A subset of chronic lymphocytic leukemia with mutated IGHV-genes express BCRs specific for an antigenic determinant of yeast and filamentous fungi, β-(1,6)-glucan.
Abstract
B cell chronic lymphocytic leukemia (CLL), the most common leukemia in adults, is a clonal expansion of CD5+CD19+ B lymphocytes. Two types of CLLs are being distinguished as carrying either unmutated or somatically mutated immunoglobulins (Igs), which are associated with unfavorable and favorable prognoses, respectively. More than 30% of CLLs can be grouped based on their expression of stereotypic B cell receptors (BCRs), strongly suggesting that distinctive antigens are involved in the development of CLL. Unmutated CLLs, carrying Ig heavy chain variable (IGHV) genes in germline configuration, express low-affinity, poly-, and self-reactive BCRs. However, the antigenic specificity of CLLs with mutated IGHV-genes (M-CLL) remained elusive. In this study, we describe a new subset of M-CLL, expressing stereotypic BCRs highly specific for β-(1,6)-glucan, a major antigenic determinant of yeasts and filamentous fungi. β-(1,6)-glucan binding depended on both the stereotypic Ig heavy and light chains, as well as on a distinct amino acid in the IGHV-CDR3. Reversion of IGHV mutations to germline configuration reduced the affinity for β-(1,6)-glucan, indicating that these BCRs are indeed affinity-selected for their cognate antigen. Moreover, CLL cells expressing these stereotypic receptors proliferate in response to β-(1,6)-glucan. This study establishes a class of common pathogens as functional ligands for a subset of somatically mutated human B cell lymphomas.
B cell chronic lymphocytic leukemia (CLL), the most common leukemia in adults in the western world (Jemal et al., 2009), is a clonal expansion of mature CD5+CD19+ B lymphocytes. Two types of CLL are being distinguished as carrying either unmutated (U-CLL) or somatically mutated Igs (M-CLL), which are associated with unfavorable and favorable prognoses, respectively (Damle et al., 1999; Hamblin et al., 1999). Despite this difference in clinical behavior, U-CLL and M-CLL share a highly similar gene expression profile (Klein et al., 2001).
Many studies allude to a role for BCR-derived signals in the pathogenesis of B cell non-Hodgkin’s lymphomas (Küppers, 2005). These signals are either antigen-independent, such as in diffuse large B cell lymphomas harboring activating mutations in CD79a and CD79b (Davis et al., 2010), or antigen-dependent, as proposed for CLL (Chiorazzi and Ferrarini, 2003; Packham and Stevenson, 2010). The Ig heavy chain variable (IGHV) gene repertoire in CLL is biased to frequent usage of IGHV1-69, IGHV3-7, and IGHV4-34 (Fais et al., 1998), and over 30% of CLLs can be grouped based on similarities of the amino acid sequences in the highly variable complementary determining region 3 (CDR3; Ghiotto et al., 2004; Messmer et al., 2004; Widhopf et al., 2004; Bende et al., 2005; Stamatopoulos et al., 2007; Murray et al., 2008). These stereotypic IGHV display biased patterns of somatic hypermutations (Murray et al., 2008) and are often paired with distinct Ig light chains (Stamatopoulos et al., 2005; Widhopf et al., 2008; Hadzidimitriou et al., 2009). Collectively, these observations suggest that distinctive antigens are involved in the development of CLL.
The majority of U-CLLs express low-affinity BCRs that are polyreactive, recognizing self- and exo-antigens, such as DNA, LPS, insulin, apoptotic cells, oxidized LDL, and the cytoskeletal antigens myosin and vimentin (Hervé et al., 2005; Catera et al., 2008; Chu et al., 2008; Lanemo Myhrinder et al., 2008; Binder et al., 2010). In contrast, M-CLL BCRs are generally not polyreactive (Hervé et al., 2005; Catera et al., 2008; Lanemo Myhrinder et al., 2008). Recently, two stereotypic subsets of M-CLL with specificity for the Fc-tail of IgG, so-called rheumatoid factors, were identified by us and by others (Hoogeboom et al., 2012; Kostareli et al., 2012); this specificity is commonly found among mucosa-associated lymphoid tissue (MALT)-lymphomas, splenic marginal zone lymphomas, and hepatitis C virus–associated lymphomas (De Re et al., 2000; Bende et al., 2005; Hoogeboom et al., 2010; Kostareli et al., 2012). In general, the specificity of M-CLLs with stereotypic BCRs remained unknown. It has been hypothesized that chronic antigenic stimulation drives CLL development (Chiorazzi and Ferrarini, 2003; Chiorazzi et al., 2005), as was also proposed for MALT lymphomas. For MALT lymphomas, this hypothesis is supported by the observation that Helicobacter pylori–associated MALT lymphomas of the stomach can be eradicated by antibiotic treatment alone (Sugiyama et al., 2001; Liu et al., 2002). Nevertheless, it was demonstrated that gastric MALT lymphoma cells themselves were not specific for H. pylori (Hussell et al., 1996). To our knowledge, expression of BCRs with high-affinity for pathogen-derived antigens has not been reported for any lymphoma entity. In this study, we provide evidence that a subset of somatically mutated lymphomas is selected for an antigenic determinant of a major class of pathogens and that these cognate ligands can drive tumor expansion.
RESULTS
Identification of a novel subset of CLL, expressing mutated BCRs
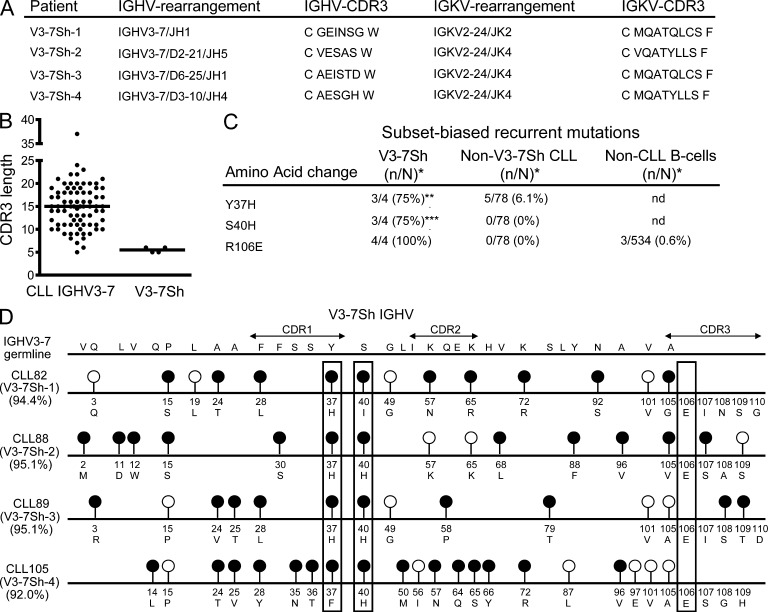
To study the antigen-specificity of M-CLL, we collected 82 CLLs expressing IGHV3-7–encoded BCRs. IGHV3-7 is overrepresented in CLL and is mutated somatically in the majority of cases (Murray et al., 2008). Among these IGHV3-7–expressing CLLs, we noted 4 with exceptionally short CDR3 sequences of 5–6 aa (Fig. 1 B), as compared with an average of ∼15 aa for normal B cells (Arnaout et al., 2011). In addition, these four cases, which we designated V3-7Short (V3-7Sh), shared a glutamic acid at codon 106 in the CDR3 instead of the highly conserved arginine present at that position in the vast majority of IGHV genes, including IGHV3-7 (Fig. 1 A). This characteristic glutamic acid at position 106 was not found in 78 other IGHV3-7-expressing CLL cases nor in 534 unique IGHV3-expressing B cell clones from healthy donors, indicating that the frequency of this feature in the normal repertoire is less than 1% (Fig. 1 C). The four V3-7Sh also harbored subset-specific replacement mutations Y37H and S40H (Fig. 1, C and D), suggesting that V3-7Sh are selected for a shared epitope. In support, these CLL also expressed near-identical IGKV2-24–encoded Ig light chains (Fig. 1 A). We conclude that these four CLLs form a thus far unidentified stereotypic subset, which represents ∼0.3% of CLL.
Figure 1.
