Abstract
Management of patients with metastatic hormone receptor-positive breast cancer poses a challenge due to the inevitable development of endocrine resistance. Hormone resistance is associated with a complex interaction of the estrogen receptor with growth factors, transmembrane receptors, and intracellular growth cascades. The PI3K/Akt/mTOR pathway plays a major role in hormone resistance and proliferation of breast cancer. Preclinical and clinical data indicate that inhibitors of human epidermal growth factor receptor-2, epidermal growth factor receptor, insulin-like growth factor-1 receptor, and the mammalian target of rapamycin pathway may act synergistically with hormone therapy to circumvent endocrine resistance. Everolimus is currently approved for combination with exemestane in postmenopausal women with advanced hormone receptor-positive breast cancer. However, we still need to unfold the full potential of targeted agents in the hormone-refractory setting and to identify the subsets of patients who will benefit from combination hormonal therapy using targeted agents.
Keywords: everolimus, estrogen receptor-positive breast cancer, hormone resistance, mammalian target of rapamycin, inhibition
Background
Breast cancer is the most common malignancy among women in the US, accounting for nearly one in three cancers diagnosed.1 It is estimated that 226,870 women will be diagnosed and 39,510 women will die of breast cancer in 2012.2 Approximately two-thirds of breast cancers are estrogen and/or progesterone receptor-positive. Hormone receptor status is determined using immunohistochemistry on paraffin-embedded tissues. The presence of at least 1% staining nuclei is required to define hormone-positive disease and predict clinical response to hormone-directed therapy.3
The natural history of hormone receptor-positive breast cancer tends to be different from hormone receptor-negative disease. The presence of hormone sensitivity is usually associated with a favorable prognosis. Use of adjuvant endocrine therapy has dramatically decreased breast cancer mortality in patients with early-stage disease, and hormone therapy is the cornerstone treatment in advanced stages. However, a subset of hormone receptor-positive breast cancers do not benefit from endocrine therapy (intrinsic resistance), and all hormone receptor-positive metastatic breast cancers ultimately develop resistance to hormonal therapies (acquired resistance). Most patients who have experienced treatment failure after several hormonal agents in the metastatic setting are treated with chemotherapy, which is associated with increased toxicity.4,5
This review focuses on new and emerging treatments for hormone receptor-positive breast cancer and particularly on the role of inhibition of mTOR (mammalian target of rapamycin) in reversing resistance to endocrine agents.
Endocrine therapy
Tamoxifen, a selective estrogen receptor modulator, had been the standard of care for all stages of hormone receptor-positive breast cancer since its initial approval by the US Food and Drug Administration in 1986.6 Aromatase inhibitors, which act by blocking the peripheral conversion of androgens to estrogen and therefore decrease levels of circulating estrogens in postmenopausal women, were approved for the treatment of metastatic breast cancer, and subsequently for early-stage cancer. The currently approved third-generation aromatase inhibitors are divided into steroidal (exemestane) and nonsteroidal (anastrozole and letrozole) agents.7
A study comparing anastrozole and tamoxifen in more than 1000 patients with advanced breast cancer showed that anastrozole was superior to tamoxifen in terms of time to progression, although there was no difference in overall survival.8,9 BIG 1-98 was a Phase III trial of letrozole versus tamoxifen in postmenopausal women with advanced breast cancer, which demonstrated that time to progression was increased from 6 to 9.4 months in the letrozole arm. The response rate and overall clinical benefit were also increased in the letrozole arm when compared with tamoxifen.10–12 Exemestane was also shown to be superior to tamoxifen in terms of clinical benefit in postmenopausal patients with breast cancer.13 The ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial demonstrated that aromatase inhibitors were superior to tamoxifen in the adjuvant setting. Aromatase inhibitors have thus become the preferred regimen in postmenopausal women.14,15
Fulvestrant, an estrogen receptor downregulator with no known agonist activity, was initially found to be equivalent to anastrozole in patients previously treated with tamoxifen.16 Fulvestrant was also compared with tamoxifen in the first-line setting in women with metastatic disease and was found to have similar efficacy in patients with hormone receptor-positive tumors.17 Fulvestrant was initially approved at a dose of 250 mg as a monthly intramuscular injection. Subsequent studies have examined the efficacy of different doses and schedules. CONFIRM (COmparisoN of Faslodex In Recurrent or Metastatic breast cancer) was a Phase III trial examining the difference in progression-free survival between the doses of 250 mg and 500 mg, and demonstrated that the higher dose improved the median progression-free survival, reducing the risk of progression by 20%.18
Combination of hormonal agents
Fulvestrant has been evaluated in combination with anastrozole in two trials with differing results. Mehta et al recently reported the results of a study combining anastrozole and fulvestrant in the metastatic setting.19 The authors hypothesized that the combination would be more effective than anastrozole alone in patients with hormone receptor-positive metastatic breast cancer. The trial randomized postmenopausal women with previously untreated metastatic disease to anastrozole alone or anastrozole plus fulvestrant. Fulvestrant was administered intramuscularly at a dose of 500 mg on day 1, 250 mg on days 14 and 28, and monthly thereafter. The median progression-free survival was 13.5 months in the anastrozole alone arm and 15.0 months in the combination arm (hazards ratio 0.80; P = 0.007). The combination therapy was generally more effective than anastrozole alone in all subgroups, with no significant interactions. Overall survival was also improved in the combination arm compared with anastrozole alone (median 47.7 versus 41.3 months, respectively). In this study, 41% of patients in the anastrozole arm crossed over to fulvestrant after progression. The study concluded that the combination of anastrozole and fulvestrant was more effective and better tolerated than anastrozole alone. It is notable that this study enrolled hormone-naïve patients who, judging from the outcomes seen in the anastrozole alone arm, included a large percentage of hormone-sensitive patients. The results of this study are in contrast with those of FACT (Fulvestrant and Anastrozole in Combination Trial), an open-label, randomized Phase III investigation of fulvestrant plus anastrozole versus anastrozole alone as first-line treatment for patients with receptor-positive postmenopausal breast cancer.20 This trial reported no significant differences in time to progression or median overall survival between the two groups. The different results reported in these two studies may be attributed to the size and choice of patient population. Combination of hormonal therapies may warrant further investigation, but it does not address the issue of hormone resistance, which eventually develops in all patients.
