Hepatic NK cells eliminate HBV-specific T cells dependent on TRAIL and TRAIL-R2 interactions to limit antiviral immunity in chronic infection.
Abstract
Antiviral T cell responses in hepatotropic viral infections such as hepatitis B virus (HBV) are profoundly diminished and prone to apoptotic deletion. In this study, we investigate whether the large population of activated NK cells in the human liver contributes to this process. We show that in vitro removal of NK cells augments circulating CD8+ T cell responses directed against HBV, but not against well-controlled viruses, in patients with chronic hepatitis B (CHB). We find that NK cells can rapidly eliminate HBV-specific T cells in a contact-dependent manner. CD8+ T cells in the liver microcirculation are visualized making intimate contact with NK cells, which are the main intrahepatic lymphocytes expressing TNF-related apoptosis-inducing ligand (TRAIL) in CHB. High-level expression of the TRAIL death receptor TRAIL-R2 is found to be a hallmark of T cells exposed to the milieu of the HBV-infected liver in patients with active disease. Up-regulation of TRAIL-R2 renders T cells susceptible to caspase-8–mediated apoptosis, from which they can be partially rescued by blockade of this death receptor pathway. Our findings demonstrate that NK cells can negatively regulate antiviral immunity in chronic HBV infection and illustrate a novel mechanism of T cell tolerance in the human liver.
T cell responses are tightly regulated to maintain immune homeostasis and limit damage to vital organs. T cells in the liver, in particular, are subjected to potent tolerizing mechanisms. Although these mechanisms prevent overzealous responses causing tissue injury, they may be exploited by hepatotropic pathogens to subvert antiviral immunity (Protzer et al., 2012). There have been major recent advances in our understanding of the multiple co-inhibitory pathways driving T cell exhaustion in the liver and perpetuating persistent viral infections (Protzer et al., 2012). However, the potential for NK cells to regulate T cell immunity has not been defined in human viral infections.
NK cells can contribute to the containment of many infections by intracellular pathogens (Orange et al., 2002; Khakoo et al., 2004; Lodoen and Lanier, 2006; Alter et al., 2011), acting though cytolytic or noncytolytic effects on target cells or by promoting adaptive immunity (Vivier et al., 2008). Accumulating data highlight the capacity of NK cells to also exert a negative regulatory effect on T cells (Su et al., 2001) through inhibition of antigen presentation (Andrews et al., 2010), production of IL-10 (Lee et al., 2009), or direct killing of T cells. Several receptor–ligand interactions between NK cells and T cells have been found to be capable of leading to autologous lysis of activated T cells (Rabinovich et al., 2003; Cerboni et al., 2007; Lu et al., 2007; Soderquest et al., 2011). More recently, NK cells have been shown to limit T cell immunity in a mouse model of chronic viral infection (Waggoner et al., 2010; Lang et al., 2012; Waggoner et al., 2012).
In this study, we sought to investigate the impact of NK cells on antiviral T cell responses in the setting of persistent infection with a human hepatotropic virus. Activated NK cells are markedly enriched in the liver microcirculation, where we hypothesized they would come into prolonged, close contact with infiltrating T cells. Although NK cells in patients with chronic hepatitis B (CHB) infection have impaired noncytolytic antiviral function, we have previously shown that they maintain their cytotoxic potential and up-regulate the death ligand TRAIL, particularly in the intrahepatic compartment (Dunn et al., 2007; Peppa et al., 2010). HBV-specific CD8+ T cells, which are essential for viral control, are profoundly depleted in these patients (Maini et al., 2000; Boni et al., 2007). Here, we demonstrate that hepatitis B virus–specific T cells up-regulate a death receptor for TRAIL and become susceptible to NK cell–mediated killing, thereby contributing to the failure of antiviral immunity in CHB.
RESULTS
Recovery of HBV-specific CD8+ T cells after depletion of NK cells
To investigate whether NK cells have the potential to regulate virus-specific CD8+ T cells, we initially determined the impact of total NK cell depletion on the magnitude of HBV-specific T cell responses. CD8+ T cell responses against a pool of peptides representing well-described HLA-A2–restricted HBV epitopes or overlapping peptides (15mers) spanning the core protein of HBV were identified by IFN-γ production after short-term culture. Fig. 1 A is a representative example of HBV responses from a patient with active CHB in the presence or absence of NK cells. Stimulation of whole PBMCs resulted in the expected low frequency of responses, in line with the well-established paucity of detectable HBV-specific T cells in CHB (Maini et al., 2000; Boni et al., 2007). Upon NK cell depletion, there was an enhancement of HBV-specific CD8+ T cells, which returned to baseline levels after re-addition of purified NK cells at a physiological ratio at the start of culture. Individual responses and summary data are depicted in Fig. 1 (B and C), showing a significant recovery of HBV-specific CD8+ T cells upon NK cell depletion from patients with CHB. To exclude any potential contribution of other lymphocyte subsets, including NKT cells, depletion experiments were also performed after flow-cytometric sorting of NK cells to 99% purity (Fig. 1 D). Removal of NK cells also promoted the expansion of a population of CD8+ T cells able to bind HLA-A2/HBV peptide multimers (Fig. 1 E). This implied that NK cells were influencing the number of HBV-specific CD8+ T cells surviving in culture, not just the function of preexisting populations.
Figure 1.
Recovery of HBV-specific CD8+ T cells after depletion of NK cells. (A) Representative FACS plots from a CHB patient. HBV-specific CD8+ T cells were identified by intracellular cytokine staining for IFN-γ after 10-d stimulation with a pool of HBV peptides of PBMCs or PBMCs depleted of NK cells (ΔNK). Where indicated, a physiological ratio of NK cells was re-added in the culture at day 0 before stimulation. (B) Individual responses of CHB patients (n = 27) and matched summary data (C). (D) NK cells were sorted to 99% purity by flow cytometry. Control PBMCs stained with the same antibodies were passed though the machine untouched. Summary bar graph of (n = 3) experiments. (E) HBV-specific CD8+ T cells were identified by MHC peptide multimer staining after short-term peptide stimulation in the absence or presence of NK cells or NK cell re-addition at day 0. Representative FACS plots from a CHB patient and summary data from (n = 9) CHB patients. Error bars represent the mean ± SEM. ND = not detected. ***, P < 0.001; **, P < 0.01.
