Mice lacking activity of the intramembrane protease SPPL2A exhibit humoral immunodeficiency and lack mature B cell subsets.
Abstract
Druggable proteins required for B lymphocyte survival and immune responses are an emerging source of new treatments for autoimmunity and lymphoid malignancy. In this study, we show that mice with an inactivating mutation in the intramembrane protease signal peptide peptidase–like 2A (SPPL2A) unexpectedly exhibit profound humoral immunodeficiency and lack mature B cell subsets, mirroring deficiency of the cytokine B cell–activating factor (BAFF). Accumulation of Sppl2a-deficient B cells was rescued by overexpression of the BAFF-induced survival protein B cell lymphoma 2 (BCL2) but not BAFF and was distinguished by low surface BAFF receptor and IgM and IgD B cell receptors. CD8-negative dendritic cells were also greatly decreased. SPPL2A deficiency blocked the proteolytic processing of CD74 MHC II invariant chain in both cell types, causing dramatic build-up of the p8 product of Cathepsin S and interfering with earlier steps in CD74 endosomal retention and processing. The findings illuminate an important role for the final step in the CD74–MHC II pathway and a new target for protease inhibitor treatment of B cell diseases.
B cell maturation starts in the bone marrow but is completed in the spleen (Hardy et al., 2007). Survival of IgM+ splenic B cells is linked to the antiapoptotic B cell lymphoma 2 (BCL2) family of proteins and their opposing proapoptotic antagonist, BCL2-interacting mediator of cell death (BIM; Enders et al., 2003), and depends on “tonic” signals from surface IgM and IgD B cell antigen receptors transmitted through spleen tyrosine kinase (SYK), Bruton’s tyrosine kinase (BTK), and phosphatidylinositol 3 kinase (Srinivasan et al., 2009). Starting from the transitional 2 (T2) stage, B cells also depend on survival signals provided by a circulating cytokine, B cell–activating factor (BAFF), engaging the BAFF receptor (BAFFR; Khan, 2009). BCRs and BAFFR signal via pathways that activate transcription factors of the NF-κB family, and these play essential roles in mediating survival of B cells (Siebenlist et al., 2005).
CD74, also called MHC II invariant chain or Ii, is a type 2 transmembrane protein with an enigmatic role in B cell survival. CD74 has a well-established function in antigen presentation: it forms nonameric complexes with nascent MHC II molecules, shields the MHC II peptide–binding groove against premature peptide binding, and directs MHC II to endocytic compartments where exogenous antigens are processed into MHC II–binding peptides (Villadangos et al., 1999). CD74–MHC II complexes reach endocytic compartments via transient appearance at the plasma membrane and rapid clathrin-mediated endocytosis (Dugast et al., 2005; McCormick et al., 2005). Once in endosomes, the lumenal domain of CD74 undergoes a series of C-terminal proteolytic cleavages (Villadangos et al., 1999). Cathepsin S is required in B cells and DCs for the penultimate CD74 cleavage, which releases the class II invariant chain peptide (CLIP) and an 8-kD N-terminal transmembrane CD74 peptide that appears to be rapidly processed by an unknown intramembrane-cleaving protease (Villadangos et al., 1999; Matza et al., 2002b).
An unexpected finding in Cd74 knockout mice was the occurrence of a modest block at the transitional stage in B cell development, characterized by a failure to up-regulate IgD and CD23 normally in H2b (Shachar and Flavell, 1996) but not in H2k mouse strains (Zimmermann et al., 1999). One line of evidence indicated this defect was caused by the absence of the final CD74 cleavage product: an unstable cytosolic N-terminal 42-aa peptide with NF-κB–promoting activity (Matza et al., 2002b). In contrast, two parallel studies indicated that deficiency of CD74’s MHC II chaperone activity was responsible for the failure to accumulate mature B cells normally (Benlagha et al., 2004; Maehr et al., 2004). Hence the role of regulated intramembrane proteolysis in B cell maturation remains unresolved.
Regulated intramembrane proteolysis (Wolfe, 2009) is best known from the role of presenilin (γ-secretase) in Alzheimer’s disease and in Notch signaling for T cell maturation and leukemia (Selkoe and Kopan, 2003). Transmembrane protein substrates are first cleaved to release their extracellular domain by an ectoprotease, followed by a second intramembrane cleavage mediated by presenilin or signal peptide peptidase (SPP) enzymes that are amenable to pharmacological inhibition (Wolfe and Kopan, 2004; Eder et al., 2007). SPPL2A is an endosome/lysosome-localized member of the SPP-like (SPPL) intramembrane cleaving aspartyl proteases (aspartyl I-CliPs; Behnke et al., 2011). SPPL2 proteases specifically degrade single pass, type II transmembrane proteins, and in vitro studies have identified several potential SPPL2A substrates, such as British precursor protein 2 (BRI2; Martin et al., 2008), membrane FAS ligand (Kirkin et al., 2007), and membrane TNF (Friedmann et al., 2006), but so far a biological role for SPPs remains largely unknown. In this study, we identify an N-ethyl-N-nitrosourea (ENU)–induced mouse mutant strain lacking SPPL2A and define a nonredundant function for SPPL2A in CD74 processing and the expression of survival receptors on B cells, revealing a critical role for regulated intramembrane proteolysis in B cells and DCs.
RESULTS AND DISCUSSION
B cell deficiency and humoral immunodeficiency in Sppl2a-deficient mice
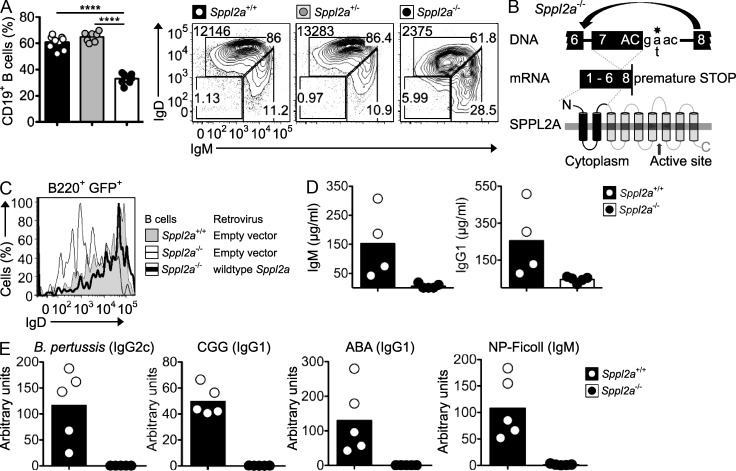
Flow cytometric screening of blood from ENU-mutagenized mouse pedigrees identified an otherwise normal strain with decreased B cells and very low expression of IgD on the IgMlow subset of mature B cells (Fig. 1 A). Meiotic mapping in (B6xCBA)F2 mice located the causative mutation to a 13-Mb interval between rs6401493 and rs3726475 on Chr2 (not depicted), whereas exome and whole genome sequencing revealed a mutation within the interval at position 2 in intron 7 of the Sppl2a gene (Fig. 1 B). The mutation caused complete skipping of exon 7 in the mRNA, introducing a frameshift and premature stop codon that deleted most of the SPPL2A protein, including seven of the nine transmembrane domains and the protease active site (Fig. 1 B). Retroviral transduction of IL-7–cultured mutant pre-B cells with wild-type Sppl2a cDNA but not with empty vector restored normal IgD expression on B cells that matured in vitro (Fig. 1 C), confirming the causative role for the truncating mutation in Sppl2a.