Mechanisms of resistance to endocrine therapy
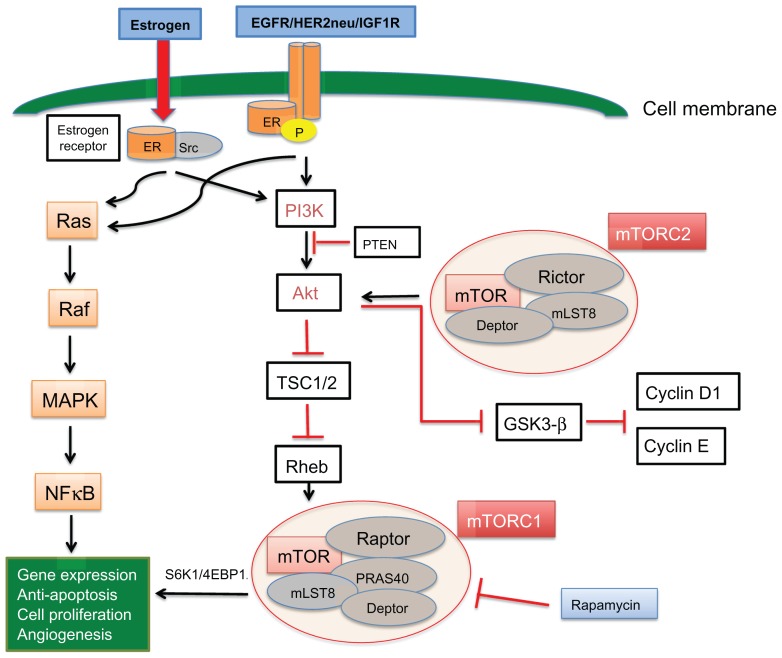
Estrogen receptor activation leads to phosphorylation, dimerization, and downstream signaling through estrogen response elements which promote cell survival, division, and growth of cancer.21,22 Clinical and preclinical data indicate that hormone receptors interact with growth factor receptors, including human epidermal growth factor receptor (HER2/neu), epidermal growth factor receptor (EGFR), and insulin-like growth factor-1 receptor (IGF1R), which likely play a role in hormone resistance.23,24 Crosstalk between the estrogen receptor and membrane tyrosine kinase receptors (EGFR, HER2, and IGF1R) can lead to gene expression and cell growth independent of hormonal activation, mainly via activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways. The estrogen receptor can also be regulated by these membrane receptors, which act as coactivators and lead to estrogen receptor phosphorylation in the absence of estrogen (ligand-independent receptor activation, Figure 1). The interaction of the estrogen receptor with growth factor receptors is complex. It is believed that the estrogen receptor can activate membrane growth factors via expression of transforming growth factor-alpha and IGF1. However at the same time, it downregulates EGFR and HER2 while inducing IGF1R. In turn, activation of MAPK and PI3K pathways by growth factor receptors downregulates estrogen receptor signaling.25
Figure 1.
Crosstalk between the estrogen receptor and EGFR/HER2/IGF1R membrane tyrosine kinase receptors can lead to gene expression and cell growth independent of hormonal activation, mainly via activation of the MAPK and PI3K pathways.
Notes: The estrogen receptor can also be regulated by these membrane receptors, which act as coactivators and lead to phosphorylation of estrogen receptors in the absence of estrogen (ligand-independent receptor activation). The PI3K/Akt/mTOR pathway is a major downstream cellular circuit, which leads to cell proliferation via the mTORC1 complex. The mTORC2 complex activates Akt, which in turn inhibits the proteolysis of cyclin D1/E.
