Follicular T helper cells are the major reservoir for HIV infection and accumulate during chronic HIV infection.
Abstract
In the present study, we have investigated the distribution of HIV-specific and HIV-infected CD4 T cells within different populations of memory CD4 T cells isolated from lymph nodes of viremic HIV-infected subjects. Four memory CD4 T cell populations were identified on the basis of the expression of CXCR5, PD-1, and Bcl-6: CXCR5−PD-1−Bcl-6−, CXCR5+PD-1−Bcl-6−, CXCR5−PD-1+Bcl-6−, and CXCR5+PD-1+Bcl-6+. On the basis of Bcl-6 expression and functional properties (IL-21 production and B cell help), the CXCR5+PD-1+Bcl-6+ cell population was considered to correspond to the T follicular helper (Tfh) cell population. We show that Tfh and CXCR5−PD-1+ cell populations are enriched in HIV-specific CD4 T cells, and these populations are significantly increased in viremic HIV-infected subjects as compared with healthy subjects. The Tfh cell population contained the highest percentage of CD4 T cells harboring HIV DNA and was the most efficient in supporting productive infection in vitro. Replication competent HIV was also readily isolated from Tfh cells in subjects with nonprogressive infection and low viremia (<1,000 HIV RNA copies). However, only the percentage of Tfh cells correlated with the levels of plasma viremia. These results demonstrate that Tfh cells serve as the major CD4 T cell compartment for HIV infection, replication, and production.
Memory CD4 T cells are the primary target of HIV (Schnittman et al., 1990). Massive depletion of this cell population occurs during primary infection (Mattapallil et al., 2005), and long-term antiretroviral therapy (ART) only partially restores the memory CD4 T cell pool (Guadalupe et al., 2003; Brenchley et al., 2004). HIV-infected activated CD4 T cells escaping from HIV-specific cytotoxic CD8 T cells and the virus cytopathic effect may enter a quiescent state and represent the major source of latently HIV-infected cells (Chun et al., 1997a,b) and the major obstacle for HIV eradication (Chun et al., 1997a,b; Finzi et al., 1997; Wong et al., 1997). Estimates of the half-life of the HIV latent reservoir in blood indicate that as long as 70 years might be required for the eradication of the latent reservoir in the presence of fully suppressive antiviral therapy (Siliciano et al., 2003). Recent studies in blood have identified central memory (defined by the CD45RA−CCR7+CD27+ phenotype) and transitional memory (CD45RA−CCR7−CD27+) CD4 T cells as the major cellular compartments of the HIV latent reservoir (Chomont et al., 2009). Although it is known that HIV replication is dependent on the state of cell activation (McDougal et al., 1985; Stevenson et al., 1990), it is not clear whether there is a memory CD4 T cell compartment predominantly responsible for active virus replication and production.
Lymphoid organs are the primary anatomical compartments for both the generation of the immune response (Allen et al., 2007) and for HIV replication and spreading (Pantaleo et al., 1991, 1993; Embretson et al., 1993; Brenchley et al., 2004). A phenotypic and functionally distinct CD4 T cell population known as T follicular helper (Tfh) cells resides within the germinal centers (GCs). It is specialized in providing help to B cells and is necessary for GC formation, Ig class switch, somatic hypermutation of antibody, and maturation of B cells into plasma cells and memory B cells (Breitfeld et al., 2000; Schaerli et al., 2000; Kim et al., 2001; Fazilleau et al., 2009a,b). The transcription factor Bcl-6 (Chtanova et al., 2004; Johnston et al., 2009) is the primary marker of Tfh cells, whereas other markers, such as CXCR5 (the chemokine receptor for CXCL13), inducible T cell co-stimulator (ICOS), and PD-1 (Breitfeld et al., 2000; Schaerli et al., 2000; Kim et al., 2001; Fazilleau et al., 2009a), are not exclusive of Tfh cells. Tfh cells produce a variety of cytokines, including IL-21 which is critical for promoting B cell maturation (Ozaki et al., 2002; Chtanova et al., 2004; Fazilleau et al., 2009a,b; Avery et al., 2010).
Recent studies have shown an expansion of Tfh cells in HIV and simian immunodeficiency virus (SIV) infection (Hong et al., 2012; Lindqvist et al., 2012; Petrovas et al., 2012) and that Tfh cells are susceptible to SIV infection (Petrovas et al., 2012) and are enriched in SIV-infected cells (Brenchley et al., 2012). However, no data are available on the HIV infection of Tfh cells and their role as potential reservoir for HIV.
In the present study, we have investigated memory CD4 T cell populations isolated from lymph nodes of 23 subjects with chronic HIV infection with CD4 T cell count >400 per mm3 and plasma HIV RNA levels >5,000 copies per ml, from 14 subjects with undetectable plasma viremia (<20 HIV RNA copies per ml) after 72 wk of ART, from 3 subjects with nonprogressive HIV disease, i.e., long-term nonprogressors (LTNPs) and low plasma HIV viremia levels, and from 13 HIV-negative subjects. Lymph nodes from the same patients were obtained at baseline (before initiation of therapy) and 72 wk after ART.
The results presented demonstrate that the memory lymph node CD4 T cell population corresponding to Tfh cells, i.e., the CXCR5+PD-1+ cell population, and the CXCR5−PD-1+ cell population were enriched in HIV-specific CD4 T cells, and that the Tfh cell population contained the highest percentage of HIV-infected cells and was the most efficient in supporting virus replication and production.
RESULTS
Characterization of memory CD4 T cell populations in lymph nodes
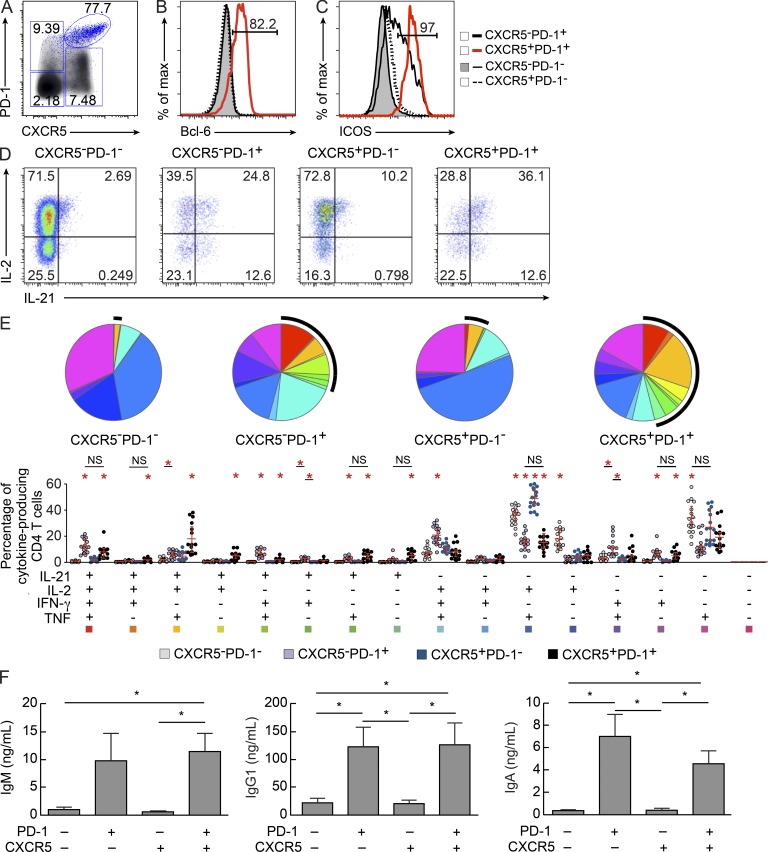
Lymph node mononuclear cells from chronically HIV-infected viremic subjects and healthy subjects (unpublished data) were stained with CD45RA, CD3, CD4, CD8, CXCR5, PD-1, ICOS, and Bcl-6 antibodies. Four populations of CD45RA− memory CD4 T cells were identified on the basis of the expression of PD-1 and CXCR5: CXCR5−PD-1−, CXCR5+PD-1−, CXCR5−PD-1+, and CXCR5+PD-1+ (Fig. 1 A). The gating strategy used was based on the expression of Bcl-6 within the four CD4 T cell populations. Bcl-6 expression was predominantly restricted to the CXCR5+PD-1+ cell population (Fig. 1, A and B). In the representative viremic HIV-infected subject shown, Bcl-6, the primary marker of Tfh cells (Chtanova et al., 2004; Johnston et al., 2009), was expressed in >80% of CXCR5+PD-1+ cells, whereas it was expressed in a minor percentage of the other CD4 T cell populations (Fig. 1 B) and 97% of CXCR5+PD-1+ cells also expressed ICOS (Fig. 1 C). Interestingly, ICOS was also expressed in a large proportion (54.9%) of CXCR5−PD-1+ CD4 T cells (Fig. 1 C). To determine the functional cytokine profile, lymph node mononuclear cells were stimulated with PMA plus ionomycin and the production of IL-21, IL-2, IFN-γ, and TNF was assessed. The expression of CXCR5 and PD-1 and the percentage of the four cell populations were not influenced by the in vitro culture (unpublished data). The representative flow cytometry cytokine profile shown in Fig. 1 (D and E) indicated that IL-21 was predominantly produced by CXCR5+PD-1+ cells (∼45% of cells), followed by the CXCR5−PD-1+ cell population (∼30% of cells), whereas <5% of the CXCR5+PD-1− and CXCR5−PD-1− cell populations produced IL-21. Interestingly, the CXCR5+PD-1+ cell populations comprised several distinct IL-21+ and IL-21− cytokine-producing cell populations: IL-21/IL-2/TNF (18%); IL-21/IL-2/TNF/IFN-γ (8%); IL-21/IL-2, IL-21/TNF, and single IL-21 (5%); and IL-2/TNF (15%) and single TNF (18%; Fig. 1 E). The cytokine profile of IL-21–producing CXCR5−PD-1+ CD4 T cells was similar to that of CXCR5+PD-1+ cells (Fig. 1 E). The most represented cytokine-producing cell populations within CXCR5−PD-1- and CXCR5+PD-1− cells were IL-2/TNF, single TNF, and single IL-2 (Fig. 1 E). Similar results were obtained in HIV-negative subjects (unpublished data). Finally, to determine the ability to support Ig production from B cells, the four sorted CD4 T cell populations were isolated from three viremic subjects and co-cultured with sorted purified GC B cells isolated from the same lymph nodes in the presence of Staphylococcus enterotoxin B (SEB) for 5 d. Only CXCR5−PD1+ and CXCR5+PD1+ cell populations supported IgM, IgG1, and IgA production from GC B cells (Fig. 1 F). Similar results were obtained with memory and naive B cells (unpublished data).
