Abstract
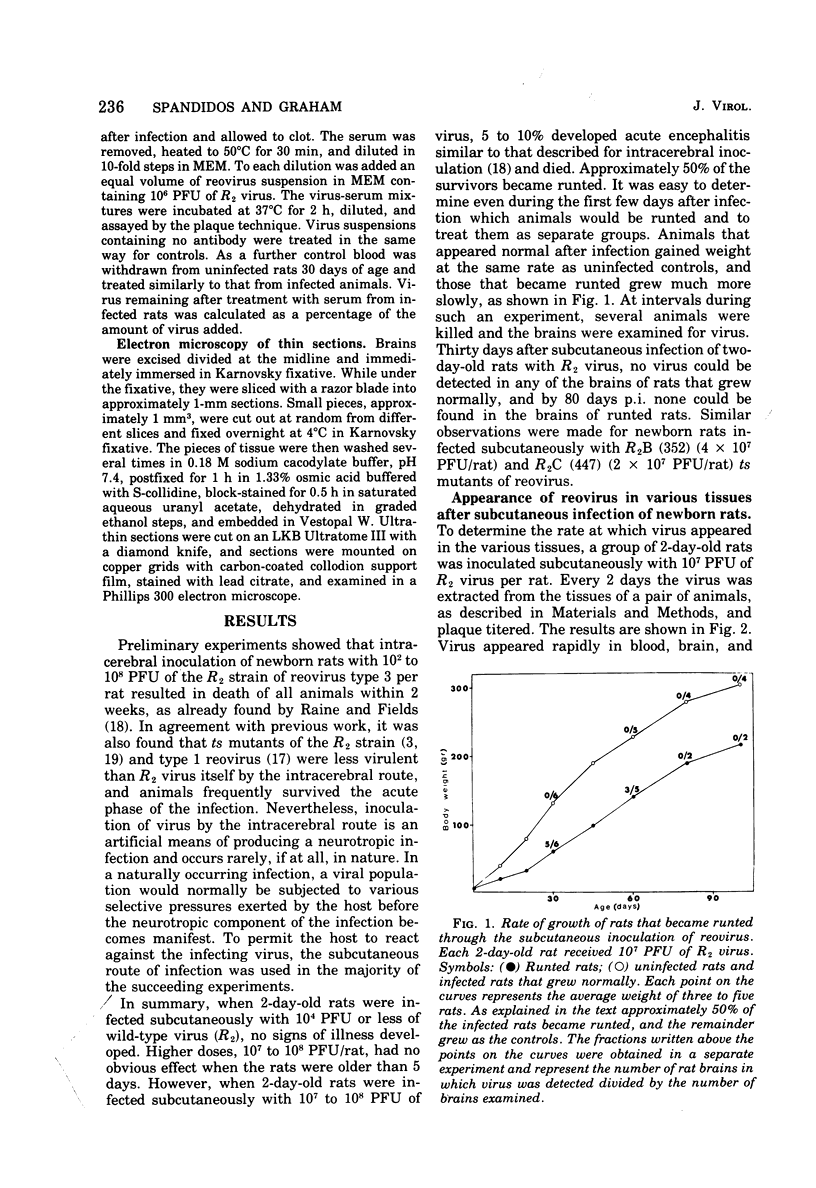
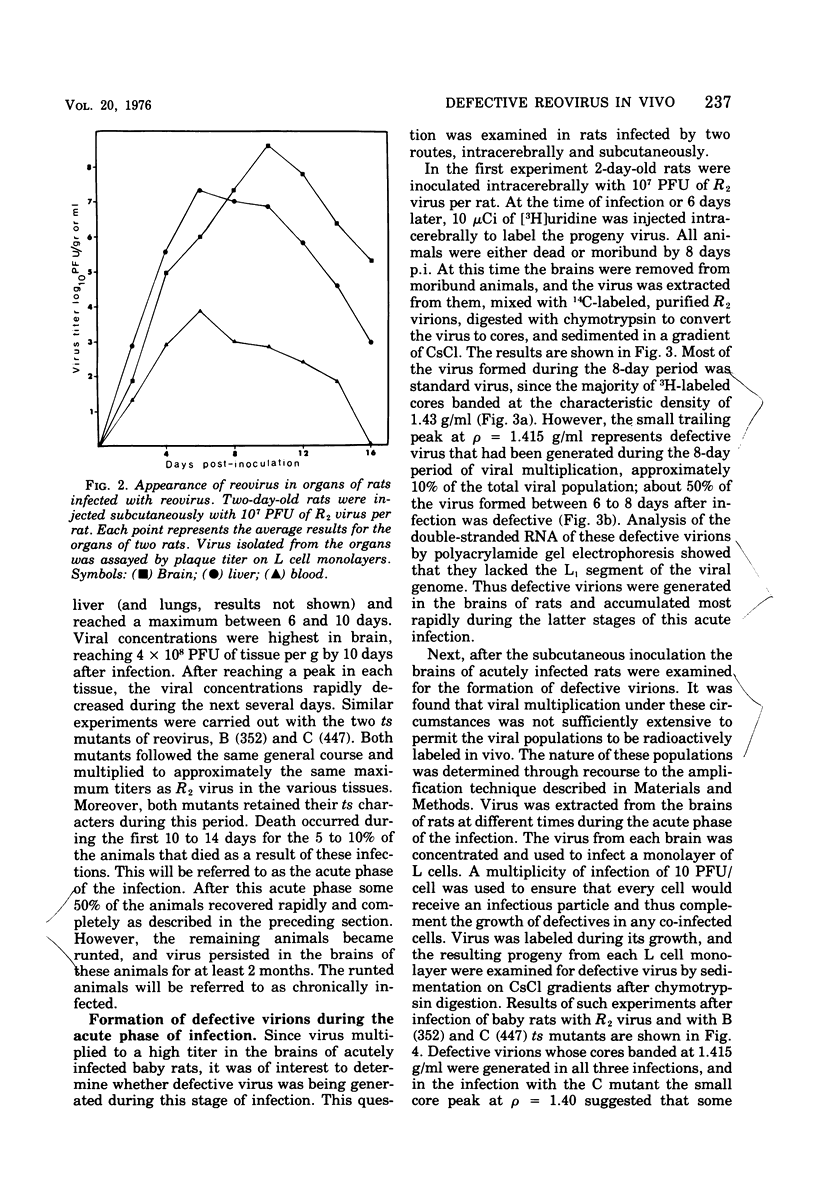
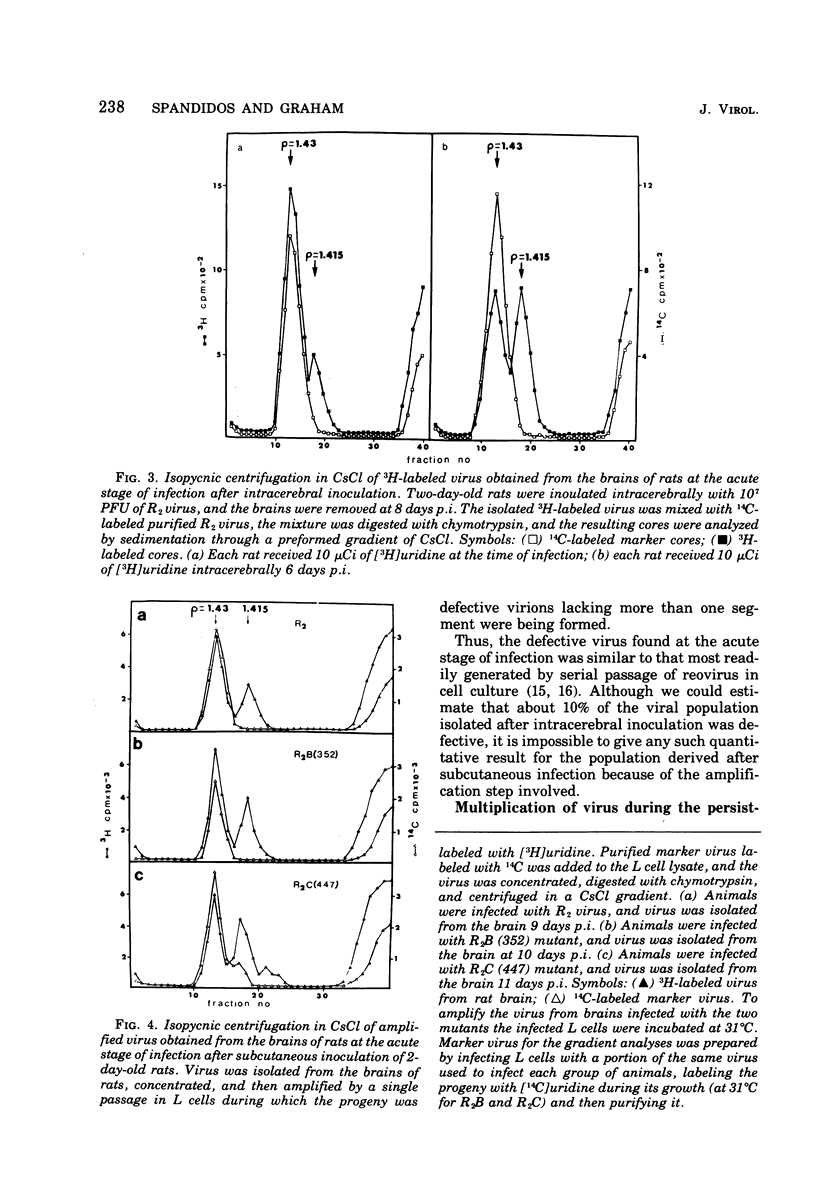
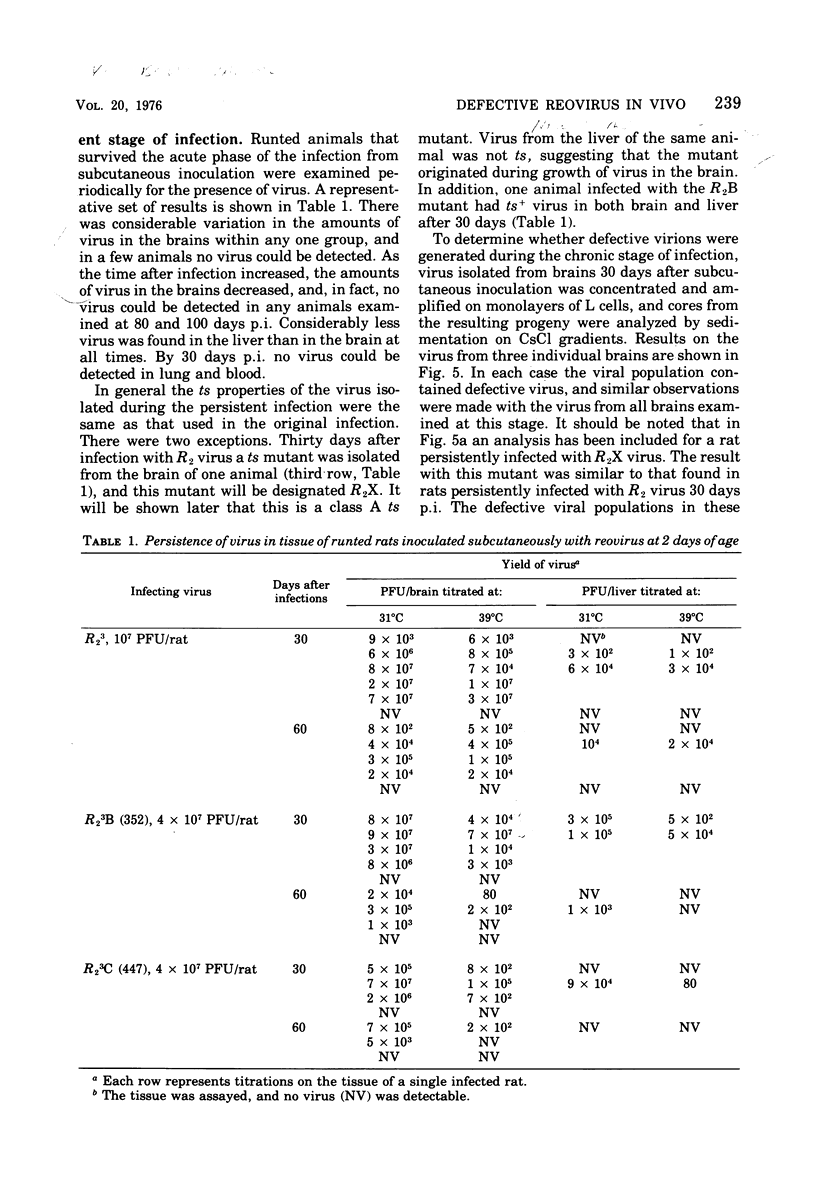
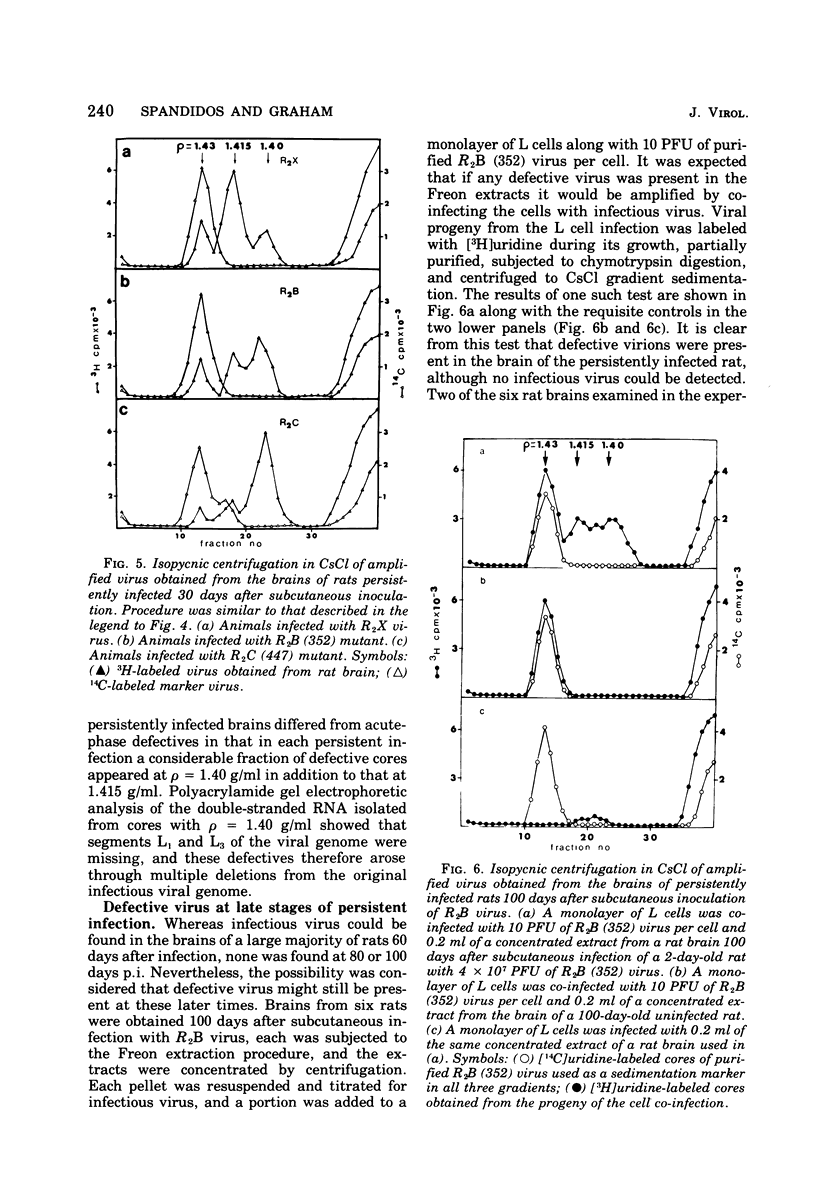
When 2-day-old rats were inoculated subcutaneously with the R2 strain of reovirus type 3 or with a class B (352) or class C (447) temperature-sensitive (ts) mutant, 5 to 10% of the animals died from acute encephalitis within 12 days. Approximately half of the survivors recovered rapidly and grew normally, but the remainder became runted. Two phases of infection are distinguished in the animals: an acute phase during which infectious virus reaches a maximum titer in brain and other tissues by 10 days p.i. and thes runting of the rats and the slow disappearance of virus from their brains over a period of 2 months or so. Virus isolated from chronically infected brains generally retained the genetic character (ts or wild type) of the inoculated virus, but two exceptions to this are described. Defective virions lacking the L1 segment of the viral genome (L1 defectives) were generated in rat brains during the acute phase of infection. Defective virus was also generated during the chronic phase, but during this period defectives were found with multiple segments deleted from the genome in addition to L1 