Abbreviations: EGFR, epidermal growth factor receptor; IGF1R, insulin-like growth factor-1 receptor; mTOR, mammalian target of rapamycin; HER2, human epidermal growth factor receptor-2; ER, estrogen receptor; TSC1/2, tuberous sclerosis complex proteins 1/2; PI3K, phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein kinase; Src, steroid receptor coactivator.
In summary, it appears that membrane growth factor receptors can phosphorylate and activate the estrogen receptor independently of estrogen and they can activate downstream pathways and induce cell growth independently of estrogen receptor activation, but can also downregulate estrogen receptor expression, leading to hormone independence.
HER2/EGFR
Breast cancers with high levels of HER2 expression are more likely to be resistant to hormonal therapy. Transfection of HER2 in estrogen receptor-positive breast cancer cells renders them resistant to tamoxifen.26,27 Further, it has been shown that selective estrogen receptor modulator-resistant breast cancer cells have increased expression of HER2 compared with selective estrogen receptor modulator-sensitive breast cancer cells.28,29 A meta-analysis by De Laurentiis et al reported that HER2-positive patients with metastatic receptor-positive breast cancer treated with tamoxifen had a shorter time to treatment failure when compared with patients having HER2-negative disease.30 These findings suggest that HER2 plays a significant role both in intrinsic and acquired hormone resistance. Preclinical evidence supports that crosstalk between HER2 and the estrogen receptor leads to tamoxifen resistance, and disruption of this crosstalk can restore tamoxifen sensitivity.31,32
A randomized Phase III, double-blind, multicenter study by Johnston et al enrolled 1286 postmenopausal patients with advanced or metastatic estrogen receptor-positive and/or progesterone receptor-positive breast cancer. No prior treatment was allowed, except for neoadjuvant/adjuvant hormonal or anti-HER2 therapy. Patients were randomly assigned to receive letrozole and lapatinib or letrozole and placebo. Median progression-free survival was significantly improved in patients with HER2-positive disease who received lapatinib plus letrozole, compared with letrozole alone (3 months in the placebo arm and 8.2 months in the combination arm, hazards ratio 0.71, P = 0.019). Among the HER2-negative patients, there was no significant improvement in progression-free survival or clinical benefit. However, a subset of patients with HER2-negative disease and estrogen receptor expression in the lowest quartile appeared to benefit from adding lapatinib to letrozole (progression-free survival 13.6 versus 6.6 months, hazards ratio 0.65, P < 0.005).33,34
Similarly, expression of EGFR in vivo and in vitro has been shown to be associated with endocrine resistance, and in preclinical models EGFR inhibition can restore sensitivity to hormone treatment.35–38 A Phase II, randomized, double-blind, placebo-controlled study by Cristofanilli et al evaluated the combination of anastrozole and gefitinib (a selective EGFR tyrosine kinase inhibitor) versus anastrozole and placebo in postmenopausal patients with hormone receptor-positive metastatic breast cancer. The study population consisted of patients who had not received prior endocrine therapy for this stage or had developed metastatic disease during/after adjuvant tamoxifen. Although the study was closed early due to slow accrual, the combination arm showed improvement in progression-free survival versus placebo (median progression-free survival 14.7 versus 8.4 months, respectively). The treatment was tolerated very well.39 Osborne et al reported a randomized Phase II trial of tamoxifen with or without gefitinib in patients with metastatic disease who had experienced treatment failure while on tamoxifen or aromatase inhibitors. The combination arm showed improved progression-free survival in patients who had relapsed after adjuvant tamoxifen. However, no clinical benefit was seen in patients who had been previously treated with aromatase inhibitors.40
IGF1R
The IGF1R pathway plays a significant role in tumor growth and inhibition of apoptosis. IGF1R can activate the estrogen receptor pathway in the absence of estrogen and thus lead to tumor growth. It appears that there is crosstalk between IGF1R and the estrogen receptor, which possibly contributes to hormone resistance. In vivo and in vitro models show that IGF1R inhibition can act synergistically with hormone therapy.41–44 However, a clinical study of AMG 479 (a human anti-IGF1R monoclonal antibody) in combination with exemestane or fulvestrant in postmenopausal women failed to show a clinical benefit or difference in progression-free survival with IGF1R inhibition.45
Steroid receptor coactivator
The steroid receptor coactivator (Src) is a nonreceptor tyrosine kinase, which plays an essential role in the life cycle of the cell.46 Breast cancer tissue has higher expression of Src than normal breast tissue. In hormone receptor-positive breast cancer cells, Src binds and phosphorylates the estrogen receptor and activates downstream signaling pathways (Figure 1). Src is thought to play a pivotal yet complex role in endocrine resistance. Elevated levels of cytoplasmic Src have been linked with an attenuated response to hormone therapy in vitro, and high expression of Src has been associated with increased metastatic potential and poor survival in the clinical setting.47,48 Preclinical studies indicate that treatment of resistant cells with Src inhibitors restores sensitivity to tamoxifen.49 However, a Phase II study of dasatinib (an oral multi-BCR/ABL and Src family tyrosine kinase inhibitor) as a single agent showed very limited activity in women with advanced HER2-positive or estrogen receptor-positive metastatic breast cancer, probably due to the complexity of the cellular circuits which ultimately lead to hormone resistance.50
