects between gray matter and white matter. Only gray matter segments were used in this analysis.
Analytic Strategy
As expected, Total PCL-YV scores were not significantly correlated with IQ, TBI, handedness, or total brain volumes, and the typical negative correlations between PCL-YV scores and age46 and substance use28 were observed (Table S1, available online). Psychopathy is frequently comorbid with substance use in adults47 and adolescents48 and substance use has been linked with GMV differences although the direction and duration of effects, and the role of related third variables (like psychopathy), is currently unsettled49-50. Thus, all analyses included the measure of substance dependence as a covariate. Age at scan was included as a covariate in all analyses, because of the developmental changes in brain structure over adolescence29. PCL-YV scores were used continuously. We also included brain volume (BV; white matter + gray matter) as an additional covariate in all analyses to account for individual variation in brain size51 and to focus on regionally-specific changes.
Whole brain analysis
Multiple regression analyses were performed on a voxel-by-voxel basis over the whole brain using the general linear model to evaluate the relationship between PCL-YV and regional GMV, including BV, age at scan, and substance dependence in the model as covariates. In multiple regression analyses evaluating the relationship between the psychopathy factors and regional GMV, both factors were included in the model simultaneously, in addition to the BV, age at scan, and substance dependence covariates.
Results from an independent adult sample28 indicated that GMV abnormalities are extensively distributed in paralimibic and limbic regions, but the effect size at each voxel is small. We utilized the two common methods in whole-brain analyses to assess for effects across voxels: (1) peak height, using a false discovery rate (FDR) correction for multiple comparisons (to test for focal peak effects); and (2) cluster size, estimating the cluster size necessary to correspond to a desired statistical threshold (to test for small, distributed effects).
Monte Carlo simulation conducted using AlphaSim52 determined that a 1643 voxel extent at height threshold of p<.05 uncorrected yielded a family-wise error rate (FWE) corrected threshold of p<.05, accounting for spatial correlations between GMVs in neighboring voxels. Peak height-based whole-brain analyses were thresholded at a FDR of p<.05.
Region of interest (ROI) analysis
In addition to the whole-brain analyses, we also tested our hypotheses in a priori regions of interest. Anatomical image masks based on ROIs (ACC, PCC, left and right parahippocampal gyrus, left and right amygdala, left and right hippocampus, left and right temporal pole, left, right, and medial OFC) identified in the adult literature were created using the Wake Forest University (WFU) Pick Atlas Toolbox in SPM5. For each region in each hemisphere, a small volume correction (SVC) was applied to the area within each mask; we report the FWE correction.
As an additional a priori analysis, we also examined the association between PCL-YV scores and brain structure at the anatomical ROI peak coordinates identified in an independent adult sample28 (N=254). We averaged the coordinates from these analyses (Table 1 in ref. 28); because the peaks associated with the amygdala, hippocampal, and parahippocampal anatomical masks were very close spatially, we averaged these peak coordinates to produce one set of coordinates for the right and for the left parahippocampal region. We then used these coordinates to conduct an SVC analysis, using 10mm diameter spheres, in our adolescent sample. All tables and figures are presented in MNI space.
Results
Were psychopathic traits associated with brain structure?
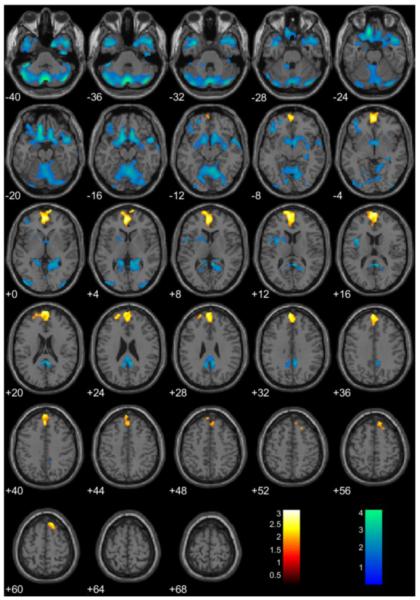
As found in adults28, cluster-extent analyses (1643 voxel threshold) across the whole brain showed that GMV in paralimbic regions was negatively associated with PCL-YV scores. Two clusters, in PCC and OFC (extending into the parahippocampal cortex and the temporal poles), were found. In addition, a significant cluster in prefrontal cortex was positively associated with PCL-YV Total scores (Figure 1). These results suggest a potentially large extent of structural abnormalities in psychopathy. In contrast, peak-height whole-brain analysis corrected for multiple comparisons (FDR p<.05) revealed no regions with GMV significantly negatively or positively associated with Total PCL-YV scores.