IGHV- and IGKV-sequences of V3-7Sh. (A) V3-7Sh CDR3 amino acid sequences and IGHV and IGKV rearrangements. (B) IGHV CDR3 lengths of 78 nonsubset IGHV3-7 and 4 V3-7Sh. (C) Frequencies of subset-biased replacement mutations in V3-7Sh and nonsubset IGHV3-7 sequences. n, number of mutated sequences; N, total number of sequences. Statistical significance analyzed by Fisher’s exact test. **, P < 0.01; ***, P < 0.001. (D) Schematic representation of V3-7Sh CLL IGHV. Lollipop-shaped symbols indicate somatic mutations as compared with the IGHV3-7 germline. Closed and open circles represent replacement and silent mutations, respectively. Boxes highlight subset-biased replacement mutations and the shared glutamic acid at position 106.
V3-7Sh BCRs recognize yeasts and filamentous fungi
To address the BCR-specificity of V3-7Sh, we produced recombinant soluble IgM (sIgM) from three V3-7Sh CLLs (designated V3-7Sh-1, -2 and -3). V3-7Sh sIgM were not polyreactive in tissue microarrays containing 21 different tissues from healthy human donors (Fig. 2 A). In contrast, recombinant sIgM from two U-CLL–stained various cell types in all tissues, in accordance with reported self- and polyreactivity (Hervé et al., 2005; Catera et al., 2008; Lanemo Myhrinder et al., 2008). V3-7Sh sIgM did not stain apoptotic lymphocytes as analyzed by flow cytometry (unpublished data). In ELISAs containing 36 different auto- and exoantigens, no binding was found of V3-7Sh sIgM, except for V3-7Sh-2, which showed some reactivity with vimentin (unpublished data). V3-7Sh-1 and V3-7Sh-3 did not bind vimentin, indicating that this is not a general feature of this subset.
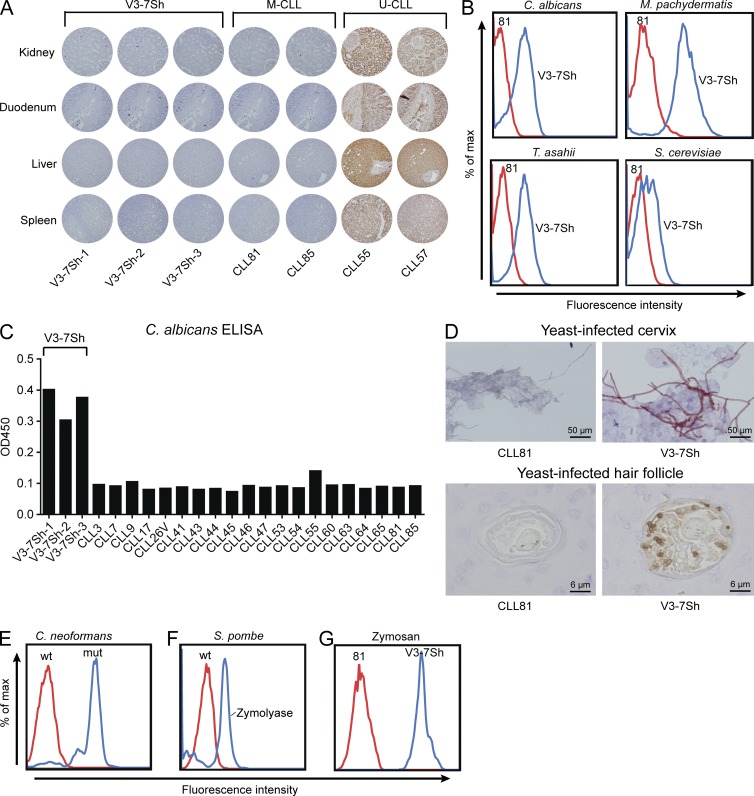
Figure 2.
Recombinant V3-7Sh sIgM are specific for yeasts. (A) Staining of tissue microarrays with sIgM of V3-7Sh, two nonsubset M-CLLs, and two polyreactive U-CLLs. Displayed are stainings of kidney, duodenum, liver, and spleen tissues of healthy human donors. Stainings are representative of at least two independent experiments. (B) Flow cytometry staining of yeast species and zymosan with V3-7Sh sIgM (blue histograms) or CLL81, a nonsubset IGHV3-7–encoded control sIgM (red histograms). Displayed stainings are representative of three V3-7Sh sIgM. (C) ELISA for C. albicans with 23 CLL-derived recombinant sIgM. Data are representative of three independent assays. (D) Staining of hyphae in cytological specimens of cervical smears (top panel) and staining of yeast in hair follicles of paraffin-embedded skin biopsies (bottom panel). Displayed stainings are representative of all three V3-7Sh sIgM. (E) Flow cytometry staining of wild-type C. neoformans (red histogram) and a mutant strain that lacks a capsule (blue histograms) with V3-7Sh sIgM. Displayed staining is representative of all three V3-7Sh sIgM. (F) Flow cytometry staining of wild-type S. pombe cells (red histogram) and zymolyase-treated S. pombe cell walls (blue histogram) with V3-7Sh sIgM. Displayed staining is representative of all three V3-7Sh sIgM. (G) Flow cytometry staining of zymosan, a cell wall preparation of S. cerevisiae with V3-7Sh sIgM (blue histograms), or control sIgM (red histograms). Displayed staining is representative of all three V3-7Sh sIgM.
Next, we tested the reactivity of these CLL-derived sIgM toward 33 microbial species by flow cytometry (Table S1). Interestingly, V3-7Sh sIgM brightly stained strains of the commensal yeast species of Candida, Trichosporon, Malassezia, and Saccharomyces (Fig. 2 B and Table S1), whereas 19 bacterial species were negative (Table S1). The specificity of V3-7Sh for fungi was confirmed in ELISAs (Fig. 2 C) and was not seen with any of 23 other recombinant CLL BCRs, including two IGHV3-7–expressing CLLs with CDR3 sequences distinct from V3-7Sh (Table S2). V3-7Sh sIgM specifically stained hyphae in cytological specimens of human cervical smears and yeasts in hair follicles in paraffin-embedded human skin biopsies (Fig. 2 D). In addition, specific staining by V3-7Sh sIgM was observed of the filamentous fungus Aspergillus in paraffin-embedded human lung tissue (Fig. 3 A) and a subset of spores and conidia of Aspergillus fumigatus, Penicillium chrysogenum, Fonsecaea pedrosoi, and Rhizopus oryzae (Fig. 3, B–E), suggesting that V3-7Sh bind epitopes conserved in ascomycetous and basidiomycetous fungi. V3-7Sh sIgM weakly stained wild-type Cryptococcus neoformans, whereas a mutant strain that lacks a capsule was stained brightly (Fig. 2 E), suggesting that the epitope is part of the cell wall and shielded from detection. V3-7Sh sIgM also bound zymolyase-treated cell walls of Schizosaccharomyces pombe (Fig. 2 F) and zymosan, a cell wall preparation of S. cerevisiae (Fig. 2 G), confirming that the antigen recognized is a cell wall component.
Figure 3.