PI3K, Akt, and mTOR
The PI3K/Akt/mTOR pathway is a major intracellular cascade, which can be regulated by nutrient availability and growth factor receptors, including EGFR, HER2, IGF1R, and the estrogen receptor. When activated, this pathway induces tumor growth, proliferation, and resistance to targeted agents and chemotherapy.51,52 The PI3K/Akt pathway can activate both estrogen-dependent and estrogen-independent estrogen receptor alpha.53
In this pathway, a central role is played by PI3K heterodimer, which consists of a p85 regulatory and p110 catalytic subunit. Activation of PI3K will phosphorylate Akt. Akt is a serine/threonine kinase, which activates major downstream intracellular effectors. Akt can directly activate the estrogen receptor by phosphorylation in the absence of estrogen, thus promoting estrogen-independent growth and resistance to hormone therapy.54–58 The PI3K/Akt pathway is often aberrantly regulated in cancer, and the PIK3CA mutation is the most common point mutation seen in breast cancer.59 Akt can be also activated by loss of PTEN, a mechanism that has been associated with a poor prognosis and increased risk of relapse after treatment with tamoxifen.60
PI3K/Akt mutations, loss of PTEN, and constitutive activation of the PI3K/Akt pathway have been associated with hormone resistance. Activation of the PI3K pathway has been associated with intrinsic and acquired hormone resistance, and preclinical data indicate that PI3K inhibitors are active when combined with endocrine therapy.56,61 Multiple clinical studies are currently evaluating PI3K inhibitors in hormone receptor-positive tumors.
Downstream of PI3K and Akt, mTOR is a serine/threonine protein kinase, which is activated by inhibition of the tuberous sclerosis complex proteins 1/2.62 mTOR exerts its effects via two very different protein complexes. The mTORC1 complex includes the regulatory-associated protein of mTOR (Raptor), mLST8, and proline-rich Akt substrate 40.63 It is irreversibly inhibited by rapamycin and exerts its action by activating S6K1 (40S ribosomal protein S6 kinase 1) and eukaryotic initiation factor 4E-binding protein, thus leading to protein production, cell activation, division, and tumor growth.64,65 mTORC2 has been traditionally thought to be insensitive to rapamycin, but there is evidence that prolonged exposure to rapamycin can induce sufficient inhibition of mTORC2.66 Although its role in the cell cycle remains largely unknown, mTORC2 is believed to modulate cell lipid metabolism and cell growth via Akt, by inhibition of glycogen synthase kinase-3β and cyclin D1/E proteolysis.63 Studies suggest that targeted inhibition of TORC2 inhibits breast cancer cells in vitro and in vivo.67
Several preclinical studies provide evidence that mTOR inhibition can restore hormone sensitivity and induce apoptosis in breast cancer cells. The mTOR inhibitor, rapamycin, can reverse resistance to endocrine therapy when combined with tamoxifen or fulvestrant.68,69 Interestingly, restoration of sensitivity to endocrine therapy can be associated with increased estrogen receptor-α protein expression levels and alteration of the phospho-ser167 estrogen receptor-α to total estrogen receptor-α ratio.69 Treatment of letrozole-resistant or fulvestrant-resistant breast cancer cells with low concentrations of the mTOR inhibitor, everolimus, reverses Akt-mediated resistance and restores responsiveness to antiestrogen treatment.70 When combined with letrozole, everolimus acts synergistically to promote cell cycle arrest and induce apoptosis.71 In summary, these preclinical data strongly support that mTOR inhibition could play a significant role in the treatment of hormone receptor-positive breast cancer, especially in resistant tumors.
Clinical studies with mTOR inhibitors
Rapamycin (sirolimus) was the first identified mTOR inhibitor, and was initially used as an immunosuppressant to prevent organ transplant rejection. The novel inhibitors, everolimus, temsirolimus, and ridaforolimus, are rapamycin analogs with improved pharmacological properties.
Temsirolimus
In a randomized, Phase II three-arm study of temsirolimus in combination with letrozole in postmenopausal women with hormone receptor-positive metastatic breast cancer, combination treatment with an intermittent schedule of temsirolimus was tolerable and showed clinical activity, with preliminary results indicating improvement in progression-free survival.72,73 A subsequent Phase III study by Chow et al who enrolled patients with metastatic breast cancer randomly assigned patients to letrozole or combination of letrozole with temsirolimus.74 The study had to be closed prematurely because there was no clinical benefit from the combination. An unplanned subset analysis suggested that patients who had been previously treated with chemotherapy might benefit from addition of the mTOR inhibitor to hormonal therapy.75 It is possible that this study failed to reach its endpoint due to suboptimal dosing and inappropriate selection of the study population.