Figure 1.
Characterization of Tfh cells in lymph nodes. Lymph node mononuclear cells were isolated from viremic HIV-infected subjects (n = 15) and stained with anti-CD3, anti-CD4, anti-CD45RA, anti-CXCR5, anti-PD-1, anti-ICOS, and anti–Bcl-6 antibodies. (A) Representative flow cytometry profile of lymph node memory (CD45RA−) CD4 T cell populations expressing CXCR5 and/or PD-1 in one viremic HIV-infected subject. Density cells in black correspond to total CD4 T cells, whereas the blue dots identify Bcl-6+CD4+ T cells. (B and C) Surface expression of Bcl-6 (B) or ICOS (C) in CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ memory CD4 T cell populations. (D) CD4 T cells were gated on total IFN-γ, TNF, IL-2, and IL-21 cytokine-producing cells, and IL-21 and IL-2 expression was analyzed. (E) Functional cytokine profile in CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cell populations from viremic HIV-infected subjects. All the possible combinations of the responses are shown on the x axis, and the percentage of the functionally distinct cell populations within the responding CD4 T cells are shown on the y axis. Responses are grouped and color-coded on the basis of the combinations of the cytokines produced. Spots correspond to the fractions of functionally distinct T cell populations within the total CD4 T cells. The pie charts above summarize the data, and black arcs identify IL-21–producing cell populations (*, P < 0.003). Error bars correspond to mean ± SD. (F) CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cell populations were sorted from three viremic HIV-infected subjects. Cells were cultured with autologous GC B cells (CD19+CD38+IgD−) in the presence of SEB. IgM, IgG1, and IgA production was assessed at day 5 by Luminex. The grade of purity of the sorted cell populations was >97% in all experiments. *, P < 0.05. Error bars correspond to mean ± SEM. Statistical significance was obtained using Student’s t test.
The expression of Bcl-6 together with the production of IL-21 and the ability to support Ig production indicate that CXCR5+PD-1+ cells likely correspond to Tfh cells and we will refer to them as Tfh cells throughout the study. Furthermore, the CXCR5−PD-1+Bcl-6− cell population expressed ICOS, produced IL-21, and was able to support Ig production. A recent study (Wang et al., 2011) in human tonsils has shown that CXCR5−PD-1+ CD4 T cells are located just outside the GC; it cannot be excluded that this PD-1+ CD4 T cell population may contain precursors of Tfh cells.
Relative frequency of memory lymph node CD4 T cell populations in HIV-infected versus healthy subjects
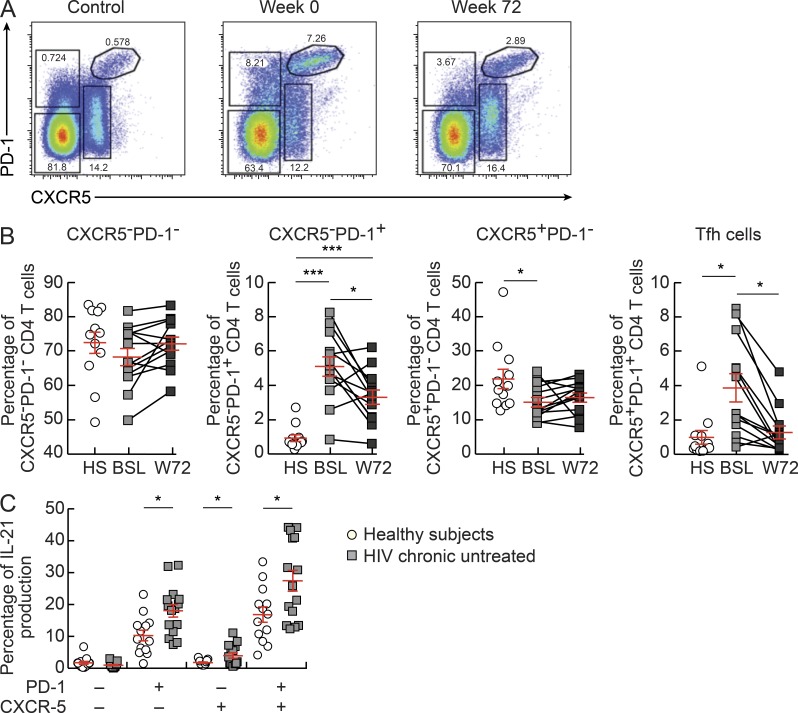
The relative frequency of the four memory CD4 T cell populations was then compared in lymph nodes of healthy subjects, viremic chronically HIV-infected subjects, and subjects treated for 72 wk with ART. No or minor differences were observed in the frequencies of CXCR5−PD1− (P > 0.05) and CXCR5+PD1− (P = 0.0406) cell populations between the three study groups (Fig. 2, A and B). Interestingly, CXCR5−PD-1+ and Tfh cell populations were significantly increased during the viremic phase of HIV infection, i.e., 5.1% (P < 0.0001) and 3.8% (P = 0.0046) as compared with healthy subjects where the percentage of each cell population was 0.9%. In addition, both cell populations were significantly decreased after ART, i.e., CXCR5−PD-1+ to 3.3% (P = 0.0078) and Tfh cells to 1.3% (P = 0.0045; Fig. 2, A and B). With regard to the cytokine profile, the frequency of IL-21–producing cells was significantly increased (P < 0.05) within the CXCR5−PD-1+, Tfh, and, to a lower extent, within CXCR5+PD-1− cell populations from viremic HIV-infected subjects as compared with healthy subjects (Fig. 2 C). No differences in the frequency of TNF, IFN-γ, and IL-2–producing Tfh cells were observed between viremic HIV-infected subjects and healthy subjects (unpublished data), whereas these cytokines were significantly reduced within the other CD4 T cell populations of viremic HIV-infected subjects (unpublished data).
Figure 2.
Frequencies of Tfh cells in lymph nodes. (A) Representative flow cytometry profiles of CXCR5 and/or PD-1 expression in CD4 T cell populations from an HIV-negative subject (control) and from one HIV-infected subject before (week 0) and after ART (week 72). (B) Frequencies of CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and Tfh CD4 T cell populations in HIV-negative subjects (HS; n = 12) and chronically HIV-1–infected subjects (n = 13) at weeks 0 (BSL) and 72 (W72). (C) Frequencies of IL-21–producing cells in HIV-negative (n = 13; open circles) and viremic HIV-infected (n = 15; closed squares) subjects. Cells were stimulated with PMA plus ionomycin for 6 h, permeabilized, and stained with anti-CD3, anti-CD4, anti–IL-21, anti–IFN-γ, anti-TNF, and anti–IL-2 antibodies. *, P < 0.05; ***, P < 0.0001. Error bars correspond to mean ± SEM. Statistical significance was obtained using one-way ANOVA (Kruskal-Wallis test) followed by Student’s t test.
Therefore, these results demonstrate that Tfh and CXCR5−PD-1+ cell populations are not depleted but increased during the viremic phase of HIV infection. In support of these results, recent studies in nonhuman primates (Hong et al., 2012; Petrovas et al., 2012) and in HIV-infected subjects (Lindqvist et al., 2012) have shown that Tfh cells were also expanded in lymph node tissues.