Differential effects of NK cells according to T cell specificity
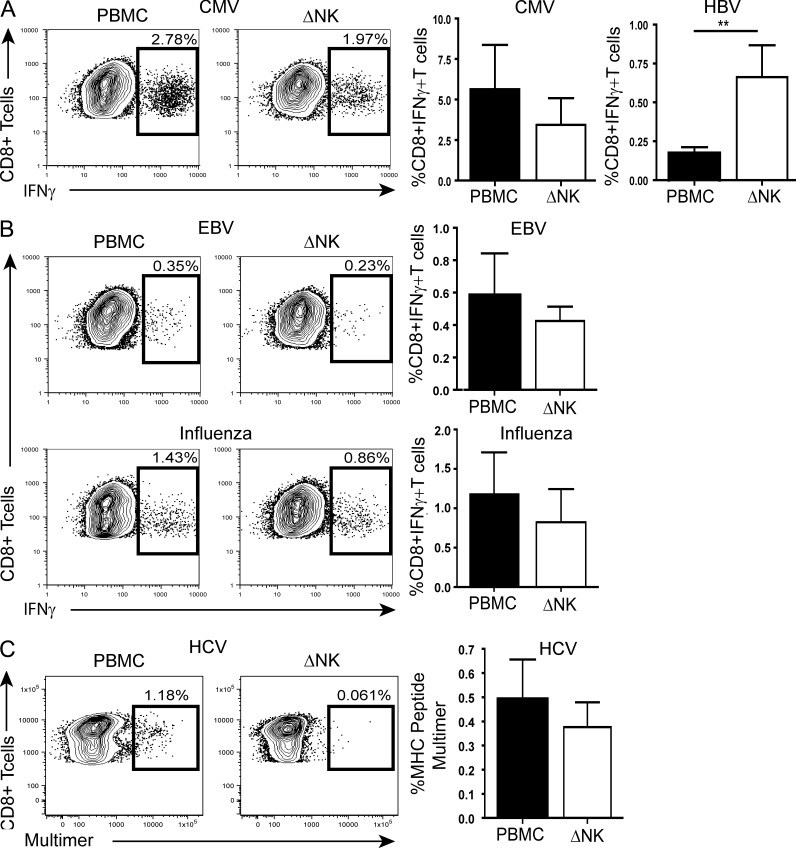
To explore whether NK cell depletion could similarly augment other virus-specific responses, we analyzed CD8+ T cells directed against immunodominant HLA-A2–restricted epitopes from cytomegalovirus (CMV), Epstein–Barr virus (EBV), and influenza in our cohort of CHB patients. In 22 patients with CHB, we were able to simultaneously compare the effect of NK depletion on HBV- and CMV-specific responses; those directed against an immunodominant CMV epitope showed a nonsignificant trend to decrease, contrasting with the increase in T cells directed against HBV in the same patients (Fig. 2 A). Similarly, EBV- and influenza-specific T cell responses in patients with CHB were not augmented upon the removal of NK cells (Fig. 2 B). In patients persistently infected with another hepatotropic virus, hepatitis C (Table S3), there was also no consistent increase in T cells able to recognize 2 immunodominant HLA-A2–restricted HCV epitopes upon NK cell depletion (Fig. 2 C). These data therefore point to differential NK cell regulation of T cells according to their antigen specificity.
Figure 2.
Differential regulation by NK cells according to T cell specificity. (A) Representative FACS plots for CMV-specific responses from a CHB patient upon PBMC stimulation and in the absence of NK cells (ΔNK) and summary bar graphs comparing CD8+ T cell responses upon HBV or CMV peptide stimulation within the same (n = 22) CHB patients ± NK cell depletion. (B) Representative FACS plots for EBV and influenza virus–specific responses from a CHB patient upon PBMC stimulation and in the absence of NK cells (ΔNK) and summary data for EBV (n = 6) and influenza (n = 7). (C) Example of HCV virus–specific CD8+ T cell identification by tetramer staining during short-term culture in the presence and absence of NK cells and summary bar graph from n = 8 HCV patients. Error bars represent the mean ± SEM. **, P < 0.01.
NK cells limit HBV-specific CD8+ T cell responses in a contact-dependent manner by inducing apoptosis
Our initial findings suggested NK cells could be suppressing HBV-specific CD8+ T cells in culture or could be actively deleting them. To investigate the latter possibility, freshly purified NK cells were re-added at day 10, just 6 h before analysis of HBV-specific CD8+ T cell numbers. A diminished HBV-specific CD8+ T cell response was observed irrespective of the timing of NK cell re-addition (representative example and summary data can be found in Fig. 3 A). The speed of this effect precluded a requirement for the inhibition of proliferative expansion of virus-specific CD8+ T cells in culture, instead indicating that these populations were amenable to rapid functional inactivation or deletion by NK cells.
Figure 3.
NK cells limit the survival of CD8+ HBV-specific T cells in a contact-dependent manner by inducing apoptosis. (A) Representative FACS plots from a CHB patient. HBV-specific CD8+ T cells were identified by intracellular cytokine staining for IFN-γ after 10-d stimulation with a pool of HBV peptides upon NK cell depletion (ΔNK) or re-addition of freshly purified NK cells (physiological ratio) at day 0 or 10. Summary data from (n = 4) CHB patients. (B) FACS plots from a CHB patient depicting HBV-specific CD8+ T cells identified by multimer staining, after depletion of NK cells at day 0, or from PBMC culture at 24 h (Day 1) and re-addition of freshly purified NK cells at a physiological ratio on day 0 or 10. Summary bar graphs of n = 5 experiments. (C, top) Representative FACS plots from a CHB patient. HBV-specific CD8+ T cells were identified by multimer staining after short-term peptide stimulation in the absence (ΔNK) or presence of NK cells. NK cells, where indicated, were re-added at a physiological ratio directly in the culture or were plated into transwells to prevent contact. (bottom) The corresponding proportions of apoptotic virus-specific cells. The degree of pancaspase activation was determined by flow cytometry using the carboxyfluorescein-FLICA apoptosis detection kit. Histograms represent early apoptotic (FLICA+7AAD−) and late apoptotic (FLICA+7AAD+) virus-specific CD8+ T cells. (D) Summary stacked bars of n = 3 experiments.
To distinguish between these two possibilities, HBV-specific CD8+ T cells were identified by HLA-A2/peptide multimer rather than IFN-γ staining after removal of NK cells at day 0 or 1 and re-addition of a physiological ratio of freshly isolated NK cells on day 0 or 10. Multimer-binding CD8+ T cells were only increased if NK cells were removed at the beginning of the culture (not on day 1), and were decreased again after re-addition of NK cells on day 0 or 10 (Fig. 3 B). These results indicated that NK cells were able to rapidly deplete the number of virus-specific CD8+ T cells surviving in culture.
The mechanism of action of NK cells on virus-specific T cell survival during short-term culture was further investigated by transwell experiments. A physiological ratio of NK cells was re-added at day 0 directly to the culture or to transwells separated from the T cells by a semipermeable membrane. When NK cells were not in contact with T cells, their survival was enhanced to a degree similar to that seen upon NK cell depletion (Fig. 3 C, top). The degree of pancaspase activation in HBV-specific T cells (indicating apoptosis induction) was reduced when NK cells were depleted or not in contact with T cells, whereas re-addition of NK cells increased the amount of early (FLICA+7AAD−) and late (FLICA+7AAD+) apoptotic events (Fig. 3, C and D, and not depicted). Thus, NK cell induction of T cell apoptosis required cell–cell contact.
Collectively, these results indicate that at least some of the impact of NK cells on HBV-specific T cells is mediated by a direct effect on their survival that is contact-dependent and results in caspase activation.
Increased TRAIL-R2 expression on T cells in CHB
Several ligand–receptor interactions could be responsible for mediating NK cell killing of T cells through caspase induction. We noted that HBV-specific CD8+ T cells stained directly ex vivo had an increase in their expression of activated caspase 8 compared with CMV-specific or global CD8+ T cells (unpublished data). This indicated a death ligand receptor pathway from the TNF superfamily that was able to induce apoptosis through caspase 8 (Sprick et al., 2000). We focused on the TRAIL pathway because we have previously found TRAIL to be up-regulated on NK cells during episodes of HBV-related liver inflammation (Dunn et al., 2007; Peppa et al., 2010). We therefore investigated whether the dysregulated T cell response in patients with CHB might become susceptible to TRAIL-mediated killing by up-regulation of TRAIL death receptors. TRAIL binding to the death receptors TRAIL-R1 and TRAIL-R2, which contain intracytoplasmic domains, triggers caspase activation and induction of apoptosis (Schneider et al., 1997; Kimberley and Screaton, 2004). TRAIL-R2 is up-regulated on the surface of hepatocytes during active flares of HBV infection (Dunn et al., 2007) and is known to have higher affinity for membrane-bound TRAIL (Schneider et al., 1998; Ichikawa et al., 2001). We therefore compared the expression of TRAIL-R2 on global T cells in CHB and healthy controls directly ex vivo. Levels of TRAIL-R2 were low on peripheral CD8+ T cells in CHB but were significantly elevated compared with the negligible levels seen in healthy controls (Fig. 4 A). No significant correlation was observed between the levels of expression of TRAIL-R2 and any virological or clinical parameters in the periphery (unpublished data).