Figure 1.
Humoral immunodeficiency in Sppl2a−/− mice. (A) Percentage of CD19+ B cells among blood lymphocytes and representative IgM/IgD flow cytometric plots gated on CD19+ B cells from mice of the indicated genotypes. Numbers in top left corner are geometric mean fluorescence intensity of IgD, and other numbers are percentage of cells in each gate. ****, P < 0.0001. (B) Schematic of Sppl2a splice-donor mutation, the resulting skipping of exon 7, and the effect on the SPPL2A protein. (C) Surface IgD on GFP+IgM+ Sppl2a−/− B cells from IL-7 bone marrow cultures transduced with a retroviral vector encoding wild-type SPPL2A and GFP (thick line) or empty vector encoding GFP alone (thin line), compared with Sppl2a+/+ B cells transduced with empty GFP vector (shaded gray). (D) ELISA analysis of total serum IgM and IgG1 in unimmunized animals. (E) ELISA analysis of serum antibodies to B. pertussis and CGG 2 wk after immunization, against the ABA 4 wk after immunization, and against NP-Ficoll 6 d after booster immunization. Bars represent the mean, and each symbol represents a single mouse. Data are representative of more than five experiments with at least three animals per group in each (A), one experiment (B and C) or two (D), and one experiment with at least five to seven mice per group (E).
Homozygous mutant animals had 25-fold less serum IgM and sixfold less IgG1 (Fig. 1 D). Almost no specific antibody was made after immunization with formalin-inactivated and heat-killed Bordetella pertussis and alum-precipitated chicken gamma globulin (CGG) coupled to p-azobenzenearsonate (ABA) hapten or after immunization with the T-independent antigen 4-hydroxy-3-nitrophenylacetyl-Ficoll (NP-Ficoll; Fig. 1 E). Thus, SPPL2A is essential for normal B cell maturation and humoral immunity.
SPPL2A is required for accumulation of mature B cells in secondary lymphoid tissues
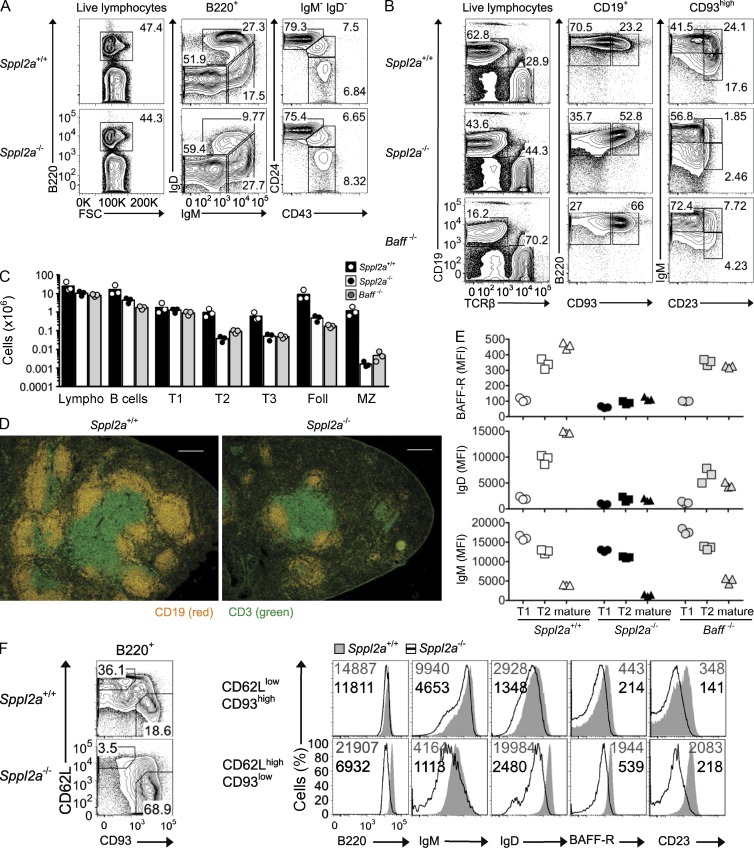
Analysis of B cell maturation in the bone marrow of Sppl2a mutant mice showed normal cellularity (not depicted), normal numbers of pro-B, pre-B, and immature B cells, and normal cell surface IgM and normal onset of IgD expression among the IgMhigh T1 subset (Fig. 2 A). Mature IgD+IgMlow recirculating B cells were selectively decreased in the bone marrow and had very low surface IgD. In the spleen, the T1 subset of recent bone marrow emigrants (CD93+IgMhighCD23−) was present in normal numbers and expressed levels of surface IgM comparable with controls (Fig. 2, B and C). Subsequent maturational stages of T2, T3, and mature CD93−IgMlow B cells were 20-fold reduced, whereas the mature marginal zone B cell subset was decreased 200-fold. Immunofluorescent staining of spleen sections showed that the residual B cells in SPPL2A-deficient mice nevertheless localized normally in primary follicles (Fig. 2 D). This developmental block resembles that caused by deficiency of BAFF or BAFFR, and we therefore directly compared Sppl2a mutants with BAFF-deficient animals, the latter serving as a control to distinguish unique traits caused by SPPL2A deficiency from general effects of mature B cell deficiency. Both defects manifested at the same developmental stage, but CD23 induction during B cell maturation, which appears to reflect NF-κB signaling (Sasaki et al., 2006), was even lower in SPPL2A-deficient than in BAFF-deficient mice (Fig. 2 B). Analysis of bone marrow chimeras confirmed that mature B cell deficiency was cell autonomous and could not be rescued by overexpression of BAFF in animals receiving Sppl2a−/− marrow (not depicted).
Figure 2.