defectives. In another type of experiment defective virus exerted a marked protective effect when inoculated intracerebrally with R2 virus. In the absence of defectives all animals died, but in their presence 17 of 23 animals survived and 15 of 23 became runted and chronically infected. The formation and evolution of defective particles in the brains of these rats were similar to those found in rats chronically infected after subcutaneous inoculation of reovirus. We conclude that the formation of defective virus particles may play a role in the initiation and maintenance of chronic neutropic infections with reovirus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Margolis G., Kilham L. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. II. Electron microscopic studies. Lab Invest. 1971 Feb;24(2):101–109. [PubMed] [Google Scholar]
- Haspel M. V., Rapp F. Measles virus: an unwanted variant causing hydrocephalus. Science. 1975 Feb 7;187(4175):450–451. doi: 10.1126/science.1111114. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- JENSON A. B., RABIN E. R., PHILLIPS C. A., MELNICK J. L. REOVIRUS ENCEPHALITIS IN NEWBORN MICE: AN ELECTRON MICROSCOPIC AND VIRUS ASSAY STUDY. Am J Pathol. 1965 Aug;47:223–239. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Herndon R. M. Virologic studies of multiple sclerosis and other chronic and relapsing neurological diseases. Prog Med Virol. 1974;18(0):214–228. [PubMed] [Google Scholar]
