Abstract
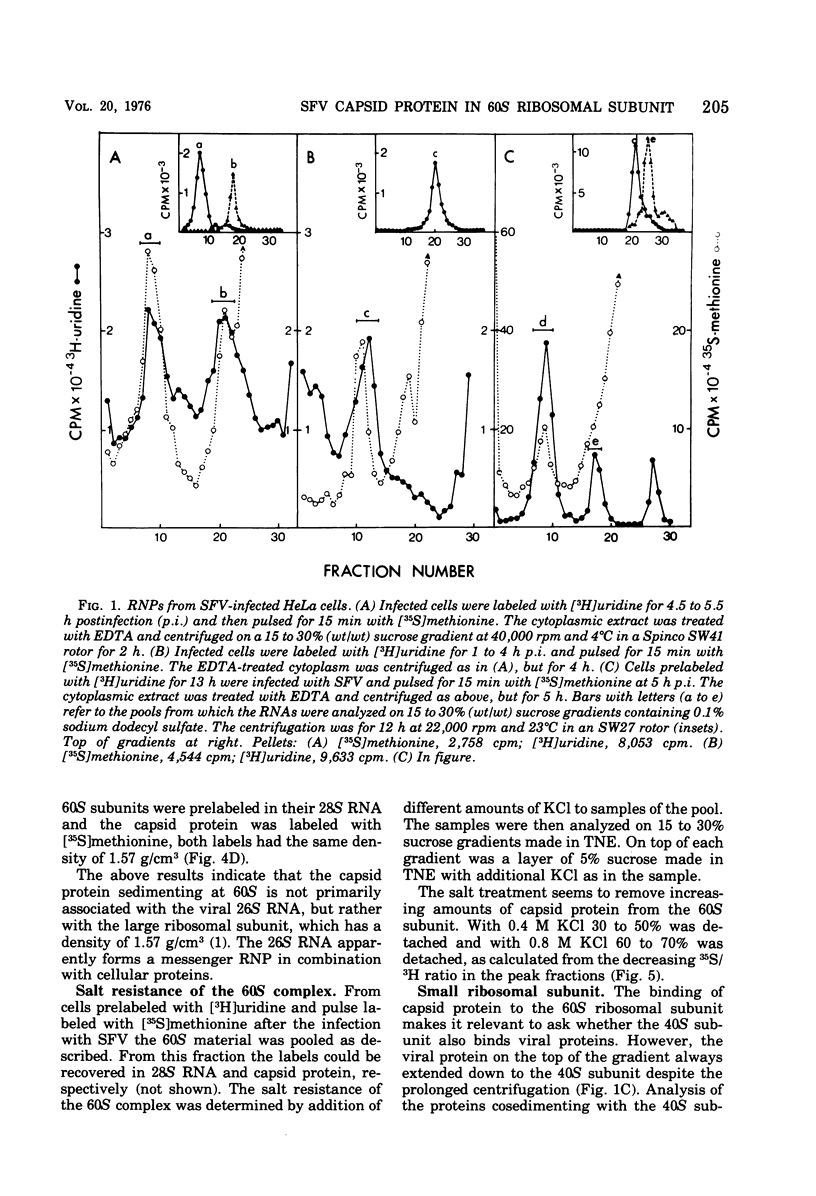
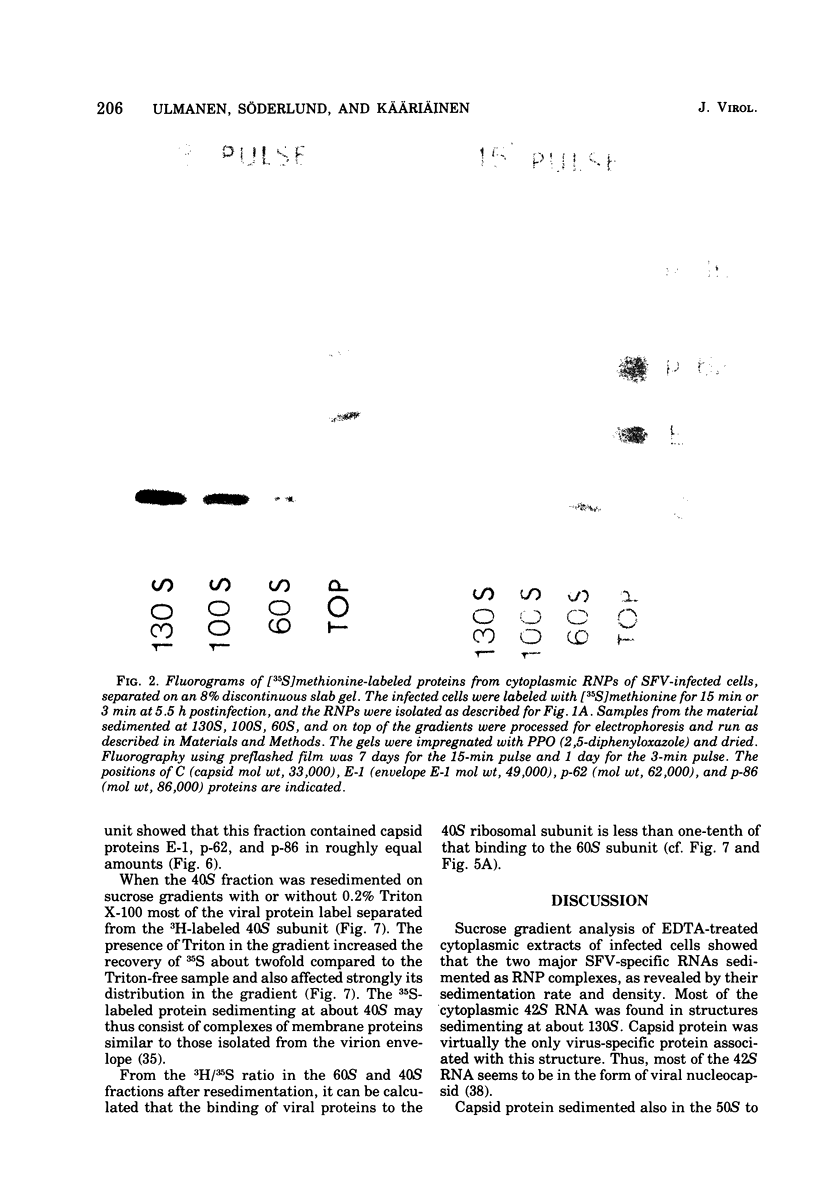
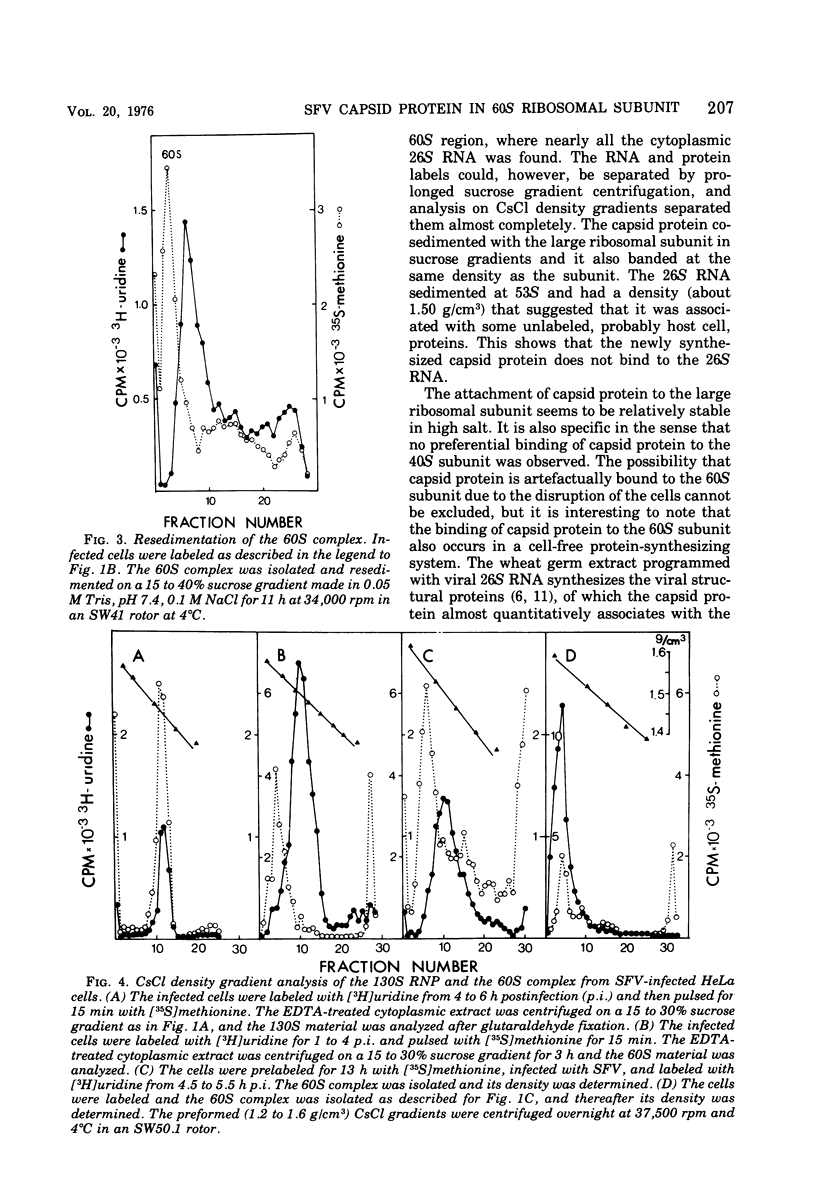
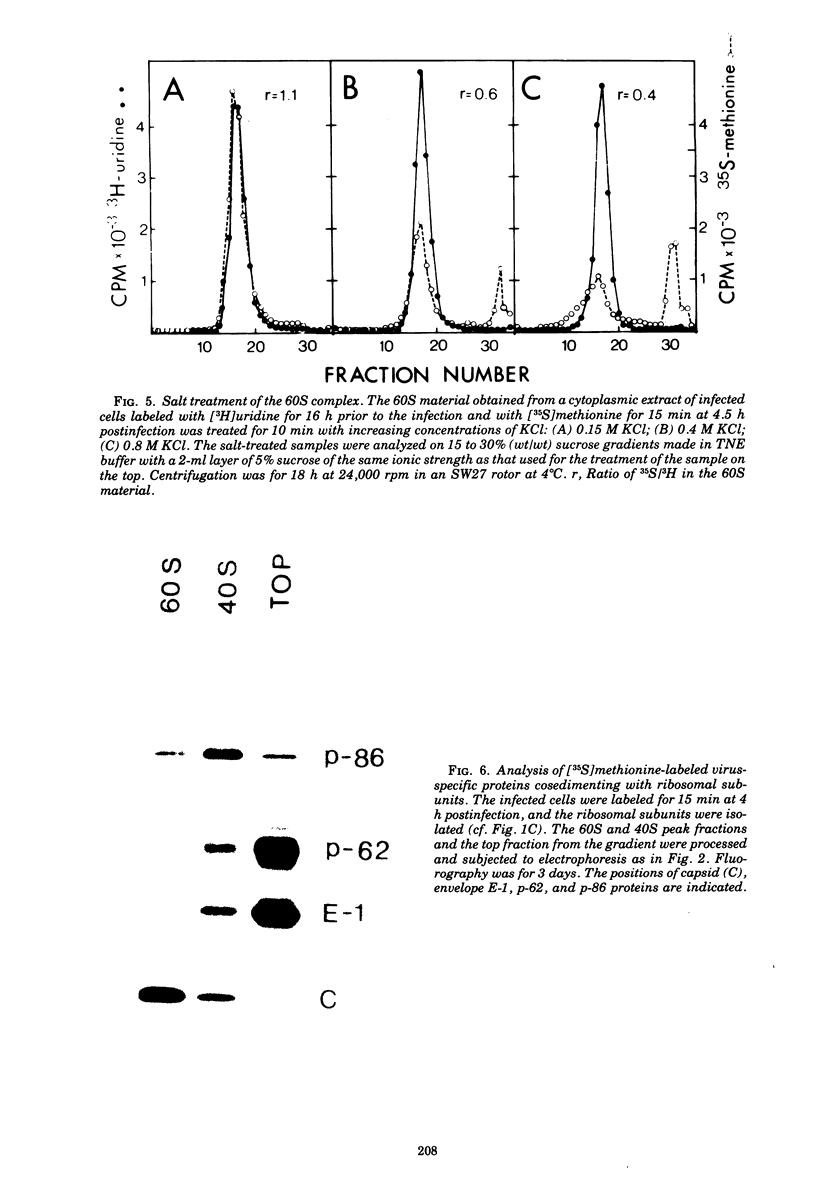
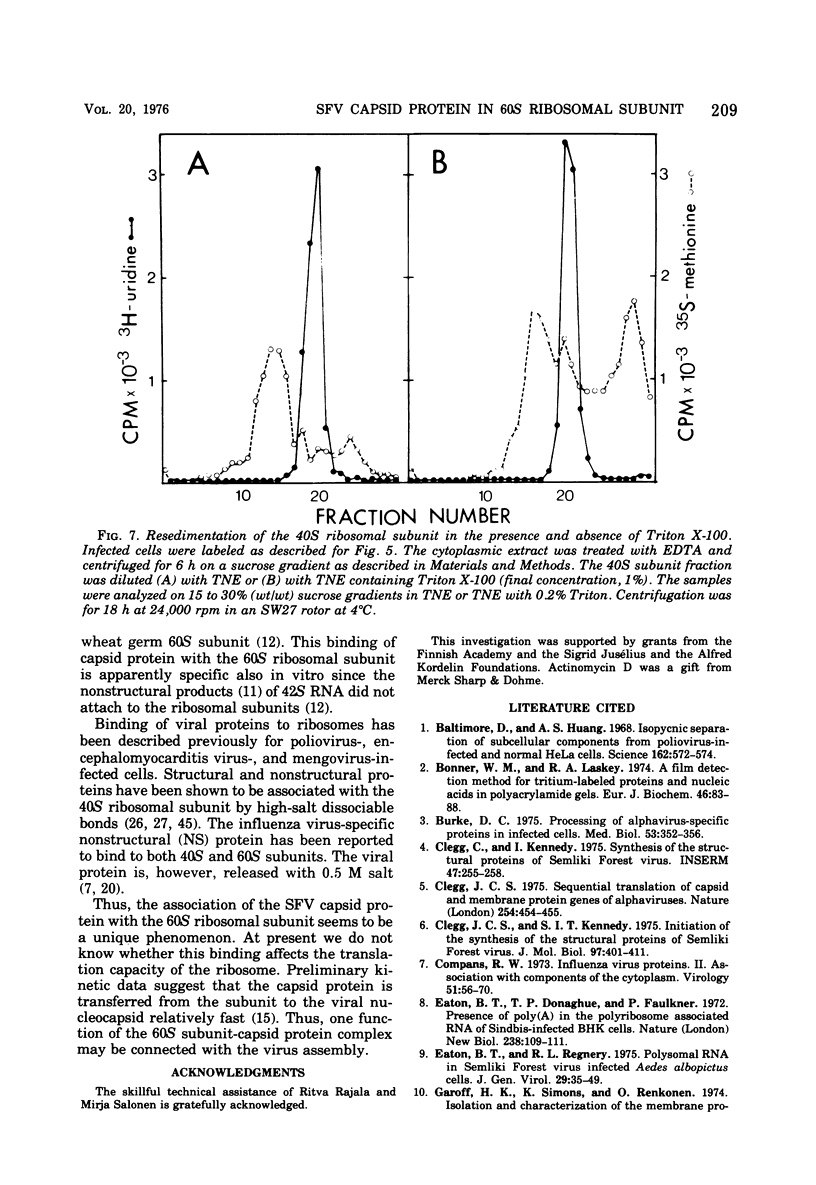
Semlike forest virus capsid protein cosedimented with the large ribosomal subunit at 60S in sucrose gradients after treatment of cytoplasm from infected cells with Triton X-100 and EDTA. In CsCl gradients the capsid protein banded with the subunit at a density of 1.56 to 1.57 g/cm3. Most of the capsid protein could be detached from the 60S structure by treatment with 0.8 M KCl. The ribonucleoprotein of the 26S RNA had a sedimentation value of 53S and a density of 1.50 g/cm3 and could thus be separated from the 60S structure. The data suggest that the capsid protein binds to the large ribosomal subunit, but not to the viral 26S RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke D. C. Processing of alphavirus-specific proteins in infected cells. Med Biol. 1975 Oct;53(5):352–356. [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Initiation of synthesis of the structural proteins of Semliki Forest virus. J Mol Biol. 1975 Oct 5;97(4):401–411. doi: 10.1016/s0022-2836(75)80050-7. [DOI] [PubMed] [Google Scholar]