Figure 1.
Regional gray matter volumes (GMV) significantly associated with Total Psychopathy Checklist– Youth Version (PCL-YV) scores, controlling for brain volume (BV), age at scan, and substance dependence. Note: These regions are significant in the whole brain at p<.05, uncorrected for multiple comparisons, with an extent threshold of 1643 voxels, yielding a corrected threshold of p<.05, accounting for spatial correlations between GMVs in neighboring voxels. Coordinates are in Montreal Neurological Institute (MNI) space. The color bar represents t-values. GMV increases are in yellow/orange/red and decreases are in green/blue. Negatively associated clusters can be found in the orbitofrontal cortex (OFC), extending into parahippocampal cortex and the temporal poles, and in the posterior cingulated cortex (PCC). There is a positively associated cluster in the prefrontal cortex.
Anatomical ROI analyses with SVC identified several paralimbic regions with GMVs significantly negatively associated with PCL-YV Total scores: the FWE p<.05 threshold was met (Table 2) within the left OFC and medial OFC. This threshold was approached in the right and left amygdala, left temporal pole, and PCC.
Table 2.
Negative associations between Total Psychopathy Checklist–Youth Version (PCL-YV) scores and gray matter volumes (GMV) in anatomical regions of interest (ROI) using small volume correction (SVC).
| Gray Matter Volumes | ||||||
|---|---|---|---|---|---|---|
| MNI Coordinates | ||||||
| Paralimbic Region | H | x | y | z | t-value | FWE |
| Lateral OFC | L | -10 | 18 | −18 | 3.11 | .175 |
| R | 12 | 32 | −24 | 3.81 | .025 | |
| Medial OFC | — | −10 | 34 | −22 | 3.84 | .011 |
| ACC | — | 16 | 18 | 14 | 1.28 | .944 |
| Temporal Pole | L | -32 | 10 | −16 | 3.08 | .140 |
| R | 52 | 6 | −34 | 3.28 | .072 | |
| Parahippocampal Gyrus | L | -24 | 6 | −18 | 2.50 | .085 |
| R | 22 | 2 | −16 | 2.47 | .084 | |
| Amygdala | L | -12 | −38 | 2 | 2.62 | .194 |
| R | 24 | −40 | 8 | 2.46 | .256 | |
| Hippocampus | L | -16 | 10 | −32 | 2.67 | .204 |
| R | 30 | 2 | −18 | 1.83 | .593 | |
| PCC | — | 4 | −46 | 12 | 3.05 | .066 |
Note: Brain volume (BV), age at scan, and substance dependence were included in the model as covariates. Montreal Neurological Institute (MNI) x, y, and z coordinates, t-values, and family wise error (FWE) rate p-values are for the peak voxel in each region. Significant regions (p<.05) are indicated in bold and marginal regions (p<.10) are indicated in italics. ACC=anterior cingulate cortex; H=Hemisphere; L=Left; OFC=orbitofrontal cortex; PCC=posterior cingulate cortex; R=Right.
A priori SVC analyses on 10mm diameter spheres around peak coordinates from an independent adult sample28 showed that PCL-YV scores were significantly (FWE p<.05) negatively associated with GMV (Table 3) in the left temporal pole, left parahippocampal cortex, and PCC, and this threshold was approached for the right temporal pole, right parahippocampal cortex, and left and right lateral OFC.
