Abstract
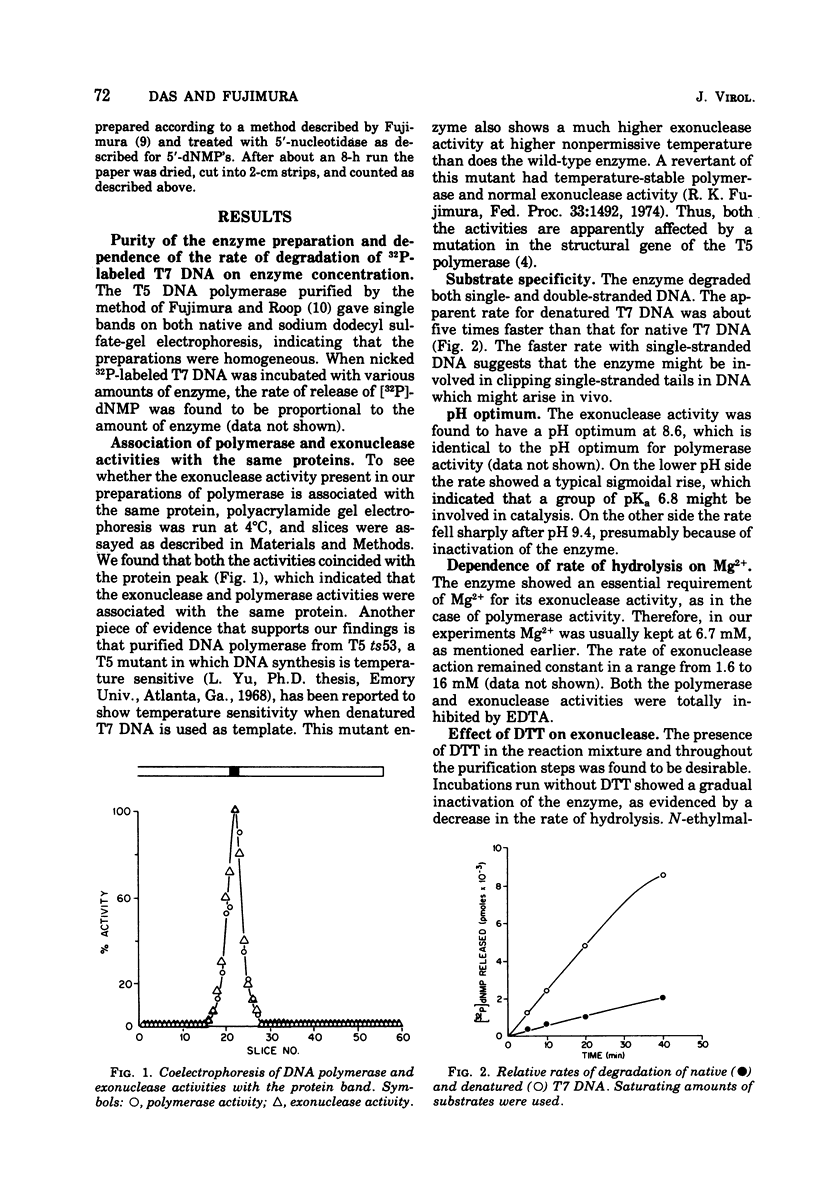
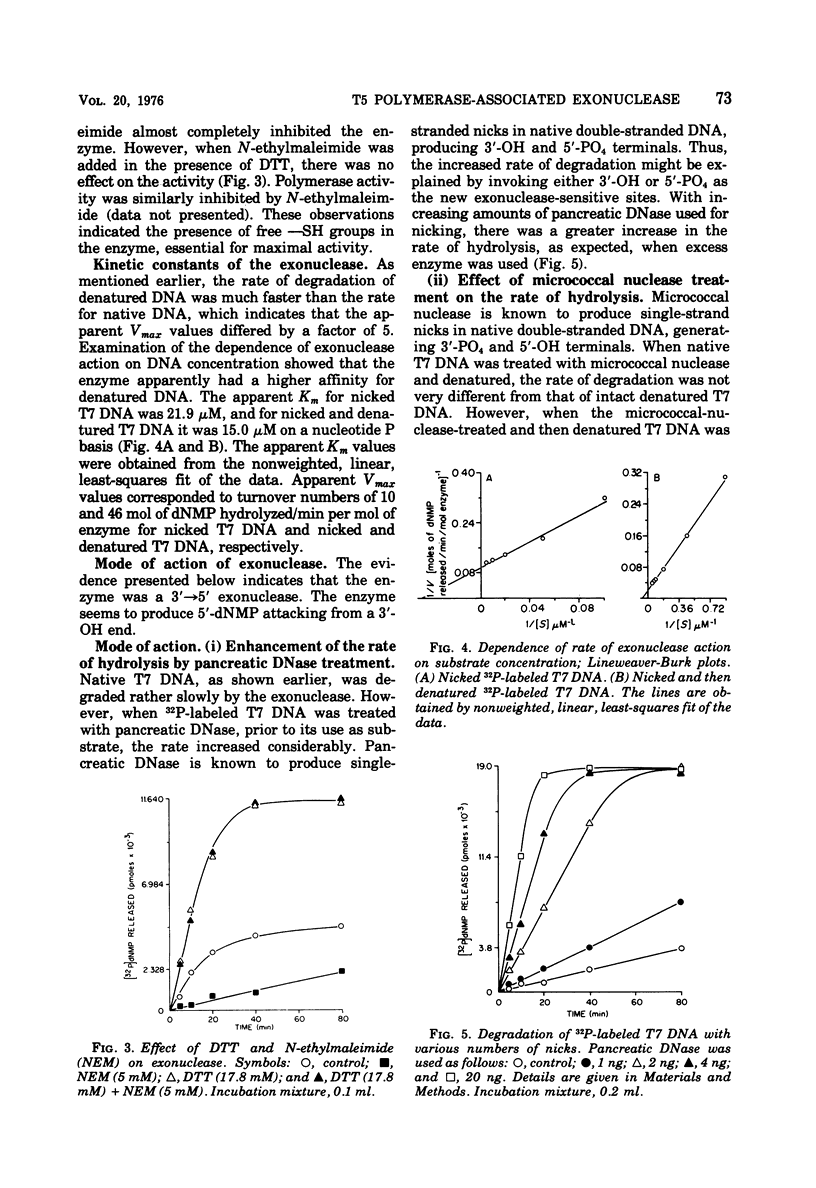
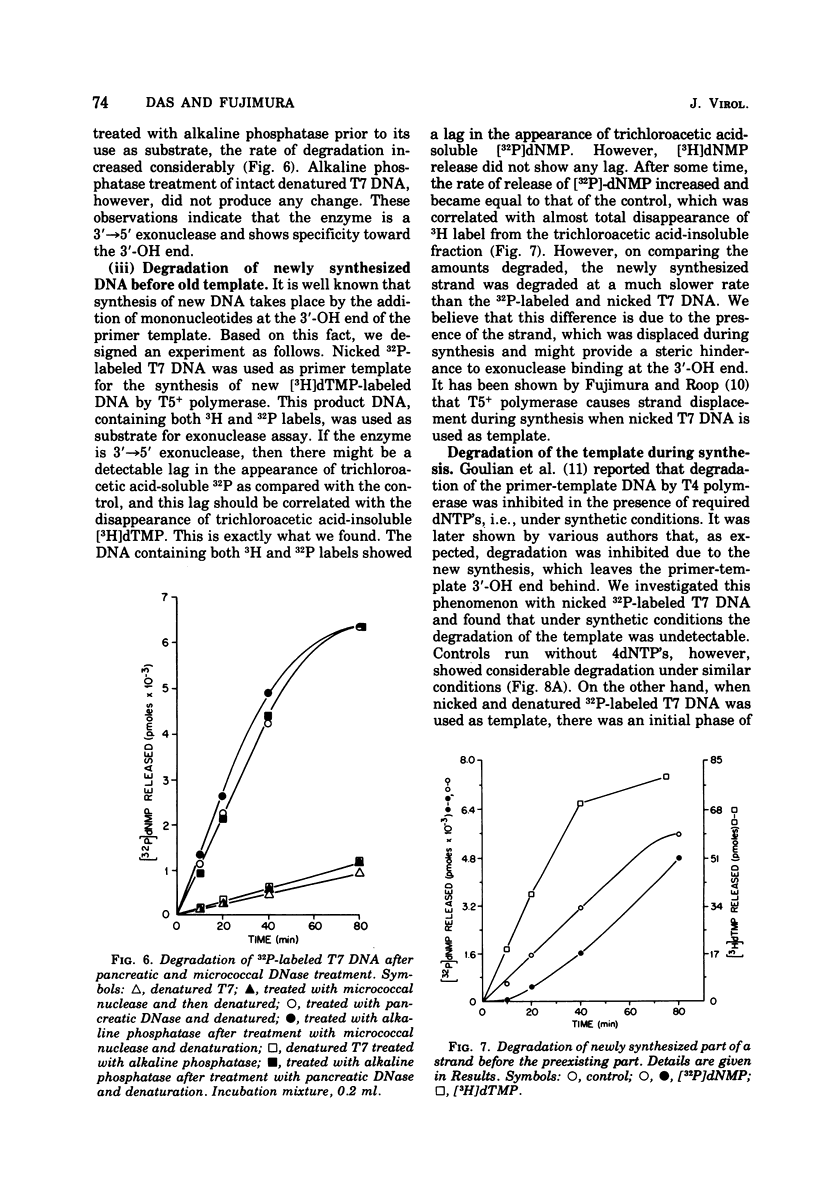
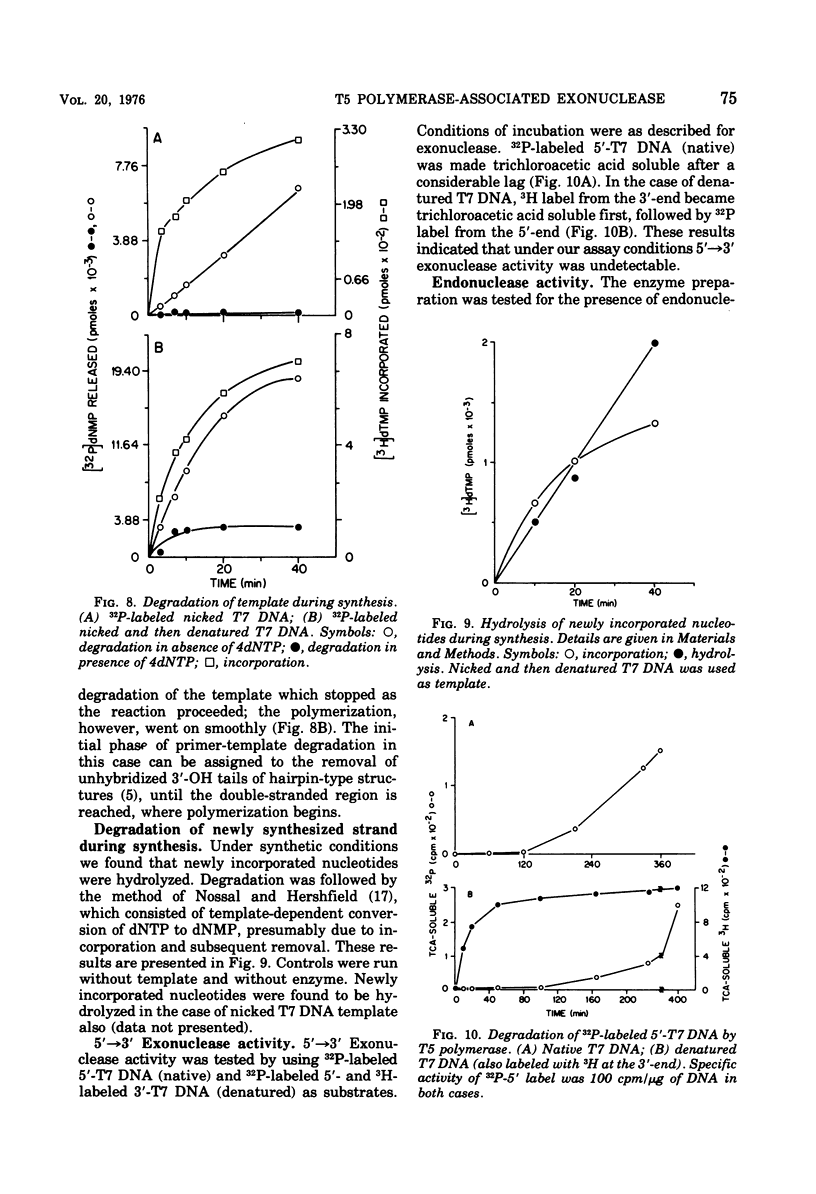
T-5-induced DNA polymerase has been shown to possess a 3' leads to 5'-exonucleolytic activity. The exonuclease acts on both native and denatured DNA, but the apparent rate of degradation of denatured DNA is about five times faster than that for native DNA. The enzyme appears to act only on 3'-OH ends and produces mainly 5'-dNMP's. Like polymerase activity, exonuclease activity shows a pH optimum around 8.6. Mg2+, dithiothreitol, and N-ethylmaleimide had identical effects on both the activities. Nicked DNA was almost totally protected from exonuclease action under synthetic conditions, i.e., in the presence of 4dNTP's. Denatured DNA was partly degraded in the early phase of incubation with 4dNTP's, presumably due to unhybridized tails at the 3'-OH primer ends. However, the