Distribution of HIV-specific CD4 T cells within the memory lymph node CD4 T cell populations
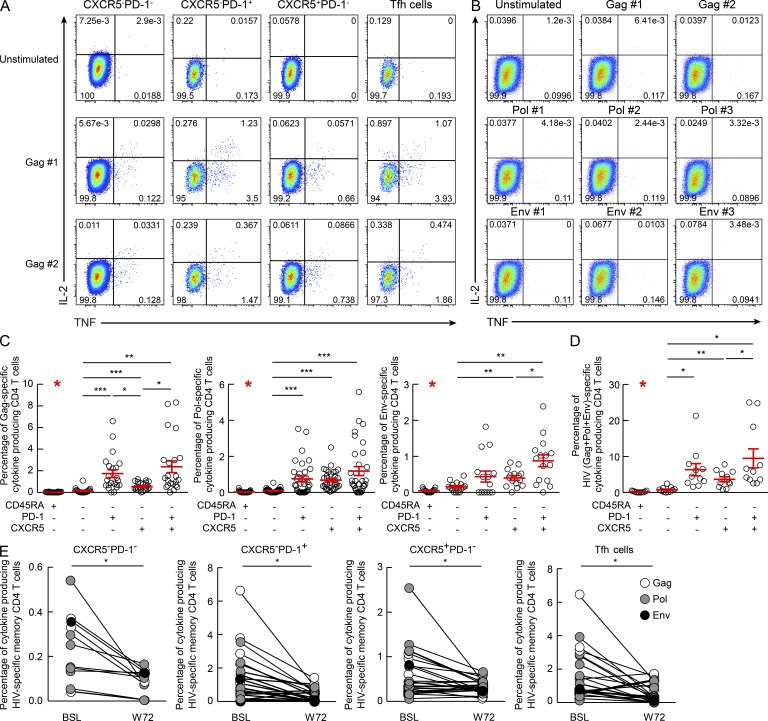
We then investigated the distribution of HIV-specific CD4 T cells within the four cell memory CD4 T cell populations. Lymph node mononuclear cells of 11 viremic subjects were stimulated with potential T cell epitope (PTE) peptide pools (Li et al., 2006) derived from Gag (2 pools), Pol (3 pools), and Env (3 pools). It has been previously shown (Betts et al., 2001) that CD4 T cell responses specific at Gag, Pol, and Env correspond to ∼80% of total HIV-specific CD4 T cells. HIV-specific CD4 T cells were identified by the ability to produce IL-2, IFN-γ, and TNF. HIV-specific CD4 T cell responses to the two Gag and three Pol PTEs peptide pools were detected within the four memory (CD45RA−) CD4 T cell populations of all the 11 subjects, whereas Env-specific responses were detected in six out of nine subjects and responses against the three Env pools in three out of six subjects (Fig. 3, A and C). Of note, HIV-specific CD4 T cells were not detected in the naive (CD45RA+) CD4 T cell population (Fig. 3, B and C). Interestingly, the Tfh cell population was the mostly enriched in Gag-, Pol-, and Env-specific CD4 T cells (Fig. 3, A and C). Tfh (9.49 ± 2.64%) and CXCR5−PD-1+ (6.32 ± 1.69%) cell populations were also significantly (P < 0.05) enriched in the total HIV-specific CD4 T cells as compared with CXCR5+PD-1− (3.69 ± 0.69%) and CXCR5−PD-1− (0.76 ± 0.21%) cell populations (Fig. 3 D). With regard to the cytokine profile, CXCR5−PD-1− and CXCR5+PD-1− cells were enriched in single TNF-producing cells and CXCR5−PD-1+ cells in single IFN-γ–producing cells as compared with Tfh cells (unpublished data). IL-21 was barely detected after antigen (Ag)-specific stimulation but consistently produced after PMA/ionomycin stimulation (unpublished data). Of note, HIV-specific CD4 T cell frequencies significantly decreased after suppression of virus replication by ART (week 72) within the four CD4 T cell populations (Fig. 3 E), whereas no major changes were observed in the cytokine profile (not depicted). No cytokine production was detected after stimulation with HIV PTE peptide pools of lymph node mononuclear cells from HIV-negative subjects (unpublished data). These further strengthen previous observations (Lindqvist et al., 2012) indicating enrichment of HIV-specific CD4 T cells within the Tfh cell population.
Figure 3.
Flow cytometry profiles of HIV-specific lymph node CD4 T cell populations. Lymph node mononuclear cells were isolated from viremic HIV-infected subjects before (week 0; n = 11) or after (week 72; n = 5) ART therapy and were stimulated or not with HIV peptide pools (Gag, Pol, and Env), and then permeabilized and stained with anti-CD3, anti-CD4, anti-CD45RA, anti–PD-1, anti-CXCR5, anti–IL-21, anti–IFN-γ, anti-TNF, and anti–IL-2 antibodies. (A and B) Representative flow cytometric cytokine profiles of Gag peptide pool #1 (Gag #1) and Gag #2–specific CD4 T cell populations producing IL-2 and/or TNF from one viremic HIV-positive subject in the memory (A) and naive T cell populations (B). (C and D) Percentage of Gag-, Pol-, and Env-specific (C) and of HIV-specific (Gag+Pol+Env; D) T cells among total CD3+CD4+ T cells making any cytokine (IFN-γ, TNF, IL-2, or IL-21) in viremic HIV-infected subjects before ART therapy (n = 11). Error bars correspond to mean ± SEM. (E) Changes in the frequencies of the HIV-specific memory CD4 T cells producing anyone cytokine in viremic HIV-infected subjects before (week 0; BSL) and after (week 72; W72) ART therapy (n = 5). *, P < 0.05; **, P < 0.001; ***, P < 0.0001. Statistical significance was obtained using Student’s t test.
Relationship between Tfh cells and B cell populations
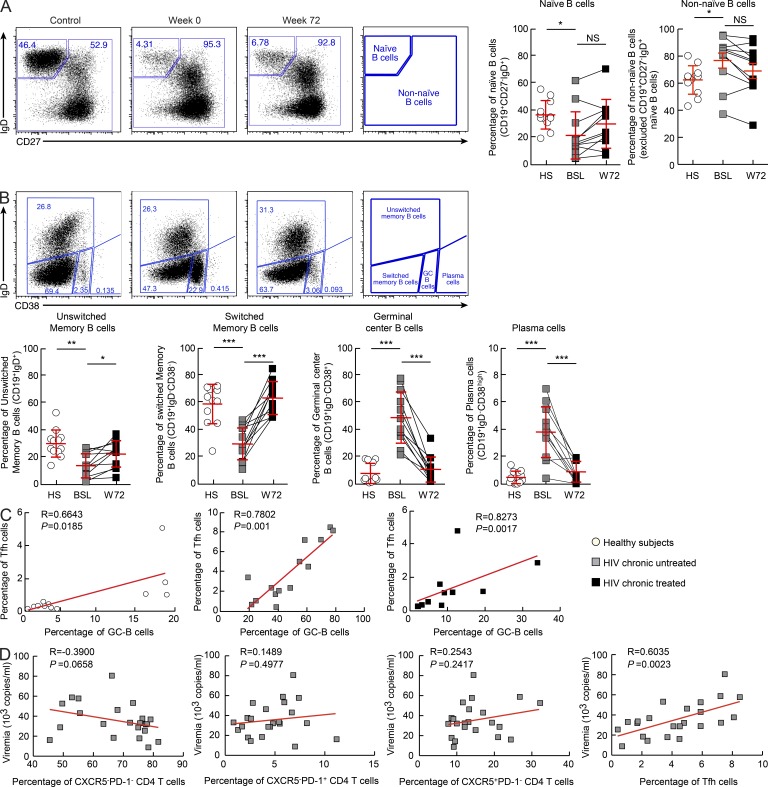
We next investigated the relationship between Tfh cells and different B cell populations. Phenotypic analysis of memory B cells is very complex, particularly with regard to the heterogeneity of the memory B cell compartment (Sanz et al., 2008). Here we have used four major markers, CD19, CD27, CD38, and IgD, to define different B cell populations (Sanz et al., 2008). On the basis of these markers, CD19+IgD+CD27− cells correspond to naive B cells and the non-naive B cells correspond to a mixed B cell population including memory, GC, and plasma cells. Of note, CD27− memory B cells are present at low percentage (5%) in healthy subjects and may expand in different pathological conditions (Ehrhardt et al., 2005; Wirths and Lanzavecchia, 2005; Wei et al., 2007). Four populations of non-naive B cells were identified by excluding naive B cells based on the expression of CD38 and IgD: unswitched memory (CD19+IgD+); switched memory (CD19+IgD−CD38−); GC (CD19+IgD−CD38+) B cells; and plasma cells (CD19+IgD B cells CD38high; Fig. 4 A). Naive (CD19+IgD+CD27− B cells), unswitched memory, and switched memory B cell populations were significantly reduced (naive: P = 0.0153; unswitched: P = 0.0003; switched: P < 0.0001), whereas GC B cells and plasma cells significantly expanded (six- and sevenfold increase, respectively) in viremic HIV-infected subjects as compared with healthy subjects (P < 0.0001; Fig. 4, A and B). Suppression of virus replication by ART was associated with an increase of both naive (not significant) and memory B cell populations (P < 0.04) and a significant reduction of GC B cells and plasma cells (P < 0.0001; Fig. 4, A and B). The percentage of Tfh cells positively correlated with the percentage of GC B cells in healthy subjects (R = 0.6643, P = 0.0185), in viremic subjects (R = 0.7802, P = 0.001), and in ART-treated subjects (R = 0.8273, P = 0.0017; Fig. 4 C). Interestingly, only the percentage of Tfh cells was significantly correlated with the levels of plasma viremia (R = 0.6035, P = 0.0023; Fig. 4 D). Therefore, consistent with previous studies (Lindqvist et al., 2012; Petrovas et al., 2012), these results further support the hypothesis that the quantitative changes in Tfh cells and different B cell populations are driven by HIV. In addition, our results have shown a strong correlation between the proportion of Tfh cells and plasma viremia levels, thus indicating a relationship between Tfh cells and HIV replication and production.