Table 3.
Negative associations between Total Psychopathy Checklist–Youth Version (PCL-YV) scores and gray matter volumes (GMV) in regions of interest (ROI) using small volume correction (SVC; 10mm diameter spheres).
| Peak for Search (from adults) |
Peak within Volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | H | x | y | z | x | y | z | t-value | p (unc.) | FWE |
| Lateral OFC | L | −26 | 32 | −20 | −28 | 28 | −22 | 2.22 | .014 | .086 |
| R | 28 | 48 | −18 | 24 | 46 | −16 | 2.21 | .014 | .088 | |
| Medial OFC | — | 4 | 52 | −20 | 6 | 50 | −24 | 1.07 | .132 | .374 |
| ACC | — | −2 | 48 | 2 | 0 | 46 | −2 | −1.63 | .612 | .631 |
| Temporal Pole | L | −38 | 12 | −40 | −42 | 14 | −38 | 2.68 | .004 | .033 |
| R | 34 | 20 | −38 | 36 | 16 | −36 | 2.45 | .008 | .056 | |
| Parahippocampal | L | −32 | −2 | −28 | −34 | 2 | −30 | 276 | .003 | .027 |
| R | 34 | −8 | −24 | 32 | −6 | −20 | 0.99 | .150 | .069 | |
| PCC | — | −6 | −54 | 32 | −8 | −52 | 28 | 2.60 | .005 | .040 |
Note: Brain volume (BV), age at scan, and substance dependence were included in the model as covariates. Search coordinates based on an independent adult sample28 (N=254). Montreal Neurological Institute (MNI) x, y, and z coordinates, t-values, and p-values are for the peak voxel in each region. Significant regions (p<.05) are indicated in bold and marginal regions (p<.10) are indicated in italics. ACC=anterior cingulate cortex; FWE=family wise error rate; H=Hemisphere; L=Left; OFC=orbitofrontal cortex; PCC=posterior cingulate cortex; R=Right; unc. = uncorrected.
How much variance in PCL-YV scores does brain structure account for?
For each of the a priori anatomical ROIs, we identified the cluster peak negatively associated with Total PCL-YV scores, controlling for BV, substance dependence, and age, and extracted the regional GMV for each subject at these peaks. To reduce multicollinearity, we scaled each regional volume by the subject’s total GMV and summed values for neighboring regions (right amygdala, right hippocampus and right parahippocampal gyrus; left amygdala, left hippocampus and left parahippocampal gyrus; right, left, and medial OFC). These regions showed acceptable independence (all tolerance values >.42) and were entered as predictors in a multiple regression. As a group, these seven a priori regions (OFC, ACC, PCC, right parahippocampal cortex, left parahippocampal cortex, right temporal pole, and left temporal pole) accounted for 19.5% of the variance in Total PCL-YV scores, F(7,183)=6.32, p<.001, adjusted R2=16.4%.
As a more stringent analysis, we extracted the regional GMV at each of the peaks (Table 3) identified in the independent adult sample28, and conducted multiple regression to predict PCL-YV scores from these volumes. As above, to reduce multicollinearity, we scaled each regional volume by the subject’s total GMV and summed values for neighboring regions (right, left, and medial OFC). These regions showed acceptable independence (all tolerance values >.46) and were entered as predictors in a multiple regression. As a group, these seven regions (OFC, ACC, PCC, right parahippocampal cortex, left parahippocampal cortex, right temporal pole, and left temporal pole) accounted for 10.6% of the variance in Total PCL-YV scores, F(7,183)=3.11, p=.004, adjusted R2=7.2%.
Were the PCL-YV factors associated with brain structure in paralimbic regions?