exonuclease activity was operative in both cases under synthetic conditions, as evidenced by template-dependent conversion of [3H]dTTP to [3H]dTMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. Enzymic synthesis of DNA. 33. Hydrolysis of a 5'-triphosphate-terminated polynucleotide in the active center of DNA polymerase. J Mol Biol. 1969 Nov 14;45(3):513–531. doi: 10.1016/0022-2836(69)90309-x. [DOI] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. The initial step of in vitro synthesis of deoxyribonucleic acid by the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1971 Sep 25;246(18):5684–5687. [PubMed] [Google Scholar]
- Forgách T., Rosdy B., Szporny L. Paper chromatographic separation and determination of nucleoside phosphates in acidic rat tissue extracts. Acta Biochim Biophys Acad Sci Hung. 1971;6(1):9–21. [PubMed] [Google Scholar]
- Frenkel G. D., Richardson C. C. The deoxyribonuclease induced after infection of Escherichia coli by bacteriophage T5. I. Characterization of the enzyme as a 5'-exonuclease. J Biol Chem. 1971 Aug 10;246(15):4839–4847. [PubMed] [Google Scholar]
- Fujimura R. K. Biochemical analysis of the naturally repaired sections of bacteriophage T5 deoxyribonucleic acid. 3. Nucleotide analysis of deoxyribonucleic acid synthesized under nonpermissive conditions. Biochemistry. 1971 Nov 23;10(24):4386–4393. doi: 10.1021/bi00800a006. [DOI] [PubMed] [Google Scholar]
- Fujimura R. K. Nucleotide analysis of deoxyribonucleic acid containing deoxybromouridylic acid. Anal Biochem. 1970 Jul;36(1):62–71. doi: 10.1016/0003-2697(70)90331-3. [DOI] [PubMed] [Google Scholar]
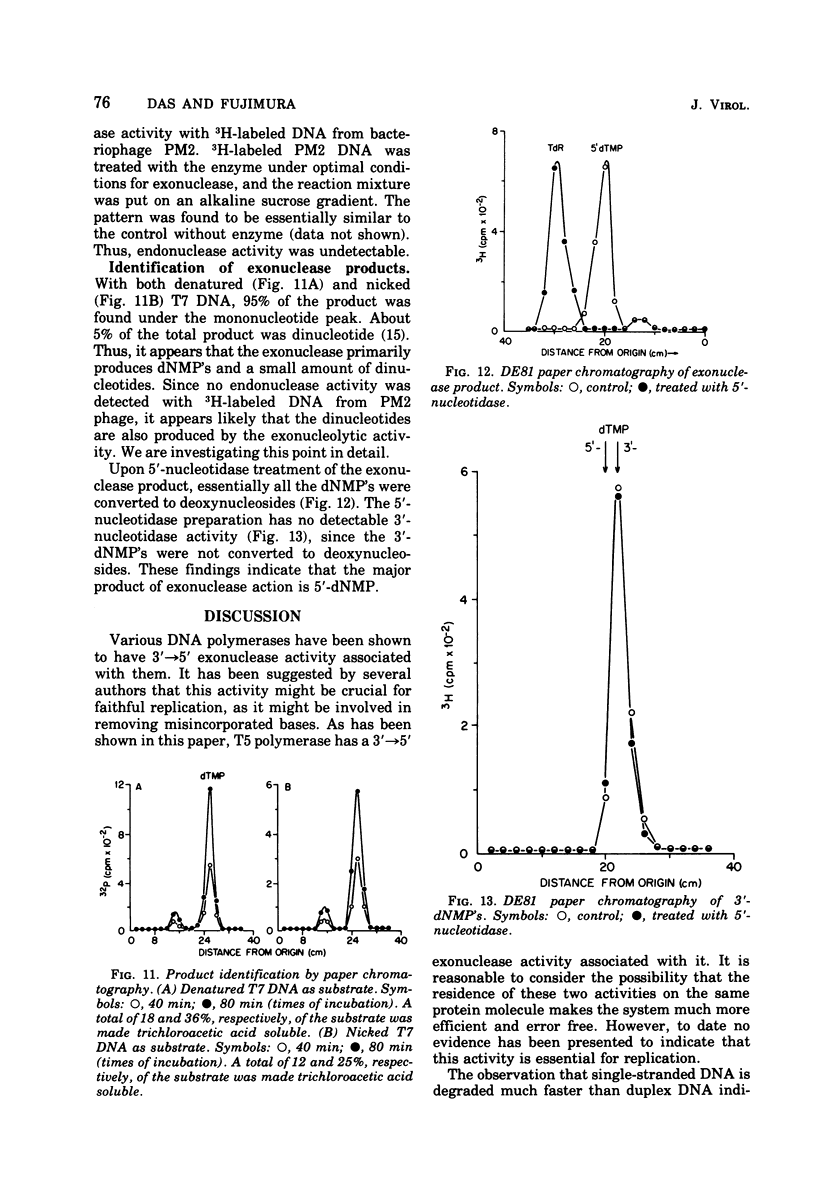
- Fujimura R. K., Roop B. C. Characterization of DNA polymerase induced by bacteriophage T5 with DNA containing single strand breaks. J Biol Chem. 1976 Apr 10;251(7):2168–2174. [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Kato T., Kondo S. Genetic and molecular characteristics of X-ray-sensitive mutants of Escherichia coli defective in repair synthesis. J Bacteriol. 