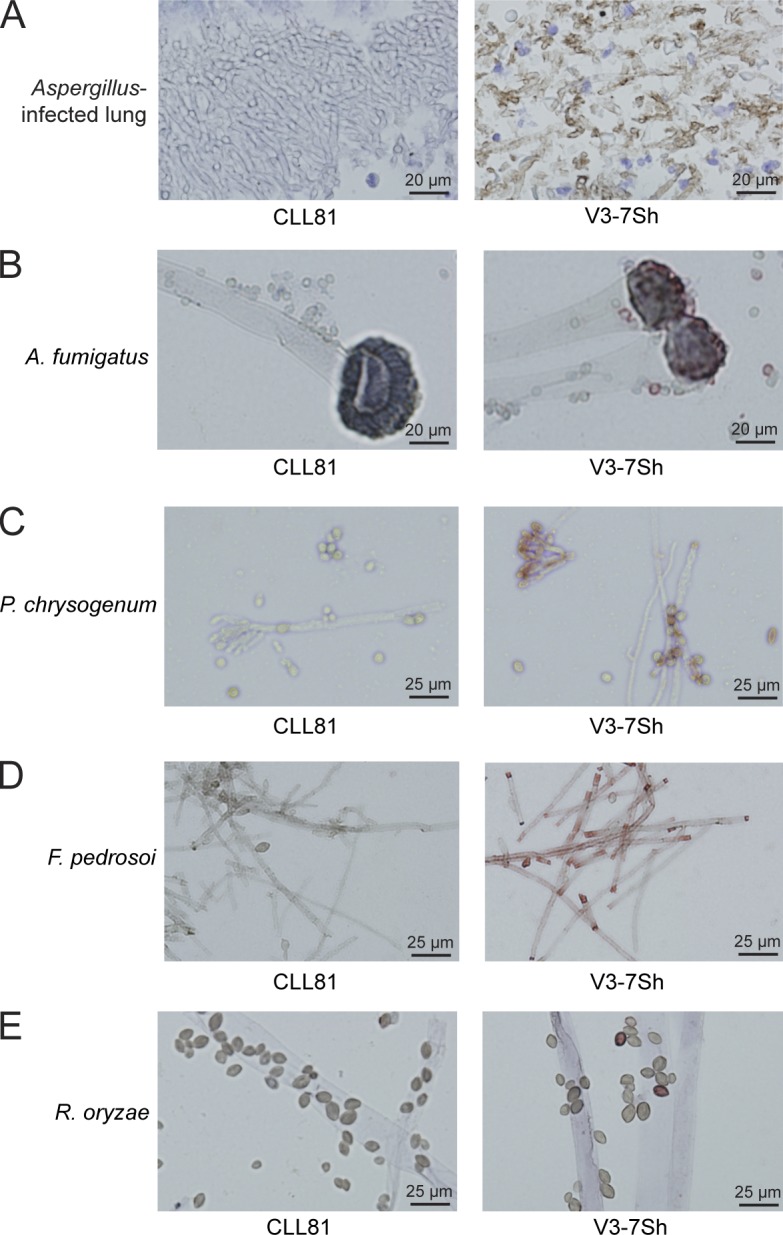
Recombinant V3-7Sh IgM stain filamentous fungi. Staining of Aspergillus in formalin-fixed, paraffin-embedded lung tissue (A), Aspergillus fumigates (B), Penicillium chrysogenum (C), F. pedrosoi (D), and R. oryzae (E) with sIgM of V3-7Sh or CLL81, a nonsubset IGHV3-7–encoded control sIgM. All displayed stainings are representative of all three V3-7Sh sIgM.
V3-7Sh BCRs bind β-(1,6)-glucan
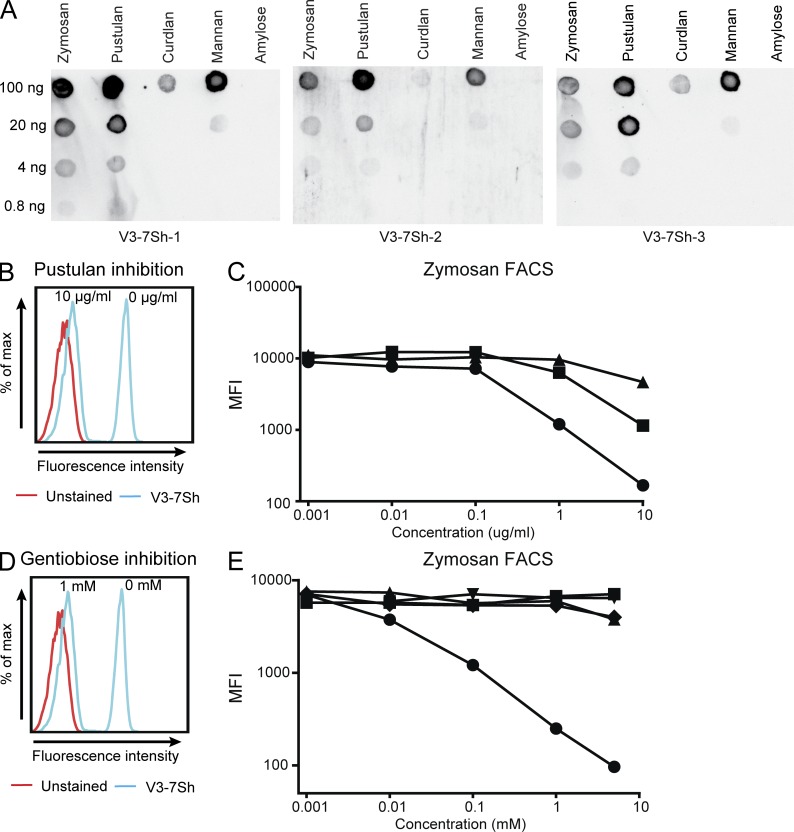
The three V3-7Sh sIgM displayed strong reactivity to dot-blotted alkali-soluble fractions of zymosan and pustulan (β-(1,6)-glucan; Fig. 4 A). A weaker reactivity was observed to preparations of mannan and curdlan (β-[1,3]-glucan) and no binding was detected to α-glucans. We confirmed reactivity to pustulan, mannan, and laminarin (β-[1,3]-glucan) preparations using ELISA (unpublished data). As all these antigen preparations are enriched but impure, we assayed their ability to block the binding of V3-7Sh sIgM to zymosan. Pustulan inhibited zymosan binding by 50% (IC50) at concentrations of ∼0.2 µg/ml (Fig. 4, B and C), whereas the IC50 values of laminarin and mannan were at least 10-fold higher (Fig. 4 C), strongly suggesting that β-(1,6)-glucan is the antigen recognized by V3-7Sh. In accordance, binding to the mannan preparations in ELISA was reduced after enzymatic removal of β-(1,6)-glucan (unpublished data) and binding to carbohydrate preparations on dot blot was abolished by periodate treatment, which degrades β-(1,6)-linkages but leaves β-(1,3)-linkages intact, as confirmed by a mouse anti–β-(1,3)-glucan antibody (unpublished data). In addition, the alkali-soluble fraction from a temperature-sensitive kre5-ts2 mutant strain of S. cerevisiae grown at restrictive temperature stained weaker by V3-7Sh sIgM on dot blot (unpublished data), in line with a reported reduction in β-(1,6)-glucan levels in this strain (Aimanianda et al., 2009). Importantly, competition by micromolar concentrations of the β-(1,6)-glucose disaccharide gentiobiose inhibited binding to zymosan (Fig. 4, D and E). We did not detect inhibition with salicin, a structural analogue of gentiobiose. Neither glucose disaccharides with alternative linkages (laminaribiose, cellobiose, and isomaltose) nor a mannose disaccharide (2α-mannobiose) inhibited binding of V3-7Sh sIgM (Fig. 4 E), demonstrating that V3-7Sh are highly specific for β-(1,6)-glucan, a structure that is not expressed by human cells.
Figure 4.
Recombinant V3-7Sh sIgM bind β-(1,6)-glucan. (A) Binding of V3-7Sh sIgM to serial dilutions of zymosan, pustulan (β-(1,6)-glucan), curdlan (β-[1,3]-glucan), mannan, and amylose (α-[1,4]-glucan) on dot blot. Blots are representative of three independent experiments. (B) Binding of V3-7Sh sIgM to zymosan in the presence of indicated concentrations of pustulan (blue histograms). Red histogram represents an unstained control. Staining is representative of three independent experiments. (C) Binding of V3-7Sh sIgM to zymosan in the presence of indicated concentrations of pustulan (•), mannan (▪), and laminarin (β-[1,3]-glucan; ▴). Displayed graph is representative of all three V3-7Sh sIgM. (D) Binding of V3-7Sh sIgM to zymosan in the presence of indicated concentrations of gentiobiose, a β-(1,6)-glucose disaccharide (blue histograms). Red histogram represents an unstained control. Staining is representative of three independent experiments. (E) Binding of V3-7Sh sIgM to zymosan in the presence of indicated concentrations of gentiobiose (•), laminaribiose (β-[1,3]-glucose disaccharide; ▾), cellobiose (β-[1,4]-glucose disaccharide; ▴), isomaltose (α-[1,6]-glucose disaccharide; ▪), and salicin (◆). Displayed graph is representative of all three V3-7Sh sIgM.