- Clegg J. C. Sequential translation of capsid and membrane protein genes of alphaviruses. Nature. 1975 Apr 3;254(5499):454–455. doi: 10.1038/254454a0. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Donaghue T. P., Faulkner P. Presence of poly (A) in the polyribosome-associated RNA of Sindbis-infected BHK cells. Nat New Biol. 1972 Jul 26;238(82):109–111. doi: 10.1038/newbio238109a0. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Regnery R. L. Polysomal RNA in Semliki Forest virus infected Aedes albopictus cells. J Gen Virol. 1975 Oct;29(1):35–49. doi: 10.1099/0022-1317-29-1-35. [DOI] [PubMed] [Google Scholar]
- Glanville N., Morser J., Uomala P., Käri5AAINEN L. Simultaneous translation of structural and nonstructural proteins from Semliki-forest-virus RNA in two eukaryotic systems in vitro. Eur J Biochem. 1976 Apr 15;64(1):167–175. doi: 10.1111/j.1432-1033.1976.tb10285.x. [DOI] [PubMed] [Google Scholar]
- Glanville N., Ulmanen I. Biological activity of in vitro synthesised protein: binding of Semliki Forest virus capsid protein to the large ribosomal subunit. Biochem Biophys Res Commun. 1976 Jul 12;71(1):393–399. doi: 10.1016/0006-291x(76)90295-3. [DOI] [PubMed] [Google Scholar]