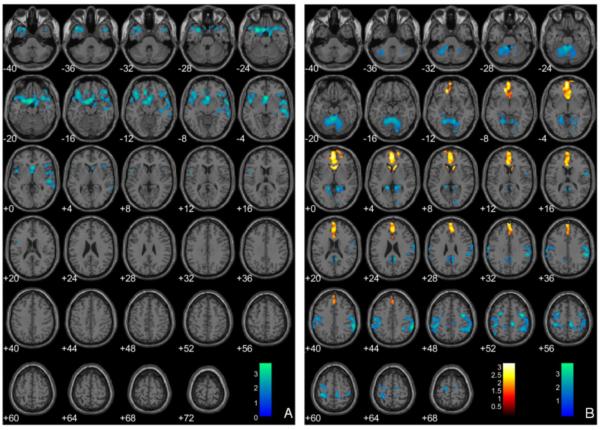
In multiple regression analyses evaluating the relationship between the PCL-YV factors and regional GMV, both factors were included in the model simultaneously, in addition to BV, substance dependence, and age covariates. Cluster extent threshold whole brain analysis (1643 voxels) showed that Factor 1 scores were negatively associated with GMV in one cluster centered in the OFC (Figure 2, Panel A). Factor 2 scores were negatively associated with GMV in clusters in the left and right inferior parietal lobule and the PCC, and positively associated with GMV in the mOFC and ACC (Figure 2, Panel B). In contrast, peak-height whole-brain analysis corrected for multiple comparisons (FDR p<.05) revealed no regions with GMV significantly negatively or positively associated with Factor 1 or Factor 2 scores.
Figure 2.
Regional gray matter volumes (GMV) negatively associated with the Hare Psychopathy Checklist–Youth Version (PCL-YV) Factor 1 scores (Panel A) and negatively (blue) and positively (red) associated with PCL-YV Factor 2 scores (Panel B), controlling for brain volume (BV), age at scan, and substance dependence. Note: These regions are significant in the whole brain at p<.05 and 1643-voxel extent. Numeric values indicate the Montreal Neurological Institute (MNI) z-coordinate of the slice, and the color bar represents t-values.
How robust are these results to model variation?
The models above used substance dependence as the measure of substance use. When regular use was used as the covariate instead, results for cluster extent analyses were substantively the same for negative associations with psychopathy scores (Figure S2; Tables S2–S3, available online). However, there was no evidence of a positive association between GMV in the medial prefrontal cortex (mPFC) and Total PCL-YV scores.
Discussion
Among adults, evidence from multiple methodologies has converged on a set of brain regions, in paralimbic cortex and limbic structures, as dysfunctional in adults with psychopathic traits. The present study adds to this literature by investigating structural abnormalities using VBM in a large sample of incarcerated male adolescents assessed for psychopathic traits using the PCL-YV. Cluster extent analysis showed that PCL-YV Total scores were associated with decreased GMV in the PCC and in the OFC, extending into the parahippocampal cortex, and the temporal poles. PCL-YV Total scores were associated with increased GMV in the medial PFC. In contrast, whole brain analyses focusing on peak height and correcting for multiple comparisons using an FDR p<.05 threshold revealed no regions with GMV significantly associated with psychopathic traits in adolescence, either negatively or positively. Given our sample size, population sampled, and methodology, this null result is unlikely to be due a lack of power. These findings suggest that structural abnormalities associated with psychopathic traits reflect distributed network(s) of subtle impairment, rather than focal lesions.
To our knowledge, this is the first structural MRI study of adolescents assessed using the PCL-YV. As such, the findings in the extant adolescent literature are not directly comparable53. However, recent research on structural MRI in incarcerated male adults, assessed using the PCL-R, demonstrates several commonalities. GMV decreases were found in the temporal poles, parahippocampal cortex, posterior cingulate, and lateral OFC in both adults and adolescents, when the same covariates were used28. In contrast to the present study in juveniles, however, no positive associations between psychopathic traits and GMVs were identified in adults. This difference may reflect the variable trajectory of brain development: male adolescents with psychopathic traits showed increased GMV in medial PFC, one of the last regions to reach developmental maturity. Notably, evidence from functional MRI suggests that medial PFC activity is abnormal in adolescents with psychopathic traits54. The differential associations of Factor 1 and Factor 2 traits on GMV in the OFC may account for this disparity between adolescents and adults. Analyses of the unique contributions of the factors (Figure 2) support the hypothesis that the positive association between GMV and PCL-YV Total scores is driven by Factor 2 traits. Some Factor 2 traits, such as impulsivity and need for stimulation, are elevated in adolescence generally and tend to decrease over the lifespan. Plausibly, this pattern is related to the maturation of the prefrontal cortex. The pattern of increased GMV in this region in adolescents with psychopathic traits may reflect a delayed trajectory of brain development55.