V3-7Sh BCRs are affinity-selected for β-(1,6)-glucan
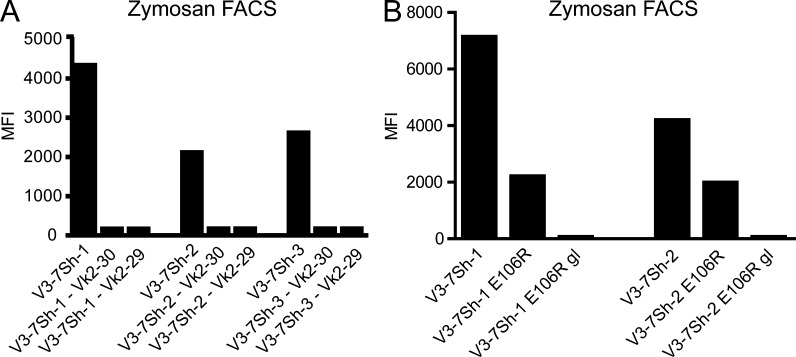
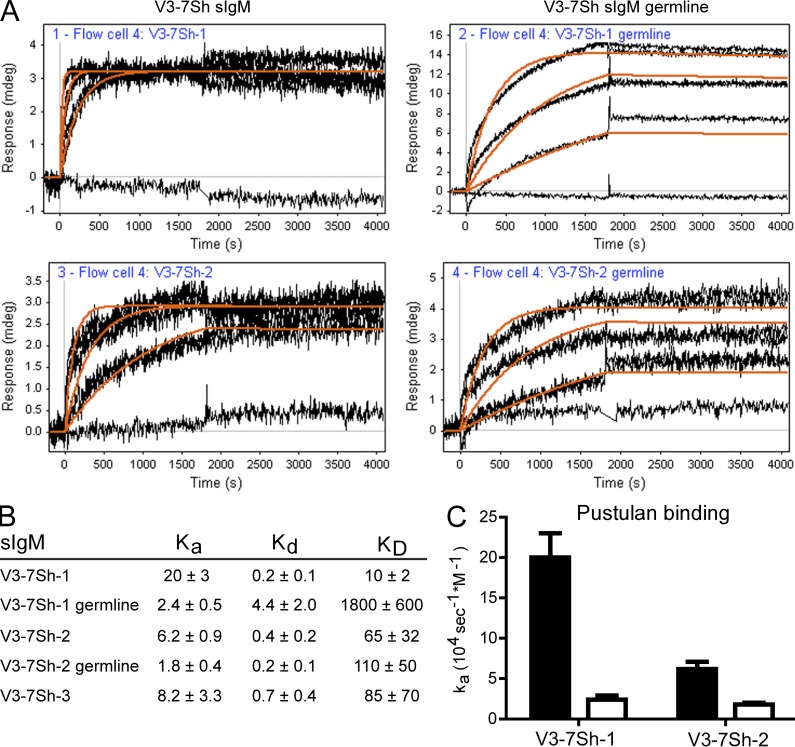
Binding to zymosan was abolished when either the endogenous Ig heavy chain or the endogenous Ig light chain was replaced with those of unrelated M-CLL (Fig. 5 A), showing that the combination of heavy and light chains in V3-7Sh CLL is essential for β-(1,6)-glucan binding. Binding of V3-7Sh sIgM to pustulan was also detected by surface plasmon resonance (SPR; Fig. 6). Association occurred very rapidly and dissociation could not accurately be detected, showing that V3-7Sh sIgM have very high affinities for β-(1,6)-glucan (KD < 0.1 nM). Reversion of the somatic mutations to IGHV3-7 germline resulted in slower association of pustulan to V3-7Sh sIgM (Fig. 6), indicating that the somatic mutations contribute to β-(1,6)-glucan affinity and that V3-7Sh are affinity-selected for β-(1,6)-glucan. Notably, the germline V3-7Sh revertants did not show polyreactive staining on tissue arrays (unpublished data), suggesting that V3-7Sh are not derived from a polyreactive precursor, in contrast to a study showing polyreactivity for a majority of reverted M-CLL (Hervé et al., 2005). Substitution of the characteristic glutamic acid in the CDR3 to an arginine (E106R) resulted in a 3.2- and 2.1-fold reduction in binding to zymosan for V3-7Sh-1 and V3-7Sh-2, respectively. A combination of E106R with reversion of somatic mutations to IGHV3-7 germline abrogated binding completely (Fig. 5 B), indicating that both the glutamic acid at position 106 and the somatic mutations determine β-(1,6)-glucan specificity and affinity.
Figure 5.
V3-7Sh BCRs are selected for β-(1,6)-glucan specificity. (A) Binding of V3-7Sh sIgM to zymosan after exchanging the IGKV2-24 light chain with nonendogenous Ig light chains. Data are representative of three independent experiments. (B) Binding of V3-7Sh sIgM to zymosan after substitution of the glutamic acid at position 106 into an arginine (E106R) and after reversion of somatic mutations to IGHV3-7 germline (gl) combined with E106R. Data are representative of two independent experiments.
Figure 6.
V3-7Sh BCR are selected for β-(1,6)-glucan affinity. (A) SPR curves of binding of pustulan (0, 0.3, 1, and 3 µg/ml) to V3-7Sh sIgM (left) and V3-7Sh sIgM after reversion of somatic mutations (V3-7Sh germline; right). The response curves were fitted to a 1:1 binding model (orange lines). Curves are representative of two independent experiments. (B) Kinetic constants for pustulan binding to V3-7Sh sIgM. ka in 104 sec−1M−1, kd in 10−5 sec−1, KD in pM. Kinetic constants were calculated with data from at least five different anti-IgM–coated spots. The error value is the deviation in the kinetic constants between different coated spots. For calculations, an estimated average molecular weight of 20 kD was used for pustulan. (C) Association constants (ka) of pustulan binding to V3-7Sh sIgM with somatic mutations (black bars) and after reversion of somatic mutations to IGHV3-7 germline determined by SPR (white bars). Data are representative of two independent experiments.
V3-7Sh CLL cells proliferate in response to β-(1,6)-glucan
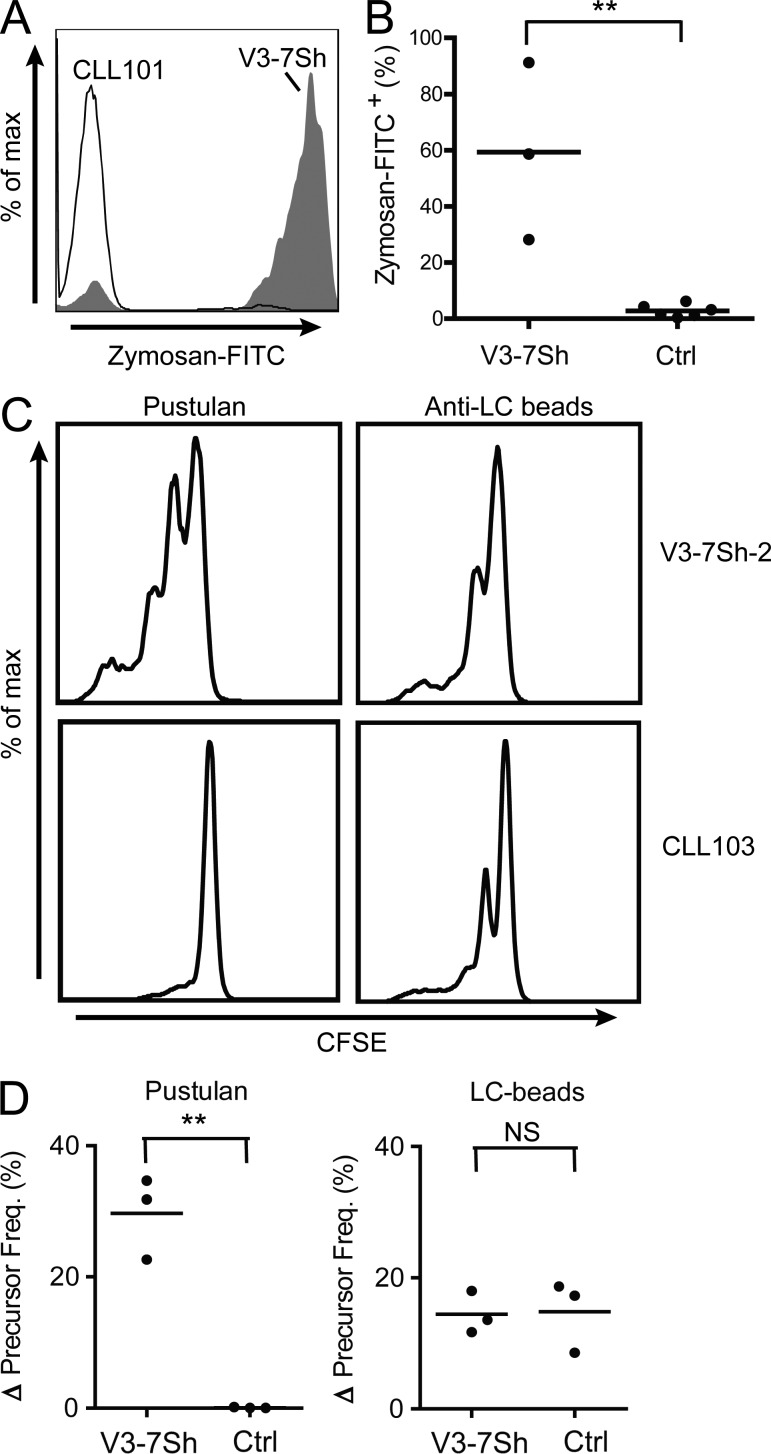
V3-7Sh CLL cells bound zymosan, confirming that membrane-bound V3-7Sh BCRs are capable of binding β-(1,6)-glucan (Fig. 7 A). Peripheral blood B cells from healthy donors and six control M-CLLs, including two nonsubset IGHV3-7–expressing CLL, did not bind zymosan (Fig. 7 B). As the natural ligands of CLL are typically not known, direct evidence for antigen-dependent CLL growth was not available. Now able to address this issue, we cultured primary CLL cells from three V3-7Sh patients on CD40L-expressing cells. In this system, addition of pustulan induced proliferation of tumor cells (Fig. 7, C and D). The proliferating cells were clonal and expressed the V3-7Sh CDR3 as demonstrated by CDR3-length spectratyping (unpublished data). Pustulan did not induce proliferation of three control M-CLL, whereas BCR-cross-linking with anti-Ig light chain antibodies induced proliferation in both V3-7Sh CLL and control M-CLL (Fig. 7, C and D).
