The protease Sppl2a cleaves the N-terminal fragment of invariant chain (CD74) and is required for efficient B cell development and function.
Abstract
B cell development requires tight regulation to allow for the generation of a diverse repertoire while preventing the development of autoreactive cells. We report, using N-ethyl-N-nitrosourea (ENU)–induced mutagenesis, the identification of a mutant mouse (chompB) with a block in early B cell development. The blockade occurs after the transitional 1 (T1) stage and leads to a decrease in mature B cell subsets and deficits in T cell–dependent antibody responses. Additionally, chompB mice have decreases in myeloid dendritic cells (DCs). The mutation was mapped to the intramembrane protease signal peptide peptidase-like 2a (Sppl2a), a gene not previously implicated in immune cell development. Proteomic analysis identified the invariant chain (CD74) as a key substrate of Sppl2a and suggests that regulated intramembrane proteolysis of CD74 by Sppl2a contributes to B cell and DC survival. Moreover, these data suggest that modulation of Sppl2a may be a useful therapeutic strategy for treatment of B cell dependent autoimmune disorders.
B cell development is a tightly controlled process that promotes the survival and expansion of B cells with affinity for invading organisms while eliminating autoreactive B cells and thereby preventing autoimmunity (Browning, 2006; Allman and Pillai, 2008). The recent clinical success of B cell targeted therapies in a multitude of autoimmune disorders has highlighted the potential role of B cells in disease pathology. Work in the last several decades has also revealed that B cells have alternative effector functions in addition to antibody production, and can play a role in the pathology of a multitude of human diseases and provide additional therapeutic intervention points (Browning, 2006; Gürcan et al., 2009).
Although much has been discovered about lymphocyte development and activation through the use of traditional gene targeting approaches, it also has its limitations. These include requirements for a gene in embryonic development, the development of a particular cell type, or a hypothesis that a gene is involved in the function of a particular cell type or pathway. Forward genetic approaches have classically proven to be valuable tools in many model organisms, and work from the last 10 years has shown that it can also be a useful tool in mice (Beutler et al., 2006; Cook et al., 2006). Random mutagenesis also provides the opportunity to identify mutants with reduced or increased activity as opposed to full ablation, which can provide insight into how a particular molecule is functioning.
To this end, we used a forward genetic screen using N-ethyl-N-nitrosourea (ENU)–induced mutagenesis in mice to identify novel regulators of immune function. We discovered a mouse strain (chompB) with a heritable recessive mutation that leads to decreased B cells and T cell–dependent antibody responses and mapped it to the gene encoding signal peptide peptidase-like 2a (Sppl2a). The chompB mutation revealed an unexpected role for Sppl2a in B cell and DC development and that Sppl2a-mediated processing of CD74 contributes to the survival of those cells during development and activation.
RESULTS AND DISCUSSION
Identification of a role for Sppl2a in B cell and DC function by ENU mutagenesis
A mutant line with a heritable recessive mutation, named chompB, was identified with decreases in peripheral blood B cells and T cell–dependent antibody responses (Fig. 1, A and B). Single nucleotide polymorphism (SNP)–based mapping identified a region of chromosome 2 that was linked to these phenotypes (Fig. S1 A). Candidate gene sequencing identified a T to A conversion in the Sppl2a gene, resulting in a methionine to lysine replacement (M252K) in the third transmembrane domain (unpublished data). Sppl2a has been shown to cleave TNF and promote inflammatory cytokine production by human DCs but its role in vivo has not been established (Fluhrer et al., 2006; Friedmann et al., 2006).
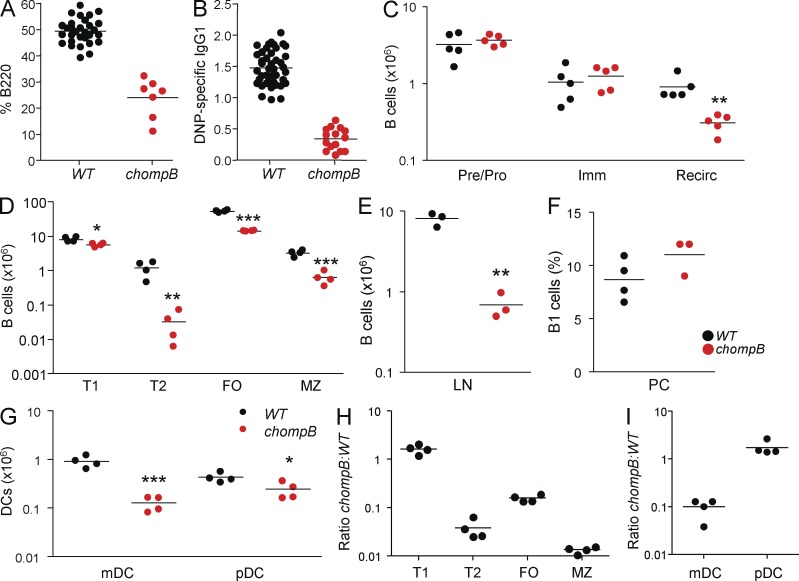
Figure 1.
chompB mice have a block in B cell and DC development. (A and B) Mutagenized mice were screened for blood B cells by B220 staining (A) and DNP-KLH–specific IgG by ELISA (B). (C) BM B cells were enumerated by flow cytometry using the following criteria: pre/pro, B220+IgM−; immature, B220+IgM+CD24+; mature/recirculating, B220+IgM+CD24−. (D) Splenic B cell subsets were enumerated using the following criteria: T1, IgMhiCD21−; T2, IgMhiCD21hiCD23+; FO, IgM+CD21lo; MZ, IgMhiCD21hiCD23−. (E) B cell numbers in the lymph nodes were determined by gating on B220+ cells. (F) B1 cell numbers from the peritoneal cavity were enumerated using CD19+B220intCD5int. (G) DC numbers were determined using the following criteria: mDC, CD19−CD3−NK1.1−CD11chi; pDC, CD19−CD3−NK1.1−CD11c+PDCA1+. (H and I) 50/50 Mixed BM chimeras were analyzed by flow cytometry 8–12 wk after reconstitution. Data are expressed as the ratio of chompB to WT cells. Analysis of the B cells (H) and DCs (I) was performed using the gating schemes as in D and E after gating on CD45 variants to determine donor. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (mean, n = 3–5 in at least two independent experiments).