Figure 4.
Frequencies of B cell populations in lymph nodes of HIV-negative and viremic untreated and treated HIV-infected subjects. Lymph node mononuclear cells were isolated from HIV-negative subjects (n = 12, control) and from HIV-infected subjects (n = 11) at weeks 0 and 72 and were stained with anti-CD19, anti-CD27, anti-CD38, and anti-IgD antibodies. (A) Representative flow cytometry profile of naive and non-naive B cells based on the expression of CD19+, CD27−, and IgD+. Graphs show cumulative percentages of naive and non-naive B cell populations in HIV-negative subjects (HS; n = 12) and HIV-positive subjects (n = 11) at weeks 0 (BSL) and 72 (W72). (B) Representative flow cytometry profile of non-naive B cell populations based on the expression of CD19, IgD, and CD38. Naive B cells (CD19+IgD+CD27−) were excluded. Plots show unswitched memory (CD19+IgD+), switched memory (CD19+IgD−CD38−), GC (CD19+IgD−CD38+), and plasma cell (CD19+IgD−CD38high) B cell populations in one HIV-negative subject (control) and one HIV-positive subject at weeks 0 and 72. Graphs show cumulative data of the different memory B cell populations of HIV-negative subjects (n = 12) and of HIV-positive subjects (HS, n = 11) at weeks 0 (BSL) and 72 (W72). (C) Correlation between the percentage of Tfh cells and GC B cells in HIV-negative subjects (n = 12; open circles) and HIV-infected subjects before (chronic untreated; n = 14; gray squares) and after ART therapy (chronic treated; n = 11; black squares). (D) Correlation between the percentage of CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, or Tfh cells and plasma viremia in viremic chronically HIV-infected subjects (n = 23). *, P < 0.05; **, P < 0.001; ***, P < 0.0001. Error bars correspond to mean ± SD. P-values were derived from Student’s t test for multiple comparisons (B), or a Spearman rank test for correlations (C and D).
Distribution of HIV-infected cells within memory lymph node CD4 T cell populations
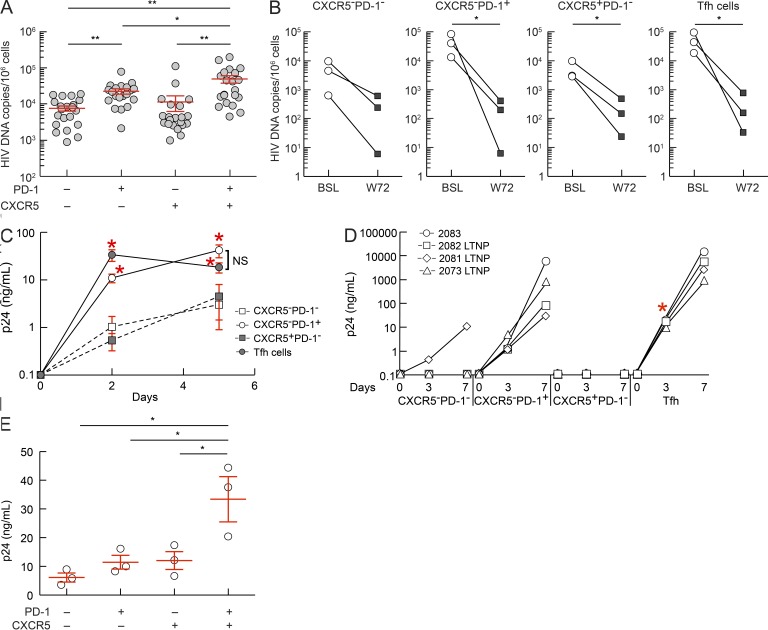
Previous studies have shown that HIV-specific CD4 T cells are preferentially infected by HIV (Douek et al., 2002). Our finding that Tfh cells are enriched in HIV-specific cells, together with the correlation of their percentage with the levels of plasma viremia, suggested that Tfh cells may contain higher numbers of HIV-infected cells and be more efficient in supporting virus replication. To test this hypothesis, the four CD4 T cell populations were sorted on the basis of the expression of CXCR5 and PD-1 from 21 viremic HIV-infected subjects and assessed for total HIV DNA. The highest number of HIV DNA copies was found in Tfh cells, i.e., mean 50,953 copies/106 mononuclear cells, thus indicating that ∼5.1% of Tfh cells were infected with HIV assuming one HIV DNA copy/per infected cell (Fig. 5 A). The CXCR5−PD-1+ cell population contained a mean 23,182 HIV DNA copies/106 cells (∼2.3% infected cells; Fig. 5 A), the CXCR5+PD-1− 11,833 HIV DNA copies/106 cells (1.1% infected cells), and the CXCR5−PD-1− cell population 8,191 HIV DNA copies/106 cells (0.8% infected cells; Fig. 5 A). The differences in viral load between Tfh and the other cell populations were significant (P < 0.05). These results are in agreement with a recent study in SIV infection (Brenchley et al., 2012). Of note, a higher frequency of HIV infection in blood PD-1+ memory CD4 T cells has been reported in a recent study (Chomont et al., 2009). A significant reduction (1.5–2.0 logs, P < 0.05) in the frequency of cells harboring HIV DNA was consistently observed 72 wk after ART (Fig. 5 B). However, HIV DNA was detected in all the four cell populations, thus indicating that may serve as cellular compartments for the HIV latent reservoir.
Figure 5.
HIV DNA load and HIV production in lymph node CD4 T cell populations. (A) HIV DNA load in sorted CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ memory (CD45RA−) CD4 T cell populations from viremic HIV-infected subjects (n = 21). (B) HIV DNA load before (week 0; BSL) and after (week 72; W72) ART therapy in three HIV-infected subjects. (C) CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ memory CD4 T cell populations were sorted from HIV-infected subjects with viremia >15,000 HIV RNA copies/ml (n = 5), and HIV replication was assessed at days 0, 2, and 5 after anti-CD3 plus anti-CD28 stimulation by measuring p24 production using an electrochemiluminescence assay. (D) CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ memory CD4 T cell populations were sorted from four HIV-infected subjects (n = 4) with low viremia (<2,000 HIV RNA copies/ml) and cultured with heterologous CD8-depleted blood mononuclear cells. HIV production was assessed at days 0, 3, and 7 after anti-CD3 plus anti-CD28 stimulation. The different symbols represent individual subjects. (E) Productive HIV infection in sorted CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ memory CD4 T cell populations of healthy subjects (n = 3) 3 d after anti-CD3 plus anti-CD28 stimulation. *, P < 0.05; **, P < 0.001. Error bars correspond to mean ± SEM. P-values were obtained using one-way ANOVA (Kruskal-Wallis test) followed by Student’s t test.
HIV replication and production within memory lymph node CD4 T cell populations
We next explored the efficiency of the four CD4 T cell populations to support active virus replication and production. We used two strategies: (1) isolation of replication competent virus from five subjects with high viremia (>15,000 HIV RNA copies/ml of plasma), from three LTNP with viremia ranging between 200 and 1,000 HIV RNA copies, from one subject experiencing a transient virus blip (1,800 HIV RNA copies), and from three ART-treated subjects with viremia <5 HIV RNA copies; and (2) HIV infection in vitro. For the first strategy, sorted purified CD4 T cell populations were isolated, stimulated with anti-CD3 plus anti-CD28 antibodies, and the production of HIV p24 was determined at days 0, 2, and 5 in the subjects with high viremia. Evidence for rapid HIV replication and significantly higher levels of HIV p24 were measured in the Tfh cell cultures already at day 2 as compared with those measured in the other cell populations (P < 0.05). CXCR5−PD-1+ cells also efficiently supported HIV replication, whereas CXCR5+PD-1− and CXCR5−PD-1− cell populations were poorly efficient (Fig. 5 C). The differences between Tfh and the other CD4 cell populations were more striking in the three LTNP and in the subject with transient virus blip (viremia was undetectable at week 84; Fig. 5 D). HIV p24 production was significantly higher in Tfh as compared with the other cell population at day 3 (Fig. 5 D). The differences were not significant between Tfh and CXCR5−PD-1+ cells at day 7, although the levels of p24 in the two LTNP with a few 100 HIV RNA copies substantially higher in the Tfh cell cultures (Fig. 5 D). HIV p24 was rarely or not detected in CXCR5+PD-1− and CXCR5−PD-1− cells cell cultures (Fig. 5 D). HIV p24 production was not detected in the cell cultures of long-term-treated subjects with viremia <20 HIV RNA copies (unpublished data). The failure in the virus isolation in the long-term-treated subjects is likely the result of the very low frequency of HIV-infected CD4 T cells harboring replication-competent virus, which may be in the range of one per million CD4 T cells in the latent pool of blood memory CD4 T cells (Chun et al., 1997a) and the very small number (5 × 104–105) of Tfh cells used in the current virus isolation experiments.
With regard to the second strategy, sorted purified CD4 T cell populations were isolated from lymph nodes of three HIV-negative subjects, inoculated with HIV, and stimulated with anti-CD3 plus anti-CD28 antibodies. HIV p24 production efficiency was measured at day 3 to minimize differences in p24 production that may be caused at later time points by differences in proliferation capacity between the four CD4 T cell populations. HIV p24 production was significantly higher in Tfh cells as compared with the other CD4 T cell populations at day 3 after infection (Fig. 5 E). Therefore, these results indicate that Tfh cells contain a higher number of HIV-infected cells during the viremic phase and are the major CD4 T cell compartment for active HIV replication and production.