Mature Sppl2a−/− B cells fail to up-regulate BAFFR or IgD and do not accumulate in the periphery. (A) Representative flow cytometric plots and percentages of bone marrow B cell subsets from homozygous mutant and wild-type mice. (B) Representative flow cytometric plots and percentages of spleen B cell subsets from Sppl2a−/−, Baff−/−, and wild-type (Sppl2a+/+) mice. (C) Number of cells of the indicated subsets in spleens from Sppl2a−/−, Baff−/−, and Sppl2a+/+ mice: Lympho, lymphocytes; Foll, follicular B cells; MZ, marginal zone B cells. Bars show mean, and each symbol is a single mouse. (D) Spleen cryosections from Sppl2a+/+ and Sppl2a−/− mice were stained with fluorescently labeled antibodies to detect B cells (CD19) and T cells (CD3). Bars, 200 µm. (E) Mean fluorescence intensity (MFI) of staining for BAFFR, IgD, and IgM on the indicated B cell subsets in spleens from Sppl2a−/−, Baff−/−, and Sppl2a+/+ mice. (F) Representative flow cytometric staining presented as overlay histograms for B220, IgM, IgD, BAFFR, and CD23 on Sppl2a+/+ (shaded gray) and Sppl2a−/− (black line) immature (CD62LlowCD93high) or mature (CD62LhighCD93low) splenic B cells. The numbers show the MFI for Sppl2a+/+ (gray; top) and Sppl2a−/− cells (black; bottom). Data are representative of at least three experiments with three mice (A–C and E), two experiments with one to three mice per group (D), or one experiment with three mice per group (F). Pregates for FACS plots are indicated above each column.
BAFFR levels on the B cell surface normally increase during maturation from the T1 to the T2 and mature stage, and this was also observed in BAFF-deficient mice (Fig. 2 E). In contrast, in the absence of SPPL2A, there was almost no up-regulation of BAFFR at either the T2 or mature stage of B cell development, resulting in receptor levels that were ∼25% of normal on mature CD93− B cells, even when the latter were further resolved from immature B cells by gating on CD62L+CD93− mature B cells (Fig. 2, E and F). Surface expression of IgD followed the same pattern as BAFFR (Fig. 2 F). Conversely, cell surface expression of IgM decreased progressively from T1 to the mature B cell stage in wild-type mice, and this measure of B cell maturation was not disrupted in BAFF-deficient mice and was even more pronounced in SPPL2A-deficient animals (Fig. 2 E). As a result, the residual mature B cells in SPPL2A-deficient mice had 12% of normal cell surface IgD and 30% of normal IgM, leading to a profound decrease in their surface BCR pool. The inability of SPPL2A-deficient B cells to maintain normal levels of surface IgM was also apparent in the mature B1 B cell subset in the peritoneal cavity, which were also reduced in numbers (not depicted).
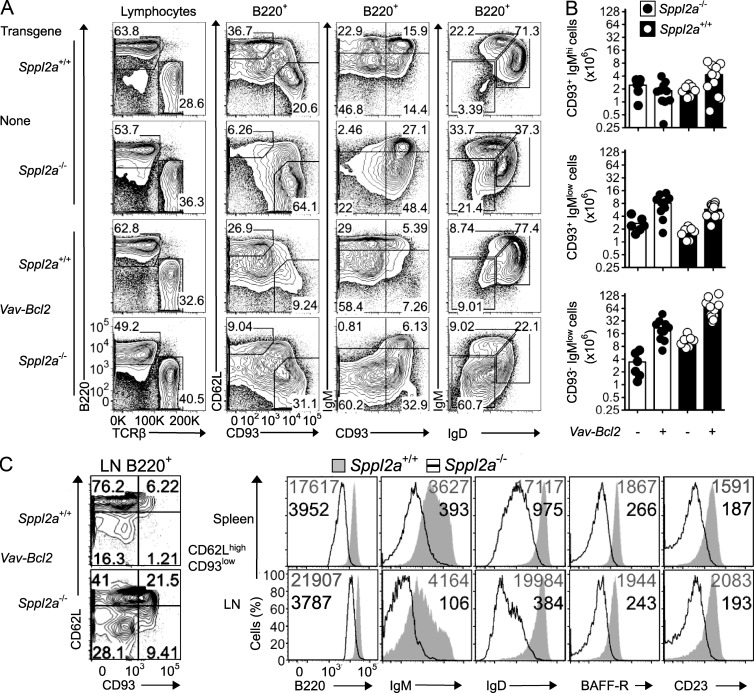
Bcl2 rescues B cell accumulation
The low BCR and BAFFR could prevent T2 and mature B cells from receiving tonic survival signals needed to suppress the BCL2-associated X protein (BAX)/BCL2 antagonist killer (BAK) mitochondrial apoptosis pathway, or conversely activation of this pathway could prevent normal BCR and BAFFR expression. To diminish mitochondrial apoptosis, the Sppl2a−/− strain was crossed with Vav-Bcl2-Tg mice overexpressing the BAX/BAK inhibitor BCL2. In the resulting Sppl2a−/− Vav-Bcl2 offspring (Fig. 3), enforced BCL2 expression increased the total number of splenic B cells to 32.2 ± 15 × 106 (mean and SD), which was greater than the number in wild-type mice (19.5 ± 4.6 × 106) or in Sppl2a−/− mice (9.4 ± 3.7 × 106). The numbers in Sppl2a−/− Vav-Bcl2 mice were nevertheless lower than in Vav-Bcl2-Tg mice with wild-type SPPL2A (107 ± 42 × 106), indicating that rescue of B cell accumulation by BCL2 did not render the B cells independent of the need for SPPL2A. BCL2 slows but does not inactivate BAX/BAK-induced B cell apoptosis upon withdrawal of survival signals (Takeuchi et al., 2005), and consequently BAFFR deficiency (Rahman and Manser, 2004), BAFF blockade (Tardivel et al., 2004), or acute loss of the BCR (Lam et al., 1997) each diminishes the accumulation of mature B cells in Bcl2 transgenic mice. Likewise, these studies have shown that BCL2 does not substitute for B cell maturation signals delivered by these receptors, and this may contribute to the lower proportion of CD93− and CD62L+ cells in Sppl2a−/− Vav-Bcl2 mice. Nevertheless, in the spleen of Sppl2a mutant mice, Vav-Bcl2 increased the number of CD93−IgMlow mature B cells by 6.5-fold but did not alter the numbers of CD93+IgMhigh T1 B cells (Fig. 3, A and B). The ratio of mature to immature B cells was also increased by BCL2, when measured comparing CD93−IgMlow with CD93+IgMhigh or comparing CD93−CD62L+ with CD93+CD62L−. In contrast, Vav-Bcl2 failed to correct the low cell surface levels of BAFFR, IgM, and IgD on the accumulating CD93− B cells in either the spleen or the LNs (Fig. 3 C), indicating that the failure to display these receptors on the cell surface is not secondary to apoptosis.