Analysis of B cells from the BM revealed a significant decrease in the number of mature recirculating B cells (Fig. 1 C). Analysis of splenic B cell populations revealed similar numbers of transitional 1 (T1) B cells but significant reductions in the numbers of T2, marginal zone (MZ), and follicular (FO) B cells in chompB mutants (Fig. 1 D). A similar reduction in mature B cells was also observed in peripheral lymph nodes of chompB mice (Fig. 1 E); however, B1 cells in the peritoneal cavity were unchanged (Fig. 1 F). Analysis of DCs in the spleen revealed a significant decrease in myeloid DCs (mDCs) in chompB mice, whereas plasmacytoid DCs (pDCs) were less affected (Fig. 1 G). Other immune cell subsets were unaffected in chompB mutants, including T cells, NK cells, and myeloid cells (unpublished results). Despite the relatively ubiquitous expression of Sppl2a (Fig. S1 B), the phenotype of chompB mice appears to be lymphoid restricted as gross analysis did not reveal any other obvious abnormalities (unpublished data; Lattin et al., 2008).
To determine the cellular origin of the chompB mutant phenotype, mixed BM chimeras were generated. Analysis of recipient mice revealed similar reductions in T2, MZ, and FO B cells and mDCs from chompB mutant BM, consistent with data from intact animals (Fig. 1, H and I). Analyses of single BM chimeras confirmed these observations (unpublished results).
Sppl2a is required for immunoglobulin production and T cell–dependent antibody responses
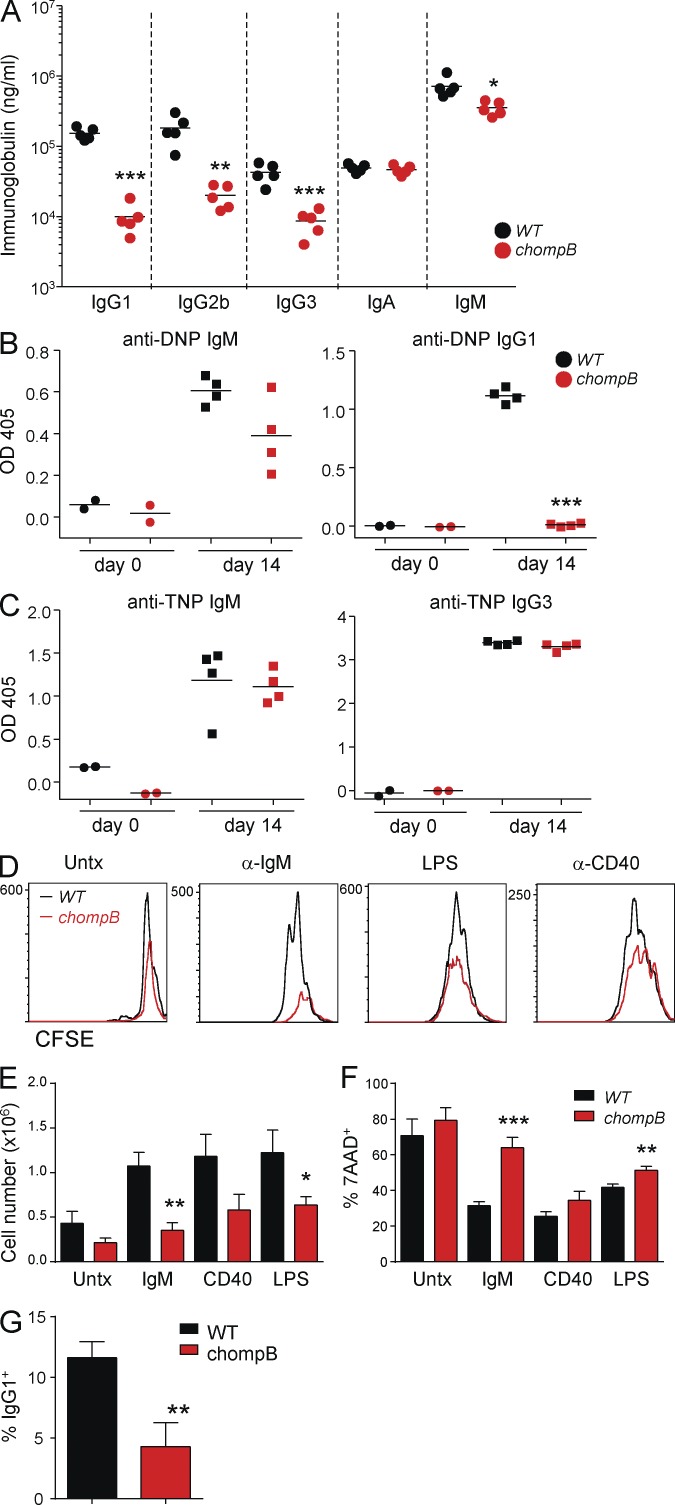
We next assessed B cell function by measuring serum immunoglobulin levels and the antigen-specific response after immunization. Significant decreases in IgG1, IgG2b, and IgG3 levels were observed in chompB mice compared with controls, whereas levels of IgA and IgM were less affected (Fig. 2 A). Consistent with the initial screening results, chompB mutant mice had significant decreases in DNP-specific IgG1 after immunization; however, DNP-specific IgM levels were unaffected (Fig. 2 B). The dramatic decrease in DNP-specific IgG1 cannot be explained solely by the threefold decrease in FO B cells, although it could be a contributing factor. Additionally, assessment of the ability of chompB B cells to respond to T cell help via class switching revealed a reduced ability to differentiate into IgG1+ cells (Fig. 2 G). The response to the T cell–independent antigen (TNP-Ficoll) was unchanged in chompB mutants, consistent with a lack of effect on the number of B1 B cells, which are known to contribute to this response (Fig. 2 C; Martin et al., 2001; Defrance et al., 2011).
Figure 2.