Unlike adults, adolescents with psychopathic traits also showed decreased GMV throughout much of the cerebellum. At present, there is limited knowledge about the precise role of the cerebellum in cognitive and emotional processes, although evidence increasingly suggests that the cerebellum may have a significant role in these areas56. Notably, the cerebellum has been implicated in other child-onset disorders such as ADHD57; however, psychopathic traits were unrelated to ADHD diagnosis in this sample (r[191]=.10, p=.17). In healthy adolescents, cerebellar volume follows an inverted-U shaped trajectory, peaking around age 15.5 in males55. The observed decreased GMV in the cerebellum in male adolescents with psychopathic traits is consistent with a delayed developmental trajectory, although other explanations remain possible.
Despite the fact that observed brain abnormalities were relatively subtle, these differences accounted for a substantial amount of variance in psychopathic traits in our sample. Together, volumes from anatomically-defined a priori paralimbic regions accounted for nearly 20% of the variance in Total PCL-YV scores. Volumes from the peak coordinates in an independent adult sample28 accounted for over 10% of the variance in Total PCL-YV scores.
Incarcerated populations in general show high comorbidities with substance problems, early life stress, and mental health problems. Thus, our results may be subject to other interpretations and it is difficult to tease out all possible moderating variables. We chose to pursue a broad sampling strategy to insure our sample was representative of incarcerated populations in general and individuals with psychopathic traits in particular. The negative associations between PCL-YV scores and GMV in paralimbic regions were robust to different specifications of substance use (Figure S2). Importantly, existing comorbidities may be risk factors for developing psychopathic traits and thus may reflect true group differences rather than introducing confounds. Nevertheless, future research comparing brain structure in typically developing youth and youth with other disorders would help clarify the specificity of these results.
VBM can be sensitive to methodological parameters (e.g., covariates), affected by atypical brains, and produce less precise localization compared to other methods44. We followed the same methodological choices in the present study as we did in our study of incarcerated adults28 (including use of the same MRI scanner), employed a study-specific template, compared brain structure across a large number of individuals, and found relatively widespread deficits across paralimbic regions. Future studies with alternative analytical techniques may identify additional abnormalities associated with psychopathic traits.
The finding that adolescents with psychopathic traits exhibit many of the same structural abnormalities as adults with psychopathy supports the hypothesis that this disorder is neurodevelopmental in nature. These results suggest that the brain abnormalities observed in adults with psychopathy are present as early as age 14. Furthermore, these results provide further support for the construct validity of psychopathic traits assessed in adolescence11 and the concept of psychopathy as a developmental disorder.
The details of the developmental process(es) of psychopathic traits are questions for future research, but one possibility is that genetic and/or environmental events perturb the normal developmental process, leading to decreased GMV in some areas and increased GMV in others. These structural abnormalities may then be directly or indirectly related to the functional deficits exhibited by both adults with psychopathic traits and adolescents with psychopathic traits.
Finally, it is unknown when the neurodevelopmental trajectory to psychopathy begins. Individuals in this study were in late adolescence, but one study with younger children also demonstrated brain abnormalities33; however, they reported increased GMVs among children with callous and unemotional traits in paralimbic and limbic regions. At this point, it is unclear whether differences between these results and the present study are to be accounted for by sample age (M=11.6 years vs. M=17.6 years), sample size (N=46 vs. N=191), population source (community vs. incarcerated), psychopathy assessment (other-report vs. expert-rater), or other factors. Future studies of younger adolescents and children that utilize the PCL-YV (or downward extension) would allow for more direct comparison with the findings from adults and older adolescents. Overall, our results strengthen the interpretation that paralimbic regions are central for understanding neural dysfunction associated with psychopathic traits and that psychopathy may best be conceptualized as a neurodevelopmental disorder.