State of activation and proliferation capacity of memory lymph node CD4 T cell populations
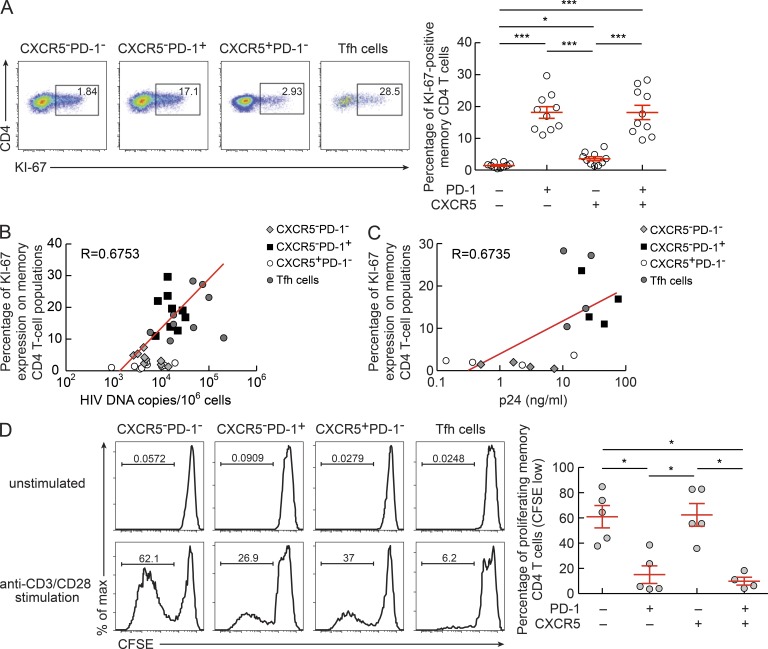
HIV integration and replication is dependent on the state of activation of the target cells (McDougal et al., 1985; Stevenson et al., 1990). We therefore investigated the relationship between the state of activation of the four cell populations and viral load content and the ability to support productive HIV infection. The four cell populations were analyzed for the expression of Ki-67, which defines cycling cells (Gerdes et al., 1984) and is also an indirect measure of the state of cell activation. Interestingly, Ki-67 was significantly more expressed in Tfh (18%) and CXCR5−PD-1+ (18%) cells as compared with CXCR5−PD-1− and CXCR5+PD-1− (P < 0.0008; Fig. 6 A). The percentage of Ki-67 cells correlated with total HIV DNA (R = 0.6753, P < 0.0001) and with the ability to support HIV replication (R = 0.6735, P = 0.004; Fig. 6, B and C). The expression of Ki-67 within the same CD4 T cell populations was ∼50% lower in lymph node CD4 T cell populations of five HIV-negative subjects (unpublished data).
Figure 6.
Ki-67 expression and cell proliferation in lymph node CD4 T cells. Lymph node mononuclear cells isolated from viremic chronically HIV-1–infected subjects (n = 10) were permeabilized and stained with anti-CD3, anti-CD4, anti-CD45RA, and anti–Ki-67 antibodies. (A) Representative flow cytometry profiles showing Ki-67 expression in CD4 T cell populations. Graph shows data from 10 viremic HIV-infected subjects. (B) Correlation between Ki-67 expression and HIV-1 DNA copies/106 cells (n = 9). (C) Correlation between Ki-67 expression and p24 production (n = 4). (D) Representative flow cytometry profile showing proliferating (CFSE low) CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cell populations. Sorted memory (CD45RA−) CD4 T cell populations from viremic HIV-infected subjects (n = 5) were labeled with CFSE and stimulated with anti-CD3 plus anti-CD28 antibodies for 5 d and acquired on an LSR SORP (BD). Flow cytometry profiles of unstimulated cells (negative control) are also shown. *, P < 0.05; ***, P < 0.0001. Error bars correspond to mean ± SEM. P-values were obtained using Student’s t test for multiple comparisons (A and D), or a Spearman rank test for correlations (B and C).
Because PD-1, which was coexpressed with Ki-67, may define cells with reduced proliferation capacity (Day et al., 2006; Trautmann et al., 2006), the four sorted purified memory CD4 T cell populations were stimulated with anti-CD3 plus anti-CD28 antibodies and cell proliferation was determined using the CFSE flow cytometry–based assay (Fig. 6 D). A significantly (P < 0.01) larger proportion (60%) of CFSE-low CXCR5−PD-1− and CXCR5+PD-1− cells was detected at day 5 of culture as compared with 10–15% of CFSE-low CXCR5−PD-1+ and Tfh cells, thus indicating that the PD-1+ T cell populations have limited proliferation capacity. These results are consistent with recent studies analyzing Ki-67 and cell cycling capacity in Tfh cells (Petrovas et al., 2012) or Ki-67 expression and proliferation capacity in Tfh cells (Rasheed et al., 2006) and in different PD-1+ CD4 T cell populations isolated from human tonsils (Wang et al., 2011).
DISCUSSION
We have investigated the distribution of HIV-specific and HIV-infected cells within different memory CD4 T cell populations isolated from lymph nodes in three study groups: (1) subjects with chronic infection and plasma viremia >5,000 HIV RNA copies; (2) ART-treated subjects with viremia <20 HIV RNA copies; and (3) LTNPs with plasma viremia in the range of a few 100 to 1,000 HIV RNA copies. The primary findings indicate that the memory lymph node CD4 T cell population corresponding to Tfh cells was: enriched in HIV-specific CD4 T cells; enriched in HIV-infected cells, i.e., harboring HIV DNA; and the most efficient in supporting virus replication and production.
Several studies (Schaerli et al., 2000; Breitfeld et al., 2000; Kim et al., 2001; Chtanova et al., 2004; Fazilleau et al., 2009a; Johnston et al., 2009; Ma et al., 2009; Bentebibel et al., 2011; Kroenke et al., 2012) using a panel of markers, including CXCR5, PD-1, ICOS, and Bcl-6—the latter being the lineage marker of Tfh cells—as well as the levels of expression of markers such as CXCR5 and PD-1, have been instrumental in distinguishing between Tfh cells and other populations of memory CD4 T cells. In the present study, Bcl-6 expression has been the strategy to identify Tfh cells, and the results obtained indicated that Bcl-6 expression was predominantly restricted to the lymph node CD4 T cell population expressing higher CXCR5 levels, PD-1, and ICOS. This CD4 T cell population also produced IL-21 and supported Ig production from B cells both functions typical of Tfh cells. Interestingly, the memory CD4 T cell population defined by the CXCR5−PD-1+Bcl-6− phenotype partially (∼50%) expressed ICOS and was also able to produce IL-21 and to support Ig production from B cells. These results are consistent with the previous study showing that the CXCR5−PD-1+ CD4 T cell population is located outside the GC (Wang et al., 2011) and that it may contain precursors of Tfh cells.
The relative percentage of Tfh cells and CXCR5−PD-1+ CD4 T cell populations significantly increased during the viremic phase of infection and decreased after suppression of viremia by ART. Consistent with this, the relative percentage of GC B cells and plasma cells was similarly modulated by the different levels of viremia. These results were obtained in a longitudinal study within the same patients. Our observations are consistent with previous studies performed in SIV infection (Hong et al., 2012; Petrovas et al., 2012) or cross-sectional studies in HIV infection (Lindqvist et al., 2012). These results indicated that the changes in the relative percentage of both the memory CD4 T cell and B cell populations were likely driven by HIV.
We show that both the Tfh and the CXCR5−PD-1+ CD4 T cell populations were enriched in HIV-specific CD4 T cells. A recent study (Lindqvist et al., 2012) has also shown enrichment of HIV-specific CD4 T cells within the Tfh cell population. The localization of these cell populations in the GCs or just outside is consistent with the critical role that HIV-specific Tfh cells play the generation of the HIV antibody response. This finding, together with the observations that the relative percentage of Tfh cells was significantly increased in viremic subjects and decreased after suppression of viremia by ART, strongly suggested that Tfh cells represented an important cellular compartment for HIV infection. In this regard, we have performed a comprehensive investigation to determine the role and the importance of Tfh and the other CD4 T cell populations as cellular compartments for HIV infection, replication, and production. These issues have not been previously investigated.