Figure 7.
β-(1,6)-glucan induces proliferation of primary V3-7Sh CLL cells. (A) Zymosan-FITC staining of CD19+CD5+ cells of V3-7Sh CLL (filled histogram) or control M-CLL (open histogram). Plot is representative of three independent experiments. (B) Frequency of CD19+CD5+Zymosan+ cells of three V3-7Sh CLL and six control M-CLLs (including two nonsubset IGHV3-7–expressing CLLs). (C) CFSE staining after 8 d of culture of V3-7Sh CLL cells (top) or control M-CLLs (bottom) in the presence of pustulan (left) or anti-light chain antibodies (right). Representative data for cell cultures from all three V3-7Sh CLL patients. (D) Precursor frequency after 8 d of culture in the presence of pustulan (left) or anti-light chain antibodies (right). Values are precursor frequencies after correction for basal proliferation. **, P < 0.01; NS; not significant. Statistical significance was analyzed by two-tailed Student’s t test.
Stimulation of V3-7Sh cells with pustulan yielded slightly more proliferating tumor cells as compared with V3-7Sh cells stimulated with anti-Ig light chain beads (Fig. 7 C), suggesting that V3-7Sh CLL cells may also receive signals through innate immune receptors recognizing fungal antigens. To date, no innate immune receptors have been described that detect β-(1,6)-glucan. However, as pustulan also contains traces of fungal antigens other than β-(1,6)-glucan (unpublished data), other innate immune receptors may also be stimulated. To analyze this possibility, we measured the expression of several innate receptors by flow cytometry. Expression of TLR2, TLR4, and DC-SIGN was not detected in V3-7Sh CLL, whereas Dectin-1, Dectin-2, and the mannose receptor were expressed (at the surface of V3-7Sh CLL cells), suggesting that V3-7Sh CLL cells may indeed receive additional signals through innate immune receptors. Expression of Dectin-1, Dectin-2, and the mannose receptor was low as compared with dendritic cells and not higher than in the three control CLLs used in our experiments (unpublished data), indicating that control CLL potentially received the same amount of signals. Control CLL cells however did not proliferate in response to pustulan stimulation, demonstrating that signaling by innate fungal immune receptors alone is insufficient to drive proliferation.
DISCUSSION
In this study, we have identified a new subset of M-CLL, designated V3-7Sh, expressing stereotypic BCRs specific for the fungal antigen β-(1,6)-glucan. V3-7Sh sIgM did not show polyreactivity and it is unlikely that V3-7Sh are cross-reactive with a self-antigen, as structures containing β-(1,6)–linked glucoses have not been identified in humans. In accordance, no specific staining was seen in any of 21 different tissues of healthy human donors in a tissue microarray. The β-(1,6)-glucan specificity of V3-7Sh BCRs depends on both the stereotypic Ig heavy and light chains, therewith excluding that this reactivity is caused by superantigen-like binding. β-(1,6)-glucan binding also depends on the characteristic glutamic acid in the IGHV-CDR3, indicating that the binding capability for this antigen accounts for the BCR stereotypy. The observation that the somatic mutations increase the affinity for β-(1,6)-glucan demonstrates that V3-7Sh BCRs are affinity-selected for β-(1,6)-glucan.
We provide unambiguous evidence that different members of a mutated CLL subgroup recognize an identical pathogen-derived epitope, supporting the hypothesis that BCR stereotypy in CLL results from stringent antigenic selection and affinity maturation. In stark contrast, Dühren-von Minden et al. (2012) have recently claimed that the growth of mutated and unmutated CLL is driven by antigen-independent, cell-autonomous Ca2+ signaling as a result of self-recognition of an intrinsic IGHV motif, based on signaling studies of retrovirally expressed CLL BCRs in mouse cells. In our view, the observations by this group, although interesting, do not explain why there are subgroups of CLL patients expressing highly similar BCRs with shared somatic mutations. Importantly, we found that primary CLL cells did not proliferate in vitro in the absence of extrinsic BCR cross-linking. In agreement, we found that rheumatoid-factor–expressing primary CLL cells proliferated in response to aggregated IgG (Hoogeboom et al., 2012). These observations are difficult to reconcile with an antigen-independent driven growth of CLL cells. Rather, antigen-independent elevation of Ca2+-levels caused by BCR self-recognition might be indicative of CLL anergy (Healy et al., 1997; Zikherman et al., 2012). Recently, an elegant study by Zikherman et al. (2012) showed that encounter of endogenous antigen during development fine-tunes the responsiveness of mouse B cells to BCR stimulation and that the level of responsiveness inversely correlates with the level of self-antigen exposure. Along these lines, it may be hypothesized that, because of superior cross-linking characteristics, extrinsic cognate antigens are essential to overcome self-antigen–induced anergy of CLL cells.
The BCR specificity of CLL cells may withhold clues concerning the B cell type of origin. Two B cell subsets have been proposed, i.e., natural antibody (Nab)–producing cells and marginal zone (MZ) B–cells (Chiorazzi and Ferrarini, 2011). Nabs are usually germline-encoded and capable of binding many antigens with low-affinity. In recent years, it has become clear that U-CLL express poly- and self-reactive BCRs of low affinities (Hervé et al., 2005; Catera et al., 2008; Lanemo Myhrinder et al., 2008), suggesting that U-CLL are derived from Nab-producing cells. M-CLLs in contrast are usually monospecific, indicating that M-CLLs are not derived from Nab-producing cells. In support, we show that V3-7Sh cells are monospecific and affinity-selected. In addition, the finding that reversion of somatic mutations in V3-7Sh did not result in polyreactivity indicates that these CLLs do not originate from a polyreactive precursor. β-(1,6)-glucan is a polyvalent carbohydrate antigen. In general, polyvalent antigens induce MZ B cell responses (Weller et al., 2004), which may point toward a MZ B cell origin for V3-7Sh CLL.