Defective T cell–dependent antibody responses and B cell activation in chompB mutants. (A) Ig levels were analyzed in 6–12-wk-old WT and chompB mutant mice. (B) 6–12-wk-old WT and chompB mice were immunized with DNP-KLH and, after 14 d, DNP-specific antibody levels were determined by ELISA. (C) 6–12-wk-old WT and chompB mice were immunized with TNP-Ficoll and, after 14 d, TNP-specific antibody levels were determined by ELISA. (D–F) Purified B cells from WT and chompB mice were labeled with CFSE and cultured with the indicated stimuli along with 10 ng/ml rIL-4 for 72 h. CFSE dilution and cell death were then assessed by flow cytometry, and total cell numbers were determined by cell counting. (G) Expression of surface IgG1 in response to anti-CD40 and IL-4 was assessed by flow cytometry after 96 h of culture (mean, n = 4–5 from two independent experiments, A–C; results are representative of at least three replicates from two independent experiments, D; mean and SEM of four independent experiments, E and F; and mean and SEM, n = 4 from two independent experiments, G). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next assessed the ability of chompB mutant B cells to respond to stimuli in vitro. chompB B cells failed to proliferate in response to anti-IgM and exhibited reduced responses to LPS and anti-CD40 stimulation as indicated by dilution of CFSE (Fig. 2 D). Additional analyses revealed significantly decreased cell expansion as well as increased cell death in response to anti-IgM stimulation and, to a lesser extent, LPS (Fig. 2, E and F). Because FO B cells comprise 60% of B cells in the spleen of chompB mice as compared with 75% in control mice (unpublished results), the inability to proliferate in response to anti-IgM cannot be explained solely by alterations in B cell subsets, although this may be a contributing factor.
The chompB allele is a loss of function mutation in Sppl2a
We next investigated the nature of the defect in the chompB mutant Sppl2a. Epitope-tagged WT, chompB, and protease dead Sppl2a were expressed at similar levels (Fig. 3 A). Both WT and chompB versions of Sppl2a colocalized with the endosome marker Rab5, ruling out misfolding or altered localization (Fig. 3 C). WT and chompB mutant Sppl2a were also capable of cleaving the previously described substrate TNF to a similar extent (Fluhrer et al., 2006; Friedmann et al., 2006; Fig. 3, A and B). These results demonstrate the localization and function of the chompB mutant Sppl2a, at least when overexpressed, is largely comparable to WT. We speculate that the function and/or localization of the mutant Sppl2a at endogenous expression levels are altered. However, because of a lack of commercially available anti-Sppl2a–specific antibodies, the endogenous expression of the chompB mutant was unable to be determined. Preliminary results confirmed that the mutation in Sppl2a was responsible for the phenotype of chompB mice, as transduction and transplant of chompB bone marrow with WT Sppl2a rescued the B cell developmental blockade (unpublished data).
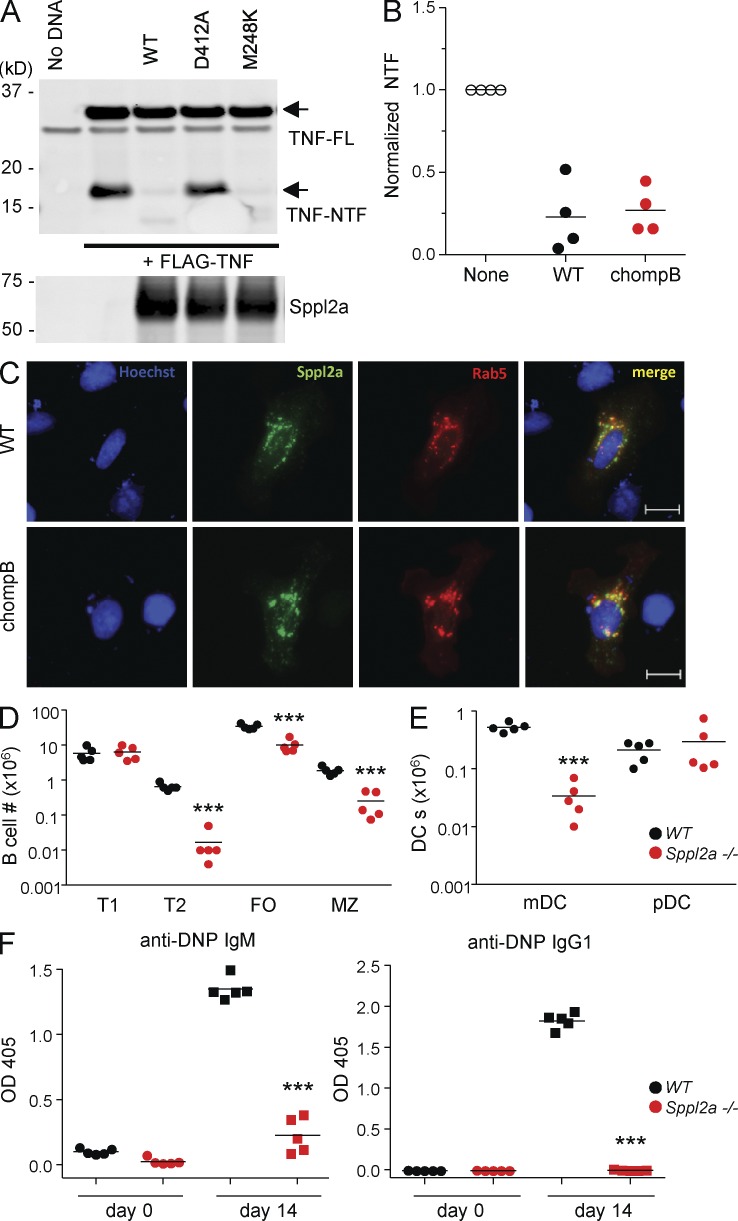
Figure 3.
Mutation in Sppl2a is responsible for the observed defects in chompB mice. (A and B) HEK293T cells were cotransfected with the indicated plasmids, and TNF and Sppl2a levels were determined by Western blot. TNF-NTF (N-terminal fragment) was first normalized to full length TNF, followed by normalization to samples without Sppl2a for quantification (representative of four independent experiments for A and mean, n = 4 from four independent experiments for B). (C) U2OS cells were cotransfected with V5-mSppl2a together with Rab5-RFP. Cells were then stained with an anti-V5 antibody and Hoechst dye and analyzed by microscopy (representative of two independent experiments). Bars, 20 µm. (D and E) Splenocytes from WT and Sppl2a−/− mice were analyzed by flow cytometry as described in Fig. 1 (mean, n = 5 from three independent experiments). (F) 6–12-wk-old WT and Sppl2a−/− mice were immunized with DNP-KLH. DNP-specific antibody levels were determined by ELISA after 14 d (mean, n = 4–5 from two independent experiments). ***, P < 0.001.
To confirm that the phenotypes observed in the chompB mutants were a result of loss of function of Sppl2a, we obtained Sppl2a−/− mice. Analysis of Sppl2a−/− mice revealed similar decreases in splenic B cells and mDCs compared with chompB mutants (Fig. 3, D and E). Also consistent with the chompB mice, DNP-specific IgG1 was decreased after immunization in Sppl2a−/− mice (Fig. 3 F).
