Abstract
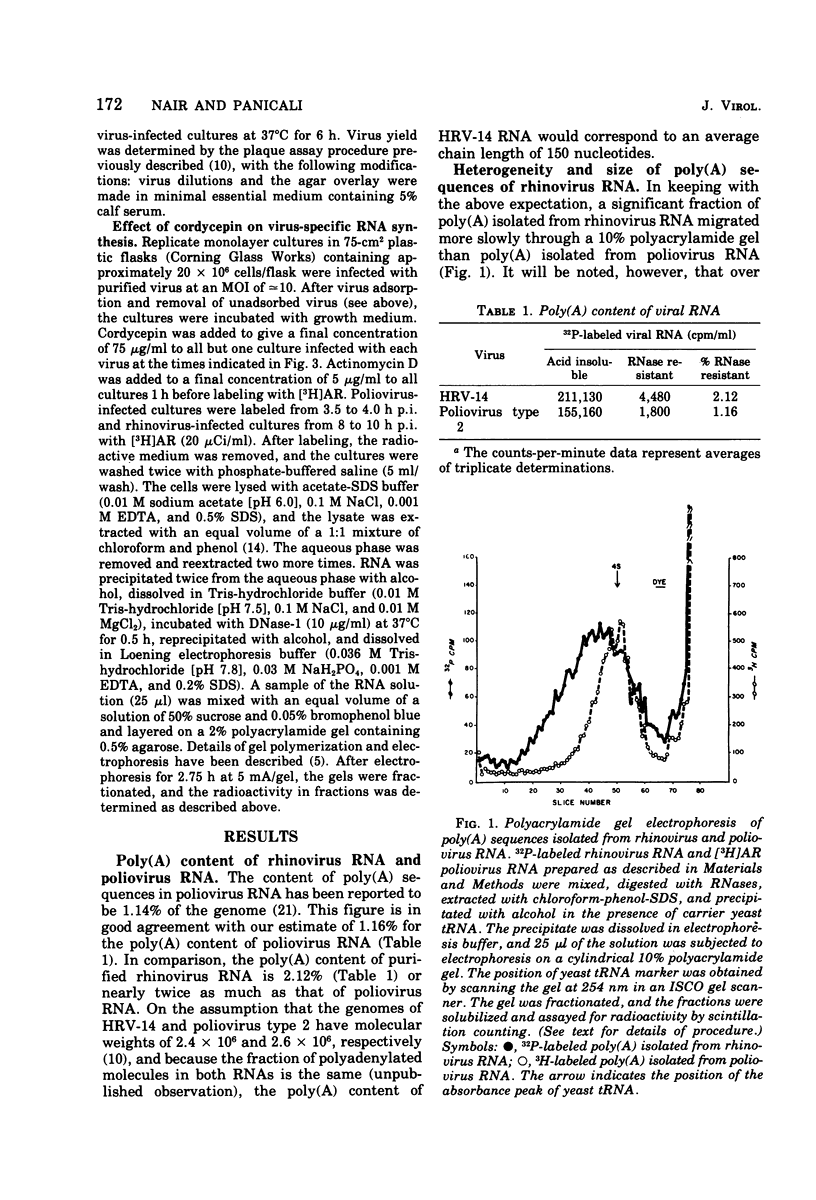
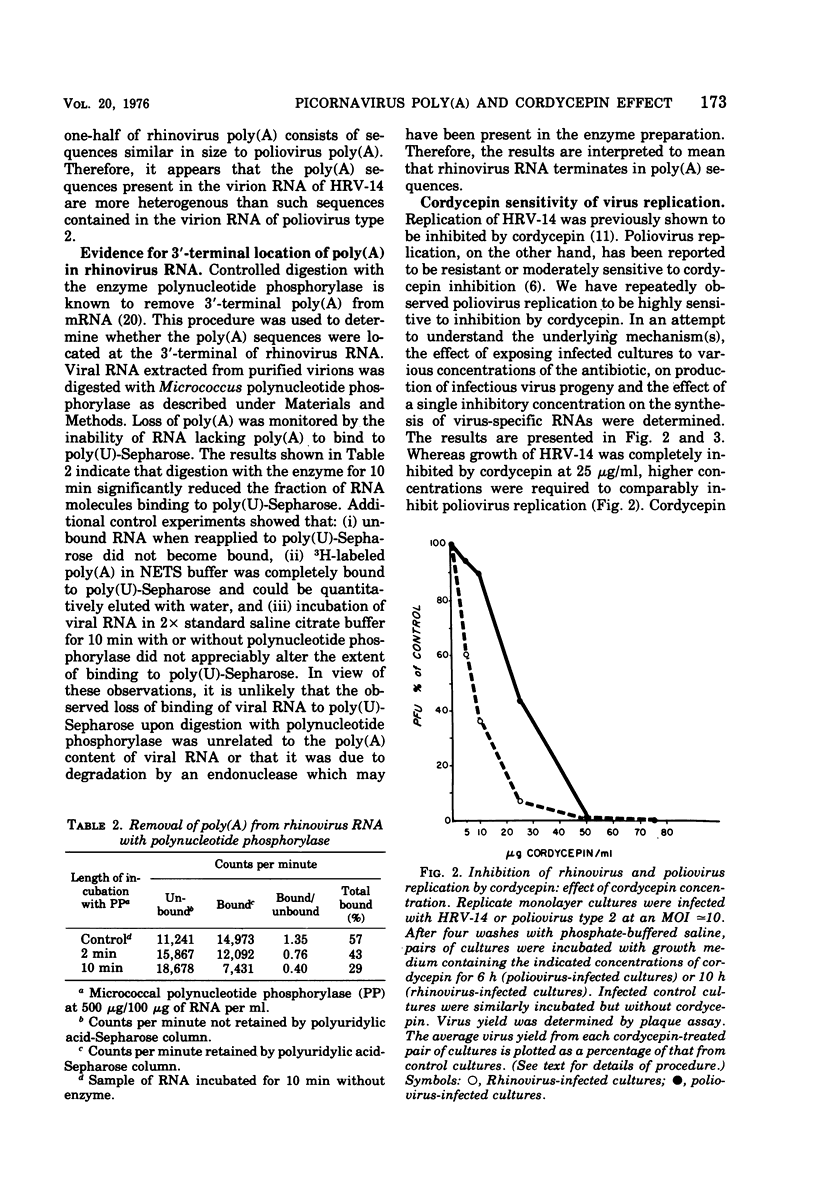
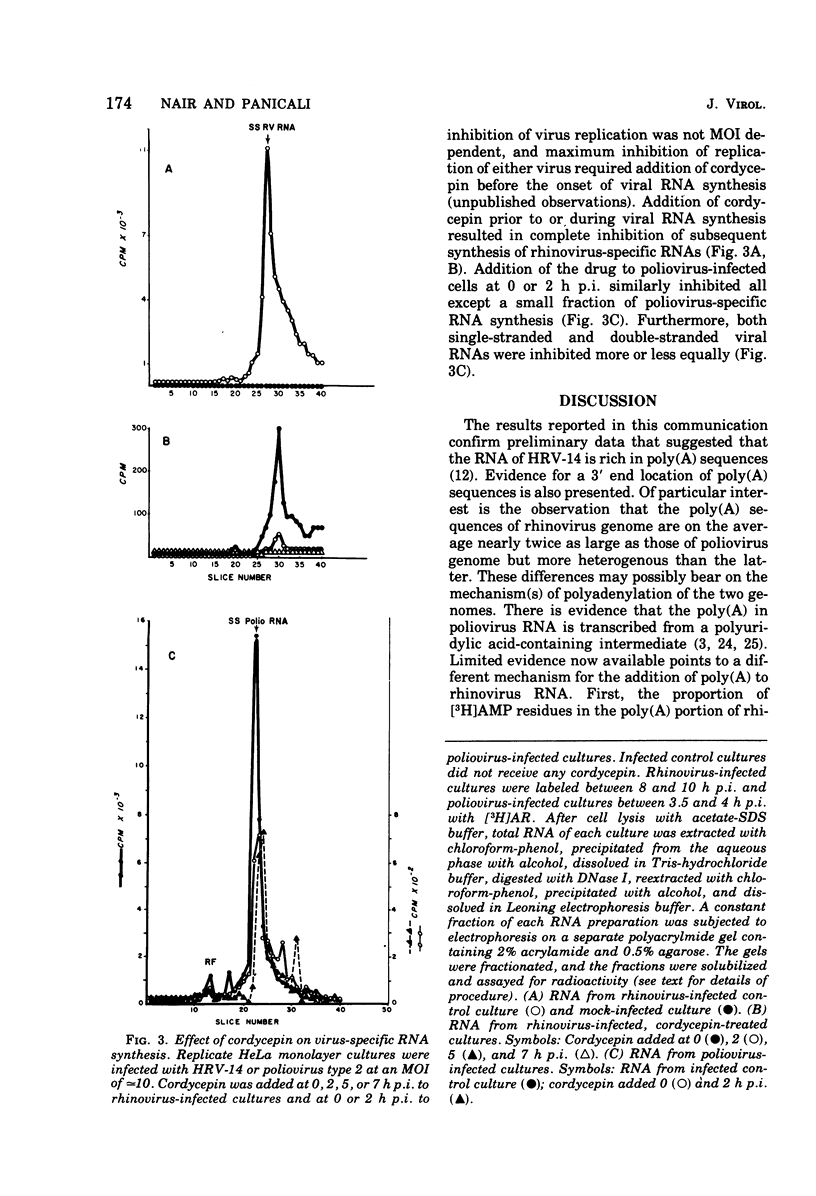
The polyadenylate [poly(A)] content of the genome RNA of human rhinovirus type 14 (HRV-14) is nearly twice as large as that of the genome RNA of poliovirus type 2. The poly(A) content of viral RNA was determined to be the RNase-resistant fraction of 32P-labeled viral RNA extracted from purified virions. Polyacrylamide gel electrophoresis indicated that the poly(A) sequences of HRV-14 are more heterogenous and on an average larger than those of poliovirus RNA. On the basis of susceptibility to micrococcal polynucleotide phosphorylase the rhinovirus genome terminates in poly(A). Replication of both viruses is almost totally inhibited by cordycepin at 50 mug/ml. At lower concentrations, rhinovirus replication is more sensitive to cordycepin than poliovirus replication. Addition of cordycepin (75 mug/ml) to infected culture prior to or during viral RNA replication results in more or less complete inhibition of virus-specific RNA synthesis. The results do not indicate that cordycepin sensitivity of either virus is due to preferential inhibition of viral poly(A) synthesis by this antibiotic.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Edmonds M., Nakazato H., Phillips B. A., Vaughn M. H. Polyadenylic acid sequences in the virion RNA of poliovirus and Eastern Equine Encephalitis virus. Science. 1972 May 5;176(4034):526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Yogo Y., Wimmer E. Replication of picornaviruses. I. Evidence from in vitro RNA synthesis that poly(A) of the poliovirus genome is genetically coded. J Virol. 