Abstract
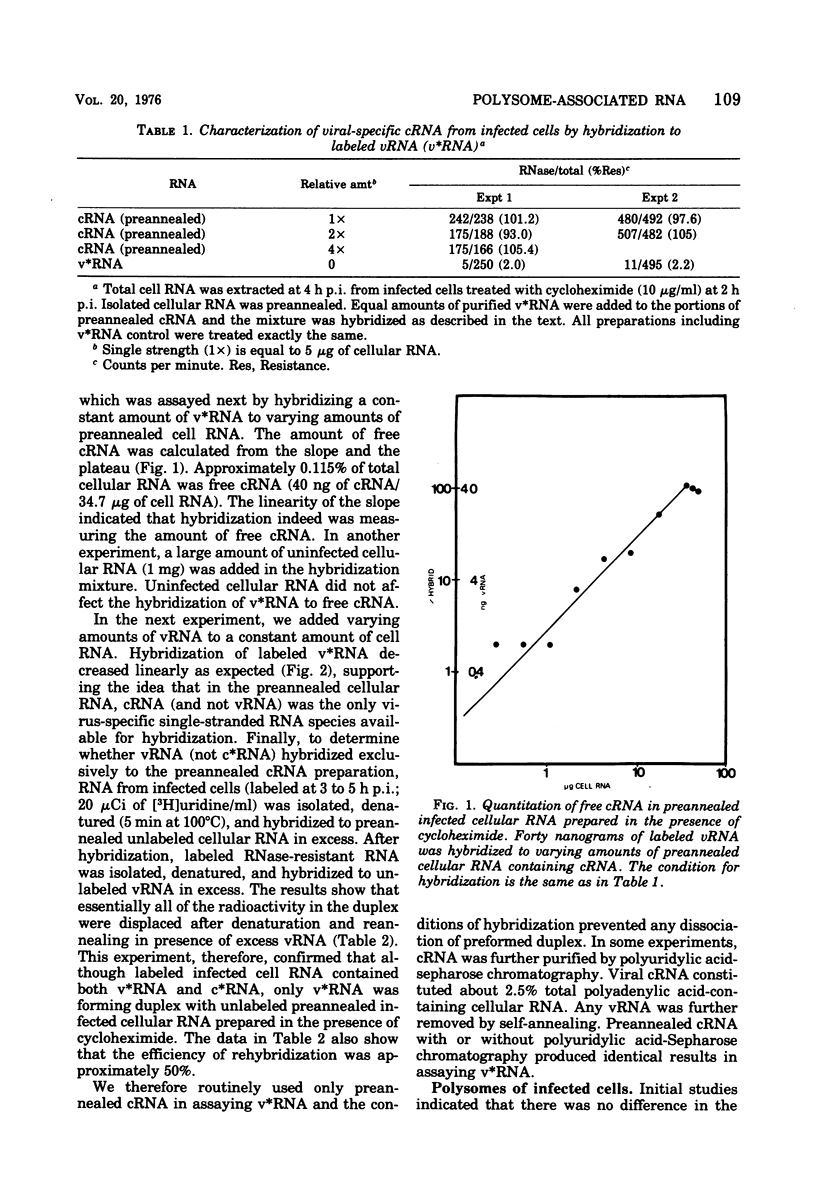
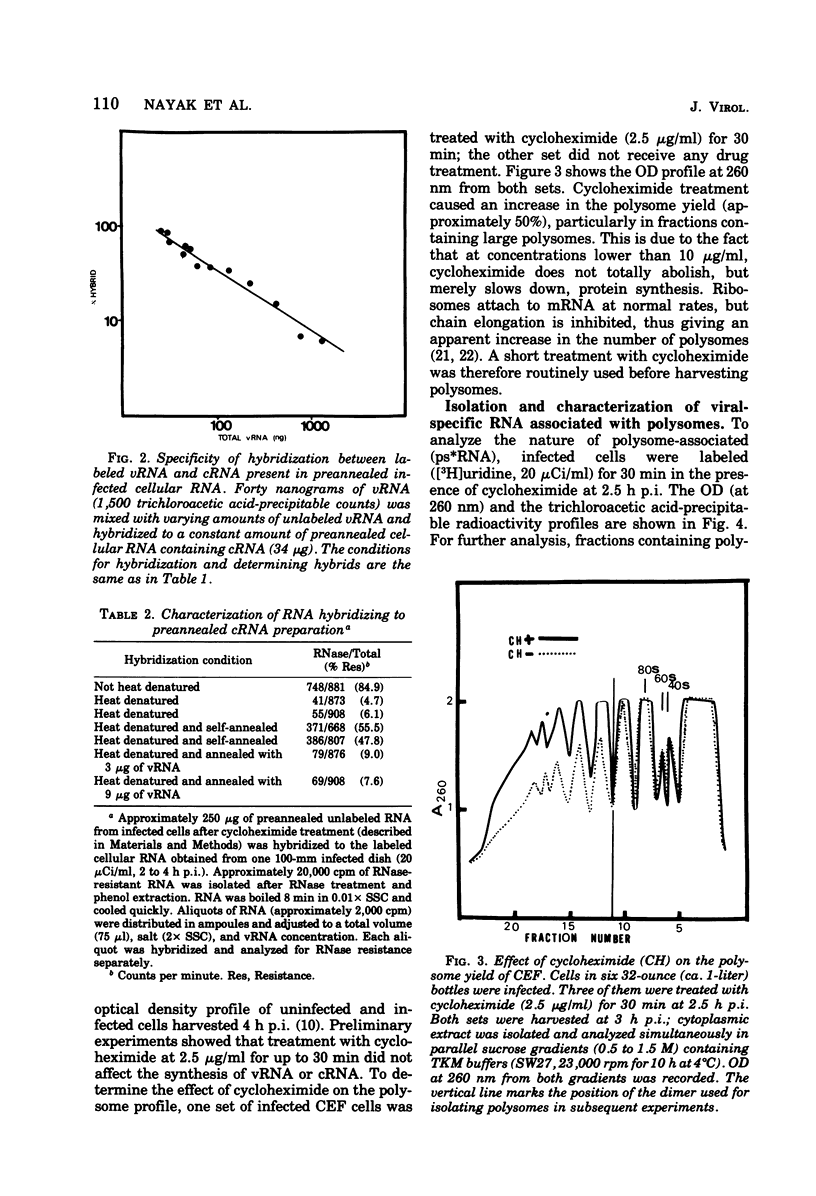
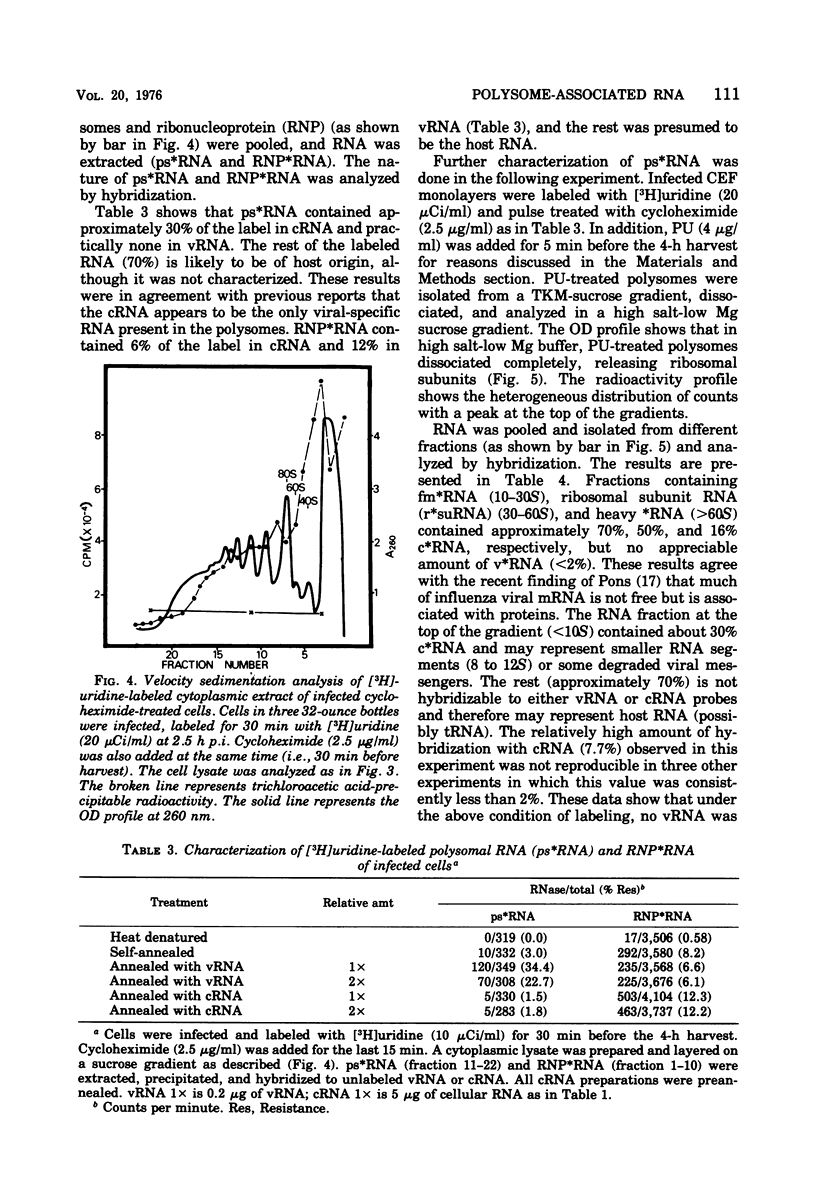
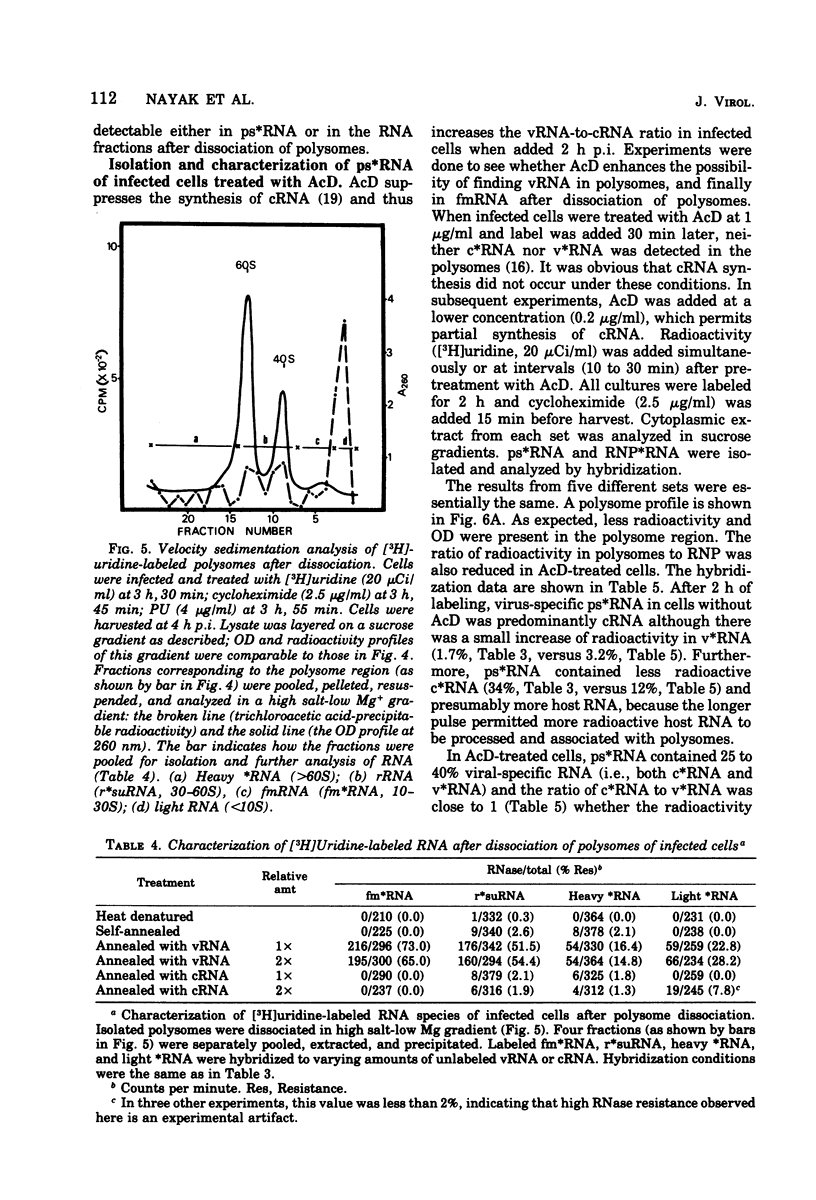
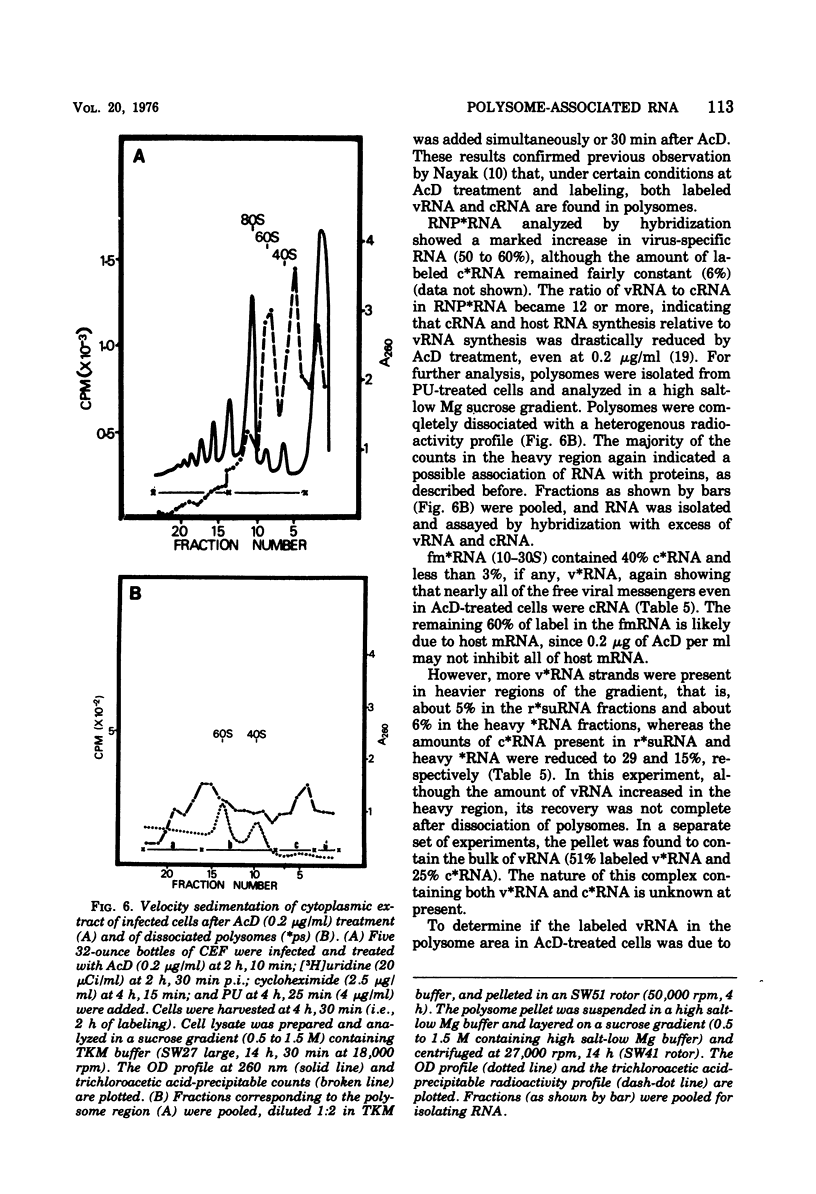
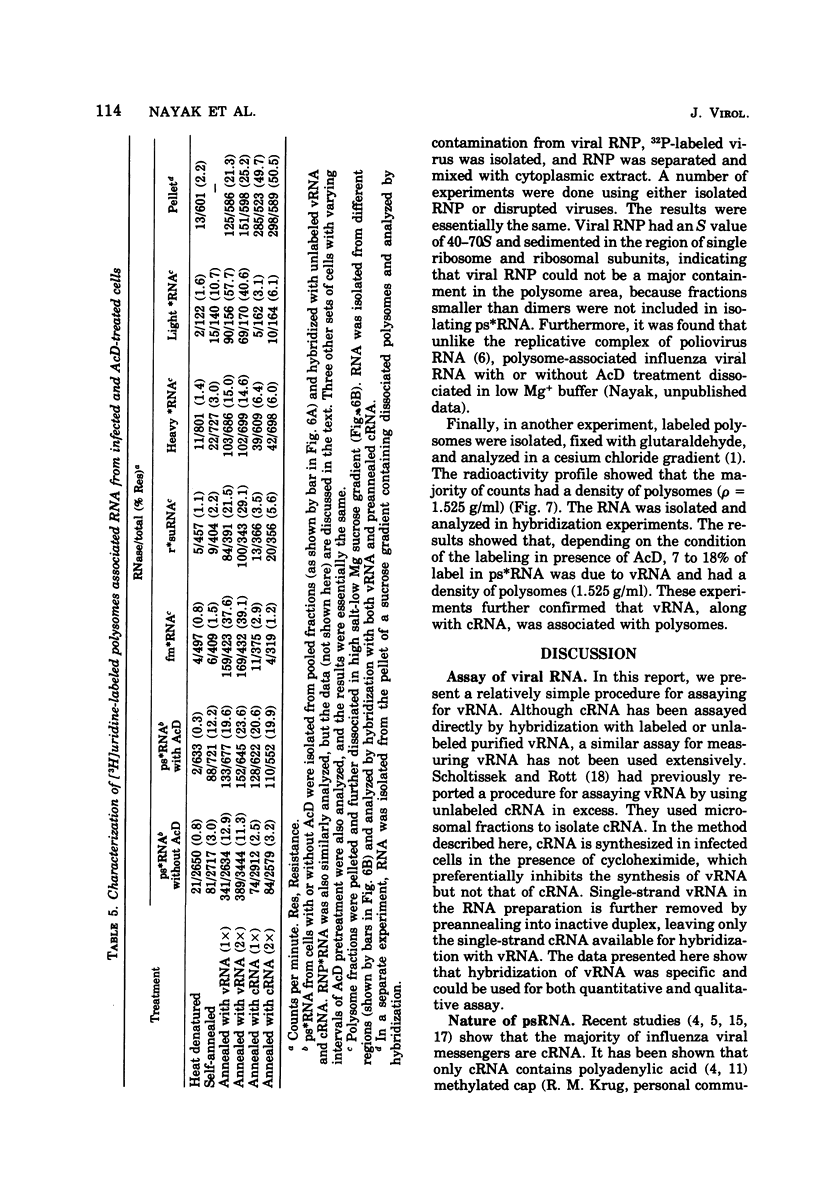
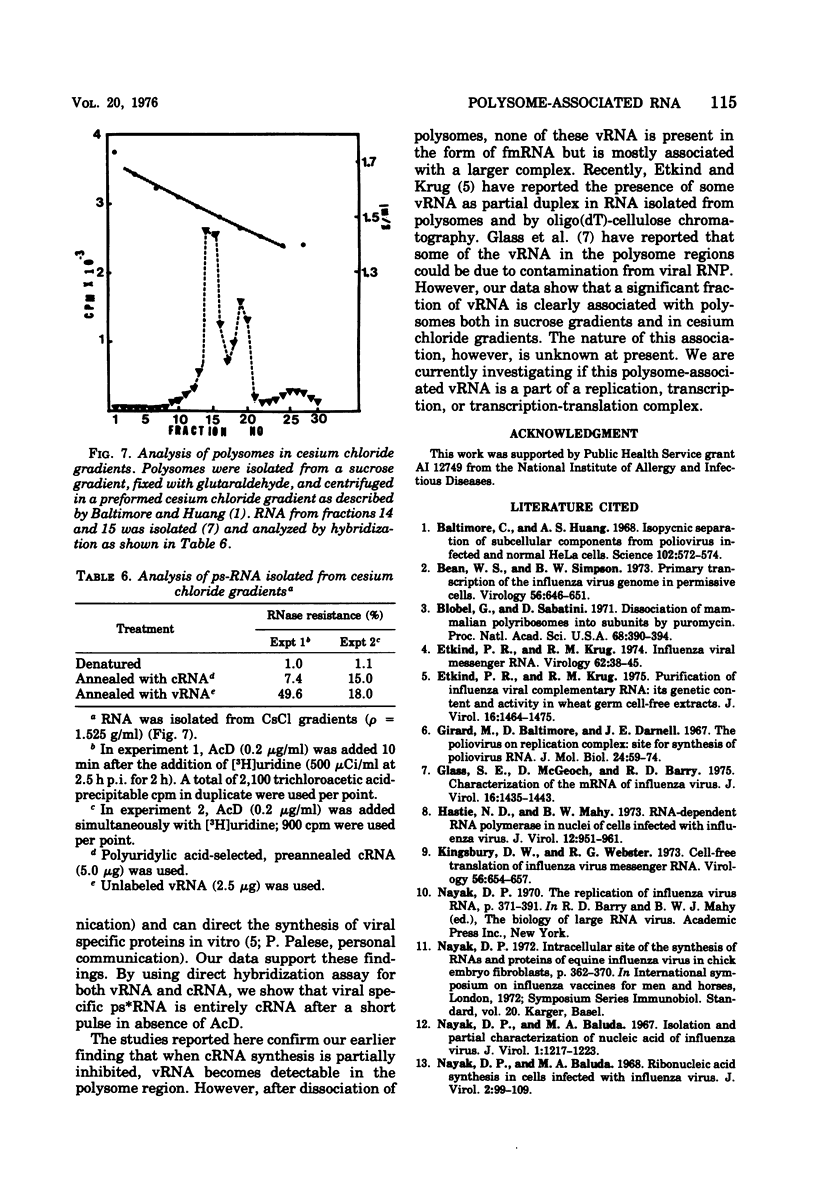
Virus-specific polysome-associated RNA (psRNA) and RNA after dissociation of polysomes were analyzed by direct hybridization with unlabeled viral RNA (vRNA) and complementary RNA (cRNA). psRNA after a 30-min pulse with [3H]uridine contained 28% labeled cRNA, 70% host RNA, and no vRNA. After dissociation, psRNA sedimented heterogeneously. Heavy RNA (greater than 60S), ribosomal subunit RNA (rsuRNA, 30-60S), free