Figure 3.
Overexpression of antiapoptotic BCL2 in Sppl2a−/− mice rescues accumulation of mature B cells. (A) Representative flow cytometric plots of lymphocytes in the spleen from mice of the indicated Sppl2a and Vav-Bcl2 genotypes. Pregates for plots are indicated above the columns. (B) Number of B cells of the indicated subsets in spleens from Sppl2a+/+ and Sppl2a−/− mice, either transgenic for Vav-Bcl2 or not. Bars show mean, and each symbol is a single mouse. (C) Representative flow cytometric plots of CD62L and CD93 expression on B cells from subcutaneous LNs in Vav-Bcl2 transgenic mice of the indicated genotype. Overlay of flow cytometric histograms for B220, IgM, IgD, BAFFR, and CD23 cell surface expression on CD62LhighCD93low mature B cells in spleen (top) or LNs (bottom) from Sppl2a+/+ (shaded gray) and Sppl2a−/− (black line) mice. Numbers in the plots indicate geometric mean fluorescence intensity for Sppl2a+/+ (gray; top) and Sppl2a−/− cells (black; bottom). Data are representative of one to three experiments with three to four mice per group (A and C) or combined from four experiments (B).
Proteolytic processing of CD74 requires SPPL2A
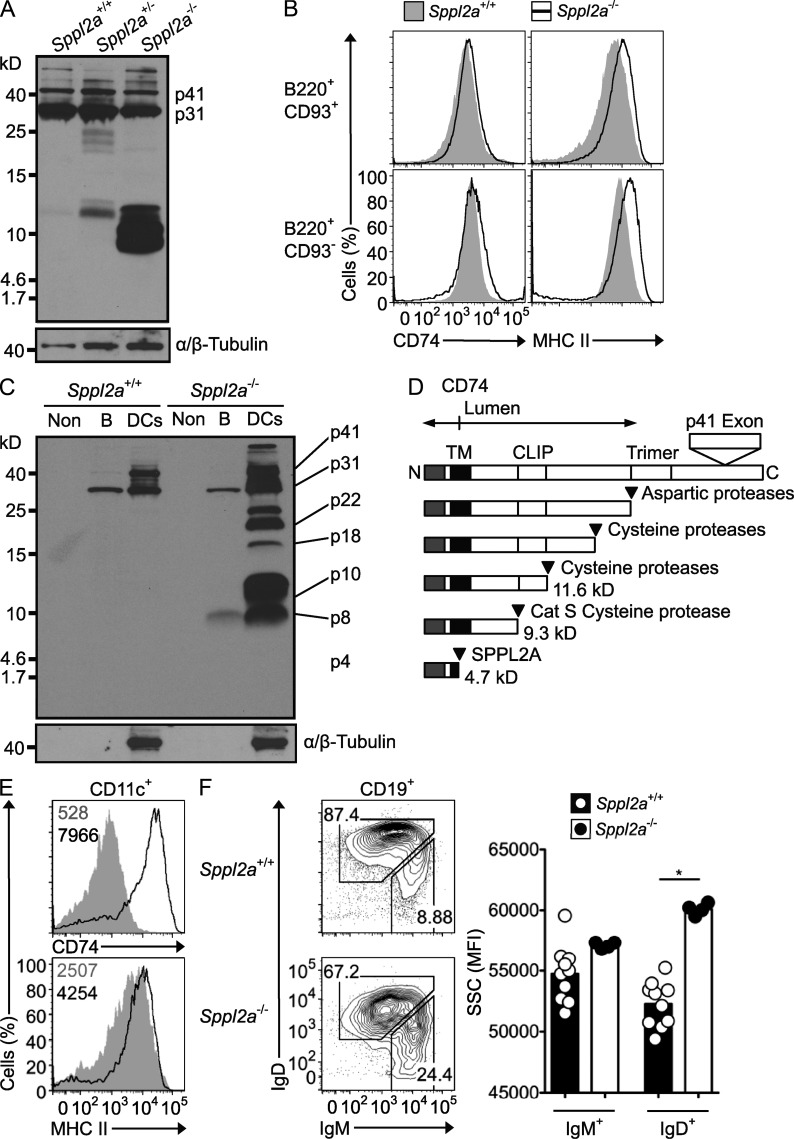
Despite ubiquitous Sppl2a expression in the immune system (Heng et al., 2008), we observed a B cell–specific phenotype in mice lacking SPPL2A. To identify possible SPPL2A targets, we performed a bioinformatic search for type II transmembrane proteins up-regulated during B cell maturation. CD74 was a particularly good candidate as CD74-deficient mice have fewer IgDhigh B cells (Shachar and Flavell, 1996; Benlagha et al., 2004; Maehr et al., 2004). Western blotting with an antibody to the CD74 N terminus revealed an accumulation of 12- and 9-kD fragments in spleen B cells from Sppl2a−/− mice and a slight accumulation in heterozygotes (Fig. 4 A). In contrast, there was no reduction in the expression of the full-length p31 and p41 isoforms of CD74 (Fig. 4 A) and a small increase in cell surface expression of full-length CD74 and MHC II on immature and mature B cells (Fig. 4 B). Western blotting of lysates from sorted CD11c+ splenic DCs revealed that SPPL2A deficiency also caused a striking accumulation of 9- and 12-kD CD74 N-terminal fragments (Fig. 4 C), corresponding to the predicted sizes for the penultimate p8 and p10 cleavage products produced by Cathepsin S in DCs and B cells (Fig. 4 D). The extraordinary accumulation of the p8 penultimate cleavage product reveals SPPL2A as the previously unknown peptidase responsible for this final cleavage of CD74 (Lipp and Dobberstein, 1986).
Figure 4.
Proteolytic processing and intracellular retention of CD74 requires SPPL2A. (A) Immunoblot analysis of splenic B cells from Sppl2a−/−, Sppl2a+/−, and Sppl2a+/+ mice. Blots were probed with In-1 antibody against the CD74 N-terminal tail and then stripped and reprobed with antibody to α/β-Tubulin. p41 and p31 isoforms of CD74 are indicated. (B) Flow cytometric staining for cell surface MHC II and CD74 on splenic B220+CD93+ immature and B220+CD93− mature B cells from Sppl2a+/+ (shaded gray) and Sppl2a−/− mice (black line). (C) Immunoblot analysis of splenic B cells and the non-DC/non-B cell (Non) fraction from Sppl2a−/− and Sppl2a+/+ mice probed as for A. (D) Schematic diagram of CD74 processing steps and products, showing estimated molecular mass (kilodaltons) and proteases involved. (E) Flow cytometric staining for cell surface MHC II and CD74 on splenic CD11c+ DCs from Sppl2a+/+ (shaded gray) and Sppl2a−/− mice (black line). Numbers in the plots indicate geometric mean fluorescence intensity (MFI). (F) Flow cytometric light SSC as a relative measure of cell vesicle content, gated on IgMhigh immature and IgD+ mature B cells in the blood as shown in the left panel of plots from Sppl2a+/+ and Sppl2a−/− mice. Bars show mean, and each symbol is data from one mouse. P-value from one-way ANOVA with Bonferroni’s Multiple Comparison posttest: *, P = 0.01–0.05. Data are representative of one experiment (A–C) or at least three experiments with three or more mice per group (E and F).