1975 Dec;16(6):1512–1517. doi: 10.1128/jvi.16.6.1512-1517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. Polyadenylation of vesicular stomatitis virus mRNA. J Virol. 1974 May;13(5):1055–1060. doi: 10.1128/jvi.13.5.1055-1060.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliais S. I., Dimmock N. J. Replication of rhinovirus RNA. J Gen Virol. 1973 Jul;20(1):1–15. doi: 10.1099/0022-1317-20-1-1. [DOI] [PubMed] [Google Scholar]
- Maale G., Stein G., Mans R. Effects of cordycepin and cordycepintriphosphate on polyadenylic and ribonucleic acid-synthesising enzymes from eukaryotes. Nature. 1975 May 1;255(5503):80–82. doi: 10.1038/255080a0. [DOI] [PubMed] [Google Scholar]
- Macnaughton M. R., Dimmock N. J. Polyadenylic acid sequences in rhinovirus RNA species from infected human diploid cells. J Virol. 1975 Sep;16(3):745–748. doi: 10.1128/jvi.16.3.745-748.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Cox N. J., Armstrong S. J., Barry R. D. Multiplication of influenza virus in the presence of cordycepin, an inhibitor of cellular RNA synthesis. Nat New Biol. 1973 Jun 6;243(127):172–174. doi: 10.1038/newbio243172a0. [DOI] [PubMed] [Google Scholar]
- Nair C. N., Lonberg-Holm K. K. Infectivity and sedimentation of rhinovirus ribonucleic acid. J Virol. 1971 Feb;7(2):278–280. doi: 10.1128/jvi.7.2.278-280.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair C. N., Owens M. J. Preliminary observations pertaining to polyadenylation of rhinovirus RNA. J Virol. 1974 Feb;13(2):535–537. doi: 10.1128/jvi.13.2.535-537.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Gratzer W. B. Gel electrophoretic analysis of poly(riboadenylic acid). Biochim Biophys Acta. 1974 Apr 27;349(1):47–52. doi: 10.1016/0005-2787(74)90007-0. [DOI] [PubMed] [Google Scholar]