It is noteworthy that the natural ligand of a M-CLL subset is a major antigenic determinant shared by an extensive group of pathogens. The V3-7Sh CLL patients studied here, do not have a history of persistent or recurrent fungal infections. However, fungi are ubiquitous (Fröhlich-Nowoisky et al., 2009; Iliev et al., 2012) and likely to chronically or intermittently stimulate V3-7Sh-expressing clones, even without clinically overt infections. We here show a crucial role for a microorganism in the pathogenesis of CLL, apparently similar to the dependence of gastric MALT lymphomas on H. pylori. However, whereas CLL growth is driven by pathogen-specific BCR signaling, MALT lymphomas were demonstrated not to be pathogen-specific but to recognize self-antigens present within the inflammatory microenvironment induced by H. pylori infection (Hussell et al., 1996; Bende et al., 2005; Craig et al., 2010; Hoogeboom et al., 2010). It is conceivable that B cells that are specific for common pathogens are particularly at risk for gradual genetic derailment during chronic or intermittent immune reactions. A major challenge now is to assess the specificity of other M-CLL subsets. Our finding that CLL cells are induced to proliferate in response to their natural ligand may reflect a general principle of somatically mutated low-grade B cell lymphomas. It is tempting to speculate on the possibilities for antigen- or pathogen-targeted therapies for this group of patients, but one might anticipate that this approach is especially challenging in case of antigens derived from commensal microorganisms, for which complete eradication may be hampered by their ubiquitous and persistent nature.
MATERIALS AND METHODS
Patients and IGHV sequences.
We obtained 21 and 40 IGHV3-7 sequences subjected to mutation status analysis according to the BIOMED protocol (van Dongen et al., 2003) from the Academic Medical Center, Amsterdam, and the Erasmus Medical Center CLL patient cohorts, respectively. An additional 21 CLL IGHV3-7 BCR sequences were obtained from GenBank. This study was conducted in accordance with the ethical standards in our institutional medical ethical committee (Medisch Etische Toetsingscommissie AMC) on human experimentation, as well as in agreement with the Helsinki Declaration of 1975, as revised in 1983. Accordingly, all patients provided informed consent.
Recombinant Ig production.
After RNA isolation from PBMCs using TRIzol Reagent (Invitrogen), cDNA was synthesized using Pd(N)6 random primers. IGHV and IGKV genes were amplified using IGHV-, IGKV-, IGHJ-, and IGKJ-specific primers. Recombinant sIgM of 23 CLL patients was produced using pIgH(μ) and pIgL(κ) expression vectors, as described previously (Bende et al., 2002). In brief, the rearranged IGHV and IGKV genes of each of these leukemias were cloned into the pIgH(μ) and pIgL(κ) vectors, respectively. For production of recombinant antibodies, 10 µg pIgH(μ) and 10 µg pIgL(κ) was linearized with PvuI and co-transfected into SP2/0 myeloma cells by electroporation, which produced soluble pentameric IgM, as confirmed by Western blotting. Subsequently, transfected cells were selected in geneticin-containing medium. To screen supernatants for sIgM, 4 µg/ml of anti–human Igκ is coated to Costar EIA 96-wells plates (Corning) in carbonate buffer overnight at 4°C, followed by blocking of the wells with 1% BSA in PBS. Subsequently, serial dilutions of supernatants containing the sIgM are incubated for 1 h at room temperature. Bound sIgM was detected by HRP-conjugated anti–human IgM and developed as described previously (Bende et al., 1992). The pIgH(μ) and pIgL(κ) expression vectors were provided by J. van Es and T. Logtenberg (Utrecht Medical Center, Utrecht, The Netherlands). Heavy and light chains were exchanged by co-transfection of nonendogenous combinations of heavy and light chains as described above.
To generate recombinant IgM of V3-7Sh with IGHV3-7 in germline configuration, naive B cells (CD19+CD27−) were sorted from a tonsil. Subsequently, the IGHV3-7 germline was amplified by PCR using an IGHV3-specific primer and a reverse IGHV3-7 primer located in FR3. The CDR3s of V3-7Sh-1 and V3-7Sh-2 were amplified with an IGHV3-7 FR3-specific forward primer and an IGHJ reverse primer. V3-7Sh-1 does not contain any replacement mutations in the IGHV-CDR3, whereas V3-7Sh-2 contains few somatic mutations in the IGHV-CDR3, which we did not revert. The IGHV3-7 germline was then ligated to the V3-7Sh CDR3 by PCR and produced as described above. V3-7Sh-1 and V3-7Sh-3 express Ig light chain variable region (IGLV) in germline configuration, indicating that the SHMs in the IGLV are not necessary for binding β-(1,6)-glucan. Therefore, somatic mutations present in the IGLV were not reverted. The glutamic acid at position 106 was substituted into an arginine using specific primers and the QuickChange II site-directed XL mutagenesis kit (Stratagene) according to the manufacturer’s instructions.
Microorganisms.
Bacteria, yeasts, and filamentous fungi (Table S1) were grown overnight on agar plates, supplemented with medium appropriate for the species. S. pombe cell walls were treated with zymolyase as described previously (Grün et al., 2005). S. cerevisiae kre5-ts2 mutant was a gift from V. Aimanianda (Institut Pasteur, Paris, France; Aimanianda et al., 2009) and was cultured in YPAD medium at a permissive temperature of 22°C and at a restrictive temperature of 37°C. Cells were lysed with glass beads and total cell lysates were adjusted to 400 µg/ml protein in 0.75 M NaOH.
Immunohistochemistry.
Fungal mycelium was separated from agar culture plates with a scalpel, chopped, and mounted on glass slides in 0.005% Tween-80 in PBS. Slides were air-dried and fixed with 5% PFA in PBS for 30 min. Cytological specimen of cervical smears, paraffin-embedded skin, and lung biopsies and healthy donor tissues were obtained from the department of Pathology at the AMC, Amsterdam, The Netherlands. Healthy donor tissues were assembled in tissue microarrays (TMA) using a manually operated TMA device (Beecher Instruments). After antigen retrieval in Citrate buffer (20 min at 120°C), slides were stained overnight with sIgM at 1 µg/ml in PBS. Mouse anti–human IgM (Clone MH15/1; Sanquin) was used as secondary antibody. Subsequently staining was visualized using Powervision+ (ImmunoVision Technologies).
Dot blot.
Zymosan and total cell lysates were dissolved in 1 M NaOH and incubated at 75°C for 1 h to extract the alkali-soluble fraction as described previously (Gilbert et al., 2010). Pustulan (EMD Millipore), curdlan (Sigma-Aldrich), C. albicans mannan (NIBSC), and amylose (Sigma-Aldrich) were dissolved in 1 M NaOH and 100, 20, 4, and 0.8 ng was spotted on PVDF-membranes and dried for 30 min. When indicated, dot blots were treated overnight with 50 mM periodic acid. Membranes were blocked with 5% milk for 1 h and subsequently stained with 2 µg/ml of sIgM or mouse β-(1,3)-glucan antibody (Biosupplies) for 30 min at room temperature. Anti–human IgM-HRP (SouthernBiotech) and rabbit anti–mouse Ig-HRP (Dako) were used as secondary antibodies. Staining was visualized with ECL (GE Healthcare). To analyze binding of V3-7Sh sIgM to the alkali-soluble fraction of the kre5-ts2 mutant total lysates, serial dilutions of protein were spotted and stained as described above.
ELISA.
Single colonies of yeasts were isolated from agar plates and suspended in PBS, and 1.5 × 106 yeast cells/well were coated overnight at 4°C in PBS in Costar EIA 96-wells plates (Corning). 10 µg/ml of purified C. albicans mannan, laminarin (Sigma-Aldrich), and pustulan were coated in carbonate buffer overnight at 4°C. After blocking with 1% BSA for 30 min at 37°C, wells were incubated with 0.4 µg/ml sIgM for 1 h at room temperature, followed by incubation with HRP-conjugated mouse anti–human IgM (clone MH15/1; Sanquin) and developed as described previously (Bende et al., 1992). Absorbance at 450 nm is plotted without subtraction of background.
Flow cytometry.
Single colonies of bacteria and yeasts were isolated from agar plates and suspended in DMEM medium. 5 × 106 bacteria, 1.5 × 106 yeast, or ∼1 × 106 zymosan particles (Sigma-Aldrich) were stained with 2 µg/ml of sIgM and, subsequently, with anti-IgM F(ab′)2-PE (Dako). For inhibition experiments, ∼1 × 106 zymosan particles were stained in the presence of ≤10 µg/ml pustulan, mannan, or laminarin and ≤5 mM of gentiobiose (Sigma-Aldrich), laminaribiose (Seikagaku), cellobiose (Sigma-Aldrich), isomaltose (Sigma-Aldrich), 2α-mannobiose (Sigma-Aldrich), or salicin (Sigma-Aldrich).