Sppl2a−/− DCs also had a dramatic accumulation of larger CD74 N-terminal fragments of ∼16, 18, and 22 kD that correspond to early Cathepsin-dependent stages in proteolytic processing of the CD74 ectodomain and a small increase in full-length CD74 p31 and p41 proteins (Fig. 4 C). Flow cytometric staining with an antibody to the full-length CD74 C-terminal domain nevertheless revealed a 20-fold increased accumulation on the cell surface of SPPL2A-deficient DCs, whereas MHC II levels were modestly increased (Fig. 4 E). The large increase in cell surface CD74 suggests a failure of this molecule to traffic to acidified endosomes. A secondary effect of SPPL2A deficiency on endosomal trafficking could account for the accumulation of partially processed 16-, 18-, and 22-kD products because these require processing of the CD74 ectodomain by cathepsin S and other proteases in acidified endosomes. Accumulation of trimerized CD74 p8 can distort the endosomal system by inducing large intracellular early endosomes with delayed maturation into late endosomes and lysosomes (Lagaudrière-Gesbert et al., 2002). As an objective measure of intracellular vacuolation, we used flow cytometry to measure the side scatter (SSC) of laser light in peripheral blood B or T cells. SSC was significantly increased selectively in mature IgD+IgMlow B cells from Sppl2a−/− mice compared with littermate controls (Fig. 4 F), and this was not observed in BAFF-deficient controls (not depicted). These data complement electron microscopic observations of increased cytoplasmic vacuoles in the accompanying paper in this issue by Schneppenheim et al. and show that this B cell abnormality arises at the transition from IgD− to IgD+ B cells and is not accounted for by increased apoptosis.
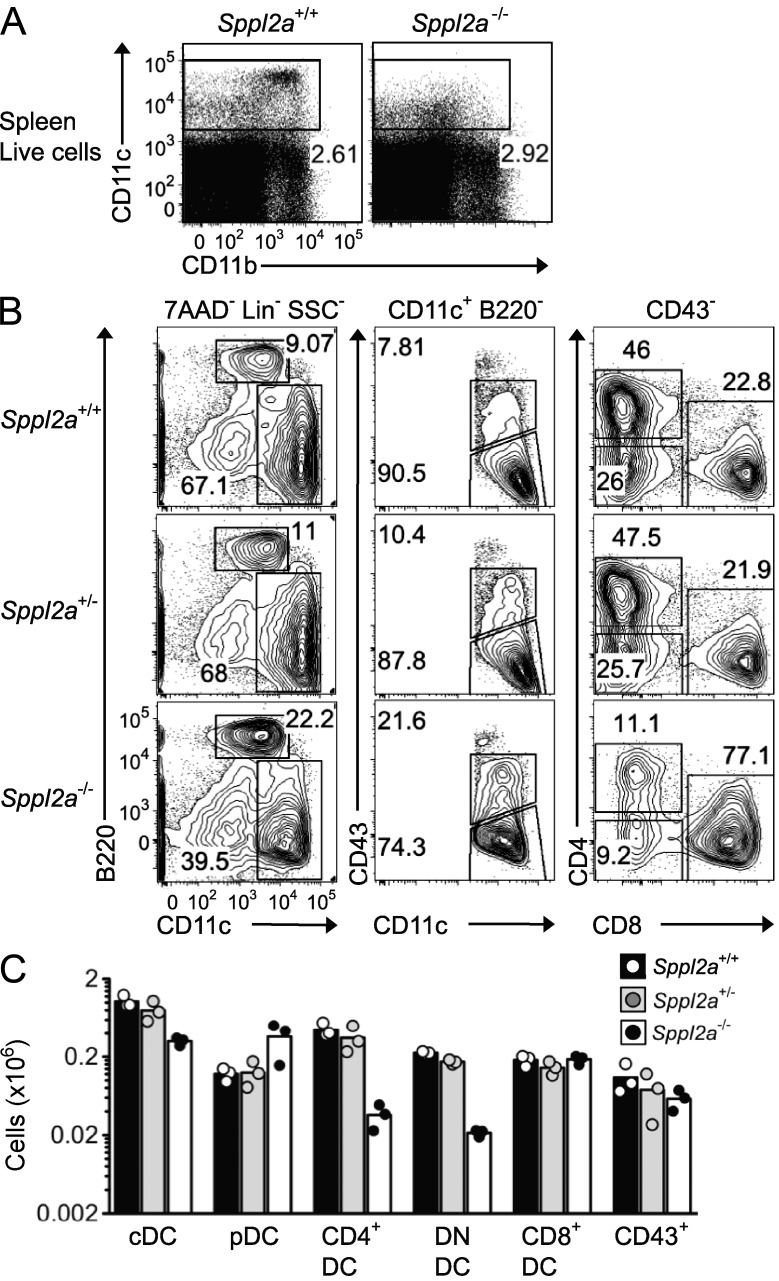
Sppl2a−/− animals have few CD8− DCs
Having shown that SPPL2A was essential for CD74 processing in DCs, we investigated the accumulation of splenic DC subsets. Sppl2a−/− mice had fewer CD11c+ DCs (Fig. 5 A) as the result of only 12% of normal numbers in the CD4+ and the CD4−CD8− double-negative (DN) DC subsets, whereas CD8+ and plasmacytoid DCs were present at normal and increased numbers, respectively (Fig. 5, B and C). Less CD11c and CD8 was present on the B220−CD8+ DCs in Sppl2a−/− animals (Fig. 5 B). Thus, SPPL2A is also critical for key populations of antigen-presenting DCs. The DC deficit may also contribute to the profound humoral immunodeficiency observed in animals lacking the enzyme.
Figure 5.
Sppl2a is critical for CD8− DCs. (A and B) Representative flow cytometric plots of total spleen cells (A) or spleen DCs (B) from Sppl2a−/−, Sppl2a+/−, and Sppl2a+/+ mice. Pregates for plots are indicated above each column. (C) Number of cells in each DC subset in spleens from mice of the indicated genotypes. cDC, conventional DCs; pDC, plasmacytoid DCs; DN, CD4− and CD8− DN DCs; CD43+, pre-DCs gated as per middle column plots in B. Bars show mean, and each symbol represents a single mouse. Data are representative of three experiments with at least three mice per group (A) or two experiments with one to three mice per group (B and C).