- Richardson L. S., Ting R. C., Gallo R. C., Wu A. M. Effect of cordycepin on the replication of type-c RNA tumor viruses. Int J Cancer. 1975 Mar 15;15(3):451–456. doi: 10.1002/ijc.2910150311. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Kates J., Kelley D. E., Perry R. P. Polyadenylic acid sequences on 3' termini of vaccinia messenger ribonucleic acid and mammalian nuclear and messenger ribonucleic acid. Biochemistry. 1972 Sep 26;11(20):3829–3834. doi: 10.1021/bi00770a023. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Kates J. Mechanism of poly(A) synthesis by vaccinia virus. J Virol. 1974 Aug;14(2):214–224. doi: 10.1128/jvi.14.2.214-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Poly(A) on mengovirus RNA. J Virol. 1975 Oct;16(4):1081–1084. doi: 10.1128/jvi.16.4.1081-1084.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Effect of cordycepin (3'-deoxyadenosine) on virus-specific RNA species synthesized in Newcastle disease virus-infected cells. J Virol. 1975 Dec;16(6):1575–1583. doi: 10.1128/jvi.16.6.1575-1583.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Crossley J., Humphries S. Translation of mouse globin messenger ribonucleic acid from which the poly(adenylic acid) sequence has been removed. Biochemistry. 1974 Feb 12;13(4):703–707. doi: 10.1021/bi00701a011. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Poly (A) and poly (U) in poliovirus double stranded RNA. Nat New Biol. 1973 Apr 11;242(119):171–174. doi: 10.1038/newbio242171a0. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Sequence studies of poliovirus RNA. III. Polyuridylic acid and polyadenylic acid as components of the purified poliovirus replicative intermediate. J Mol Biol. 1975 Mar 5;92(3):467–477. doi: 10.1016/0022-2836(75)90292-2. [DOI] [PubMed] [Google Scholar]