Figure 4.
Higher levels of TRAIL-R2 on T cells in patients with CHB. (A) Representative FACS plots and isotype control from a healthy individual and a CHB patient showing expression of TRAIL-R2 on global peripheral CD8+ T cells, and summary data from n = 18 healthy and n = 27 CHB patients. (B) FACS plots depicting identification of virus-specific CD8+ T cells ex vivo via multimer staining from a representative CHB patient, and gating strategy showing expression of TRAIL-R2 on virus-specific cells. A control multimer was used to help identify the virus-specific populations. (C) Paired data showing expression of TRAIL-R2 on HBV versus CMV virus-specific CD8+ T cells from (n = 9) CHB patients. (D) Representative FACS plot from an individual with resolved HBV infection, showing gating for HBV-specific CD8+ T cells and TRAIL-R2 expression. (E) Summary bar graphs of TRAIL-R2 expression on HBV-specific CD8+ T cells from seven individuals with resolved HBV infection and nine CHB individuals. Error bars represent the mean ± SEM. *, P < 0.05; **, P < 0.01.
TRAIL-R2 expression was then compared on CD8+ T cells directed against CMV or HBV within the same CHB patients, identified directly ex vivo by MHC/peptide multimer staining (Fig. 4 B). A significantly higher proportion of HBV-specific CD8+ T cells expressed TRAIL-R2 compared with CMV-specific responses (Fig. 4 C), in line with their differential susceptibility to NK cell modulation. No up-regulation of TRAIL-R2 was observed on HBV-specific CD8+ T cells obtained from individuals who had previously resolved HBV infection (Fig. 4, D and E). These data indicated that TRAIL-R2 was preferentially induced on HBV-specific CD8+ T cells encountering their cognate antigen.
Further up-regulation of TRAIL-R2 on intrahepatic CD8+ T cells in CHB
Because the TRAIL-R2 death receptor was up-regulated in CHB, particularly on HBV-specific T cells, we hypothesized that its expression might be further enriched in the liver, the site of HBV replication. To determine whether TRAIL-R2 was expressed on CD8+ T cells infiltrating the liver, we initially examined paraffin-embedded sections of HBV-infected livers by immunofluorescence. Using this approach, we noted clear co-staining of CD8+ T cells in the liver with TRAIL-R2 (Fig. 5 A). We expanded on these data by extracting liver-infiltrating lymphocytes from surplus liver biopsy tissue, allowing flow cytometric comparison of TRAIL-R2 expression on paired circulating and intrahepatic CD8+ T cell samples. In 19 out of 21 patients with CHB, the expression of TRAIL-R2 on CD8+ T cells was further increased in the liver compared with the peripheral compartment (Fig. 5 B, C). There was considerable variability in TRAIL-R2 expression levels on intrahepatic CD8+ T cells (Fig. 5 C), and this was found to correlate positively with HBV viral load (Fig. 5 D). These data suggested that the liver environment and HBV may both be factors driving up-regulation of this death receptor. To address this, we obtained surplus liver tissue from seven patients with HCV and eight individuals without viral hepatitis. Liver-infiltrating CD8+ T cells from controls without viral hepatitis expressed more TRAIL-R2 than peripheral CD8+ T cells from healthy donors, but much less than those from HBV-infected livers (Fig. 5 E). CD8+ T cells extracted from HCV-infected livers showed a level of TRAIL-R2 expression that was intermediate between control and HBV-infected livers (Fig. 5 E). Levels of TRAIL-R2 were lower on CD8+ T cells from HCV-infected as opposed to HBV-infected livers, despite the former group tending to be more inflamed (based on ALT levels; Tables S1 and S2). Levels of TRAIL-R2 were not simply a reflection of the degree of T cell activation; significantly more TRAIL-R2 was coexpressed by the HLA-DR+ (activated) fraction of CD8+ T cells extracted from HBV-infected livers compared with HCV or control livers (Fig. 5 F).
Figure 5.
Intrahepatic CD8+ T cells in CHB patients have up-regulated expression of TRAIL-R2. (A) Representative example of immunostaining of paraffin-embedded liver tissue (derived from a CHB patient undergoing diagnostic biopsy) showing CD8+ T cells and TRAIL-R2 colocalization by immunofluorescence. Bars, 20 µm. (B) Representative FACS plots from a CHB patient showing ex vivo expression of TRAIL-R2 on global peripheral and intrahepatic CD8+ T cells. (C) Ex vivo TRAIL-R2 expression on paired peripheral and intrahepatic global CD8+ T cells from (n = 21) patients with CHB. (D) Correlation of TRAIL-R2 expression on intrahepatic CD8+ T cells and HBV viral load. Spearman r = 0.4870, P value (two-tailed) = 0.02 (E) Comparison of TRAIL-R2 expression on intrahepatic CD8+ T cells from 8 patients with nonviral hepatitis (control), 7 HCV-infected patients, and 21 CHB patients. (F) Co-staining for TRAIL-R2 and HLADR gated on intrahepatic global CD8+ T cells directly ex vivo from a CHB patient, a HCV patient, and a nonviral hepatitis control patient; and summary data from control (n = 5), HCV (n = 6), and CHB (n = 10) patients. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
To further explore the characteristics of global intrahepatic CD8+ T cells bearing TRAIL-R2, we compared the expression of a panel of phenotypic markers on TRAIL-R2 high, low, and negative fractions (Fig. 6, A and B). TRAIL-R2 expressing CD8+ T cells were enriched for the activation marker CD38 (in line with the co-staining with HLA-DR demonstrated in Fig. 5 F) and the apoptosis marker Annexin V, but not for the exhaustion marker PD-1 (Fig. 6 A). Cells expressing high levels of TRAIL-R2 were less likely to be found in the CD57+ and CD27-CD45RA+ subsets and were instead mainly of a central memory phenotype (Fig. 6, A and B). These data suggest that TRAIL-R2–expressing CD8+ T cells are activated, apoptotic responses rather than exhausted or senescent populations.
Figure 6.
TRAIL-R2 expression is a feature of CD8+ T cells encountering antigen in the HBV-infected liver. (A) Representative gating strategy identifying intrahepatic TRAIL-R2+ high (red), TRAIL-R2+ low (black), and TRAIL-R2 negative (gray) CD8+ T cells from a CHB patient. Histograms and bars depict the proportions of CD38 (n = 4), Annexin V (n = 3), PD1 (n = 6), and CD57 (n = 8) expressed by each subset directly ex vivo. (B) The maturation status of intrahepatic TRAIL-R2+ high (red), TRAIL-R2+ low (black), and TRAIL-R2 negative (gray) CD8+ T cells were analyzed by co-staining for CD27 and CD45RA. Representative FACS plot and summary bars of frequencies of expression of TRAIL-R2+ high (red), TRAIL-R2+ low (black), and TRAIL-R2 negative (gray) CD8+ T cells with naive, central memory (CM), effector memory (EM) and revertant (EMRA) phenotypes from n = 4 CHB patients. (C) Summary bar graphs comparing expression of TRAIL-R2 on global intrahepatic CD8+ T cells, CMV-specific and HBV-specific CD8+ T cells. Virus-specific CD8+ T cells were identified directly ex vivo by multimer staining in (n = 5) HLA-A2+ CHB patients with available liver biopsies. (D) Representative examples of the gating strategy from a control patient with no evidence of viral hepatitis (control liver) and a CHB patient (HBV liver) showing expression of TRAIL-R2 on intrahepatic global CD8+ T cells and virus-specific CD8+ T cells identified via IFN-γ staining after overnight stimulation with CMV peptide and HBV overlapping peptides (OLP), respectively. Summary bar graphs comparing expression of TRAIL-R2 on CMV-specific CD8+ T cells from control livers (n = 5) versus CMV and HBV-specific CD8+ T cells from (n = 5) CHB patients. Bars represent the mean ± SEM. *, P < 0.05.