For zymosan staining of PBMCs, 2 × 105 cells were incubated with anti-CD19-APC (BD), anti-CD5-PE (BD), and zymosan-FITC (Invitrogen). To generate apoptotic cells, Nalm-6 cells were irradiated by γ-irradiation (20 Gy) and cultured overnight in IMDM supplemented with 10% FCS. 2 × 105 cells were incubated with 2 µg/ml sIgM and subsequently with anti-IgM F(ab′)2-PE (Dako) and 7-AAD (eBioscience). Staining was visualized on a FACSCanto II (BD) and analysis was done using FlowJo software (Tree Star).
SPR.
Serial dilutions (0.1–10 µg/ml) of mouse anti–human IgM (MH15/1; Sanquin) were spotted on an amine-specific Easy2Spot gold-film gel-type SPRchip (Ssens), using a Continuous Flow Microspotter (Wasatch Microfluidics). Pustulan binding was analyzed on an IBIS MX96 (IBIS Technologies) by performing cycles of concatenated injections of recombinant sIgM and pustulan on the anti-IgM coated chip. In each concatenated injection, first sIgM was captured on the anti-IgM–coated spots for 30 min. Subsequently, pustulan (0.3–3 µg/ml in binding buffer, 0.1% BSA and 0.03% Tween-20 in PBS) was injected and incubated for 30 min, followed by thorough washing with binding buffer to detect pustulan dissociation. Injections with binding buffer were used as reference. After each concatenated injection, the chip was regenerated with 10 mM glycine-HCl, pH 2.0. Experimental data were processed with SPRint software (IBIS Technologies) and kinetic parameters were determined using Scrubber2 software (Biological).
Cells isolation and cell culture.
PBMCs were isolated using Ficoll Hypaque (GE Healthcare) according to manufacturer’s instructions. Cells were stained with CFSE supplied by Invitrogen. 4 × 105 cells were cultured in the presence of CD40L expressing 3T3 fibroblasts (Tromp et al., 2010) in wells coated with pustulan (EMD Millipore) or in the presence of biotinylated anti-kappa or anti-lambda F(ab′)2 (Southern Biotech) coupled to anti-biotin antibody-coated MicroBeads (Miltenyi Biotec; 40 µg/ml). After 8 d, CFSE staining was measured in combination with CD19-APC (BD) and CD5-PE (BD) by flow cytometry. Precursor frequency, defined as the proportion of cells that have responded in the original population, was calculated by FlowJo software as described previously (Givan et al., 1999). The precursor frequency of cells cultured with CD40L expressing fibroblasts was subtracted as background.
Online supplemental material.
Table S1 lists the microorganisms used in this study and Table S2 lists IGHV- and IGLV-rearrangements of the CLL used in this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20121801/DC1.
Supplementary Material
Acknowledgments
We thank Ludo M. Evers (Hematology, AMC, Amsterdam, The Netherlands) and Anton W. Langerak (Molecular Immunology, Erasmus MC, Rotterdam, The Netherlands) for kindly providing CLL IGHV3-7 sequences. We thank Teun Boekhout (CBS-KNAW, Fungal Biodiversity Centre, Utrecht and Department of Internal Medicine and Infectious Diseases, University Medical Center, Utrecht, The Netherlands) for supplying fungal cells.
This work was financially supported by the Dutch Cancer Society, grant UVA 2006-3644.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- CLL
- B cell chronic lymphocytic leukemia
- IGHV
- Ig heavy chain variable region
- IGLV
- Ig light chain variable region
- M-CLL
- CLL expressing mutated IGHV
- MZ
- marginal zone
- SHM
- somatic hypermutation
- U-CLL
- CLL expressing unmutated IGHV
References
- Aimanianda V., Clavaud C., Simenel C., Fontaine T., Delepierre M., Latgé J.P. 2009. Cell wall β-(1,6)-glucan of Saccharomyces cerevisiae: structural characterization and in situ synthesis. J. Biol. Chem. 284:13401–13412 10.1074/jbc.M807667200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout R., Lee W., Cahill P., Honan T., Sparrow T., Weiand M., Nusbaum C., Rajewsky K., Koralov S.B. 2011. High-resolution description of antibody heavy-chain repertoires in humans. PLoS ONE. 6:e22365 10.1371/journal.pone.0022365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bende R.J., Jochems G.J., Frame T.H., Klein M.R., van Eijk R.V.W., van Lier R.A., Zeijlemaker W.P. 1992. Effects of IL-4, IL-5, and IL-6 on growth and immunoglobulin production of Epstein-Barr virus-infected human B cells. Cell. Immunol. 143:310–323 10.1016/0008-8749(92)90028-N [DOI] [PubMed] [Google Scholar]
- Bende R.J., Aarts W.M., Pals S.T., van Noesel C.J. 2002. Immunoglobulin diversification in B cell malignancies: internal splicing of heavy chain variable region as a by-product of somatic hypermutation. Leukemia. 16:636–644 10.1038/sj.leu.2402405 [DOI] [PubMed] [Google Scholar]
- Bende R.J., Aarts W.M., Riedl R.G., de Jong D., Pals S.T., van Noesel C.J. 2005. Among B cell non-Hodgkin’s lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 201:1229–1241 10.1084/jem.20050068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M., Léchenne B., Ummanni R., Scharf C., Balabanov S., Trusch M., Schlüter H., Braren I., Spillner E., Trepel M. 2010. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS ONE. 5:e15992 10.1371/journal.pone.0015992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catera R., Silverman G.J., Hatzi K., Seiler T., Didier S., Zhang L., Hervé M., Meffre E., Oscier D.G., Vlassara H., et al. 2008. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol. Med. 14:665–674 10.2119/2008-00102.Catera [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N., Ferrarini M. 2003. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu. Rev. Immunol. 21:841–894 10.1146/annurev.immunol.21.120601.141018 [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., Ferrarini M. 2011. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 117:1781–1791 10.1182/blood-2010-07-155663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N., Rai K.R., Ferrarini M. 2005. Chronic lymphocytic leukemia. N. Engl. J. Med. 352:804–815 10.1056/NEJMra041720 [DOI] [PubMed] [Google Scholar]
- Chu C.C., Catera R., Hatzi K., Yan X.J., Zhang L., Wang X.B., Fales H.M., Allen S.L., Kolitz J.E., Rai K.R., Chiorazzi N. 2008. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 112:5122–5129 10.1182/blood-2008-06-162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig V.J., Cogliatti S.B., Arnold I., Gerke C., Balandat J.E., Wündisch T., Müller A. 2010. B-cell receptor signaling and CD40 ligand-independent T cell help cooperate in Helicobacter-induced MALT lymphomagenesis. Leukemia. 24:1186–1196 10.1038/leu.2010.76 [DOI] [PubMed] [Google Scholar]
- Damle R.N., Wasil T., Fais F., Ghiotto F., Valetto A., Allen S.L., Buchbinder A., Budman D., Dittmar K., Kolitz J., et al. 1999. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 94:1840–1847 [PubMed] [Google Scholar]
- Davis R.E., Ngo V.N., Lenz G., Tolar P., Young R.M., Romesser P.B., Kohlhammer H., Lamy L., Zhao H., Yang Y., et al. 2010. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 463:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Re V., De Vita S., Marzotto A., Rupolo M., Gloghini A., Pivetta B., Gasparotto D., Carbone A., Boiocchi M. 2000. Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus-associated non-Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood. 96:3578–3584 [PubMed] [Google Scholar]
- Dühren-von Minden M., Übelhart R., Schneider D., Wossning T., Bach M.P., Buchner M., Hofmann D., Surova E., Follo M., Köhler F., et al. 2012. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 489:309–312 10.1038/nature11309 [DOI] [PubMed] [Google Scholar]