- Henshaw E. C. Messenger RNA in rat liver polyribosomes: evidence that it exists as ribonucleoprotein particles. J Mol Biol. 1968 Sep 28;36(3):401–411. doi: 10.1016/0022-2836(68)90164-2. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Isolation and identification of the virus-specified RNA species found on membrane-bound polyribosomes of chick embryo cells infected with Semliki Forest virus. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1254–1258. doi: 10.1016/0006-291x(72)90846-7. [DOI] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Proteins synthesized by Semliki Forest virus and its 16 temperature-sensitive mutants. J Virol. 1975 Aug;16(2):388–396. doi: 10.1128/jvi.16.2.388-396.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Keränen S., Lachmi B., Söderlund H., Tuomi K., Ulmanen I. Replication of Semliki Forest virus. Med Biol. 1975 Oct;53(5):342–351. [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Kärläinen L., Lachmi B. E., Glanville N. Transitional control in Semliki forest virus infected cells. Ann Microbiol (Paris) 1976 Jan;127A(1):197–203. [PubMed] [Google Scholar]
- Lachmi B. E., Glanville N., Keränen S., Läriäinen L. Tryptic peptide analysis on nonstructural and structural precursor proteins from Semliki Forest virus mutant-infected cells. J Virol. 1975 Dec;16(6):1615–1629. doi: 10.1128/jvi.16.6.1615-1629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Medvedkina O. A., Scarlat I. V., Kalinina N. O., Agol V. I. Virus-specific proteins associated with ribosomes of Krebs-II cells infected with encephalomyocarditis virus. FEBS Lett. 1974 Feb 1;39(1):4–8. doi: 10.1016/0014-5793(74)80003-7. [DOI] [PubMed] [Google Scholar]
- Mowshowitz D. Identification of polysomal RNA in BHK cells infected by sindbis virus. J Virol. 1973 Apr;11(4):535–543. doi: 10.1128/jvi.11.4.535-543.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Rosemond H., Sreevalsan T. Viral RNAs associated with ribosomes in Sindbis virus-infected HeLa cells. J Virol. 1973 Mar;11(3):399–415. doi: 10.1128/jvi.11.3.399-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol. 1973 Oct 15;80(1):119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Wheeler T., Glanville N., Käriäinen Translation of Semliki-Forest-virus 42-S RNA in a mouse cell free system to give virus-coat proteins. Eur J Biochem. 1974 Nov 1;49(1):101–110. doi: 10.1111/j.1432-1033.1974.tb03815.x. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Käriäinen L., von Bonsdorff C. H. Properties of Semliki Forest virus nucleocapsid. Med Biol. 1975 Oct;53(5):412–417. [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]
- Tuomi K., Kädäridäinen L., Söderlund H. Quantitation of Semlike Forest virus RNAs in infected cells using 32-P equilibrium labelling. Nucleic Acids Res. 1975 Apr;2(4):555–565. doi: 10.1093/nar/2.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Beato M., Hackemack B. A. Translation of 26 S virus-specific RNA from Semliki Forest virus-infected cells in vitro. Virology. 1974 Sep;61(1):120–128. doi: 10.1016/0042-6822(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Studies on the polyribosome-associated RNA in BHK21 cells infected with Semliki Forest virus. Virology. 1974 May;59(1):21–35. doi: 10.1016/0042-6822(74)90202-5. [DOI] [PubMed] [Google Scholar]
- Wright P. J., Cooper P. D. Poliovirus proteins associated with ribosomal structures in infected cells. Virology. 1974 May;59(1):1–20. doi: 10.1016/0042-6822(74)90201-3. [DOI] [PubMed] [Google Scholar]