The large proportion of total intrahepatic CD8+ T cells bearing TRAIL-R2 in CHB led us to question whether its expression was restricted to HBV-specific T cells. In five HLA-A2+ individuals from whom sufficient liver-infiltrating lymphocytes could be obtained, responses directed against HBV or CMV were compared directly ex vivo by HLA-A2/peptide multimer staining. The expression of TRAIL-R2 was higher on intrahepatic CMV-specific CD8+ T cells compared with the low levels seen on CD8+ T cells of this specificity in the periphery. However, TRAIL-R2 was further enriched on intrahepatic HBV-specific CD8+ T cells (Fig. 6 C). Upon recognition of their cognate antigen after overnight stimulation with HBV peptides, the majority of virus-specific CD8 from HBV-infected livers expressed TRAIL-R2 (Fig. 6 D). An up-regulation of TRAIL-R2 was also observed after stimulation with CMV peptide on intrahepatic virus-specific CD8+ T cells from HBV-infected patients (Fig. 6 D). In contrast, CMV-specific CD8+ T cells from control livers without hepatitis failed to express any TRAIL-R2 upon peptide stimulation (Fig. 6 D). Thus, high TRAIL-R2 expression appeared to be a hallmark of T cells encountering their cognate antigen in the milieu of an HBV-infected liver.
TRAIL blocking partially recovers HBV-specific CD8+ T cells
We next sought functional evidence for the involvement of the TRAIL pathway in NK-cell mediated deletion of virus-specific T cells. To do this, we examined the impact of TRAIL blockade at the time of peptide stimulation of PBMCs on HBV-specific CD8+ T cell responses in vitro. TRAIL blocking experiments were performed with a TRAIL-R2 Fc, compared with a control IgG1 Fc to exclude a nonspecific effect (Fig. 7 A). Fig. 7 A presents representative FACS plots from a CHB patient and summary data from nine CHB patients during short-term culture, which shows enhanced responses upon TRAIL blockade. However, the effect of TRAIL blockade was less striking than that of NK cell depletion in the same patients (Fig. 7 A), suggesting that NK cells were using additional pathways for deleting T cells. Although T cells can express TRAIL in some circumstances (Mirandola et al., 2004; Janssen et al., 2005), in our cohort, TRAIL expression was confined to NK cells (unpublished data), and we could therefore assume that TRAIL blockade of PBMCs was acting on NK cells. In support of this approach, TRAIL blocking of PBMCs depleted of NK cells did not show any further increase in the responses rescued above that observed upon NK depletion alone (Fig. 7 A).
Figure 7.
Partial recovery HBV-specific CD8+ T cells to TRAIL blockade. (A) Representative FACS plots from a CHB patient after short-term peptide stimulation of PBMCs in thepresence or absence of NK cells and in the presence of TRAIL-R2/Fc blocking or IgG1-Fc control. Plotted are summary paired data from (n = 9) CHB patients. (B) Representative FACS plot and summary data from n = 6 CHB patients showing the effect of TRAIL-R2 Fc addition at the time of PMBC stimulation with CMV peptide during short-term culture. (C) Representative example from a CHB patient demonstrating the expression of TRAIL-R2 on global CD8+ T cells in the presence or absence (ΔNK) of NK cells after short-term culture with HBV peptides and summary data from (n = 19) CHB patients. Bars represent the mean ± SEM ***, P < 0.001; **, P < 0.01; *, P < 0.05.
No significant recovery of CMV-specific CD8+ T cells from the same patients with CHB was observed upon TRAIL blockade (Fig. 7 B), in keeping with the low levels of TRAIL-R2 expressed on these cells in the circulation and the lack of their reconstitution upon NK cell depletion. Further support for the preferential deletion of a selective subset of TRAIL-R2-bearing T cells by NK cells came from the finding that the percentage of global CD8+ T cells expressing TRAIL-R2 was increased after depletion of NK cells from HBV peptide-stimulated PBMC cultures (Fig. 7 C).
Overnight rescue of intrahepatic HBV-specific T cells by TRAIL blockade
NK cells constitute up to 40% of the lymphocytic infiltrate within the liver, the site of HBV replication. We have previously shown that intrahepatic NK cells in patients with HBV-related inflammation are highly activated and express increased levels of TRAIL compared with circulating NK cells, whereas intrahepatic CD3+ T cells express little TRAIL (Dunn et al., 2007). The extensive sinusoidal network in the liver forms a unique vascular bed with a narrow lumen and sluggish blood flow. We postulated that this would facilitate close associations between NK cells and T cells as they infiltrated the liver. To investigate this, we performed staining of paraffin-embedded liver sections from patients with CHB by immunohistochemistry. Using this approach we were able to visualize intimate contact between NK cells and T cells in the HBV-infected liver sinusoids, as exemplified in Fig. 8 A. This supported the concept that the intrahepatic predominance of TRAIL-expressing NK cells, in conjunction with the unique architecture of the liver encouraging close contact between lymphocyte subsets, would promote the deletion of TRAIL-R2–bearing T cells.
Figure 8.
Overnight recovery of intrahepatic HBV-specific T cells by TRAIL blockade. (A) Examples of NK cells (NKp46 in blue) in intimate contact with CD3+ T cells (red) in the sinusoidal spaces of a representative HBV-infected liver. Immunohistochemistry was performed on paraffin-embedded HBV tissue. Bars 20 µm. (B) Representative FACS plot from a CHB patient and gating strategy showing expression of caspase 8 in intrahepatic TRAIL-R2+ CD8+ T cells ex vivo and summary bar graphs comparing expression of caspase 8 in global CD8+ T cells versus TRAIL-R2+ CD8+ T cells (n = 5). Only live events were analyzed. (C) Representative FACS plots showing response to TRAIL blockade after overnight incubation of intrahepatic cells with overlapping (OLP) HBV peptides and individual intrahepatic responses from nine CHB patients with available liver tissue.
To test whether the T cells infiltrating HBV-infected livers were subject to death receptor-mediated apoptosis, we stained them for caspase 8, which is activated by these pathways from the TNF superfamily (Sprick et al., 2000). Caspase 8 was detectable in a proportion of CD8+ T cells isolated from HBV-infected liver biopsies; its expression was enriched in the expanded population of intrahepatic CD8+ T cells expressing TRAIL-R2 on their surface (Fig. 8 B), in line with their enhanced expression of Annexin V (Fig. 6 A). These findings support the capacity of TRAIL-R2 to deliver an apoptotic signal to the large proportion of intrahepatic CD8+ T cells on which it is expressed in CHB.
To assess whether TRAIL blocking was capable of restoring virus-specific T cell responses in the liver, we examined paired samples from nine HLA-A2− CHB patients from whom sufficient liver biopsy tissue was available for functional experiments. Overlapping peptides (15mers) spanning the core protein of HBV were used for overnight stimulation, and both peripheral and intrahepatic virus-specific T cell responses were assessed for IFN-γ production in the presence or absence of TRAIL blocking. Despite the fact that many of these cells were already poised to die, with activation of intracellular caspases, intrahepatic HBV-specific CD8+ T cell responses could be augmented after just overnight TRAIL blockade in four out of nine patients (Fig. 8 C). In contrast, the expansion of detectable HBV-specific T cell responses from PBMCs could only be achieved after 10 d rather than overnight TRAIL blockade (unpublished data). These results underscore the susceptibility of the enriched population of HBV-specific T cells in the liver compartment to NK cell TRAIL-mediated deletion.