The chompB mutation is a hypomorphic allele of Sppl2a
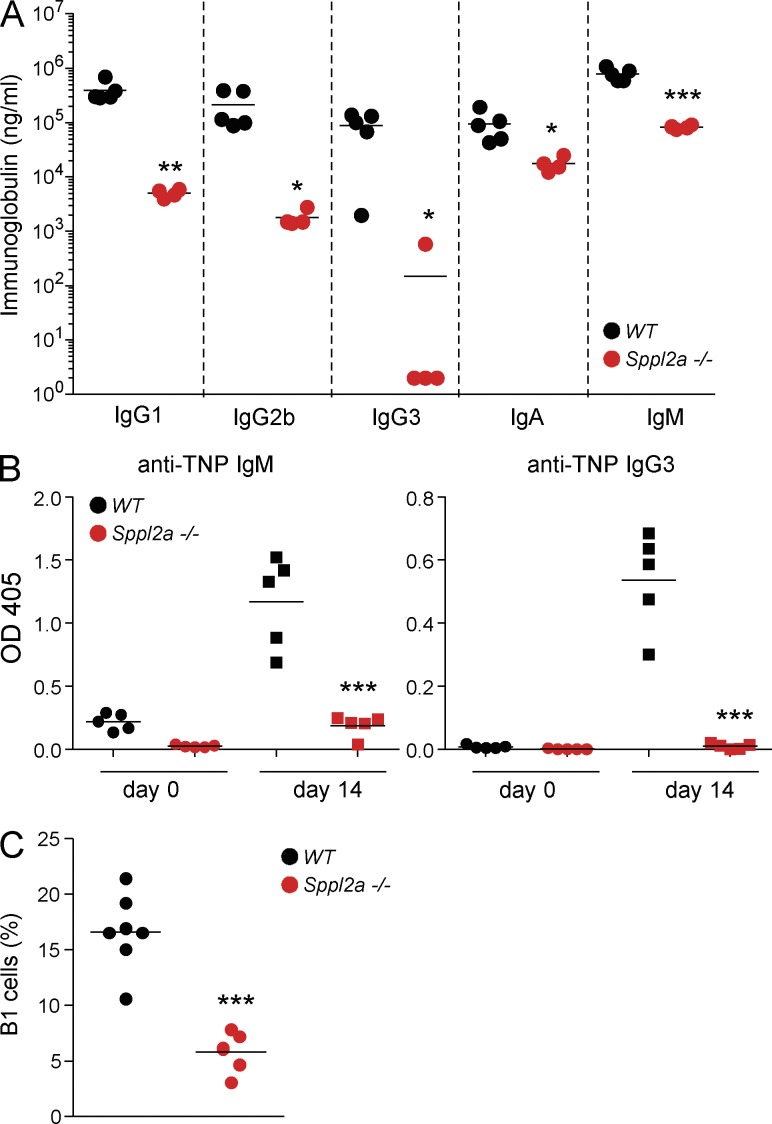
Despite the similarities, there were also differences between chompB mutant and Sppl2a−/− mice. Unlike the chompB mice, the levels of DNP-specific IgM were decreased in Sppl2a−/− mice after immunization (Fig. 3 F). Analysis of steady-state Ig levels also revealed decreases in all isotypes tested in the Sppl2a−/− mice (Fig. 4 A). Additionally, the response to TNP-Ficoll revealed a decrease in TNP-specific IgM and IgG3 in the Sppl2a−/− mice (Fig. 4 B). Unlike the chompB mutants, there was a significant decrease in B1 B cells in the Sppl2a−/− mice and this likely explains some of the observed differences between the two strains (Fig. 4 C). The similarities between the chompB and Sppl2a−/− mice suggest that the chompB mutation in Sppl2a is a loss of function allele. However, the intact T cell–independent responses, B1 cell numbers, intact IgA and IgM levels, and assessment of activity when overexpressed together suggest that the chompB mutation is a hypomorphic allele that maintains some level of activity in vivo.
Figure 4.
The chompB mutation in Sppl2a is a hypomorphic allele. (A) Plasma Ig levels were determined from WT and Sppl2a−/− mice (mean, n = 4–5 from two independent experiments). (B) 6–12-wk-old WT and Sppl2a−/− mice were immunized with TNP-Ficoll and TNP-specific antibody levels were determined by ELISA 14 d later (mean, n = 4–5, two independent experiments). (C) B1 cell numbers from the peritoneal cavity were defined as in Fig. 1 (mean, n = 5–7 from three independent experiments). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Sppl2a-mediated cleavage of the N terminus of CD74 is required for B cell survival
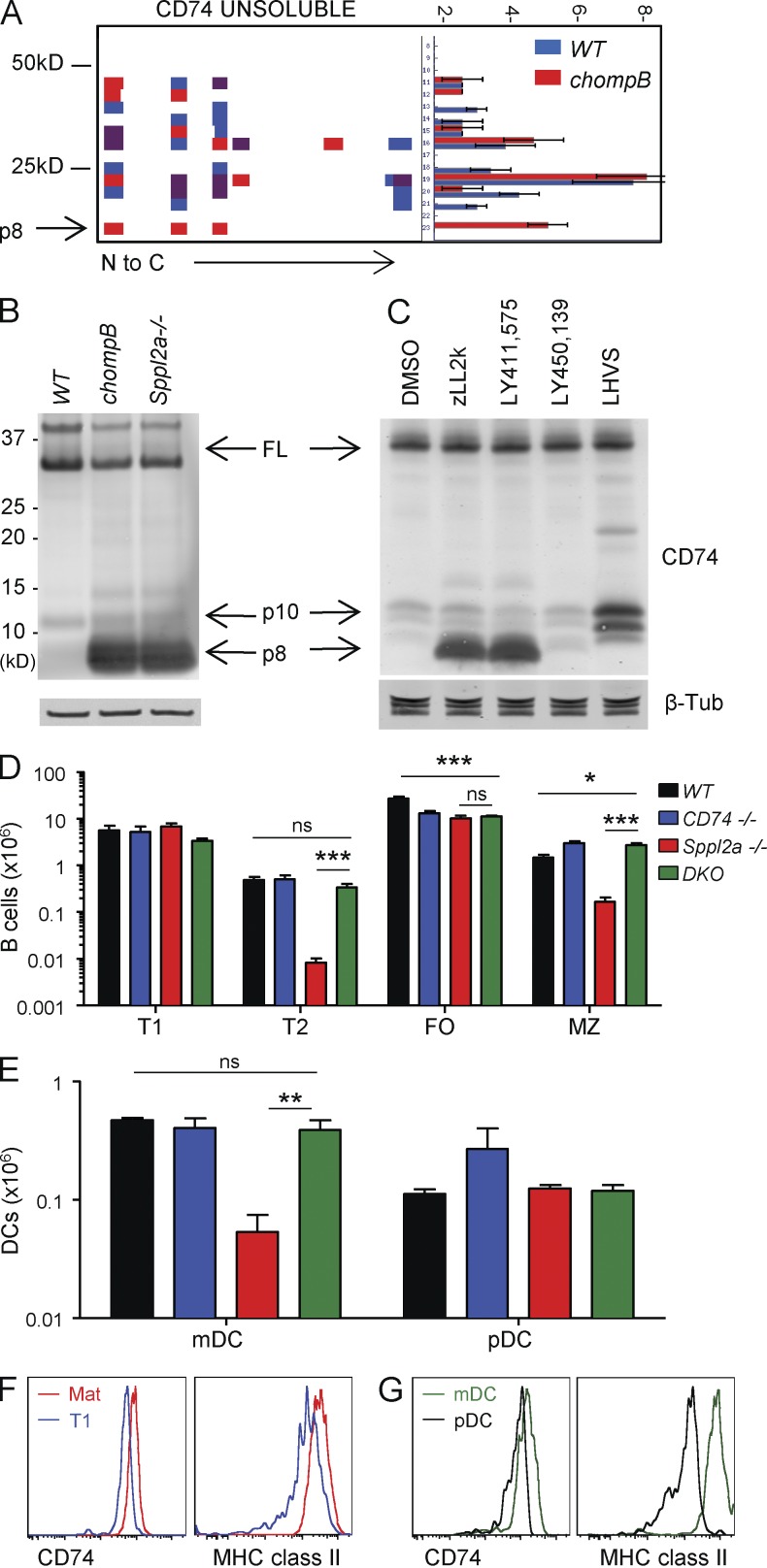
In addition to TNF, Sppl2a has been shown to cleave several substrates in vitro; however, none of these have been implicated in B cell development (Kirkin et al., 2007; Martin et al., 2008). To identify additional substrates, an unbiased proteomics approach was used to compare the proteomes from WT and chompB B cells (Dix et al., 2008). This analysis identified accumulation of a fragment of CD74 in membrane-enriched fractions from chompB but not WT B cells (Fig. 5 A). Although CD74 has been well studied for its role in MHC class II antigen presentation, it has also been implicated in B cell development (Kenty and Bikoff, 1999; Matza et al., 2002b; Stumptner-Cuvelette and Benaroch, 2002). Analysis of CD74 by Western blot revealed accumulation of an 8-kD fragment (p8) in both Sppl2a−/− and chompB cells consistent with the observations in the proteomics approach (Fig. 5 B). Cleavage of the p8 fragment by Sppl2a should generate a 4-kD fragment (p4) but it is not detectible by Western blot. CD74 processing was further analyzed in A20 mouse B cells using protease inhibitors (Fig. 5 C). Cathepsin inhibition (LHVS) led to accumulation of the CLIP-containing 10-kD fragment consistent with previous results (Riese et al., 1996). Treatment of B cells with Spp family active inhibitors (zLL2k and LY411,575) also led to accumulation of CD74 p8 (Nyborg et al., 2004). Because LY411,575 also has γ-secretase activity, we tested a γ-secretase selective inhibitor (LY450,139) which failed to block the processing of CD74 p8. These data are in contrast with a previous study demonstrating a role for γ-secretase, but not the Spp family, in CD74 processing (Becker-Herman et al., 2005). The reasons for the discrepancies are unclear but could be a result of the differences in the systems used.