1970 Nov;104(2):871–881. doi: 10.1128/jb.104.2.871-881.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., ROUSSOS G. G., PRATT E. A. The deoxyribonucleases of Escherichia coli. II. Purification and properties of a ribonucleic acid-inhibitable endonuclease. J Biol Chem. 1962 Mar;237:819–828. [PubMed] [Google Scholar]
- Nossal N. G. A T4 bacteriophage mutant which lacks deoxyribonucleic acid polymerase but retains the polymerase-associated nuclease. J Biol Chem. 1969 Jan 10;244(1):218–220. [PubMed] [Google Scholar]
- Nossal N. G., Hershfield M. S. Nuclease activity in a fragment of bacteriophage T4 deoxyribonucleic acid polymerase induced by the amber mutant am B22. J Biol Chem. 1971 Sep 10;246(17):5414–5426. [PubMed] [Google Scholar]
- Orr C. W., Herriott S. T., Bessman M. J. The enzymology of virus-infected bacteria. VII. A new deoxyribonucleic acid polymerase induced by bacteriophage T5. J Biol Chem. 1965 Dec;240(12):4652–4658. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuart C. D., Anand S. R., Bessman M. J. Studies on the synthesis of deoxyribonucleic acid. I. Further purification and properties of the deoxyribonucleic acid polymerase induced by infection of Escherichia coli with bacteriophage T5. J Biol Chem. 1968 Oct 25;243(20):5308–5318. [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Deoxyribonucleic acid synthesis in Escherichia coli infected with some deoxyribonucleic acid polymerase-less mutants of bacteriophage T4. Virology. 1966 Jan;28(1):100–107. doi: 10.1016/0042-6822(66)90310-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]
- Wu R., Padmanabhan R., Bambara R. Nucleotide sequence analysis of bacteriophage DNA. Methods Enzymol. 1974;29:231–253. doi: 10.1016/0076-6879(74)29025-6. [DOI] [PubMed] [Google Scholar]