Previous studies have shown that HIV-specific cells were enriched in HIV-infected cells (Douek et al., 2002). We demonstrate that Tfh cells contained the higher proportion of CD4 T cells harboring HIV DNA. Our results are in agreement with a recent study in SIV infection (Brenchley et al., 2012). Furthermore, Hufert et al. (1997) showed before the discovery of Tfh cells that CD4 T cells residing within GCs and expressing CD57, which is expressed by a subset of Tfh cells, were preferentially infected by HIV. Our finding, although important, was not sufficient to delineate the role of Tfh in HIV infection. We therefore assessed the ability of Tfh cells to support HIV replication and production after HIV inoculation of different memory CD4 T cell populations in vitro and isolation of replication competent HIV from the different memory CD4 T cell populations. Tfh cells were clearly the most efficient in supporting both HIV replication and production under the above experimental conditions. In this regard, it should be underscored that the differences between Tfh cells and the other memory CD4 T cell populations were even more striking when replication competent HIV was isolated from patients with low viremia levels, such as LTNP, and from one patient experiencing a transient viral blip. Limit dilution experiments of the different cell populations would have been instrumental to quantify more precisely the differences in virus replication and production between the different memory CD4 T cell populations. However, these experiments are not feasible, particularly for Tfh and CXCR5−PD-1+ cell populations as a result of the limited total number of cells (range 30 to 100 million mononuclear cells) per lymph node, which prevents purification of large cell numbers from these minority cell populations. Of note, we have also shown that the memory CXCR5−PD-1+ CD4 T cell population was also enriched in HIV-specific CD4 T cells and second only to Tfh cells for HIV DNA load and ability to support virus replication and production. Collectively, the results presented here provide evidence that Tfh cells serve as the major CD4 T cell compartment for HIV infection, replication, and production.
The state of activation of Tfh and CXCR5−PD-1+ cells is consistent with the expression of PD-1 and their localization in anatomical sites of high levels of cell activation such as GCs. The likely enrichment of Ag-specific CD4 T cells (HIV and non-HIV specific) within the Tfh cell population and the state of activation of Tfh cells may render Tfh cells more susceptible to infection with HIV. Finally, the localization of HIV-specific Tfh in GCs and their exposure to the high virion load associated with follicular dendritic cells (Embretson et al., 1993; Pantaleo et al., 1993) may warrant infection of Tfh cells. These are all factors that may explain why Tfh cells represent the major CD4 T cell compartment for HIV infection, virus replication, and production. Because Tfh cells are resident with GCs, an important issue to address is why they are not eliminated by HIV-specific CD8 T cells. In this regard, CD8 T cells have generally limited access into GCs (Connick et al., 2007; King and Sprent, 2012), and it has been shown that the CD8 T cells entering GCs have limited cytotoxic capacity (Quigley et al., 2007). These are all potential factors contributing to create a unique microenvironment for HIV persistence within GCs. The limited proliferation of Tfh and CXCR5−PD-1+ cells supports the hypothesis that the expression of PD-1 in Tfh cells dissociates TCR signaling from proliferation (Kroenke et al., 2012) and also indicates that the state of activation associated with PD-1 expression in Tfh and CXCR5−PD-1+ cells is sufficient for supporting HIV replication but not cell proliferation.
The existence of an HIV latent cellular reservoir composed of long-lived resting memory CD4 T cells or the existence of residual virus replication that replenishes the HIV latent cell reservoir are the two main hypotheses proposed to explain HIV persistence after suppression of HIV replication by ART (Chun et al., 1997a,b; Finzi et al., 1997; Wong et al., 1997; Siliciano et al., 2003; Chomont et al., 2009). Residual HIV replication is supported by studies showing that low level plasma viremia, i.e., <20 HIV RNA copies/ml, can be measured using more sensitive assays in a large number of subjects (Dornadula et al., 1999; Palmer et al., 2003). The cells responsible for residual virus replication during ART have not yet been identified. Our results suggest that Tfh cells can be good candidates for supporting residual virus replication and eventually for the origin of viral blips (Di Mascio et al., 2003). We speculate that any time an infection occurs in an ART-treated HIV-infected subject, activation will occur in GCs within secondary lymphoid organs, such as lymph nodes and gut-associated lymphoid tissues, to generate the immune response against the encountered pathogen. Because of their fundamental role in the generation of the antibody response, Tfh cells will be stimulated, and either preexisting Tfh-infected cells shift to an activation state supporting higher levels of HIV replication or HIV spreading occurs in the de novo generated Tfh cells. This sudden boost in HIV replication remains a limited entity, i.e., viral blips in the range of a few 100 HIV RNA copies (Di Mascio et al., 2003), because antiviral therapy prevents efficient spreading of HIV. By supporting residual virus replication and viral blips, Tfh cells contribute to the replenishment of the latent reservoir. This possibility is also suggested by a recent study (Lüthje et al., 2012) demonstrating that Tfh cells can give rise to other populations of conventional memory CD4 T cells after the end of GC response. Other HIV cellular reservoirs, such as macrophages and dendritic cells (Stevenson, 2003), have not been investigated here and, together with Tfh cells, may represent additional candidates for supporting residual virus replication.
The development of immunological intervention strategies aimed toward a functional HIV cure, i.e., virus control in the absence of antiviral therapy, and ultimate HIV eradication has recently been the object of extensive debate in the scientific community. Among these immunological interventions, anti–PD-1 antibodies represent an attractive strategy because this treatment may both reactivate HIV replication in latently infected CD4 T cells, causing their death via HIV-mediated apoptosis, and rescue the function of HIV-specific CD8 T cells, thus leading to killing of infected CD4 T cells. Recent studies in nonhuman primates have shown partial control of viral load after anti–PD-1 treatment (Finnefrock et al., 2009; Velu et al., 2009). The identification of Tfh (and, to a lower extent, PD-1+CXCR5−) cells as the major CD4 T cell compartment for HIV replication and production may help to design more selective strategies to tackle the cells that are either responsible for residual virus replication or that support high levels of virus replication and production. Development of bi-specific antibodies to CD4 and PD-1 coupled to toxins may represent an effective strategy to purge HIV-infected Tfh and CXCR5−PD-1+ cells.
MATERIALS AND METHODS
Study groups and isolation of mononuclear cells.
Lymph node biopsies were performed in HIV-infected subjects with CD4 T cell counts >400 cells/mm3 and plasma viremia >5,000 HIV RNA copies/ml enrolled in two therapeutic studies evaluating different antiviral drugs combinations. 26 subjects were investigated in the present study. Lymph node biopsies were performed at week 0 before the initiation of therapy and week 72 after therapy. Lymph node biopsies were also obtained from three LTNP. The criteria for defining nonprogressive disease included: documented HIV infection for >8 yr, CD4 T cell counts >500 per µl, and plasma viremia <5,000 HIV RNA copies/ml. With regard to HIV-negative subjects, lymph node biopsies (inguinal lymph nodes) were performed in 13 subjects who underwent vascular (varicose vein stripping) and general (uncomplicated bilateral inguinal herniorrhaphy) surgery. These studies were approved by the Institutional Review Board of the Centre Hospitalier Universitaire Vaudois, and all subjects gave written informed consent.
Isolation of lymph node mononuclear cells.
Lymph node mononuclear cells were isolated by mechanical disruption as previously described (Pantaleo et al., 1991) and cells were cryopreserved in liquid nitrogen.
Antibodies.
The following antibodies were used: PerCP-Cy5.5–conjugated anti-CD278 (ICOS; clone C398.4A), PerCP-Cy5.5–conjugated anti–IL-2 (clone MQ1-17H12), PE-conjugated anti–IL-21 (clone 3A3-N2), and PE-conjugated anti–Ki-67 (clone Ki-67; BioLegend); PeCy7-conjugated anti-CD279 (PD-1; clone EH12.1), Alexa Fluor 647– or Alexa Fluor 488–conjugated anti-CXCR5 (clone RF8B2), APC-H7–conjugated anti-CD3 (clone SK7), PerCP-Cy5.5–conjugated anti-CD8 (clone SK1), PB-, APC-, or PE-CF594–conjugated anti-CD4 (clone RPA-T4), APC-conjugated anti-TNF (clone MabII), Alexa Fluor 700–conjugated anti–IFN-γ (clone B27), PE-conjugated anti-IgD (clone IA6-2), Alexa Fluor 488–conjugated anti–Bcl-6 (clone K112-91), and PeCy5-conjugated anti-CD45RA (clone HI100; BD); PE-conjugated anti-CXCR5 (clone 51505.111; R&D Systems); EFluor 625NC-conjugated anti-CD8 (clone RPA-T8) and PeCy7-conjugated anti-CD27 (clone O323; eBioscience); ECD-conjugated anti-CD45RA (clone 2H4) and ECD-conjugated anti-CD4 (clone T4; Beckman Coulter); APC–Alexa Fluor 750–conjugated anti-CD19 (clone SJ25-C1) and PE–Texas red–conjugated anti-CD38 (clone HIT2; Life Technologies).
Flow cytometry.
Data were acquired on LSR SORP four lasers (405, 488, 532, and 633 nm), analyzed using FlowJo (v9.4.11; Tree Star) and SPICE (v5.21; developed by M. Roederer, National Institutes of Health; Roederer et al., 2011). At least 100,000 events were acquired for each sample.
Intracellular cytokine staining.
Cryopreserved lymph node mononuclear cells were thawed and resuspended in complete RPMI medium (Gibco; Life Technologies; 10% heat-inactivated FBS [Institut de Biotechnologies Jacques Boy], 100 IU/ml penicillin, and 100 µg/ml streptomycin [BioConcept]) at 106 cells/ml and stimulated with 100 ng/ml PMA (Sigma-Aldrich) and 1 µg/ml ionomycin (Sigma-Aldrich) in the presence of Brefeldin A (BD) for 6 h at 37°C or with 1 µg/ml of broad spectrum subtype B HIV-1 PTE peptide pools encompassing Gag, Pol, and Env proteins (Comprehensive T Cell Vaccine Immune Monitoring Consortium) in the presence of Brefeldin A (BD) for 18 h at 37°C (Li et al., 2006). Dead cells were excluded using the Aqua LIVE/DEAD stain kit at 4°C for 25 min (Life Technologies). Cells were stained at 4°C for 25 min with anti-CD3 APC-H7, anti-CD4 ECD, or PE-CF594, anti-CD8 EFluor 625NC, anti-CXCR5 Alexa Fluor 488, and anti-PD-1 PeCy7, permeabilized for 20 min at 20°C with Cytofix/Cytoperm (BD), washed and stained at 4°C for 25 min with anti–IL-2 PerCP-Cy5.5, anti-TNF APC, anti–IFN-γ Alexa Fluor 700, and anti–IL-21 PE. Frequencies of cytokine-producing CD4 T cell population were assessed by flow cytometry.