Everolimus
BOLERO-2 (Breast Cancer Trials of Oral Everolimus) is a randomized Phase III investigation by Baselga et al which evaluated a combination of everolimus with the steroidal aromatase inhibitor, exemestane, in postmenopausal patients with advanced estrogen receptor-positive breast cancer who had recurrence or progression while receiving a nonsteroidal aromatase inhibitor.76 In total, 724 patients were randomized to receive exemestane 25 mg daily plus everolimus 10 mg daily or exemestane 25 mg daily plus placebo. At the interim analysis, with a median follow-up of 12.5 months, the patients treated with everolimus had a significant improvement in progression-free survival compared with the placebo arm (by local assessment, 6.9 months versus 2.8 months respectively, hazards ratio 0.43, P < 0.001; by central assessment 10.6 versus 4.1 months respectively, hazards ratio 0.36, P < 0.001). More serious adverse events were reported in the combination group, and a higher percentage of patients discontinued everolimus (19% versus 4%). The most common grade 3 or 4 adverse events were stomatitis, anemia, dyspnea, hyperglycemia, fatigue, and pneumonitis.
TAMRAD (tamoxifen and RAD001) was an open-label Phase II study that randomized patients to tamoxifen alone or tamoxifen in combination with everolimus 10 mg daily.77 The clinical benefit rate, which was the primary endpoint, was significantly improved in patients receiving tamoxifen plus everolimus versus tamoxifen alone (61% versus 42%, respectively, P = 0.045). Time to progression was also significantly improved in patients treated with tamoxifen and everolimus compared with tamoxifen alone (8.6 versus 4.5 months, respectively). Preliminary analysis demonstrated that the risk of death was also reduced by 55% with everolimus. Patients with secondary resistance seemed to benefit more from the addition of everolimus to tamoxifen than patients with primary resistance. The main toxicities seen in the everolimus arm were fatigue, stomatitis, rash, anorexia, and diarrhea.
Baselga et al reported a neoadjuvant study of everolimus plus letrozole versus placebo plus letrozole in estrogen receptor-positive disease.78 Two hundred and seventy postmenopausal women with operable estrogen receptor-positive breast cancer were randomly assigned to receive 4 months of neoadjuvant treatment with letrozole 2.5 mg/day and either everolimus 10 mg/day or placebo. The primary endpoint was clinical response by palpation. The response rate was higher in the everolimus arm (68% versus 59%). Biopsies were obtained at baseline and after 2 weeks of treatment. Progesterone receptor and cyclin D1 expression were decreased in both treatment arms, but phospho-S6 was downregulated significantly in the everolimus arm. Ki67 expression also decreased more dramatically in the everolimus arm compared with placebo. This study showed that everolimus significantly increased the efficacy of letrozole in the neoadjuvant setting.
These clinical studies (Table 1) continue to support that everolimus has synergistic anticancer activity and improves outcomes when combined with hormonal therapy in hormone receptor-positive breast cancer.
Table 1.
Phase II and III trials of everolimus in patients with hormone receptor-positive, HER2-negative breast cancer
| Trial | Phase | Patients (n) | Study treatment | Primary endpoint | Outcomes |
|---|---|---|---|---|---|
| BOLERO-2 Baselga et al76 | III | 724 | Exemestane 25 mg/day + everolimus 10 mg/day versus exemestane 25 mg/day + placebo | Progression-free survival | Median PFS 10.6 months versus 4.1 months, P < 0.001 |
| TAMRAD Bachelot et al77 | II | 111 | Tamoxifen 20 mg/day + everolimus 10 mg/day versus tamoxifen 20 mg/day | Clinical benefit rate | CBR 61% versus 42%, P = 0.046 |
| Neoadjuvant Baselga et al78 | II | 270 | Everolimus 10 mg/day + letrozole 2.5 mg/day versus placebo + letrozole 2.5 mg/day | Clinical response by palpation | CR 68.1% versus 59.1%, P = 0.062 |
Abbreviations: CR, complete response; CBR, clinical benefit rate; BOLERO-2, Breast Cancer Trials of Oral Everolimus; TAMRAD, tamoxifen and RAD001; PFS, progression-free survival.