Our results identify SPPL2A as the long-sought protease responsible for the final step in CD74 invariant chain processing and reveal its critical role in B and DC accumulation. A key question arising from our findings is how SPPL2A deficiency results in Bcl2-inhibitable loss of B cells. One explanation is failure to release the CD74 p4 intracellular domain, which can serve as a transcriptional coactivator for NF-κB and B cell maturation (Matza et al., 2002a; Becker-Herman et al., 2005). This appears ruled out for three reasons. First, there was much greater loss of T2 and mature B cells in SPPL2A deficiency than CD74 deficiency. Second, CD74 deficiency rescued accumulation of T2 and mature B cells in Sppl2a mutant mice (see accompanying papers in this issue by Beisner et al. and Schneppenheim et al. [2013]), establishing that loss of SPPL2A protease activity accounts for the B cell developmental defect via accumulation of unprocessed CD74. Third, the residual B cell deficiency in mice lacking CD74 was corrected in Cd74−/−Ia−/− double-deficient mice, implying that unchaperoned MHC II inhibits B cell maturation in CD74-deficient mice (Zimmermann et al., 1999; Benlagha et al., 2004; Maehr et al., 2004).
Interference with intracellular trafficking may explain the defects observed here. In mature B cells, MHC II and full-length CD74 are primarily on the cell surface until the BCR is engaged by antigen. BCR signals induce CD74 association with myosin light chain kinase and activate CD74-dependent formation of large, perinuclear, MHC II–rich intracellular vesicles containing processed CD74 p10, BCRs, and partially processed antigen (Siemasko et al., 1998; Zimmermann et al., 1999; Kim et al., 2006; Vascotto et al., 2007). In the absence of SPPL2A, the accumulation of CD74 p8 or p12 trimers in mature B cells may modify endosomal trafficking to interfere with IgM, IgD, and BAFFR transport or recycling to the cell surface, depriving the B cells of survival signals. The selective dependence of mature B cells and DC subsets on functional SPPL2A and the existence of pharmacological inhibitors of SPPL proteases (Eder et al., 2007) make this enzyme an interesting drug target for immunosuppression, particularly for B cell malignancies that often express high levels of CD74 (Stein et al., 2007).
MATERIALS AND METHODS
Mice and procedures.
Sppl2a and Tnfsf13b (BAFF) mutant strains were identified by flow cytometry screening of blood lymphocytes in third generation offspring of C57BL/6 mice treated with 3 × 90 mg/kg ENU. The Tnfsf13b nonsense mutation converts Tyrosine 27 codon (TAT) to STOP (TAA). Bone marrow chimeras and immunizations were performed as described previously (Yabas et al., 2011). All ENU mutant mice were generated and maintained on a C57BL/6 background, and the Rag1−/− and Vav-Bcl2-Tg were backcrossed for at least 10 generations to the C57BL/6 background. For all mouse experiments, littermate controls were used. All mice were housed in specific pathogen–free conditions at the Australian National University Bioscience Research Services facility, and all animal procedures were approved by the Australian National University Animal Ethics and Experimentation Committee.
For retroviral transduction, mouse Sppl2a cDNA was cloned into pMXs-IRES-EGFP (provided by T. Kitamrua, The University of Tokyo, Tokyo, Japan) and transduced into retroviral producer cell line GP+E86 (a gift from D. Vignali, St. Jude Children’s Research Hospital, Memphis, TN). MACS-purified B220+ B cells from bone marrow were cultured in 10 ng/ml IL-7 (R&D Systems) for 2 d and co-cultured with retroviral producer cells for 48 h in the presence of IL-7 and Polybrene (Sigma-Aldrich). The cells were washed, cultured for 8 d with 10 ng/ml BAFF (R&D Systems) and without IL-7 to facilitate B cell maturation, and analyzed by flow cytometry.
Exome and whole genome sequencing.
A fragmented Illumina library was prepared from the DNA of a single affected mouse using the Illumina paired end genomic DNA sample pre kit (Illumina). Exome capture of this library was then performed using Agilent Mouse SureSelect kit (early access) according to protocol v1, September 2009, and the DNA was sequenced on a GAIIx sequencer (Illumina). Alignment to the UCSC mm9 assembly of the reference mouse genome and SNP calling and filtering were performed as described previously (Andrews et al., 2012). Whole genome 100-bp paired-end sequencing of a single affected mouse was performed on an Illumina HiSeqII machine to a mean coverage depth of 3.4x. Reads were mapped to the MGSCv37 mouse genome using Stampy and an in-house variant caller (www.well.ox.ac.uk/platypus) and filtered for known variants in dbSNP or other ENU pedigrees in addition to quality, strand bias, and coverage filters using in-house python scripts.
cDNA sequencing.
Total RNA was isolated from splenic lymphocytes using the mirVana micro RNA isolation kit (Ambion) according to the manufacturer’s instructions. cDNA was synthesized using the SuperScript First Strand Synthesis System (Invitrogen) using oligo (dT) primers. PCR amplification of parts of the Sppl2a cDNA used primers in exon 5 (5′-CCATCCTGGCCTAACTTTGA-3′) and exon 9 (5′-CATGAAATTGGGTAACTTCATTGT-3′) and AccuPrime High Fidelity Taq (Invitrogen). After gel purification, PCR products were sequenced on an ABI 3730 Sequencer (Applied Biosystems).
Flow cytometry and Western blotting.
Spleen and bone marrow cells were prepared, stained, and analyzed by flow cytometry according to published methods (Yabas et al., 2011). For DC isolation, DC-enriched light density fractions of spleens were used for analysis as described previously (Aliberti et al., 2003). In brief, spleens were injected with warmed Collagenase D (Roche)–RPMI (Invitrogen) and incubated for 30 min at 37°C, after which all steps were performed at 0–4°C. After collagenase incubation, spleens were forced through cell strainer followed by several washes in EDTA-PBS, before cell suspension was layered over 30% BSA-PBS, and centrifuged for 15 min at 500 g. The resulting interface was collected and stained for DC analysis by flow cytometry, using antibodies mentioned below, including lineage markers CD19, TCRβ, TCRγ/δ, CD90.2, Ter119, and NK1.1.