Ki-67 and Bcl-6 expression.
Lymph node mononuclear cells were thawed and dead cells were excluded using the aqua LIVE/DEAD stain kit at 4°C for 25 min. Cells were stained with anti-CD3 APC-H7, anti-CD8 PerCp-Cy5.5, anti-CD4 PB, anti-CD45RA PeCy5, anti-CXCR5 APC, and anti–PD-1 PeCy7 at 4°C for 25 min, and then permeabilized for 1 h at 4°C with Foxp3 Fixation/Permeabilization kit (eBioscience), washed, and stained with anti–Ki-67 PE and anti–Bcl-6 Alexa Fluor 488 at 4°C for 25 min. Expression of Ki-67 on CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cell populations was assessed by flow cytometry.
Sorting of CD4 T cell populations.
Cryopreserved lymph node mononuclear cells were thawed and stained with the violet LIVE/DEAD stain kit (4°C; 15 min) and then with anti-CD3 APC-H7, anti-CD4 APC, anti-CD45RA ECD, anti-CXCR5 PE, and anti–PD-1 PeCy7 at 4°C for 25 min, and the CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cell populations were sorted using a FACSAria (BD). In all sorting experiments, the grade of purity of the sorted cell populations was >97%.
B cell/T cell co-culture assay.
B cells from lymph node mononuclear cells were enriched using CD19-positive selection (STEMCELL Technologies), and dead cells were excluded using the violet LIVE/DEAD stain kit at 4°C for 15 min and stained at 4°C for 25 min with the anti-CD19 APC–Alexa Fluor 750, anti-IgD PE, and anti-CD38 PE–Texas red mAbs. Naive B cells (CD19+IgD+CD38−), memory B cells (CD19+IgD−CD38−), and GC B cells (CD19+IgD−CD38+) were sorted from enriched B cell fraction (CD19 positive) using a FACSAria. The CD19-negative fraction was stained with the violet LIVE/DEAD stain kit at 4°C for 15 min and stained at 4°C for 25 min with the anti-CD3 APC-H7, anti-CD4 ECD, anti-CXCR5 Alexa Fluor 488, and anti–PD-1 PeCy7. CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cell populations were sorted using a FACSAria. Sorted CD4 T cell populations (105 cells) were co-cultured with sorted autologous B cell populations (105 cells) in the presence of 250 ng/ml SEB (Sigma-Aldrich) in 96-well U-bottom plates as previously described (Morita et al., 2011). As positive controls, each B cell population was cultured alone in presence of 5 × 104 pfu/ml of inactivated Staphylococcus aureus and 25 µg/ml CpG. Secretion of IgM, IgG1, and IgA was assessed at day 5 by Luminex (Bio-Rad Laboratories).
CD4 T cell proliferation.
Sorted CD4 T cell populations (CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cells; 2 × 105 cells) were labeled with CFSE at 37°C for 7min (Life Technologies) and cultured for 5 d at 37°C and 5% CO2 in 96-well U-bottom plates coated or not with 50 µg/ml anti-CD3 (BD) and 5 µg/ml anti-CD28 (BD) in complete RPMI. The proliferation of the four CD4 T cell populations was assessed by quantifying the percentage of CFSE low cells on a LSR SORP cell analyzer (BD).
Assessment of p24 production of CD4 T cell populations from HIV-infected subject with high HIV viremia (viremia >15,000 HIV RNA copies/ml; n = 5).
Sorted CD4 T cell populations (CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cells; >5 × 104 cells) were stimulated for 2 d at 37°C and 5% CO2 in 96-well U-bottom plates coated with 50 µg/ml anti-CD3 (BD) and 5 µg/ml anti-CD28 (BD) in complete RPMI. Supernatants were collected at days 0, 2, and 5 and analyzed for the presence of p24 Ag by electrochemiluminescence (ECL; HIVAg; Eleciys and Cobas e analyzers; Roche).
Assessment of p24 production of CD4 T cell populations from HIV-infected subjects with low HIV viremia (viremia <2,000 HIV RNA copies/ml; n = 7).
Sorted CD4 T cell populations (CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cells; >5 × 104 cells) were stimulated for 2 d at 37°C and 5% CO2 in 96-well U-bottom plates coated with 50 µg/ml anti-CD3 (BD) and 5 µg/ml anti-CD28 (BD) and cultured with allogeneic CD8-depleted blood mononuclear cells (Miltenyi Biotec) of HIV-uninfected subjects (1:1 ratio) in complete RPMI containing 50 U/ml IL-2. Supernatants were collected at days 0, 3, and 7 and analyzed for the presence of p24 Ag by ECL.
Assessment of HIV infectivity of CD4 T cell populations of HIV uninfected subjects.
Sorted CD4 T cell populations (>5 × 104 cells) were exposed to 300 pg HIVLAV virus for 3 h at 37°C and 5% CO2 and extensively washed. Cells were then stimulated for 2 d at 37°C and 5% CO2 in 96-well U-bottom plates coated with 50 µg/ml anti-CD3 (BD) and 5 µg/ml anti-CD28 (BD) and cultured in complete RPMI containing 50 U/ml IL-2. Supernatants were collected at day 3 and analyzed for the presence of p24 Ag by ECL.
HIV DNA quantification.
DNA from sorted CD4 T cell populations (CXCR5−PD-1−, CXCR5−PD-1+, CXCR5+PD-1−, and CXCR5+PD-1+ CD4 T cells; 105 cells) was purified using QIAmpDNA Micro kit (QIAGEN). HIV-1 DNA levels were determined by a TaqMan real-time PCR assay targeting HIV-1 gag gene using the StepOnePlus Real-Time PCR System (Applied Biosystems). qPCR was performed in a final volume of 25 µl using 5 µl of DNA, 2.5 µl of 10× PCR buffer (Invitrogen), 1.75 µl of 50 mM MgCl2 (Invitrogen), 0.5 µl of 10 mM dNTPs (Invitrogen), 0.5 µM of each primer and 0.15 µM of probe, 0.5 µl ROX reference dye (Invitrogen), and 0.1 µl Platinum Taq (Invitrogen). To quantify the number of cells in each reaction, qPCR for albumin gene was performed in the same plate using the same PCR conditions. Previously published primer-probe combinations were used for both albumin and HIV-gag amplification (Douek et al., 2002). Two standard curves were included in each run: the first ranging from 3 to 3 × 106 copies of a plasmid containing the full-length HIV-1 genome and the second ranging from 20 to 2 × 106 copies of a plasmid containing albumin amplicon. Extraction and quantification were validated on 10-fold dilutions of Ach2 cells diluted into an equivalent number of uninfected cells (5 × 104 total cells). HIV-DNA load was expressed as number of copies per 106 cells.
Statistical analyses.
Statistical significance (p-values) was obtained using one-way ANOVA (Kruskal-Wallis test), followed by Student’s t test for multiple comparisons or a Spearman rank test for correlations. Statistical analyses of global cytokine profiles (pie charts) were performed by partial permutation tests using the SPICE software as previously described (Roederer et al., 2011).
Acknowledgments
We are grateful to Nicole Grandchamp and Gregory Gonzalez for technical assistance and to Alexandre Harari for discussion.