Supplementary Material
Figure S1. Distribution of Psychopathy Checklist–Youth Version (PCL-YV) Total scores (n=191).
Figure S2. Regional gray matter volumes significantly associated with Total Psychopathy Checklist–Youth Version (PCL-YV) scores, controlling for brain volume (BV), age at scan, and regular substance use. Note: These regions are significant in the whole brain at p<.05, uncorrected for multiple comparisons, with an extent threshold of 1,643 voxels, yielding a corrected threshold of p<.05, accounting for spatial correlations between gray matter volumes (GMVs) in neighboring voxels. Coordinates are in Montreal Neurological Institute (MNI) space. The color bar represents t-values.
Table S1. Zero-order correlations (and two-tailed p-values) among Psychopathy Checklist–Youth Version (PCL-YV) Total, Factor 1 scores, Factor 2 scores, and control variables.
Table S2. Negative associations between Total Psychopathy Checklist–Youth Version (PCL-YV) scores and gray matter volumes (GMV) in anatomical regions of interest (ROI) using small volume correction (SVC).
Table S3. Negative associations between Total Psychopathy Checklist–Youth Version (PCL-YV) scores and gray matter volumes (GMV) in regions of interest (ROI) using small volume correction (SVC; 10mm diameter spheres).
Acknowledgments
Disclosure: Dr. Calhoun has received research support from the National Institutes of Health (NIH), NIMH, the National Institute of Drug Abuse (NIDA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Center for Research Resources, the National Science Foundation, the Defense Advanced Research Projects Agency, and the Department of Energy. He has served as a legal consultant, performed grant reviews for NIH, guest-edited journal sections, given academic lectures in various scientific venues, and generated books or book chapters for publishers of various texts. Dr. Kiehl has received grant support from NIH, NIMH, NIDA, NIBIB, NIAAA, and the John D. and Catherine T. MacArthur Foundation. He has performed grant reviews for NIH and other agencies, given academic lectures in various scientific venues and universities, and served as a consultant on judicial education for the states of Arizona, California, Illinois, Missouri, New Hampshire, and Nevada.
This study was funded by the National Institute of Mental Health (NIMH) grant MH071896 (K.A.K., PI) and by NIMH National Research Service Award F32 MH086247 (E.E.).
The authors are grateful to the staff and clients (and parents) at the Youth Diagnostic and Detention Facility and the New Mexico Children, Youth and Families Department for their support and assistance in making this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental content cited in this article is available online.
This article is discussed in an editorial by Dr. Tonya J.H. White on page xxx.
Dr. Ermer, Ms. Cope, and Mr. Nyalakanti report no biomedical financial interests or potential conflicts of interest.