Figure 5.
Sppl2a-mediated cleavage of CD74 is responsible for the B cell developmental blockade in chompB mice. (A) Peptograph of CD74 from WT and chompB membrane-enriched fractions. The left side depicts the weight in kD and the right side depicts the frequency. (B) Western blot analysis of sorted mature B cells (CD24loCD21int) using antibodies to CD74 and β-tubulin (representative of two independent experiments). (C) CD74 cleavage in A20 cells assessed by Western blot (results are representative of at least three independent experiments). (D and E) B cell and DC numbers were determined using the same parameters in Fig. 1 (mean, n = 4 from three independent experiments). (F and G) Intracellular CD74 and MHC class II levels were determined using the gating schemes in Fig. 1 for the indicated B cell (F) and DC (G) populations (n = 3, from two independent experiments). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Deletion of CD74 prevents B cell and mDC loss in Sppl2a−/− mice
The accumulation of CD74 p8 in the absence of Sppl2a could affect B cell and DC development/survival in several ways. Two possibilities are either that Sppl2a cleavage of p8 releases p4 which mediates cell signaling, or that accumulation of p8 in the endosome is toxic. To differentiate between these possibilities, Sppl2a:CD74 double KO mice (DKO) were generated. If signaling via p4 were the predominant function of Sppl2a, DKO mice should have a similar phenotype as the Sppl2a−/− mice. Alternatively, if the primary function of Sppl2a is to clear p8, the Sppl2a phenotype should be rescued in DKO mice. Analysis of B cell populations in the spleen revealed a restoration of T2 and MZ B cells to WT levels in DKO mice (Fig. 5 D). Analysis of DCs also revealed a restoration in the mDC population to WT levels in DKO mice (Fig. 5 E). FO B cells were not restored in DKO mice, as CD74−/− mice have decreased FO B cell numbers. Collectively, these data demonstrate that removal of CD74 p8 from endosomal membranes is the primary function of Sppl2a and that CD74 is the key substrate of Sppl2a with respect to B cell and DC development/survival.
One open question relates to why post-T1 B cells and mDCs are particularly sensitive to loss of Sppl2a. One hypothesis is that the sensitivity could be driven by increased expression of Sppl2a or CD74 in these cell types. Analysis of Sppl2a mRNA expression revealed similar levels in T1 and mature B cells (Fig. S1 C). In contrast, analysis of intracellular levels of CD74 and MHC class II on WT B cell subsets revealed an increase between T1 and mature B cells, consistent with timing of the B cell developmental blockade in Sppl2a−/− mice (Fig. 5 F). Analysis of DCs also revealed elevated levels of both proteins in mDCs compared with pDCs (Fig. 5 G). Collectively, these data suggest that Sppl2a is especially important in cells that contain high levels of MHC II and CD74 and that Sppl2a-mediated processing of CD74 p8 is important for B cell and DC survival.