DISCUSSION
Persistent viral infection with HBV is associated with several T cell–intrinsic and –extrinsic defects, culminating in profound depletion of the HBV-specific CD8+ T cell responses that constitute a critical component of antiviral defense (Boni et al., 2007; Lopes et al., 2008; Protzer et al., 2012). In this study, we addressed the role of NK cells, one of the main effectors of the innate immune response, in HBV-specific T cell modulation. Our results demonstrate that NK cells can limit virus-specific T cell responses, contributing to the apoptotic predilection of these cells. NK cells from patients with CHB can mediate these effects without prior activation and at physiological ratios. NK cell–mediated regulation of T cell responses was found to be partly mediated through TRAIL. We have previously found TRAIL to be up-regulated on NK cells in flares of HBV-related liver inflammation and enriched in the intrahepatic compartment (Dunn et al., 2007). Here, we show that HBV-specific CD8+ T cells, particularly those infiltrating the liver, up-regulate the TRAIL-R2 death-inducing receptor. Therefore, NK cell–mediated deletion of virus-specific T cells via TRAIL may perpetuate viral persistence and immunopathology, especially within the inflammatory milieu of the intrahepatic compartment.
There is accumulating evidence suggesting that NK cells have an important immunoregulatory role (Zingoni et al., 2004; Martín-Fontecha et al., 2004; Morandi et al., 2006; Andrews et al., 2010), in addition to a direct antiviral function in the setting of the early response to infection (Vivier et al., 2008). Our data are the first to reveal a pathway for direct NK cell regulation of T cells in a human persistent viral infection. They are supported by recent studies highlighting a detrimental role of NK cells on the antiviral response in a murine model of persistent viral infection (Waggoner et al., 2010, 2012; Lang et al., 2012). Activated NK cells have been described to target activated T cells in a cytolytic manner, resulting in a significant loss of lymphocytic choriomeningitis virus (LCMV)–specific CD8+ T effector cells, which leads to impaired virus control and altered immunopathology (Waggoner et al., 2010; Lang et al., 2012). A follow-up study further highlighted a role for NK cells as rheostats, regulating CD4+ T cell–mediated support of CD8+ T cells, and thereby controlling LCMV pathogenesis and persistence (Waggoner et al., 2012). Thus, depending on the context of infection and viral kinetics, NK cells may dictate viral clearance or immunopathology.
NK cell activation is the result of the balance of signals from a complex array of activatory and inhibitory receptors, combined with the cytokine milieu. The fact that NK cells can potentially have either a beneficial or deleterious effect on autologous T cells implies that the protective effect of MHC in maintaining self-tolerance may be supported or over-ridden according to the type of infection. This concept is supported by population genetic studies that have demonstrated that KIR haplotypes are under balancing selection and can be associated with resistance to infection (Khakoo et al., 2004; Alter et al., 2011) but also autoimmunity and immunopathology (Parham, 2005; Kulkarni et al., 2008). Although speculative, it is possible that reported associations between KIR haplotypes and outcome of infections with HIV, HCV and HTLV-1 (Khakoo et al., 2004; Alter et al., 2011; Seich Al Basatena et al., 2011) may in part be determined by differential NK cell regulation of antiviral T cell responses. In CHB, the protective effect of MHC class I molecule expression may be insufficient for self tolerance in the face of selective up-regulation of receptors/ligands superseding control of self-killing.
Although we have delineated a role for the TRAIL pathway, our data imply that additional receptor–ligand interactions could contribute to driving NK cell lysis of T cells in CHB. The capacity of NK cells to lyse LCMV-specific CD8+ T cells has been shown to be regulated by 2B4 (Waggoner et al., 2010) or NKG2D (Lang et al., 2012); the impact of HBV infection on relevant pathways regulating NK cell cytotoxicity merits investigation. The recent studies in LCMV show that NK cells can regulate CD8+ T cell responses not only by direct killing (Waggoner et al., 2010; Lang et al., 2012), as demonstrated in our experiments, but also via elimination of CD4+ T cell responses (Waggoner et al., 2012). This finding may also be pertinent to the liver, where CD4+ T cell numbers are already low and priming is defective (Wuensch et al., 2010).
The contrasting effects of NK cell removal on HBV-specific T cells and responses to unrelated viruses (CMV, EBV, and influenza) within the same patients pointed toward differential NK cell–T cell receptor–ligand interactions depending on their antigenic exposure. In line with this, TRAIL-R2 was found to be substantially higher on the surface of HBV-specific cells compared with CMV-specific cells, suggesting that the features of HBV-specific CD8+ T cells that target them for NK cell–mediated deletion are not shared by T cells of all virus specificities. The expression of TRAIL-R2 was increased globally on activated, apoptotic intrahepatic CD8+ T cells, although more so in CHB than HCV-infected or other control liver samples. It is conceivable that antigen presentation in the tolerogenic liver environment is able to impose a TRAIL-R2-expressing phenotype on T cells, as described for the pro-apoptotic molecule Bim (Holz et al., 2008). The hepatic cytokine milieu (Dunn et al., 2007) and HBV antigens (Janssen et al., 2003) can up-regulate TRAIL-R2 expression on hepatocytes, and could likewise affect its expression on intrahepatic T cells. In addition, reactive oxygen species (ROS) have been shown to regulate T cell apoptosis (Hildeman et al., 1999) and influence the expression of TRAIL receptors (Kwon et al., 2008) and may represent an important mechanism in the liver.
Although healthy T cells are normally protected against the apoptotic effects of soluble TRAIL (Mirandola et al., 2004), several studies suggest that viral infections can render lymphoid cells susceptible to TRAIL-mediated cytotoxicity (Katsikis et al., 1997; Jeremias et al., 1998; Miura et al., 2001). We find that cells expressing high levels of TRAIL-R2 in the liver are poised to die, with elevated levels of intracellular caspase 8. We would therefore anticipate that receptor blockade would be more effective in vivo than in vitro, allowing for rescue of newly generated antiviral CD8+ T cells before they are driven to their apoptotic fate. We have previously demonstrated that pretreatment of PBMCs from HBV patients with a pancaspase inhibitor before peptide stimulation can rescue HBV-specific CD8+ T cells that have up-regulated Bim (Lopes et al., 2008). TRAIL signaling can also lead to Bim up-reglation (Han et al., 2006; Cummins and Badley, 2009) and it is likely that the two pathways converge (Bouillet and O’Reilly, 2009), increasing the apoptotic propensity of virus-specific cells. TRAIL expression on NK cells is increased during disease flares (Dunn et al., 2007), which would be predicted to result in an accelerated deletion of T cells expressing TRAIL receptors. Ongoing high-level expression of TRAIL-R2–bearing T cells in the HBV-infected liver is consistent with the constant renewal of antigen-primed T cells in persistent viral infections (Vezys et al., 2006) and with our phenotypic characterization of these populations.
In the inflamed HBV-infected liver, the prominence of activated NK cells bearing high levels of TRAIL may have the dual effect of both contributing to death of hepatocytes (Dunn et al., 2007) and of promoting apoptosis of T cells with up-regulated TRAIL receptors. However it is important to note that TRAIL-bearing NK cells have also been suggested to protect against tumors in the murine liver (Takeda et al., 2001) and to counteract liver fibrosis in chronic hepatitis C (Glässner et al., 2012). With these considerations in mind, the timing of TRAIL blockade in the liver would need to be carefully considered. As a short-term adjuvant to the immunotherapy of CHB, TRAIL blockade could promote antiviral responses while minimizing liver inflammation.