- Johnson R. T. Subacute sclerosing panencephalitis. J Infect Dis. 1970 Feb;121(2):227–230. doi: 10.1093/infdis/121.2.227. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. I. Virologic studies. Lab Invest. 1969 Sep;21(3):183–188. [PubMed] [Google Scholar]
- Margolis G., Kilham L., Gonatas N. K. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. I. Light microscopy studies. Lab Invest. 1971 Feb;24(2):91–100. [PubMed] [Google Scholar]
- Mims C. A. Factors in the mechanism of persistence of viral infections. Prog Med Virol. 1974;18(0):1–14. [PubMed] [Google Scholar]
- Nonoyama M., Graham A. F. Appearance of defective virions in clones of reovirus. J Virol. 1970 Nov;6(5):693–694. doi: 10.1128/jvi.6.5.693-694.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Watanabe Y., Graham A. F. Defective virions of reovirus. J Virol. 1970 Aug;6(2):226–236. doi: 10.1128/jvi.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. A., Alpers M. P., Stanley N. F. Hydrocephalus in mice inoculated neonatally by the oronasal route with reovirus type 1. Science. 1970 May 15;168(3933):858–859. doi: 10.1126/science.168.3933.858. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Neurotropic virus-host relationship alterations due to variation in viral genome as studied by electron microscopy. Am J Pathol. 1974 Apr;75(1):119–138. [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Reovirus type 3 encephalitis--a virologic and ultrastructural study. J Neuropathol Exp Neurol. 1973 Jan;32(1):19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- STANLEY N. F., LEAK P. J., WALTERS M. N., JOSKE R. A. MURINE INFECTIONS WITH REOVIRUS. II. THE CHRONIC DISEASE FOLLOWING REOVIRUS TYPE 3 INFECTION. Br J Exp Pathol. 1964 Apr;45:142–149. [PMC free article] [PubMed] [Google Scholar]
- Schuerch A. R., Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. VI. Mutant ts 447 and ts 556 particles that lack either one or two genome RNA segments. Intervirology. 1974;3(1-2):36–46. doi: 10.1159/000149740. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Complementation between temperature-sensitive and deletion mutants of reovirus. J Virol. 1975 Dec;16(6):1444–1452. doi: 10.1128/jvi.16.6.1444-1452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Complementation of defective reovirus by ts mutants. J Virol. 1975 Apr;15(4):954–963. doi: 10.1128/jvi.15.4.954-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Infectious center assay for complementation and recombination between mutant of reovirus. J Virol. 