mRNA (fmRNA, 10-30S), and light RNA (less than 10S) contained 16%, 54%, 70% and 28% cRNA, respectively, but no vRNA. When actinomycin D (AcD) was added at 2 h postinfection, the nature of the psRNA depended on the concentration of AcD and the condition of the labeling. At AcD concentrations of 1 mug or more per ml, no detectable vRNA or cRNA was associated with polysomes. At 0.2 mug of AcD per ml (a concentration that partially inhibited cRNA synthesis) and 2 h of labeling at 2.5 h postinfection, psRNA contained 40% viral-specific RNA, which included both vRNA and cRNA in almost equal amounts. When polysomes were dissociated, however, viral-specific fm RNA from AcD-treated cells contained exclusively cRNA and no detectable vRNA. Increasing amounts of labeled vRNA were present in the heavy region of the gradient (and in the pellet), which also contained varying amounts of cRNA. The labeled vRNA appears to be associated with polysomes in a cesium chloride density gradient (rho = 1.525 g/ml). Although we have ruled out the trivial explanation of viral ribonucleoprotein contamination,the nature of the complex containing both polysomes and vRNA is unknown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Mahy B. W. RNA-dependent RNA polymerase in nuclei of cells infected with influenza virus. J Virol. 1973 Nov;12(5):951–961. doi: 10.1128/jvi.12.5.951-961.