In summary, we provide evidence of a novel pathway whereby activated NK cells may excessively down-modulate the antiviral immune response in CHB. NK cell–mediated deletion of T cells may represent an important homeostatic control mechanism to prevent exuberant T cell responses in the liver that has been hijacked by HBV to assist it in its ongoing battle to evade immune control.
MATERIALS AND METHODS
Patients and healthy controls.
Patients were recruited from Mortimer Market Clinic (London), the Royal Free Hospital (London), and the Royal London Hospital. Full ethical approval was obtained and each patient gave written informed consent. All CHB patients were anti-HCV and anti-HIV antibody negative and treatment naive. Seven patients who had resolved previous HBV infection and 18 age-matched healthy volunteers donated blood for the study. Patient characteristics are included in Table I. Liver samples were obtained from 21 HLAA2− patients with CHB and paired peripheral and intrahepatic samples. Surplus liver tissue was available from five HLAA2+ and four HLA-A2− patients with CHB undergoing diagnostic liver biopsies (Table S1). Characteristics of patients with HCV and controls without viral hepatitis from whom liver tissue was available are outlined in Table S2. Characteristics of HCV patients from whom only PBMCs were used shown in Table S3.
Table 1.
Characteristics of patients from whom PBMCs alone were available
| Age in years median (range) | Sex (female:male) | HBeAg+ | HBV DNA IU/ml median (range) | ALT IU/L: median (range) | |
| CHB patients (n = 27) | 34 (23-51) | 4:23 | 3/27 | 1,500 (36–7.3 × 107) | 38 (17–378) |
| Resolved HBV (n = 7) | 39 (29-69) | 1:6 | NA | NA | NA |
| Healthy controls (n = 18) | 32 (21-49) | 6:12 | NA | NA | NA |
NA, not applicable.
PBMC and intrahepatic lymphocyte isolation.
PBMCs were isolated by gradient centrifugation on Ficoll-Hypaque and frozen or immediately studied as described later. Sera were collected and frozen for later use. Intrahepatic lymphocytes were isolated as previously described (Peppa et al., 2010) In brief, liver tissue was suspended in RPMI-1640 (Sigma-Aldrich) and macerated with a plunger from a 25-ml syringe and a scalpel in a Petri dish. The cell suspension was then passed several times through a 70-mm cell strainer (BD), washed three times, and resuspended in RPMI complete medium with 10% fetal bovine serum for counting. Lymphocytes were identified under a high magnification by their size, shape, and granularity.
Purification of NK cells.
Freshly purified NK cells from PBMCs of CHB patients were isolated (>96% purity and viability; NK isolation kit; Miltenyi Biotec) as per the manufacturer’s instructions. NK cells were depleted by CD56 MACS microbeads (Miltenyi Biotec); TRAIL expression on NK cell–enriched fractions was confirmed by flow cytometry. Where indicated, separated NK cells were plated into transwells (1 µm pore size; Polycarbonated Membrane; Corning Costar). FACS of NK cells (99% purities) was performed on the basis of CD56 expression (CD56+CD3−) by FACSAria (BD). Control PBMCs stained with the same antibodies were passed though the machine untouched.
Flow cytometric analysis.
For phenotypic analysis, PBMCs isolated from HBV patients and healthy donors were washed in PBS, and surface stained at 4°C for 20 min with saturating concentrations of monoclonal anti-CD3 PE-Cy7, CD8 Alexa Fluor 700, HLADR V500, CD19 V450, CD4 APC-Cy7 (eBioscience), CD56-TEXAS Red (Beckman Coulter), and TRAIL-R2 (R&D Systems) in the presence of fixable live/dead stain (Invitrogen). Where stated, the degree of activated caspase 8 and activated pancaspases were determined using the FAM-LETD-FMK or the carboxyfluorescein-(FAM-VAD-FMK) FLICA kit (Serotec) according to the manufacturer’s instructions. For further phenotypic analysis of intrahepatic CD8+ T cells, the following antibodies or isotype-matched controls were used: CD38-FITC (BD), CD45RA V450 (eBioscience), CD27 V500 (BD), PD1-PERCP (eBioscience), CD57-APC (BD) in the presence of fixable live/dead stain (Invitrogen). Where indicated, the viability of CD8+ T cells was further assessed by staining for Annexin V (BioLegend) according to the manufacturer’s protocol in the presence of 7AAD viability staining solution. The frequencies of HBV peptide-specific cells from HLA-A2+ individuals were evaluated directly ex vivo or after short-term culture by multimer staining as previously described. In brief, total PBMCs were stained with APC-labeled HBV c18-27, envelope 183–191, envelope 335–343, envelope 348–357, and polymerase 508–510 dextramers (Immudex) at 37°C for 15 min in complete RPMI plus 10% FCS. The cells were then pelleted and stained as above. A control dextramer was used to identify the population of positive cells. For the analysis of HCV-specific CD8+ T cells PE-labeled HLA-A2–restricted MHC class I tetrameric complexes specific for HCV NS3 1406–1415 (KLSGLGINAV) and HCV NS3 1435–1443 (CVNGVCWTV) were used (a gift from E. Barnes, Oxford University, Oxford, England, UK). Tetramer staining was considered positive if a distinct population (>0.02%) could be discriminated. Cells were acquired on a LSRII (BD) and analyzed using FlowJo (Tree Star).
Peptide stimulation.
PBMCs or PBMCs depleted of NK cells were stimulated with the following peptides representing HLA-A2–restricted viral epitopes: HBV envelope epitopes: FLLTRILTI, WLSLLVPFV, LLVPFVQWFV, and GLSPTVWLSV; HBV core epitope: FLPSDFFPSV; HBV polymerase epitopes: GLSRYVARL, KLHLYSHPI); CMV pp65 immunodominant epitope: NLVPMVATV; EBV BMLF1 immunodominant epitope: GLCTLVAML and influenza MP 58–66 immunodominant epitope: GILGFVFTL0 (Proimmune). For stimulation of HLA-A2− patients, a pool of 15mer peptides overlapping by 10 residues spanning the core protein of HBV genotype D (JPT Peptide Techonologies) was used. For control viral responses in HLA-A2− patients, CMV peptides spanning the pp65 protein were used (JPT Peptide Technologies). PBMCs from HCV-infected patients were stimulated with HCV peptides. Amino acid sequences of the specific antigenic HCV peptides were identical to those of the respective MHC class I tetrameric complexes used (provided by E. Barnes). Peptides were dissolved in sterile endotoxin-free DMSO. Final DMSO concentration during culture was <0.1%.
Short-term culture.