We have provided evidence that chompB mutant B cells have survival defects as a result of accumulation of CD74 p8. In contrast, previous work has suggested a role for CD74 in B cell development via p4 signaling (Matza et al., 2002a,b). However, the observation that mature B cell numbers are restored in MHC class II:CD74 double-deficient mice suggests that the loss of FO B cells in CD74−/− mice results from altered MHC class II trafficking rather than lack of p4 signaling (Maehr et al., 2004). These data, together with the current work, provide strong support for the hypothesis that p8 accumulation, rather than p4 loss, leads to B cell and mDC defects in Sppl2a−/− mice. Two accompanying manuscripts in this issue by Schneppenheim et al. and Bergmann et al. support the general conclusions we have reached.
We speculate that Sppl2a permits higher levels of CD74 and MHC class II expression and thereby facilitates effective sampling of the immune environment. Rapid turnover of MHC–peptide complexes ensures that antigen-presenting cells are able to present the most current range of foreign antigens to ensure a rapid response to invading pathogens. However, a byproduct of this rapid turnover is high levels of membrane-bound p8 which are toxic to the cell. By degrading p8, Sppl2a prevents accumulation of this otherwise toxic byproduct of MHC Class II antigen processing and permits higher levels of MHC Class II and CD74 needed for efficient antigen sampling in specialized antigen-presenting cells.
The past decade of clinical experience with B cell depleting antibodies, such as Rituximab, has revealed a critical role for B cells in the pathogenesis of several autoimmune diseases. Our results raise the possibility that targeting Sppl2a may prove useful for the treatment of autoimmune disease. However, given the essential role of Sppl2a in mediating B and DC development, a thorough assessment of the therapeutic potential of Sppl2a will require the generation of appropriate inducible models that allow elimination of Sppl2a in mature cells.
MATERIALS AND METHODS
Mice.
All mice used were 6–12 wk of age and were maintained in the specific pathogen-free facility of the Genomics Institute of the Novartis Research Foundation. C57BL/6 ENU-mutagenized mice were generated as described elsewhere (Miosge et al., 2002). B cell percentages in whole blood were measured using antibodies to B220 (eBioscience) and cells were analyzed by flow cytometry. SNP mapping of the affected mice was performed as described elsewhere (Wiltshire et al., 2003). Sppl2a−/− mice were purchased from Deltagen and backcrossed to C57BL/6 for five generations to >99% pure background using speed congenics. CD74−/− mice (B6.129S6-Cd74tm1Liz/J; The Jackson Laboratory) have been described previously (Bikoff et al., 1993). All procedures were approved by the Genomics Institute of the Novartis Research Foundation Institutional Animal Care and Use Committee. Littermates were used as controls in the majority of experiments, but in some instances, C57BL/6 mice from our internal colony were used as well.
Bone marrow transplant.
Traditional mixed and single bone marrow chimeras were set up as previously described except the cells were transferred into the retro-orbital sinus region. (Miller et al., 2007).
Immunoglobulin levels.
Immunoglobulin levels were measured from mouse plasma using an isotyping kit (Millipore) according to the manufacturer’s instructions.
Plasmids and cell transfection.
Cell transfections in 293T cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In brief, 2 × 105 293T cells were cultured in 24-well plates (Costar), followed by additions of 1 µg of the indicated plasmids together with Lipofectamine in Opti-MEM (Invitrogen). 24 h after transfection, cells were released from the plate using 0.05% Trypsin (Invitrogen) and whole cell lysates, and Western blotting procedures were performed as described below. Plasmids for human Sppl2a (pCI-hSPPL2a) were described previously (Friedmann et al., 2006), and the chompB equivalent mutant (pCI-hSPPL2aM248K) and protease inactive mutant (pCI-hSPPL2aD412A) were generated by site-directed mutagenesis of the appropriate base pairs. Plasmids for mouse Sppl2a were generated by inserting the full length cDNA into the pCDNA3.1/V5-His-TOPO vector (Invitrogen) by PCR. The chompB version was generated using site-directed mutagenesis of the appropriate base pairs. N-terminally tagged human TNF (pBICEP-CMV-2-TNF) was generated by introducing the full length cDNA into the pBICEP-CMV-2 vector (Sigma-Aldrich) using SalI and NotI sites. pCMV6-AC-RFP_Rab5 (expressing RFP-labeled endosome marker Rab5) was purchased from Origene.
Immunofluorescence.
For immunofluorescence studies of transiently expressed proteins in U2OS cells, DNA (0.06 µg/well; 96-well plate) was diluted in Opti-MEM (Invitrogen) and incubated at room temperature for 5 min before addition of PEI (Polysciences) solution and 10-min incubation at room temperature. 104 cells/well were added to the preformed complexes and incubated for 20 min at room temperature before the mixture was transferred into a clear-bottom 96-well plate. 24 h after transfection, cells were fixed with 4% paraformaldehyde for 15 min at room temperature, followed by 1 h of treatment with blocking solution (4% BSA in PBS/0.1% Tween-20). Primary antibody directed against the V5 tag (Sigma-Aldrich) was added in PBS/0.1% Tween-20/1% BSA for 1 h and washed three times with PBS/0.1% Tween-20. The secondary antibody goat anti–mouse IgG Alexa Fluor 488 (Invitrogen) and Hoechst dye (1:10,000) were added in PBS/0.1% Tween-20/1% BSA for 1 h, followed by three washes with PBS/0.1% Tween-20. Fluorescence images were taken with an Axio fluorescence microscope (Carl Zeiss).
Proteomics approach for substrate identification.
Purified WT and chompB mutant B cells were resuspended in 50 mM Tris, pH 8.0, with appropriate amount of protease inhibitors (lysis solution) and probe was sonicated 3× 10 bursts on ice. Protein samples were centrifuged at 100,000 g for 45 min as 4°C. The supernatant, the soluble proteome, was moved to a new tube and the pellet, the insoluble proteome, was washed three times with lysis solution and resuspended back into lysis solution by probe sonication. Proteins were quantified and 100 µg was mixed with protein loading dye for separation by SDS-PAGE. The rest of the procedure was performed as described previously (Dix et al., 2008).
B cell isolation and cell culture.