Sirolimus
Bhattacharyya et al have reported a Phase I/II trial that evaluated the combination of tamoxifen and sirolimus 2 mg daily in hormone receptor-positive and HER2-negative breast cancer.79 The study was divided into two groups and included a total of 400 patients. The first group included patients who could not afford aromatase inhibitors and thus were hormone-naïve. The second group included patients who had experienced treatment failure on aromatase inhibitors or tamoxifen. Response rates and time to progression were the primary endpoints. The Phase II study showed a response rate of 4% versus 40% and time to progression of 3 versus 11 months in the tamoxifen alone versus the tamoxifen plus sirolimus arm, respectively. The patients who had progression of disease within 6 months had less benefit (2.2 versus 7.4 months), and both hormone-naïve and hormone-resistant patients seemed to benefit from the combination therapy. This study concluded that the combination of sirolimus and tamoxifen was effective and well tolerated.80
Resistance to mTOR inhibitors
Drug resistance is a potential challenge that may arise with the use of mTOR inhibitors, and can be mediated by dysregulation of p27, feedback activation of PI3K/Akt by S6K, activation of the ERK, PIM, and PDK1 pathways, alterations in protein synthesis, and increased bcl-2.81,82 Resistance can potentially be overcome by a combination of agents that target the mTOR pathway and PI3K/Akt, EGFR, or mTORC2 inhibitors. There are ongoing Phase I and II studies which are investigating the combination of these agents.83 BELLE-3 (clinicaltrials.gov, NCT01633060) is an ongoing Phase III study evaluating the combination of BKM120 and fulvestrant in previously treated, hormone receptor-positive, HER2-negative patients who have progressed on or after an mTOR inhibitor. BKM120 is an oral pan-class PI3K inhibitor, which can potentially overcome mTOR resistance by targeting upstream PI3K signaling.61
Conclusion and future directions
The development of resistance to hormonal agents represents a significant challenge in the management of advanced hormone receptor-positive breast cancer. Several cellular pathways have been investigated as potential targets in an effort to bypass the estrogen receptor and block tumor growth. Preclinical data suggest that there is crosstalk between the estrogen receptor and membrane growth factors, which can stimulate cell growth independent of hormonal activation. HER2, EGFR and mTOR inhibitors appear to have activity and act synergistically with hormonal therapy. On the other hand, a recent review reports that it may be possible to identify a subset of patients who are HER2-positive and estrogen receptor-positive who will benefit from the combination of HER2 and hormone inhibition, while patients with low hormone expression may not benefit from hormonal therapy and should be treated with chemotherapy and HER2-directed therapy instead.84
Recent studies have shown that everolimus is active in combination with hormonal therapy in the metastatic and neoadjuvant setting, with an acceptable side effect profile. Based on the progression-free survival data in the control arm of the BOLERO-2 trial, as well as the data from the TAMRAD trial, it is possible that mTOR inhibitors may be more effective in tumors that have developed secondary resistance to endocrine agents.
It is unclear why the temsirolimus trials did not produce the clinical benefit that was seen with everolimus, but it may be attributed to the selection of hormone-naïve patients.
Ridaforolimus is another rapamycin analog, which is currently being studied in multiple tumors. In hormone receptor-positive and HER2-negative breast cancer, ridaforolimus is currently being evaluated in combination with dalotozumab (an IGF1R inhibitor) in one study that has completed recruitment (clinicaltrials.gov, NCT01234857). Another study, which is comparing the combination of ridaforolimus plus dalotozumab plus exemestane versus ridaforolimus plus exemestane, is currently recruiting patients (clinicaltrials.gov, NCT01605396). Another study which is currently recruiting patients is comparing trastuzumab or everolimus in combination with endocrine therapy in patients with hormone-refractory, HER2-negative metastatic breast cancer (clinical trials.gov, NCT00912340).
The most common side effects of mTOR inhibitors include stomatitis, rash, pneumonitis, hyperglycemia, and hyperlipidemia. Pneumonitis may warrant interruption of treatment and dose reduction if moderate or severe. The clinician should be aware that use of mTOR inhibitors is associated with an increased cost and side effects, especially in elderly patients with multiple comorbidities. In future research, we need to define biomarkers to help us identify better those patients who will benefit from addition of mTOR inhibitors, such as those with known mutations of the PI3K pathway. Use of gene expression profiling might also identify subsets of patients who will benefit from a combination of targeting therapies, such as patients with luminal B tumors, which are associated with high recurrence rates and poor survival.85,86
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Ahmedin Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER statistic fact sheet: breast cancer. [Accessed August 6, 2012]. Available from: http://seer.cancer.gov/statfacts/html/breast.html.