Samples were analyzed using an LSR II or LSRFortessa flow cytometer or sorted using a FACSAria II cell sorter (BD). For analysis or sorting, the following antibodies (clones) were used and purchased from the indicated companies. From BD: anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD19 (1D3), anti–CD21-CD35 (7G6), anti-CD23 (B3B4), anti-CD43 (S7), anti-CD45.2 (104), anti-B220 (RA3-6B2), anti-CD90.2 (53–2.1), anti–TCR-γ/δ (GL3), anti–Gr-1 (RB6-8C5), anti-IgM (II/41), anti-NK1.1 (PK136), and anti-CD11c (HL3). From eBioscience: anti-CD5 (53–7.3), anti-CD8 (53–6.7), anti-CD19 (eBio1D3), anti-CD93 (AA4.1), anti-B220 (RA3-6B2), anti-IgD (11-26), anti-IgM (II/41), and anti-Ter119 (TER-119). From BioLegend: anti-CD3 (17A2), anti–CD21-CD35 (7E9), anti-CD23 (B3B4), anti-CD24 (M1/69), anti-CD43 (1B11), anti-CD45.1 (A20), anti-IgD (11-26c.2a), anti–Mac-1 (M1/70), anti-TCRβ (H57-597), and anti-BAFFR (7H22-E16). From Invitrogen: 7-AAD and Qdot 605 streptavidin conjugate. From Santa Cruz Biotechnology, Inc.: goat anti–rabbit IgG and F(ab′). Anti–CD74-JV11 rabbit polyclonal serum was a gift from J.A. Villadangos (University of Melbourne, Melbourne, Victoria, Australia).
For Western blotting, sorted B cells, DCs, and non-B cell–DC fractions were counted and resuspended at equal cell numbers in 2-mercaptoethanol containing SDS sample buffer, incubated at 37°C for 60 min, sonicated at intervals to achieve 15 min of total sonication time, separated by Tricine SDS-PAGE on 16% acrylamide gel with 6% cross-linking, and transferred to nitrocellulose membrane. For immunoblotting and detection of N-terminal CD74 and α/β-Tubulin, antibodies from BD (In-1) and Cell Signaling Technology were used.
Immunofluorescence microscopy.
10-µm spleen cryosections were fixed in acetone and blocked with 5% pig serum, before detecting B cell follicles and T cell zones with anti–mouse CD19-PE (1D3) and anti–mouse CD3-FITC (145-2C11) antibodies from BD. Slides were analyzed on an IX 71 fluorescence microscope (Olympus).
Statistical analysis.
Data were statistically analyzed using Prism 5.0d for Mac OS X (GraphPad Software). For comparison of multiple groups, ANOVA with Bonferroni’s Multiple Comparison posttest was used.
Acknowledgments
We thank the staff of the Australian National University Bioscience Research Services, the Australian Phenomics Facility, Debbie Howard, Michelle Townsend, the John Curtain School of Medical Research Microscopy and Cytometry Resource Facility, the Australian Cancer Research Foundation Biomolecular Research Facility, and the Australian Genome Research Facility and the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics for expert technical services and Dr. Jose A. Villadangos for anti-CD74 antibody.
This work was supported by grants to C.C. Goodnow from the National Institutes of Health National Institute of Allergy and Infectious Diseases, Wellcome Trust, and Australian Research Council; grants to C.C. Goodnow and A. Enders from the National Health and Medical Research Council and Ramaciotti Foundation; and grants to R. Cornall from the Medical Research Council.
The authors have no financial conflicts of interest.
Footnotes
Abbreviations used:
- BAFF
- B cell–activating factor
- BAFFR
- BAFF receptor
- BAK
- BCL2 antagonist killer
- BAX
- BCL2-associated X protein
- DN
- double negative
- ENU
- N-ethyl-N-nitrosourea
- SSC
- side scatter
References
- Aliberti J., Schulz O., Pennington D.J., Tsujimura H., Reis e Sousa C., Ozato K., Sher A. 2003. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 101:305–310 10.1182/blood-2002-04-1088 [DOI] [PubMed] [Google Scholar]
- Andrews T.D., Whittle B., Field M.A., Balakishnan B., Zhang Y., Shao Y., Cho V., Kirk M., Singh M., Xia Y., et al. 2012. Massively parallel sequencing of the mouse exome to accurately identify rare, induced mutations: an immediate source for thousands of new mouse models. Open Biol. 2:120061 10.1098/rsob.120061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Herman S., Arie G., Medvedovsky H., Kerem A., Shachar I. 2005. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol. Biol. Cell. 16:5061–5069 10.1091/mbc.E05-04-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke J., Schneppenheim J., Koch-Nolte F., Haag F., Saftig P., Schröder B. 2011. Signal-peptide-peptidase-like 2a (SPPL2a) is targeted to lysosomes/late endosomes by a tyrosine motif in its C-terminal tail. FEBS Lett. 585:2951–2957 10.1016/j.febslet.2011.08.043 [DOI] [PubMed] [Google Scholar]
- Beisner D.R., Langerak P., Parker A.E., Dahlberg C., Otero F.J., Sutton S.E., Poirot L., Barnes W., Young M.A., Niessen S., et al. 2013. The intramembrane protease Sppl2a is required for B cell and DC development and survival via cleavage of the invariant chain. J. Exp. Med. 210:23–30 10.1084/jem.20121072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K., Park S.-H., Guinamard R., Forestier C., Karlsson L., Chang C.-H., Bendelac A. 2004. Mechanisms governing B cell developmental defects in invariant chain-deficient mice. J. Immunol. 172:2076–2083 [DOI] [PubMed] [Google Scholar]
- Dugast M., Toussaint H., Dousset C., Benaroch P. 2005. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 280:19656–19664 10.1074/jbc.M501357200 [DOI] [PubMed] [Google Scholar]
- Eder J., Hommel U., Cumin F., Martoglio B., Gerhartz B. 2007. Aspartic proteases in drug discovery. Curr. Pharm. Des. 13:271–285 10.2174/138161207779313560 [DOI] [PubMed] [Google Scholar]
- Enders A., Bouillet P., Puthalakath H., Xu Y., Tarlinton D.M., Strasser A. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation–induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119–1126 10.1084/jem.20030411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann E., Hauben E., Maylandt K., Schleeger S., Vreugde S., Lichtenthaler S.F., Kuhn P.-H., Stauffer D., Rovelli G., Martoglio B. 2006. SPPL2a and SPPL2b promote intramembrane proteolysis of TNFalpha in activated dendritic cells to trigger IL-12 production. Nat. Cell Biol. 8:843–848 10.1038/ncb1440 [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Kincade P.W., Dorshkind K. 2007. The protean nature of cells in the B lymphocyte lineage. Immunity. 26:703–714 10.1016/j.immuni.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Heng T.S.P., Painter M.W.; Immunological Genome Project Consortium 2008. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9:1091–1094 10.1038/ni1008-1091 [DOI] [PubMed] [Google Scholar]