Where indicated, PBMCs or PBMCs depleted of NK cells were stimulated with 1 mM peptide (or a pool of the seven HBV peptides) in the presence of 20 IU IL-2 in RPMI complete medium for 10 d at 37°C. IL-2 and medium were refreshed on day 4 of culture. In selected experiments, NK cells were depleted from PBMC culture at 24 h (day 1). On day 9, PBMCs were restimulated with 1 mM peptide overnight in the presence of Brefeldin A (added 1 h into the incubation). In selected experiments, a physiological ratio (based on the patient-specific circulating NK cell frequency) of freshly isolated NK cells was re-added in the culture either at the onset of stimulation or at day 10. Where indicated, isolated NK cells were plated into transwells (1 µm pore size; Polycarbonated Membrane; Corning Costar) at onset of culture. Virus-specific T cells were identified either via dextramer staining as previously described or via ICS for IFN-γ. In brief, cells were surface stained, fixed, and permeabilized, followed by intracellular staining for IFN-γ APC (BD). To examine the effect of blocking TRAIL on virus-specific CD8+ T cells, 1 µg/ml of TRAIL-R2/Fc (R&D Systems), or IgG1-Fc (R&D) control antibody was added with peptide at onset of culture, and cells were treated as described above. TRAIL-R2/Fc is a dimeric receptor that binds TRAIL ligand with higher affinity than the natural receptor, thereby blocking this interaction. The degree of pancaspase activation was determined using the carboxyfluorescein-(FAM-VAD-FMK) FLICA apoptosis detection kit (Serotec) according to the manufacturer’s protocol for detection by flow cytometry. FLICA reagent was added at day 10 of culture, for 1 h before staining. PBMCs from HCV-infected patients were stimulated with 1 mM pooled peptides and expanded as above. HCV-specific T cells were identified via tetramer staining as described above.
Overnight stimulation.
For overnight stimulation of PBMCs or IHL, 10 mM peptide was added for 12 h, and the cells were incubated at 37°C in the presence of Brefeldin A (added 1 h into the incubation). To examine the effect of TRAIL blocking these experiments were repeated in the presence of a combination of TRAIL-R2/Fc and TRAIL-R1/Fc chimeras (R&D Systems) added at the time of peptide stimulation. Virus-specific T cells were identified by ICS for IFN-γ.
Immunohistochemistry and immunofluorescence.
Sections of archival paraffin-embedded HBV tissues were dewaxed and rehydrated, and epitope retrieval was performed as previously described (Dunn et al., 2007). All washes for immunostaining techniques and dilution of antibodies were done with EnVision FLEX wash buffer (Dako). After 2 min in Harris hematoxylin to block autofluorescence, immunostaining was performed on a Shandon Sequenza. After a 10-min endogenous protein block in 2% Casein solution (Vector), primary antibodies mouse anti-CD8 (Vector) at 1/50 dilution and polyclonal rabbit anti-DR5 (Abcam) at 1/100 were applied for 1 h. Visualization was performed with anti–mouse DyLight 488 and anti–rabbit DyLight 594 (Vector) at 1/200 dilution for 15 min. Sections were mounted with VECTASHIELD, with DAPI (Vector), and captured on a Zeiss Axiovision microscope with 1,000× magnification. Immunohistochemistry was performed on a Dako autostainer. After a 10-min peroxidase block (Dako), a 10-min endogenous protein block in 2% Casein solution was applied. Sections were incubated with mouse anti-CD3 (Dako) at 1/100 dilution for 30 min, visualized with mouse ImmPRESS (Vector), 30 min and ImmPACT NovaRED (Vector) for 5 min. After a water wash, 2% Casein solution was reapplied. Sections were then incubated in goat polyclonal anti-NKp46 (R&D Systems) at 1/100 dilution for 1 h, followed by biotinylated anti–goat secondary (Vector) and strept-ABC alkaline phosphatase (Vector), as per manufacturer’s recommendations. Visualization was completed with Vector blue for 20 min. After counterstaining with Mayer’s hematoxylin (Leica), slides were rapidly dehydrated, cleared, and mounted in VectaMount (Vector Systems). Images were captured on a Leica DM with Nikon Coolpix camera at 1,260× magnification.
Statistical analysis.
Statistical significance was performed between paired samples using the Wilcoxon signed rank test and between HBV patients and healthy controls using the Mann-Whitney U test. The nonparametric Spearman test was used for correlation analysis. P < 0.05 was considered to be significant for all tests.
Online supplemental material.
Tables S1–S3 detail clinical characteristics of patients from whom liver biopsies or HCV-specific responses were acquired. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20121172/DC1.
Supplementary Material
Acknowledgments
We thank all the patients and staff who generously helped with the study, and Prof. Ian Weller for his invaluable mentoring.
This work was funded by the Medical Research Council (Clinical Research Training Fellowship to DP and grant G0801213 to M.K. Maini).
M.K. Maini filed a patent (PCT/GB2008/000811) for the use of TRAIL blocking agents in viral hepatitis. The authors have no additional financial interests.
Footnotes
Abbreviations used:
- CHB
- chronic hepatitis B
- CMV
- cytomegalovirus
- EBV
- Epstein–Barr virus
- HBV
- hepatitis B virus
- LCMV
- lymphocytic choriomeningitis virus
- TRAIL
- TNF-related apoptosis-inducing ligand
References
- Alter G., Heckerman D., Schneidewind A., Fadda L., Kadie C.M., Carlson J.M., Oniangue-Ndza C., Martin M., Li B., Khakoo S.I., et al. 2011. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 476:96–100 10.1038/nature10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D.M., Estcourt M.J., Andoniou C.E., Wikstrom M.E., Khong A., Voigt V., Fleming P., Tabarias H., Hill G.R., van der Most R.G., et al. 2010. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J. Exp. Med. 207:1333–1343 10.1084/jem.20091193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni C., Fisicaro P., Valdatta C., Amadei B., Di Vincenzo P., Giuberti T., Laccabue D., Zerbini A., Cavalli A., Missale G., et al. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225 10.1128/JVI.02844-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., O’Reilly L.A. 2009. CD95, BIM and T cell homeostasis. Nat. Rev. Immunol. 9:514–519 10.1038/nri2570 [DOI] [PubMed] [Google Scholar]
- Cerboni C., Zingoni A., Cippitelli M., Piccoli M., Frati L., Santoni A. 2007. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 110:606–615 10.1182/blood-2006-10-052720 [DOI] [PubMed] [Google Scholar]
- Cummins N., Badley A. 2009. The TRAIL to viral pathogenesis: the good, the bad and the ugly. Curr. Mol. Med. 9:495–505 10.2174/156652409788167078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C., Brunetto M., Reynolds G., Christophides T., Kennedy P.T., Lampertico P., Das A., Lopes A.R., Borrow P., Williams K., et al. 2007. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 204:667–680 10.1084/jem.20061287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glässner A., Eisenhardt M., Krämer B., Körner C., Coenen M., Sauerbruch T., Spengler U., Nattermann J. 2012. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab. Invest. 92:967–977 10.1038/labinvest.2012.54 [DOI] [PubMed] [Google Scholar]
- Han J., Goldstein L.A., Gastman B.R., Rabinowich H. 2006. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J. Biol. Chem. 281:10153–10163 10.1074/jbc.M510349200 [DOI] [PubMed] [Google Scholar]
- Hildeman D.A., Mitchell T., Teague T.K., Henson P., Day B.J., Kappler J., Marrack P.C. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 10:735–744 10.1016/S1074-7613(00)80072-2 [DOI] [PubMed] [Google Scholar]
- Holz L.E., Benseler V., Bowen D.G., Bouillet P., Strasser A., O’Reilly L., d’Avigdor W.M., Bishop A.G., McCaughan G.W., Bertolino P. 2008. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 135:989–997 10.1053/j.gastro.2008.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa K., Liu W., Zhao L., Wang Z., Liu D., Ohtsuka T., Zhang H., Mountz J.D., Koopman W.J., Kimberly R.P., Zhou T. 2001. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 7:954–960 10.1038/91000 [DOI] [PubMed] [Google Scholar]