Cell culture and isolation was performed as described elsewhere (Beisner et al., 2003). Resting splenic B cells were purified magnetically by depletion with CD43, Thy1.2, and CD11b MACS beads (Miltenyi Biotec). The purity of these negatively selected cells was determined to be >95% B220+ by flow cytometry. For assessment of proliferation, purified B cells were labeled with 5 µM CFSE (Life Sciences) and cultured for 72 h in the presence of the indicated stimuli, together with 10 ng/ml rIL-4 (eBioscience). After 72 h, cells were stained with B220 APC and 7AAD, and proliferation and cell death was assessed by flow cytometry. For assessment of cell numbers from proliferation experiments, cell counts were determined using a Vicell (Beckman Coulter). For analysis of class switching, purified B cells were stimulated for 96 h with anti-CD40 and 10 ng/ml rIL-4 (eBioscience). IgG1 surface staining on B220+ cells was assessed by flow cytometry.
Flow cytometry.
Cells were stained for 20 min at 4°C using FITC-, PE-, PerCP-, allophycocyanin-, Pacific blue–, APC-Cy7–, APC-Cy5–, and biotin-conjugated Abs against B220, CD21, CD23, IgM, IgD, CD4, CD8, CD11c, CD19, CD3, NK1.1, PDCA1, CD95, GL7, CD45.1, CD45.2, Gr1, CD11b, MHC class II, and Ter119 (BD, eBioscience, and BioLegend). Cells were collected on either an LSR-II or Fortessa (BD) flow cytometer and data were analyzed using FlowJo (Tree Star). Cell sorting was performed on a FACSARIA. For intracellular staining of CD74 and MHC class II, surfaced-stained cells were fixed, permeabilized, and stained for each marker using a kit from eBioscience according to the manufacturer’s instructions.
Western blotting.
Cells were harvested in 1× lysis buffer (Cell Signaling Technology) containing protease inhibitors, phosphatase inhibitors (Sigma-Aldrich), and 1 mM PMSF. Samples were electrophoresed through NuPAGE Novex 10% Bis-Tris precast polyacrylamide gels in MOPS buffer (Invitrogen) and transferred to nitrocellulose membranes using the iBlot Dry Blotting System (Invitrogen). Membranes were probed with antibodies against mouse CD74 (BD), β-tubulin (Millipore), FLAG (Sigma-Aldrich), and HA (Invitrogen), followed by IRDye 800– (Rockland Immunochemical) or Alexa Fluor 680 (Invitrogen)–conjugated secondary antibodies. Membranes were scanned and analyzed with the Odyssey system (LI-COR Biosciences).
Immunizations and antigen-specific antibody determination.
Mice were immunized intraperitoneally with 25 µg TNP-Ficoll or 100 µg DNP-KLH (Biosearch Technologies) in ALUM (Thermo Fisher Scientific). Levels DNP or TNP-specific Ig were assayed by ELISA as described previously (Schopf et al., 1999). HRP-conjugated rat anti–mouse IgM, IgG1, and IgG3 were purchased from BD. Plates were developed using the Super AquaBlue ELISA substrate system (eBioscience).
Quantitative mRNA expression analysis.
RNA was isolated from mature and T1 B cells using the RNeasy kit (QIAGEN). cDNA was generated with the QuantiTect Rev. Transcription kit (QIAGEN). mGAPDH and mSppl2a were amplified using SYBR Greener (Life Technologies) on the ABI 7900HT. Primer sequences are as follows: mGAPDH forward: 5′-CATGGCCTTCCGTGTTCCTA-3′, mGAPDH reverse: 5′-CCTGCTTCACCACCTTCTTGAT-3′; mSppl2a forward: 5′-CCATCCTGGCCTAACTTTGA-3′, and mSppl2a reverse: 5′-TCTTCTGCGTCTTCCACTGA-3′. The following program was used: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. mSppl2a mRNA levels shown in the graph are normalized to GAPDH levels.
Statistics.
All statistical analysis was performed with Prism software using an unpaired two-tailed Student’s t test.
Online supplemental material.
Fig. S1 shows identification of Sppl2a by ENU mutagenesis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20121072/DC1.
Supplementary Material
Acknowledgments
The authors would like to thank Evelyn Rodrigo, Carie Jackson, Alejandra Rocha, Chris Trussel, Marion Kamke, and Cynthia Cienfeugos for technical assistance. The authors would like to thank Deb Nguyen, Ben Wen, and Jonathan Deane for review of the manuscript.
Competing financial interests: D.R. Beisner, A.E. Parker, C. Dahlberg, P. Langerak, S.E. Sutton, W. Barnes, F.J. Otero, M.A. Young, U. Bodendorf, B. Martoglio, and M.P. Cooke work for The Novartis Institute for Biomedical Research. The authors have no other conflicts of interest.