- 3.Harvey JM, Clark GM, Osborne CK, Alfred DC. Estrogen receptor status by immunohistochemistry is superior to ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 4.Colleoni M, Gelber S, Coates AS, et al. Influence of endocrine-related factors on response to perioperative chemotherapy for patients with node-negative breast cancer. J Clin Oncol. 2001;19:4141–4149. doi: 10.1200/JCO.2001.19.21.4141. [DOI] [PubMed] [Google Scholar]
- 5.Winer EP. Optimizing endocrine therapy for breast cancer. J Clin Oncol. 2005;23:1609–1610. doi: 10.1200/JCO.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Jaiyesimi IA, Buzedar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol. 1995;13:513–529. doi: 10.1200/JCO.1995.13.2.513. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso F, Bischoff J, Brain E, et al. A review of the treatment of endocrine responsive metastatic breast cancer in postmenopausal women. Cancer Treat Rev. 2012 Jul 25; doi: 10.1016/j.ctrv.2012.06.011. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 8.Nabholtz JM, Bonneterre J, Buzdar A, Robertson JF, Thurlimann B. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results. Eur J Cancer. 2003;39:1684–1689. doi: 10.1016/s0959-8049(03)00326-5. [DOI] [PubMed] [Google Scholar]
- 9.Bonneterre J, Buzdar A, Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–2258. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a Phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 11.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 12.Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. BIG 1-98 Collaborative Group. N Engl J Med. 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paridaens R, Dirix L, Lohrisch C, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol. 2003;14:1391–1398. doi: 10.1093/annonc/mdg362. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 15.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 16.Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003;98:229–238. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 17.Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 18.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergh J, Jönsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–1925. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 21.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 22.Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res. 2001;7:4338s–4342s. [PubMed] [Google Scholar]
- 23.Massarweh S, Schiff R. Unraveling the mechanism of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res. 2007;13:1950–1954. doi: 10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 24.Pietras RJ, Arboleda J, Reese DM, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 25.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 27.Mackey JR, Kaufman B, Clemens M, et al. Trastuzumab prolongs progression free survival in hormone-dependent and HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2006;100(Suppl 1) Abstract 3. [Google Scholar]
- 28.Schafer JM, Bentrem DJ, Takei H, et al. A mechanism of drug resistance to tamoxifen in breast cancer. J Steroid Biochem Mol Biol. 2002;83:75–83. doi: 10.1016/s0960-0760(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 29.Kaklamani VG, Cicconi J, Gradishar W, et al. Increased HER2 expression in women with recurrent ER-positive breast cancer. Proc Am Soc Clin Oncol. 2007;25:569s. [Google Scholar]
- 30.De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER2 expression and response to endocrine therapy in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 31.Shou J, Massarweh S, Osborne C, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor- HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 32.Leary AF, Drury S, Detre S, et al. Lapatinib restores hormone sensitivity with differential effects on ER signaling in cell models of HER2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16:1486–1497. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 33.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 34.Finn RS, Press M, Dering J, et al. Progression-free survival (PFS) of patients with HER2-negative, estrogen receptor (ER)-low metastatic breast cancer (MBC) with the addition of lapatinib to letrozole: biomarker results of EGF30008. Proc Am Soc Clin Oncol. 2009;27:45s. [Google Scholar]
- 35.Nicholson RI, McClelland RA, Finlay P, et al. Relationship between EGF-R, c-erb B-2 protein expression and Ki67 immunostaining in breast cancer and hormone sensitivity. Eur J Cancer. 1993;7:1018–1023. doi: 10.1016/s0959-8049(05)80215-1. [DOI] [PubMed] [Google Scholar]
- 36.Gee JM, Harper ME, Hutcheson IR, et al. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology. 2003;144:5105–5117. doi: 10.1210/en.2003-0705. [DOI] [PubMed] [Google Scholar]
- 37.Massarweh S, Shou J, DiPietro M, et al. Targeting the epidermal growth factor receptor pathway improves the anti-tumor effect of tamoxifen and delays acquired resistance in a xenograft model of breast cancer. Breast Cancer Res Treat. 2002;76:S33. Abstract 18. [Google Scholar]
- 38.McClelland RA, Barrow D, Madden TA, et al. Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex) Endocrinology. 2001;142:2776–2788. doi: 10.1210/endo.142.7.8259. [DOI] [PubMed] [Google Scholar]
- 39.Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 40.Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB. Role of insulin-like growth factor-I in regulating estrogen receptor-a gene expression. J Cell Biochem. 2000;76:605–614. doi: 10.1002/(sici)1097-4644(20000315)76:4<605::aid-jcb9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 42.Hou X, Huang F, Macedo LF, et al. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 2011;71:7597–7607. doi: 10.1158/0008-5472.CAN-11-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox EM, Miller TW, Balko JM, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71:6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Moerkens M, Ramaiahgari S, et al. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufman PA, Ferrero JM, Bourgeois H, et al. A randomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC) Cancer Res. 2010;70(Suppl 2) Abstract S1–S4. [Google Scholar]