1976 Jun;18(3):1151–1154. doi: 10.1128/jvi.18.3.1151-1154.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Physical and chemical characterization of an avian reovirus. J Virol. 1976 Sep;19(3):968–976. doi: 10.1128/jvi.19.3.968-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley N. F., Joske R. A. Animal model of human disease. Active chronic hepatitis; Animal model: Chronic murine hepatitis induced by Reovirus type 3. Am J Pathol. 1975 Jul;80(1):181–184. [PMC free article] [PubMed] [Google Scholar]
- Stanley N. F. The reovirus murine models. Prog Med Virol. 1974;18(0):257–272. [PubMed] [Google Scholar]
- Stanners C. P., Goldberg V. J. On the mechanism of neurotropism of vesicular stomatitis virus in newborn hamsters. Studies with temperature-sensitive mutants. J Gen Virol. 1975 Dec;29(3):281–296. doi: 10.1099/0022-1317-29-3-281. [DOI] [PubMed] [Google Scholar]
- WALTERS M. N., JOSKE R. A., LEAK P. J., STANLEY N. F. MURINE INFECTION WITH REOVIRUS: I. PATHOLOGY OF THE ACUTE PHASE. Br J Exp Pathol. 1963 Aug;44:427–436. [PMC free article] [PubMed] [Google Scholar]
- Walters M. L., Stanley N. F., Dawkins R. L., Alpers M. P. Immunological assessment of mice with chronic jaundice and runting induced by reovirus 3. Br J Exp Pathol. 1973 Jun;54(3):329–345. [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]