- Khan W.N. 2009. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J. Immunol. 183:3561–3567 10.4049/jimmunol.0800933 [DOI] [PubMed] [Google Scholar]
- Kim Y.-M., Pan J.Y.-J., Korbel G.A., Peperzak V., Boes M., Ploegh H.L. 2006. Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc. Natl. Acad. Sci. USA. 103:3327–3332 10.1073/pnas.0511315103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V., Cahuzac N., Guardiola-Serrano F., Huault S., Lückerath K., Friedmann E., Novac N., Wels W.S., Martoglio B., Hueber A.-O., Zörnig M. 2007. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ. 14:1678–1687 10.1038/sj.cdd.4402175 [DOI] [PubMed] [Google Scholar]
- Lagaudrière-Gesbert C., Newmyer S.L., Gregers T.F., Bakke O., Ploegh H.L. 2002. Uncoating ATPase Hsc70 is recruited by invariant chain and controls the size of endocytic compartments. Proc. Natl. Acad. Sci. USA. 99:1515–1520 10.1073/pnas.042688099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.P., Kühn R., Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 90:1073–1083 10.1016/S0092-8674(00)80373-6 [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. 1986. The membrane-spanning segment of invariant chain (I gamma) contains a potentially cleavable signal sequence. Cell. 46:1103–1112 10.1016/0092-8674(86)90710-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R., Kraus M., Ploegh H.L. 2004. Mice deficient in invariant-chain and MHC class II exhibit a normal mature B2 cell compartment. Eur. J. Immunol. 34:2230–2236 10.1002/eji.200425246 [DOI] [PubMed] [Google Scholar]
- Martin L., Fluhrer R., Reiss K., Kremmer E., Saftig P., Haass C. 2008. Regulated intramembrane proteolysis of Bri2 (Itm2b) by ADAM10 and SPPL2a/SPPL2b. J. Biol. Chem. 283:1644–1652 10.1074/jbc.M706661200 [DOI] [PubMed] [Google Scholar]
- Matza D., Kerem A., Medvedovsky H., Lantner F., Shachar I. 2002a. Invariant chain-induced B cell differentiation requires intramembrane proteolytic release of the cytosolic domain. Immunity. 17:549–560 10.1016/S1074-7613(02)00455-7 [DOI] [PubMed] [Google Scholar]
- Matza D., Lantner F., Bogoch Y., Flaishon L., Hershkoviz R., Shachar I. 2002b. Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc. Natl. Acad. Sci. USA. 99:3018–3023 10.1073/pnas.052703299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick P.J., Martina J.A., Bonifacino J.S. 2005. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl. Acad. Sci. USA. 102:7910–7915 10.1073/pnas.0502206102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z.S.M., Manser T. 2004. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J. Immunol. 173:6179–6188 [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Derudder E., Hobeika E., Pelanda R., Reth M., Rajewsky K., Schmidt-Supprian M. 2006. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 24:729–739 10.1016/j.immuni.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Schneppenheim J., Dressel R., Hüttl S., Lüllmann-Rauch R., Engelke M., Dittmann K., Wienands J., Eskelinen E.-L., Hermans-Borgmeyer I., Fluhrer R., et al. 2013. The intramembrane protease SPPL2a promotes B cell development and controls endosomal traffic by cleavage of the invariant chain. J. Exp. Med. 210:41–58 10.1084/jem.20121069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D., Kopan R. 2003. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 26:565–597 10.1146/annurev.neuro.26.041002.131334 [DOI] [PubMed] [Google Scholar]
- Shachar I., Flavell R.A. 1996. Requirement for invariant chain in B cell maturation and function. Science. 274:106–108 10.1126/science.274.5284.106 [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Brown K., Claudio E. 2005. Control of lymphocyte development by nuclear factor-kappaB. Nat. Rev. Immunol. 5:435–445 10.1038/nri1629 [DOI] [PubMed] [Google Scholar]
- Siemasko K., Eisfelder B.J., Williamson E., Kabak S., Clark M.R. 1998. Cutting edge: signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J. Immunol. 160:5203–5208 [PubMed] [Google Scholar]
- Srinivasan L., Sasaki Y., Calado D.P., Zhang B., Paik J.H., DePinho R.A., Kutok J.L., Kearney J.F., Otipoby K.L., Rajewsky K. 2009. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 139:573–586 10.1016/j.cell.2009.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Mattes M.J., Cardillo T.M., Hansen H.J., Chang C.-H., Burton J., Govindan S., Goldenberg D.M. 2007. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin. Cancer Res. 13:5556s–5563s 10.1158/1078-0432.CCR-07-1167 [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Fisher J., Suh H., Harada H., Malynn B.A., Korsmeyer S.J. 2005. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc. Natl. Acad. Sci. USA. 102:11272–11277 10.1073/pnas.0504783102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardivel A., Tinel A., Lens S., Steiner Q.-G., Sauberli E., Wilson A., Mackay F., Rolink A.G., Beermann F., Tschopp J., Schneider P. 2004. The anti-apoptotic factor Bcl-2 can functionally substitute for the B cell survival but not for the marginal zone B cell differentiation activity of BAFF. Eur. J. Immunol. 34:509–518 10.1002/eji.200324692 [DOI] [PubMed] [Google Scholar]
- Vascotto F., Lankar D., Faure-André G., Vargas P., Diaz J., Le Roux D., Yuseff M.-I., Sibarita J.-B., Boes M., Raposo G., et al. 2007. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J. Cell Biol. 176:1007–1019 10.1083/jcb.200611147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J.A., Bryant R.A., Deussing J., Driessen C., Lennon-Duménil A.M., Riese R.J., Roth W., Saftig P., Shi G.P., Chapman H.A., et al. 1999. Proteases involved in MHC class II antigen presentation. Immunol. Rev. 172:109–120 10.1111/j.1600-065X.1999.tb01360.x [DOI] [PubMed] [Google Scholar]
- Wolfe M.S. 2009. Intramembrane proteolysis. Chem. Rev. 109:1599–1612 10.1021/cr8004197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.S., Kopan R. 2004. Intramembrane proteolysis: theme and variations. Science. 305:1119–1123 10.1126/science.1096187 [DOI] [PubMed] [Google Scholar]
- Yabas M., Teh C.E., Frankenreiter S., Lal D., Roots C.M., Whittle B., Andrews D.T., Zhang Y., Teoh N.C., Sprent J., et al. 2011. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat. Immunol. 12:441–449 10.1038/ni.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann V.S., Rovere P., Trucy J., Serre K., Machy P., Forquet F., Leserman L., Davoust J. 1999. Engagement of B cell receptor regulates the invariant chain-dependent MHC class II presentation pathway. J. Immunol. 162:2495–2502 [PubMed] [Google Scholar]