References
- 1.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior. Psychol Rev. 1993;100:674–701. [PubMed] [Google Scholar]
- 2.Hemphill JF, Hare RD, Wong S. Psychopathy and recidivism. Legal Criminological Psychol. 1998;3:139–170. [Google Scholar]
- 3.Singh JP, Grann M, Fazel S. A comparative study of violence risk assessment tools. Clin Psychol Rev. 2011;31:499–513. doi: 10.1016/j.cpr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Wong SCP, Coid J. The efficacy of violence prediction. Psychol Bull. 2010;136:740–767. doi: 10.1037/a0020473. [DOI] [PubMed] [Google Scholar]
- 5.Cleckley H. The mask of sanity. 5th ed Mosby; St. Louis, MO: 1976. [Google Scholar]
- 6.Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2nd ed Multi-Health Systems; Toronto: 2003. [Google Scholar]
- 7.Diagnostic and statistical manual of mental health disorders. 4th ed American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 8.Barry CT, Frick PJ, DeShazo TM, McCoy M, Ellis M, Loney BR. The importance of callous– unemotional traits for extending the concept of psychopathy to children. J Abnorm Psychol. 2000;109:335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- 9.Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. J Abnorm Child Psychol. 2003;31:457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- 10.Forth AE, Kosson DS, Hare RD. The psychopathy checklist: youth version. Multi-Health Systems; Toronto, ON, Canada: 2003. [Google Scholar]
- 11.Salekin RT, Rosenbaum J, Lee Z, Lester WS. Child and adolescent psychopathy. Youth Violence Juvenile Justice. 2009;7:239–255. [Google Scholar]
- 12.Salekin RT. Psychopathy and therapeutic pessimism. Clin Psychol Rev. 2002;22:79–112. doi: 10.1016/s0272-7358(01)00083-6. [DOI] [PubMed] [Google Scholar]
- 13.Seagrave D, Grisso T. Adolescent development and the measurement of juvenile psychopathy. Law Hum Behav. 2002;26:219–239. doi: 10.1023/a:1014696110850. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell M, Skeem J, Salekin R, van Rybroek G. Treatment response of adolescent offenders with psychopathy features. Crim Justice Behav. 2006;33:571–596. [Google Scholar]
- 15.Lynam DR, Miller DJ, Vachon D, Loeber R, Stouthamer-Loeber M. Psychopathy in adolescence predicts official reports of offending in adulthood. Youth Violence Juvenile Justice. 2009;7:189–207. doi: 10.1177/1541204009333797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice ME, Harris GT, Cormier CA. An evaluation of a maximum security therapeutic community for psychopaths and other mentally disordered offenders. Law Hum Behav. 1992;16:399–412. [Google Scholar]
- 17.Edens JF, Campbell JS, Weir JM. Youth psychopathy and criminal recidivism. Law Hum Behav. 2006;31:53–75. doi: 10.1007/s10979-006-9019-y. [DOI] [PubMed] [Google Scholar]
- 18.Gretton HM, Hare RD, Catchpole REH. Psychopathy and offending from adolescence to adulthood. J Counseling Clinical Psychol. 2004;72:636–645. doi: 10.1037/0022-006X.72.4.636. [DOI] [PubMed] [Google Scholar]
- 19.Kiehl KA, Hare RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Bio Psychiatry. 1999;45:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 20.Kiehl KA, Hare RD, McDonald JJ, Brink J. Semantic and affective processing in psychopaths. Psychophysiology. 1999;36:765–774. [PubMed] [Google Scholar]
- 21.Birbaumer N, Viet R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 22.Kiehl KA, Smith AM, Hare RD, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Bio Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- 23.Malloy P, Bihrle A, Duffy J, Cimino C. The orbitomedial frontal syndrome. Archives of Clinical Neuropsychology. 1993;8:185–201. [PubMed] [Google Scholar]
- 24.Kiehl KA. A cognitive neuroscience perspective on psychopathy. Psychiatric Res. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 26.Tiihonen J, Rossi R, Laakso MP, et al. Brain anatomy of persistent violent offenders. Psychiatry Res Neuroimaging. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Muller JL, Ganssbauer S, Sommer M, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Psychiatry Res Neuroimaging. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 30.Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 32.Huebner T, Vloet TD, Marx I, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:540–7. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 33.De Brito SA, Mechelli A, Wilke M, et al. Size matters. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 34.Hare RD. Manual for the Hare Psychopathy Checklist. Multi-Health Systems; Toronto: 1991. [Google Scholar]