This work was funded by the Swiss National Science Foundation.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Ag
- antigen
- ART
- antiretroviral therapy
- ECL
- electrochemiluminescence
- GC
- germinal center
- ICOS
- inducible T cell co-stimulator
- LTNP
- long-term nonprogressor
- PTE
- potential T cell epitope
- SEB
- Staphylococcus enterotoxin B
- SIV
- simian immunodeficiency virus
References
- Allen C.D., Okada T., Cyster J.G. 2007. Germinal-center organization and cellular dynamics. Immunity. 27:190–202 10.1016/j.immuni.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery D.T., Deenick E.K., Ma C.S., Suryani S., Simpson N., Chew G.Y., Chan T.D., Palendira U., Bustamante J., Boisson-Dupuis S., et al. 2010. B cell–intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207:155–171 10.1084/jem.20091706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel S.E., Schmitt N., Banchereau J., Ueno H. 2011. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Natl. Acad. Sci. USA. 108:E488–E497 10.1073/pnas.1100898108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts M.R., Ambrozak D.R., Douek D.C., Bonhoeffer S., Brenchley J.M., Casazza J.P., Koup R.A., Picker L.J. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983–11991 10.1128/JVI.75.24.11983-11991.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Förster R. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192:1545–1552 10.1084/jem.192.11.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., Douek D.C. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 10.1084/jem.20040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J.M., Vinton C., Tabb B., Hao X.P., Connick E., Paiardini M., Lifson J.D., Silvestri G., Estes J.D. 2012. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 120:4172–4181 10.1182/blood-2012-06-437608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N., El-Far M., Ancuta P., Trautmann L., Procopio F.A., Yassine-Diab B., Boucher G., Boulassel M.R., Ghattas G., Brenchley J.M., et al. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15:893–900 10.1038/nm.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T., Tangye S.G., Newton R., Frank N., Hodge M.R., Rolph M.S., Mackay C.R. 2004. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173:68–78 [DOI] [PubMed] [Google Scholar]
- Chun T.W., Carruth L., Finzi D., Shen X., DiGiuseppe J.A., Taylor H., Hermankova M., Chadwick K., Margolick J., Quinn T.C., et al. 1997a. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 387:183–188 10.1038/387183a0 [DOI] [PubMed] [Google Scholar]
- Chun T.W., Stuyver L., Mizell S.B., Ehler L.A., Mican J.A., Baseler M., Lloyd A.L., Nowak M.A., Fauci A.S. 1997b. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 94:13193–13197 10.1073/pnas.94.24.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick E., Mattila T., Folkvord J.M., Schlichtemeier R., Meditz A.L., Ray M.G., McCarter M.D., Mawhinney S., Hage A., White C., Skinner P.J. 2007. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J. Immunol. 178:6975–6983 [DOI] [PubMed] [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- Di Mascio M., Markowitz M., Louie M., Hogan C., Hurley A., Chung C., Ho D.D., Perelson A.S. 2003. Viral blip dynamics during highly active antiretroviral therapy. J. Virol. 77:12165–12172 10.1128/JVI.77.22.12165-12172.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornadula G., Zhang H., VanUitert B., Stern J., Livornese L., Jr, Ingerman M.J., Witek J., Kedanis R.J., Natkin J., DeSimone J., Pomerantz R.J. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 282:1627–1632 10.1001/jama.282.17.1627 [DOI] [PubMed] [Google Scholar]
- Douek D.C., Brenchley J.M., Betts M.R., Ambrozak D.R., Hill B.J., Okamoto Y., Casazza J.P., Kuruppu J., Kunstman K., Wolinsky S., et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 417:95–98 10.1038/417095a [DOI] [PubMed] [Google Scholar]
- Ehrhardt G.R., Hsu J.T., Gartland L., Leu C.M., Zhang S., Davis R.S., Cooper M.D. 2005. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 202:783–791 10.1084/jem.20050879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J.L., Burke A., Racz P., Tenner-Racz K., Haase A.T. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 362:359–362 10.1038/362359a0 [DOI] [PubMed] [Google Scholar]
- Fazilleau N., Mark L., McHeyzer-Williams L.J., McHeyzer-Williams M.G. 2009a. Follicular helper T cells: lineage and location. Immunity. 30:324–335 10.1016/j.immuni.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N., McHeyzer-Williams L.J., Rosen H., McHeyzer-Williams M.G. 2009b. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10:375–384 10.1038/ni.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnefrock A.C., Tang A., Li F., Freed D.C., Feng M., Cox K.S., Sykes K.J., Guare J.P., Miller M.D., Olsen D.B., et al. 2009. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J. Immunol. 182:980–987 [DOI] [PubMed] [Google Scholar]
- Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 278:1295–1300 10.1126/science.278.5341.1295 [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab U., Stein H. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710–1715 [PubMed] [Google Scholar]
- Guadalupe M., Reay E., Sankaran S., Prindiville T., Flamm J., McNeil A., Dandekar S. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717 10.1128/JVI.77.21.11708-11717.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.J., Amancha P.K., Rogers K., Ansari A.A., Villinger F. 2012. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J. Immunol. 188:3247–3256 10.4049/jimmunol.1103138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufert F.T., van Lunzen J., Janossy G., Bertram S., Schmitz J., Haller O., Racz P., von Laer D. 1997. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 11:849–857 10.1097/00002030-199707000-00003 [DOI] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 325:1006–1010 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Rott L.S., Clark-Lewis I., Campbell D.J., Wu L., Butcher E.C. 2001. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center–localized subset of CXCR5+ T cells. J. Exp. Med. 193:1373–1381 10.1084/jem.193.12.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C., Sprent J. 2012. Emerging cellular networks for regulation of T follicular helper cells. Trends Immunol. 33:59–65 10.1016/j.it.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Kroenke M.A., Eto D., Locci M., Cho M., Davidson T., Haddad E.K., Crotty S. 2012. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 188:3734–3744 10.4049/jimmunol.1103246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Malhotra U., Gilbert P.B., Hawkins N.R., Duerr A.C., McElrath J.M., Corey L., Self S.G. 2006. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 24:6893–6904 10.1016/j.vaccine.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Lindqvist M., van Lunzen J., Soghoian D.Z., Kuhl B.D., Ranasinghe S., Kranias G., Flanders M.D., Cutler S., Yudanin N., Muller M.I., et al. 2012. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 122:3271–3280 10.1172/JCI64314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthje K., Kallies A., Shimohakamada Y., TBelz G.T., Light A., Tarlinton D.M., Nutt S.L. 2012. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 13:491–498 10.1038/ni.2261 [DOI] [PubMed] [Google Scholar]
- Ma C.S., Suryani S., Avery D.T., Chan A., Nanan R., Santner-Nanan B., Deenick E.K., Tangye S.G. 2009. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol. Cell Biol. 87:590–600 10.1038/icb.2009.64 [DOI] [PubMed] [Google Scholar]
- Mattapallil J.J., Douek D.C., Hill B., Nishimura Y., Martin M., Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 434:1093–1097 10.1038/nature03501 [DOI] [PubMed] [Google Scholar]
- McDougal J.S., Mawle A., Cort S.P., Nicholson J.K., Cross G.D., Scheppler-Campbell J.A., Hicks D., Sligh J. 1985. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J. Immunol. 135:3151–3162 [PubMed] [Google Scholar]
- Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G., Foucat E., Dullaers M., Oh S., Sabzghabaei N., et al. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 34:108–121 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Feng C.G., Qi C.F., Cheng J., Sher A., Morse H.C., III, Liu C., Schwartzberg P.L., Leonard W.J. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science. 298:1630–1634 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]
- Palmer S., Wiegand A.P., Maldarelli F., Bazmi H., Mican J.M., Polis M., Dewar R.L., Planta A., Liu S., Metcalf J.A., et al. 2003. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41:4531–4536 10.1128/JCM.41.10.4531-4536.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P.A., Schnittman S.M., Kotler D.P., Fauci A.S. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA. 88:9838–9842 10.1073/pnas.88.21.9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J.F., Butini L., Montroni M., Fox C.H., Orenstein J.M., Kotler D.P., Fauci A.S. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 362:355–358 10.1038/362355a0 [DOI] [PubMed] [Google Scholar]
- Petrovas C., Yamamoto T., Gerner M.Y., Boswell K.L., Wloka K., Smith E.C., Ambrozak D.R., Sandler N.G., Timmer K.J., Sun X., et al. 2012. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 122:3281–3294 10.1172/JCI63039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M.F., Gonzalez V.D., Granath A., Andersson J., Sandberg J.K. 2007. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol. 37:3352–3362 10.1002/eji.200636746 [DOI] [PubMed] [Google Scholar]
- Rasheed A.U., Rahn H.P., Sallusto F., Lipp M., Müller G. 2006. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 36:1892–1903 10.1002/eji.200636136 [DOI] [PubMed] [Google Scholar]
- Roederer M., Nozzi J.L., Nason M.C. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 79:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I., Wei C., Lee F.E., Anolik J. 2008. Phenotypic and functional heterogeneity of human memory B cells. Semin. Immunol. 20:67–82 10.1016/j.smim.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P., Willimann K., Lang A.B., Lipp M., Loetscher P., Moser B. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192:1553–1562 10.1084/jem.192.11.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S.M., Lane H.C., Greenhouse J., Justement J.S., Baseler M., Fauci A.S. 1990. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci. USA. 87:6058–6062 10.1073/pnas.87.16.6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B., Kovacs C., Gange S.J., Siliciano R.F. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727–728 10.1038/nm880 [DOI] [PubMed] [Google Scholar]
- Stevenson M. 2003. HIV-1 pathogenesis. Nat. Med. 9:853–860 10.1038/nm0703-853 [DOI] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T.L., Dempsey M.P., Lamonica C.A. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S., et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
- Velu V., Titanji K., Zhu B., Husain S., Pladevega A., Lai L., Vanderford T.H., Chennareddi L., Silvestri G., Freeman G.J., et al. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 458:206–210 10.1038/nature07662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Hillsamer P., Kim C.H. 2011. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunol. 12:53 10.1186/1471-2172-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Anolik J., Cappione A., Zheng B., Pugh-Bernard A., Brooks J., Lee E.H., Milner E.C., Sanz I. 2007. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178:6624–6633 [DOI] [PubMed] [Google Scholar]
- Wirths S., Lanzavecchia A. 2005. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 35:3433–3441 10.1002/eji.200535364 [DOI] [PubMed] [Google Scholar]
- Wong J.K., Hezareh M., Günthard H.F., Havlir D.V., Ignacio C.C., Spina C.A., Richman D.D. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 278:1291–1295 10.1126/science.278.5341.1291 [DOI] [PubMed] [Google Scholar]