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Webster R. G. Cell-free translation of influenza virus messenger RNA. Virology. 1973 Dec;56(2):654–657. doi: 10.1016/0042-6822(73)90069-x. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Baluda M. A. Isolation and partial characterization of nucleic acid of influenza virus. J Virol. 1967 Dec;1(6):1217–1223. doi: 10.1128/jvi.1.6.1217-1223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Baluda M. A. Ribonucleic acid synthesis in cells infected with influenza virus. J Virol. 1968 Feb;2(2):99–109. doi: 10.1128/jvi.2.2.99-109.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. Influenza virus messenger ribonucleoprotein. Virology. 1975 Sep;67(1):209–218. doi: 10.1016/0042-6822(75)90418-3. [DOI] [PubMed] [Google Scholar]
- Pons M. W. Isolation of influenza virus ribonucleoprotein from infected cells. Demonstration of the presence of negative-stranded RNA in viral RNP. Virology. 1971 Oct;46(1):149–160. doi: 10.1016/0042-6822(71)90014-6. [DOI] [PubMed] [Google Scholar]
- Pons M. W. Studies on the replication of influenza virus RNA. Virology. 1972 Mar;47(3):823–832. doi: 10.1016/0042-6822(72)90574-0. [DOI] [PubMed] [Google Scholar]
- Pons M. W. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973 Jan;51(1):120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Hybridization studies with influenza virus RNA. Virology. 1969 Nov;39(3):400–407. doi: 10.1016/0042-6822(69)90088-9. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Synthesis in vivo of influenza virus plus and minus strand RNA and its preferential inhibition by antibiotics. Virology. 1970 Apr;40(4):989–996. doi: 10.1016/0042-6822(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Siegert W., Bauer G., Hofschneider P. H. Direct evidence for messenger activity of influenza virion RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2960–2963. doi: 10.1073/pnas.70.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H., PENMAN S. EFFECT OF CYCLOHEXIMIDE ON RIBOSOMAL AGGREGATES ENGAGED IN PROTEIN SYNTHESIS IN VITRO. Biochim Biophys Acta. 1964 Jul 22;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]