- 35.Harpur TJ, Hare RD, Hakstian AR. Two-factor conceptualization of psychopathy. Psychol Assessment. 1989;1:6–17. [Google Scholar]
- 36.Wechsler D. Wechsler Adult Intelligence Scale. Psychological Corporation; New York: 1997. [Google Scholar]
- 37.Ryan R, Lopez S, Werth T. Development and preliminary validation of a Satz-Mogel short form of the WAIS-III in a sample of persons with substance abuse disorders. Int J Neurosci. 1999;98:131–140. doi: 10.3109/00207459908994796. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. Wechlser Intelligence Scale for Children—Fourth Edition. Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 39.Sattler JM, Dumont R. Assessment of Children: WISC-IV and WPPSIIII Supplement. Jerome M. Sattler Publishing Company; San Diego, CA: 2004. [Google Scholar]
- 40.Lynam D, Moffitt T, Stouthamer-Loeber M. Explaining the relation between IQ and delinquency. J Abnorm Psychol. 1993;102:187–196. doi: 10.1037//0021-843x.102.2.187. [DOI] [PubMed] [Google Scholar]
- 41.King NS, Crawford S, Wenden FJ, Moss NEG, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children Present and Lifetime version (K-SADS-PL) J Am Acad Child Adolesc Psychiatry. 1995;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 43.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subs Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 44.Ashburner J, Friston KJ. Voxel-based morphometry. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 45.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Harpur TJ, Hare RD. Assessment of psychopathy as a function of age. J Abnorm Psychol. 1994;103:604–609. doi: 10.1037//0021-843x.103.4.604. [DOI] [PubMed] [Google Scholar]
- 47.Smith SS, Newman JP. Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. J Abnorm Psychol. 1990;99:430–439. doi: 10.1037//0021-843x.99.4.430. [DOI] [PubMed] [Google Scholar]
- 48.O’Neill ML, Lidz V, Heilbrun K. Adolescents with psychopathic characteristics in a substance abusing cohort. Law Hum Behav. 2003;27:299–313. doi: 10.1023/a:1023435924569. [DOI] [PubMed] [Google Scholar]
- 49.Tanabe J, Tregellas JR, Dalwani M, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Bio Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Bio Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 51.Pell GS, Briellmann RS, Chan CH, Pardoe H, Abbott DF, Jackson GD. Selection of the control group for VBM analysis. Neuroimage. 2008;41:1324–1335. doi: 10.1016/j.neuroimage.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 52.Ward DB. Simultaneous inference for fMRI data. Author; Milwaukee, WI: 2000. [Google Scholar]
- 53.Fink B, Tant A, Tremba K, Kiehl KA. Assessment of psychopathic traits in a youth forensic sample. J Abnorm Child Psychol. 2012;40:971–986. doi: 10.1007/s10802-012-9614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finger EC, Marsh AA, Mitchell DG, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–94. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shannon BJ, Raichle ME, Snyder AZ, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci USA. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiemeier J, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence. NeuroImage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siedman LJ, Valera EM, Markis N. Structural brain imaging of attention deficit/hyperactivity disorder. Bio Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of Psychopathy Checklist–Youth Version (PCL-YV) Total scores (n=191).
Figure S2. Regional gray matter volumes significantly associated with Total Psychopathy Checklist–Youth Version (PCL-YV) scores, controlling for brain volume (BV), age at scan, and regular substance use. Note: These regions are significant in the whole brain at p<.05, uncorrected for multiple comparisons, with an extent threshold of 1,643 voxels, yielding a corrected threshold of p<.05, accounting for spatial correlations between gray matter volumes (GMVs) in neighboring voxels. Coordinates are in Montreal Neurological Institute (MNI) space. The color bar represents t-values.
Table S1. Zero-order correlations (and two-tailed p-values) among Psychopathy Checklist–Youth Version (PCL-YV) Total, Factor 1 scores, Factor 2 scores, and control variables.
Table S2. Negative associations between Total Psychopathy Checklist–Youth Version (PCL-YV) scores and gray matter volumes (GMV) in anatomical regions of interest (ROI) using small volume correction (SVC).
Table S3. Negative associations between Total Psychopathy Checklist–Youth Version (PCL-YV) scores and gray matter volumes (GMV) in regions of interest (ROI) using small volume correction (SVC; 10mm diameter spheres).