- Fais F., Ghiotto F., Hashimoto S., Sellars B., Valetto A., Allen S.L., Schulman P., Vinciguerra V.P., Rai K., Rassenti L.Z., et al. 1998. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J. Clin. Invest. 102:1515–1525 10.1172/JCI3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich-Nowoisky J., Pickersgill D.A., Després V.R., Pöschl U. 2009. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA. 106:12814–12819 10.1073/pnas.0811003106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiotto F., Fais F., Valetto A., Albesiano E., Hashimoto S., Dono M., Ikematsu H., Allen S.L., Kolitz J., Rai K.R., et al. 2004. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia. J. Clin. Invest. 113:1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N.M., Donlin M.J., Gerik K.J., Specht C.A., Djordjevic J.T., Wilson C.F., Sorrell T.C., Lodge J.K. 2010. KRE genes are required for β-1,6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol. Microbiol. 76:517–534 10.1111/j.1365-2958.2010.07119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan A.L., Fisher J.L., Waugh M., Ernstoff M.S., Wallace P.K. 1999. A flow cytometric method to estimate the precursor frequencies of cells proliferating in response to specific antigens. J. Immunol. Methods. 230:99–112 10.1016/S0022-1759(99)00136-2 [DOI] [PubMed] [Google Scholar]
- Grün C.H., Hochstenbach F., Humbel B.M., Verkleij A.J., Sietsma J.H., Klis F.M., Kamerling J.P., Vliegenthart J.F.G. 2005. The structure of cell wall α-glucan from fission yeast. Glycobiology. 15:245–257 10.1093/glycob/cwi002 [DOI] [PubMed] [Google Scholar]
- Hadzidimitriou A., Darzentas N., Murray F., Smilevska T., Arvaniti E., Tresoldi C., Tsaftaris A., Laoutaris N., Anagnostopoulos A., Davi F., et al. 2009. Evidence for the significant role of immunoglobulin light chains in antigen recognition and selection in chronic lymphocytic leukemia. Blood. 113:403–411 10.1182/blood-2008-07-166868 [DOI] [PubMed] [Google Scholar]
- Hamblin T.J., Davis Z., Gardiner A., Oscier D.G., Stevenson F.K. 1999. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 94:1848–1854 [PubMed] [Google Scholar]
- Healy J.I., Dolmetsch R.E., Timmerman L.A., Cyster J.G., Thomas M.L., Crabtree G.R., Lewis R.S., Goodnow C.C. 1997. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 6:419–428 10.1016/S1074-7613(00)80285-X [DOI] [PubMed] [Google Scholar]
- Hervé M., Xu K., Ng Y.S., Wardemann H., Albesiano E., Messmer B.T., Chiorazzi N., Meffre E. 2005. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J. Clin. Invest. 115:1636–1643 10.1172/JCI24387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeboom R., Bende R.J., van Noesel C.J. 2010. MALT lymphoma-derived rheumatoid factors are nonpolyreactive high-affinity antibodies. Blood. 116:1818–1819, author reply :1819–1820 10.1182/blood-2010-03-274613 [DOI] [PubMed] [Google Scholar]
- Hoogeboom R., Wormhoudt T.A., Schipperus M.R., Langerak A.W., Dunn-Walters D.K., Guikema J.E.J., Bende R.J., van Noesel C.J.M. 2012. A novel chronic lymphocytic leukemia subset expressing mutated IGHV3-7-encoded rheumatoid factor B-cell receptors that are functionally proficient. Leukemia. In press [DOI] [PubMed] [Google Scholar]
- Hussell T., Isaacson P.G., Crabtree J.E., Spencer J. 1996. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J. Pathol. 178:122–127 [DOI] [PubMed] [Google Scholar]
- Iliev I.D., Funari V.A., Taylor K.D., Nguyen Q., Reyes C.N., Strom S.P., Brown J., Becker C.A., Fleshner P.R., Dubinsky M., et al. 2012. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 336:1314–1317 10.1126/science.1221789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. 2009. Cancer statistics, 2009. CA Cancer J. Clin. 59:225–249 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- Klein U., Tu Y., Stolovitzky G.A., Mattioli M., Cattoretti G., Husson H., Freedman A., Inghirami G., Cro L., Baldini L., et al. 2001. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J. Exp. Med. 194:1625–1638 10.1084/jem.194.11.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostareli E., Gounari M., Janus A., Murray F., Brochet X., Giudicelli V., Pospisilova S., Oscier D., Foroni L., di Celle P.F., et al. 2012. Antigen receptor stereotypy across B-cell lymphoproliferations: the case of IGHV4-59/IGKV3-20 receptors with rheumatoid factor activity. Leukemia. 26:1127–1131 10.1038/leu.2011.311 [DOI] [PubMed] [Google Scholar]
- Küppers R. 2005. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer. 5:251–262 10.1038/nrc1589 [DOI] [PubMed] [Google Scholar]
- Lanemo Myhrinder A., Hellqvist E., Sidorova E., Söderberg A., Baxendale H., Dahle C., Willander K., Tobin G., Bäckman E., Söderberg O., et al. 2008. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 111:3838–3848 10.1182/blood-2007-11-125450 [DOI] [PubMed] [Google Scholar]
- Liu H., Ye H., Ruskone-Fourmestraux A., De Jong D., Pileri S., Thiede C., Lavergne A., Boot H., Caletti G., Wündisch T., et al. 2002. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 122:1286–1294 10.1053/gast.2002.33047 [DOI] [PubMed] [Google Scholar]
- Messmer B.T., Albesiano E., Efremov D.G., Ghiotto F., Allen S.L., Kolitz J., Foa R., Damle R.N., Fais F., Messmer D., et al. 2004. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J. Exp. Med. 200:519–525 10.1084/jem.20040544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray F., Darzentas N., Hadzidimitriou A., Tobin G., Boudjogra M., Scielzo C., Laoutaris N., Karlsson K., Baran-Marzsak F., Tsaftaris A., et al. 2008. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: implications for the role of antigen selection in leukemogenesis. Blood. 111:1524–1533 10.1182/blood-2007-07-099564 [DOI] [PubMed] [Google Scholar]
- Packham G., Stevenson F. 2010. The role of the B-cell receptor in the pathogenesis of chronic lymphocytic leukaemia. Semin. Cancer Biol. 20:391–399 10.1016/j.semcancer.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Stamatopoulos K., Belessi C., Hadzidimitriou A., Smilevska T., Kalagiakou E., Hatzi K., Stavroyianni N., Athanasiadou A., Tsompanakou A., Papadaki T., et al. 2005. Immunoglobulin light chain repertoire in chronic lymphocytic leukemia. Blood. 106:3575–3583 10.1182/blood-2005-04-1511 [DOI] [PubMed] [Google Scholar]
- Stamatopoulos K., Belessi C., Moreno C., Boudjograh M., Guida G., Smilevska T., Belhoul L., Stella S., Stavroyianni N., Crespo M., et al. 2007. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood. 109:259–270 10.1182/blood-2006-03-012948 [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Asaka M., Nakamura T., Nakamura S., Yonezumi S., Seto M. 2001. API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in low-grade gastric MALT lymphoma. Gastroenterology. 120:1884–1885 10.1053/gast.2001.25305 [DOI] [PubMed] [Google Scholar]
- Tromp J.M., Tonino S.H., Elias J.A., Jaspers A., Luijks D.M., Kater A.P., van Lier R.A.W., van Oers M.H.J., Eldering E. 2010. Dichotomy in NF-kappaB signaling and chemoresistance in immunoglobulin variable heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9 triggering. Oncogene. 29:5071–5082 10.1038/onc.2010.248 [DOI] [PubMed] [Google Scholar]
- van Dongen J.J.M., Langerak A.W., Brüggemann M., Evans P.A.S., Hummel M., Lavender F.L., Delabesse E., Davi F., Schuuring E., García-Sanz R., et al. 2003. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 17:2257–2317 10.1038/sj.leu.2403202 [DOI] [PubMed] [Google Scholar]
- Weller S., Braun M.C., Tan B.K., Rosenwald A., Cordier C., Conley M.E., Plebani A., Kumararatne D.S., Bonnet D., Tournilhac O., et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 104:3647–3654 10.1182/blood-2004-01-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhopf G.F., II, Rassenti L.Z., Toy T.L., Gribben J.G., Wierda W.G., Kipps T.J. 2004. Chronic lymphocytic leukemia B cells of more than 1% of patients express virtually identical immunoglobulins. Blood. 104:2499–2504 10.1182/blood-2004-03-0818 [DOI] [PubMed] [Google Scholar]
- Widhopf G.F., II, Goldberg C.J., Toy T.L., Rassenti L.Z., Wierda W.G., Byrd J.C., Keating M.J., Gribben J.G., Rai K.R., Kipps T.J. 2008. Nonstochastic pairing of immunoglobulin heavy and light chains expressed by chronic lymphocytic leukemia B cells is predicated on the heavy chain CDR3. Blood. 111:3137–3144 10.1182/blood-2007-02-073130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikherman J., Parameswaran R., Weiss A. 2012. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 489:160–164 10.1038/nature11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.