- 46.Frame MC. Src in cancer: deregulation and consequences for cell behavior. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 47.Morgan L, Gee J, Pumford S, et al. Elevated Src kinase activity attenuates tamoxifen response in vitro and is associated with poor prognosis clinically. Cancer Biol Ther. 2009;8:1550–1558. doi: 10.4161/cbt.8.16.8954. [DOI] [PubMed] [Google Scholar]
- 48.McBryan J, Theissen SM, Byrne C, et al. Metastatic progression with resistance to aromatase inhibitors is driven by steroid receptor coactivator SRC-1. Cancer Res. 2012;72:548–559. doi: 10.1158/0008-5472.CAN-11-2073. [DOI] [PubMed] [Google Scholar]
- 49.Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer EL, Baurain JF, Sparano J, et al. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin Cancer Res. 2011;17:6897–6904. doi: 10.1158/1078-0432.CCR-11-0070. [DOI] [PubMed] [Google Scholar]
- 51.Fedele P, Calvani N, Marino A, et al. Targeted agents to reverse resistance to endocrine therapy in metastatic breast cancer: where are we now and where are we going? Crit Rev Oncol Hematol. 2012;84:243–251. doi: 10.1016/j.critrevonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Carraway H, Hidalgo M. New targets for therapy in breast cancer: mammalian target of rapamycin (mTOR) antagonists. Breast Cancer Res. 2004;6:219–224. doi: 10.1186/bcr927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun M, Paciga J, Feldman R, et al. Phosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor α (ERα) via interaction between ERα and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 54.Hernandez-Aya LF, Gonzalez-Angulo AM. Targeting the phosphatidylinositol 3-kinase signaling pathway in breast cancer. Oncologist. 2011;16:404–414. doi: 10.1634/theoncologist.2010-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 56.Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for antiestrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 57.Sheri A, Martin LA, Johnston S. Targeting endocrine resistance: is there a role for mTOR inhibition? Clin Breast Cancer. 2010;10(Suppl 3):S79–S85. doi: 10.3816/CBC.2010.s.016. [DOI] [PubMed] [Google Scholar]
- 58.Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;28:407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachman KE, Argani P, Samuels Y. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 60.Shoman N, Klassen S, McFadden A, Bickis MG, Torlakovic E, Chibbar R. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod Pathol. 2005;18:250–259. doi: 10.1038/modpathol.3800296. [DOI] [PubMed] [Google Scholar]
- 61.Miller TW, Balko JM, Fox EM, et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kenerson HL, Aicher LD, True LD, et al. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- 63.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dowling RJ, Topisirovic I, Fonseca BD, et al. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:43–49. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Holtz MK. The role of S6K1 in ER-positive breast cancer. Cell Cycle. 2012;11:3159–3165. doi: 10.4161/cc.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Lin J, Wang X, et al. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Cancer Res Treat. 2012;134:1057–1066. doi: 10.1007/s10549-012-2036-2. [DOI] [PubMed] [Google Scholar]
- 68.Chang S, Miron P, Miron A, et al. Rapamycin inhibits proliferation of estrogen-receptor-positive breast cancer cells. J Surg Res. 2007;138:37–44. doi: 10.1016/j.jss.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Ghayad SE, Bieche I, Vendrell JA, et al. mTOR inhibition reverses acquired endocrine therapy resistance of breast cancer cells at the cell proliferation and gene-expression levels. Cancer Sci. 2008;99:1992–2003. doi: 10.1111/j.1349-7006.2008.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beeram M, Tan Q, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- 71.Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 72.Baselga J, Fumoleau P, Gil M. Phase II, 3 arm study of CCI-779 in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. Preliminary results. J Clin Oncol. 2004;22(Suppl 14):544. [Google Scholar]
- 73.Carpenter JT, Roché H, Campone M, et al. Randomized 3-arm, phase 2 study of temsirolimus (CCI-779) in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:16S:564. [Google Scholar]
- 74.Chow LWC, Sun Y, Jassem J, et al. Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2006;100(Suppl 1) Abstract 6091. [Google Scholar]
- 75.Rugo HS, Keck S. Reversing hormone resistance: have we found the golden key? J Clin Oncol. 2012;30:2707–2709. doi: 10.1200/JCO.2012.42.1271. [DOI] [PubMed] [Google Scholar]
- 76.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients with hormone-receptor positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 78.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharyya GS, Biswas J, Singh JK, et al. Reversal of tamoxifen resistance (hormone resistance) by addition of sirolimus (mTOR inhibitor) in metastatic breast cancer. 2011 European Multidisciplinary Cancer Congress; Edition Stockholm, Sweden: 2011. [Google Scholar]
- 80.Villarreal-Garza C, Cortes J, Andre F, Verma S. mTOR inhibitors in the management of hormone receptor-positive breast cancer: the latest evidence and future directions. Ann Oncol. 2012;23:2526–2535. doi: 10.1093/annonc/mds075. [DOI] [PubMed] [Google Scholar]
- 81.Carew JS, Kelly KR, Nawrocki ST. Mechanisms of mTOR inhibitor resistance in cancer therapy. Target Oncol. 2011;6:17–27. doi: 10.1007/s11523-011-0167-8. [DOI] [PubMed] [Google Scholar]
- 82.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Margariti N, Fox SB, Bottini A, Generali D. Overcoming breast cancer drug resistance with mTOR inhibitors. Could it be a myth or a real possibility in the short-term future? Breast Cancer Res Treat. 2011;128:599–606. doi: 10.1007/s10549-010-0986-9. [DOI] [PubMed] [Google Scholar]
- 84.Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat. 2012;135:39–48. doi: 10.1007/s10549-012-2067-8. [DOI] [PubMed] [Google Scholar]
- 85.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harichand-Herdt S, Zelnak A, O’Regan R. Endocrine therapy for the treatment of postmenopausal women with breast cancer. Expert Rev Anticancer Ther. 2009;9:187–198. doi: 10.1586/14737140.9.2.187. [DOI] [PubMed] [Google Scholar]