Footnotes
Abbreviations used:
- DKO
- double KO
- ENU
- N-ethyl-N-nitrosourea
- FO
- follicular
- mDC
- myeloid DC
- MZ
- marginal zone
- NTF
- N-terminal fragment
- pDC
- plasmacytoid DC
- SNP
- single nucleotide polymorphism
- Sppl2a
- signal peptide peptidase-like 2a
- T1
- transitional 1
References
- Allman D., Pillai S. 2008. Peripheral B cell subsets. Curr. Opin. Immunol. 20:149–157 10.1016/j.coi.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Herman S., Arie G., Medvedovsky H., Kerem A., Shachar I. 2005. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol. Biol. Cell. 16:5061–5069 10.1091/mbc.E05-04-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner D.R., Chu I.H., Arechiga A.F., Hedrick S.M., Walsh C.M. 2003. The requirements for Fas-associated death domain signaling in mature T cell activation and survival. J. Immunol. 171:247–256 [DOI] [PubMed] [Google Scholar]
- Bergmann H., Yabas M., Short A., Miosge L., Barthel N., Teh C.E., Roots C.M., Bull K.R., Jeelall Y., Horikawa K., et al. 2013. B cell survival, surface BCR and BAFFR expression, CD74 metabolism, and CD8− dendritic cells require the intramembrane endopeptidase SPPL2A. J. Exp. Med. 210:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Jiang Z., Georgel P., Crozat K., Croker B., Rutschmann S., Du X., Hoebe K. 2006. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353–389 10.1146/annurev.immunol.24.021605.090552 [DOI] [PubMed] [Google Scholar]
- Bikoff E.K., Huang L.Y., Episkopou V., van Meerwijk J., Germain R.N., Robertson E.J. 1993. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J. Exp. Med. 177:1699–1712 10.1084/jem.177.6.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J.L. 2006. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat. Rev. Drug Discov. 5:564–576 10.1038/nrd2085 [DOI] [PubMed] [Google Scholar]
- Cook M.C., Vinuesa C.G., Goodnow C.C. 2006. ENU-mutagenesis: insight into immune function and pathology. Curr. Opin. Immunol. 18:627–633 10.1016/j.coi.2006.07.011 [DOI] [PubMed] [Google Scholar]
- Defrance T., Taillardet M., Genestier L. 2011. T cell-independent B cell memory. Curr. Opin. Immunol. 23:330–336 10.1016/j.coi.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Dix M.M., Simon G.M., Cravatt B.F. 2008. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 134:679–691 10.1016/j.cell.2008.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhrer R., Grammer G., Israel L., Condron M.M., Haffner C., Friedmann E., Böhland C., Imhof A., Martoglio B., Teplow D.B., Haass C. 2006. A gamma-secretase-like intramembrane cleavage of TNFalpha by the GxGD aspartyl protease SPPL2b. Nat. Cell Biol. 8:894–896 10.1038/ncb1450 [DOI] [PubMed] [Google Scholar]
- Friedmann E., Hauben E., Maylandt K., Schleeger S., Vreugde S., Lichtenthaler S.F., Kuhn P.H., Stauffer D., Rovelli G., Martoglio B. 2006. SPPL2a and SPPL2b promote intramembrane proteolysis of TNFalpha in activated dendritic cells to trigger IL-12 production. Nat. Cell Biol. 8:843–848 10.1038/ncb1440 [DOI] [PubMed] [Google Scholar]
- Gürcan H.M., Keskin D.B., Stern J.N., Nitzberg M.A., Shekhani H., Ahmed A.R. 2009. A review of the current use of rituximab in autoimmune diseases. Int. Immunopharmacol. 9:10–25 10.1016/j.intimp.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Kenty G., Bikoff E.K. 1999. BALB/c invariant chain mutant mice display relatively efficient maturation of CD4+ T cells in the periphery and secondary proliferative responses elicited upon peptide challenge. J. Immunol. 163:232–241 [PubMed] [Google Scholar]
- Kirkin V., Cahuzac N., Guardiola-Serrano F., Huault S., Lückerath K., Friedmann E., Novac N., Wels W.S., Martoglio B., Hueber A.O., Zörnig M. 2007. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ. 14:1678–1687 10.1038/sj.cdd.4402175 [DOI] [PubMed] [Google Scholar]
- Lattin J.E., Schroder K., Su A.I., Walker J.R., Zhang J., Wiltshire T., Saijo K., Glass C.K., Hume D.A., Kellie S., Sweet M.J. 2008. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 4:5 10.1186/1745-7580-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R., Kraus M., Ploegh H.L. 2004. Mice deficient in invariant-chain and MHC class II exhibit a normal mature B2 cell compartment. Eur. J. Immunol. 34:2230–2236 10.1002/eji.200425246 [DOI] [PubMed] [Google Scholar]
- Martin F., Oliver A.M., Kearney J.F. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 14:617–629 10.1016/S1074-7613(01)00129-7 [DOI] [PubMed] [Google Scholar]
- Martin L., Fluhrer R., Reiss K., Kremmer E., Saftig P., Haass C. 2008. Regulated intramembrane proteolysis of Bri2 (Itm2b) by ADAM10 and SPPL2a/SPPL2b. J. Biol. Chem. 283:1644–1652 10.1074/jbc.M706661200 [DOI] [PubMed] [Google Scholar]
- Matza D., Kerem A., Medvedovsky H., Lantner F., Shachar I. 2002a. Invariant chain-induced B cell differentiation requires intramembrane proteolytic release of the cytosolic domain. Immunity. 17:549–560 10.1016/S1074-7613(02)00455-7 [DOI] [PubMed] [Google Scholar]
- Matza D., Lantner F., Bogoch Y., Flaishon L., Hershkoviz R., Shachar I. 2002b. Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc. Natl. Acad. Sci. USA. 99:3018–3023 10.1073/pnas.052703299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.T., Sandberg M., Huang Y.H., Young M., Sutton S., Sauer K., Cooke M.P. 2007. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat. Immunol. 8:514–521 10.1038/ni1458 [DOI] [PubMed] [Google Scholar]
- Miosge L.A., Blasioli J., Blery M., Goodnow C.C. 2002. Analysis of an ethylnitrosourea-generated mouse mutation defines a cell intrinsic role of nuclear factor kappaB2 in regulating circulating B cell numbers. J. Exp. Med. 196:1113–1119 10.1084/jem.20020959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg A.C., Jansen K., Ladd T.B., Fauq A., Golde T.E. 2004. A signal peptide peptidase (SPP) reporter activity assay based on the cleavage of type II membrane protein substrates provides further evidence for an inverted orientation of the SPP active site relative to presenilin. J. Biol. Chem. 279:43148–43156 10.1074/jbc.M405879200 [DOI] [PubMed] [Google Scholar]
- Riese R.J., Wolf P.R., Brömme D., Natkin L.R., Villadangos J.A., Ploegh H.L., Chapman H.A. 1996. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 4:357–366 10.1016/S1074-7613(00)80249-6 [DOI] [PubMed] [Google Scholar]
- Schneppenheim J., Dressel R., Hüttl S., Lüllmann-Rauch R., Engelke M., Dittmann K., Wienands J., Eskelinen E.-L., Hermans-Borgmeyer I., Fluhrer R., Saftig P., Schröder B. 2013. The intramembrane protease SPPL2a promotes B cell development and controls endosomal traffic by cleavage of the invariant chain. J. Exp. Med. 210:41–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf L.R., Bliss J.L., Lavigne L.M., Chung C.L., Wolf S.F., Sypek J.P. 1999. Interleukin-12 is capable of generating an antigen-specific Th1-type response in the presence of an ongoing infection-driven Th2-type response. Infect. Immun. 67:2166–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumptner-Cuvelette P., Benaroch P. 2002. Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta. 1542:1–13 10.1016/S0167-4889(01)00166-5 [DOI] [PubMed] [Google Scholar]
- Wiltshire T., Pletcher M.T., Batalov S., Barnes S.W., Tarantino L.M., Cooke M.P., Wu H., Smylie K., Santrosyan A., Copeland N.G., et al. 2003. Genome-wide single-nucleotide polymorphism analysis defines haplotype patterns in mouse. Proc. Natl. Acad. Sci. USA. 100:3380–3385 10.1073/pnas.0130101100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.