- Janssen H.L., Higuchi H., Abdulkarim A., Gores G.J. 2003. Hepatitis B virus enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity by increasing TRAIL-R1/death receptor 4 expression. J. Hepatol. 39:414–420 10.1016/S0168-8278(03)00265-4 [DOI] [PubMed] [Google Scholar]
- Janssen E.M., Droin N.M., Lemmens E.E., Pinkoski M.J., Bensinger S.J., Ehst B.D., Griffith T.S., Green D.R., Schoenberger S.P. 2005. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 434:88–93 10.1038/nature03337 [DOI] [PubMed] [Google Scholar]
- Jeremias I., Herr I., Boehler T., Debatin K.M. 1998. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 28:143–152 [DOI] [PubMed] [Google Scholar]
- Katsikis P.D., Garcia-Ojeda M.E., Torres-Roca J.F., Tijoe I.M., Smith C.A., Herzenberg L.A., Herzenberg L.A. 1997. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 186:1365–1372 10.1084/jem.186.8.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakoo S.I., Thio C.L., Martin M.P., Brooks C.R., Gao X., Astemborski J., Cheng J., Goedert J.J., Vlahov D., Hilgartner M., et al. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 305:872–874 [DOI] [PubMed] [Google Scholar]
- Kimberley F.C., Screaton G.R. 2004. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 14:359–372 10.1038/sj.cr.7290236 [DOI] [PubMed] [Google Scholar]
- Kulkarni S., Martin M.P., Carrington M. 2008. The Yin and Yang of HLA and KIR in human disease. Semin. Immunol. 20:343–352 10.1016/j.smim.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D., Choi K., Choi C., Benveniste E.N. 2008. Hydrogen peroxide enhances TRAIL-induced cell death through up-regulation of DR5 in human astrocytic cells. Biochem. Biophys. Res. Commun. 372:870–874 10.1016/j.bbrc.2008.05.148 [DOI] [PubMed] [Google Scholar]
- Lang P.A., Lang K.S., Xu H.C., Grusdat M., Parish I.A., Recher M., Elford A.R., Dhanji S., Shaabani N., Tran C.W., et al. 2012. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. USA. 109:1210–1215 10.1073/pnas.1118834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kim K.S., Fodil-Cornu N., Vidal S.M., Biron C.A. 2009. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J. Exp. Med. 206:2235–2251 10.1084/jem.20082387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen M.B., Lanier L.L. 2006. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18:391–398 10.1016/j.coi.2006.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes A.R., Kellam P., Das A., Dunn C., Kwan A., Turner J., Peppa D., Gilson R.J., Gehring A., Bertoletti A., Maini M.K. 2008. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J. Clin. Invest. 118:1835–1845 10.1172/JCI33402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Ikizawa K., Hu D., Werneck M.B., Wucherpfennig K.W., Cantor H. 2007. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 26:593–604 10.1016/j.immuni.2007.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini M.K., Boni C., Lee C.K., Larrubia J.R., Reignat S., Ogg G.S., King A.S., Herberg J., Gilson R., Alisa A., et al. 2000. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269–1280 10.1084/jem.191.8.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Fontecha A., Thomsen L.L., Brett S., Gerard C., Lipp M., Lanzavecchia A., Sallusto F. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260–1265 10.1038/ni1138 [DOI] [PubMed] [Google Scholar]
- Mirandola P., Ponti C., Gobbi G., Sponzilli I., Vaccarezza M., Cocco L., Zauli G., Secchiero P., Manzoli F.A., Vitale M. 2004. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood. 104:2418–2424 10.1182/blood-2004-04-1294 [DOI] [PubMed] [Google Scholar]
- Miura Y., Misawa N., Maeda N., Inagaki Y., Tanaka Y., Ito M., Kayagaki N., Yamamoto N., Yagita H., Mizusawa H., Koyanagi Y. 2001. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193:651–660 10.1084/jem.193.5.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi B., Bougras G., Muller W.A., Ferlazzo G., Münz C. 2006. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur. J. Immunol. 36:2394–2400 10.1002/eji.200636290 [DOI] [PubMed] [Google Scholar]
- Orange J.S., Fassett M.S., Koopman L.A., Boyson J.E., Strominger J.L. 2002. Viral evasion of natural killer cells. Nat. Immunol. 3:1006–1012 10.1038/ni1102-1006 [DOI] [PubMed] [Google Scholar]
- Parham P. 2005. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 5:201–214 10.1038/nri1570 [DOI] [PubMed] [Google Scholar]
- Peppa D., Micco L., Javaid A., Kennedy P.T., Schurich A., Dunn C., Pallant C., Ellis G., Khanna P., Dusheiko G., et al. 2010. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 6:e1001227 10.1371/journal.ppat.1001227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzer U., Maini M.K., Knolle P.A. 2012. Living in the liver: hepatic infections. Nat. Rev. Immunol. 12:201–213 10.1038/nri3169 [DOI] [PubMed] [Google Scholar]
- Rabinovich B.A., Li J., Shannon J., Hurren R., Chalupny J., Cosman D., Miller R.G. 2003. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J. Immunol. 170:3572–3576 [DOI] [PubMed] [Google Scholar]
- Schneider P., Thome M., Burns K., Bodmer J.L., Hofmann K., Kataoka T., Holler N., Tschopp J. 1997. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 7:831–836 10.1016/S1074-7613(00)80401-X [DOI] [PubMed] [Google Scholar]
- Schneider P., Holler N., Bodmer J.L., Hahne M., Frei K., Fontana A., Tschopp J. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205–1213 10.1084/jem.187.8.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seich Al Basatena N.K., Macnamara A., Vine A.M., Thio C.L., Astemborski J., Usuku K., Osame M., Kirk G.D., Donfield S.M., Goedert J.J., et al. 2011. KIR2DL2 enhances protective and detrimental HLA class I-mediated immunity in chronic viral infection. PLoS Pathog. 7:e1002270 10.1371/journal.ppat.1002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquest K., Walzer T., Zafirova B., Klavinskis L.S., Polić B., Vivier E., Lord G.M., Martín-Fontecha A. 2011. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J. Immunol. 186:3304–3308 10.4049/jimmunol.1004122 [DOI] [PubMed] [Google Scholar]
- Sprick M.R., Weigand M.A., Rieser E., Rauch C.T., Juo P., Blenis J., Krammer P.H., Walczak H. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 12:599–609 10.1016/S1074-7613(00)80211-3 [DOI] [PubMed] [Google Scholar]
- Su H.C., Nguyen K.B., Salazar-Mather T.P., Ruzek M.C., Dalod M.Y., Biron C.A. 2001. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 31:3048–3055 [DOI] [PubMed] [Google Scholar]
- Takeda K., Hayakawa Y., Smyth M.J., Kayagaki N., Yamaguchi N., Kakuta S., Iwakura Y., Yagita H., Okumura K. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7:94–100 10.1038/83416 [DOI] [PubMed] [Google Scholar]
- Vezys V., Masopust D., Kemball C.C., Barber D.L., O’Mara L.A., Larsen C.P., Pearson T.C., Ahmed R., Lukacher A.E. 2006. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 203:2263–2269 10.1084/jem.20060995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. 2008. Functions of natural killer cells. Nat. Immunol. 9:503–510 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- Waggoner S.N., Taniguchi R.T., Mathew P.A., Kumar V., Welsh R.M. 2010. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J. Clin. Invest. 120:1925–1938 10.1172/JCI41264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner S.N., Cornberg M., Selin L.K., Welsh R.M. 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 481:394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuensch S.A., Spahn J., Crispe I.N. 2010. Direct, help-independent priming of CD8+ T cells by adeno-associated virus-transduced hepatocytes. Hepatology. 52:1068–1077 10.1002/hep.23745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingoni A., Sornasse T., Cocks B.G., Tanaka Y., Santoni A., Lanier L.L. 2004. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J. Immunol. 173:3716–3724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.