Abstract
Many of the failures of total joint replacements are related to tribology, i.e., wear of the cup, head and liner. Accumulation of wear particles at the implants can be linked to osteolysis which leads to bone loss and in the end aseptic implant loosening. Therefore it is highly desirable to reduce the generation of wear particles from the implant surfaces.
Silicon nitride (Si3N4) has shown to be biocompatible and have a low wear rate when sliding against itself and is therefore a good candidate as a hip joint material. Furthermore, wear particles of Si3N4 are predicted to slowly dissolve in polar liquids and they therefore have the potential to be resorbed in vivo, potentially reducing the risk for aseptic loosening.
In this study, it was shown that α-Si3N4-powder dissolves in PBS. Adsorption of blood plasma indicated a good acceptance of Si3N4 in the body with relatively low immune response. Si3N4 sliding against Si3N4 showed low wear rates both in bovine serum and PBS compared with the other tested wear couples. Tribofilms were built up on the Si3N4 surfaces both in PBS and in bovine serum, controlling the friction and wear characteristics.
Keywords: friction, hip joint replacement, silicon nitride, solubility, wear
Introduction
Total hip joint replacement is one of the most commonly performed orthopedic operations.1 A total hip joint replacement has an average life span of about 15 years.2 Implant failures can be due to several factors but one of the most critical is release of wear particles from the bearing surface of the implant. Accumulation of wear particles at the implant has been linked to osteolysis which leads to bone loss and eventually aseptic implant loosening.2,3 The chemistry and particle size have been found to be of high importance to the inflammation response.2,4 Therefore, it is highly desirable to reduce the generation of wear particles from the implant surfaces. One strategy to overcome the problem is to develop a material combining wear resistance, biocompatibility and biodegradability. Ideally, such material would release very few and very small wear particles, which would readily resorb without being detrimental to the tissue or even stimulate bone formation. This paper describes a first step toward such a material strategy and pin points some important future steps.
A commonly used material in the acetabular cup is ultra-high molecular weight polyethylene (UHMWPE), often combined with a femoral head of cobalt-chromium (CoCr). UHMWPE against either a metal or a ceramic material has exhibited a relatively low coefficient of friction but the UHMWPE liner often gets worn and produces a relatively large amount of wear particles.5,6 CoCr based materials have been shown to have adequate properties for use as load-bearing implants. Experiments have however shown that CoCr particles released due to wear and corrosion could restrain the bone growth similar to UHMWPE.7 Titanium alloys and stainless steels have also been frequently used in joint implants.2 The main risk with metal alloy implants is the release of metal ions due to corrosion and wear and the potential negative effect of these ions.4 Alumina (Al2O3) and zirconia (ZrO2) ceramics have been used due to their high wear and corrosion resistance.6,8,9 In general, bulk ceramic materials have a poor fracture toughness and e.g., alumina components in joint replacements have been shown to release wear particles due to this short coming.10 However, the ceramic wear particles may be less bioreactive than those of UHMWPE.10 Other interesting ceramic materials include silicon nitride (Si3N4) that has a higher fracture toughness and is more resistant to micro crack propagation than alumina.10-12 The most common crystalline forms of Si3N4 are the α-phase and β-phase. The β-phase has longer grains, tougher and softer than the α-phase.13 Silicon nitride sliding against itself in a water based lubricated environment has shown a very low coefficient of friction and also low wear.14 It has been reported that the wear of silicon nitride in water occurs by tribochemical dissolution of the material without release of any solid wear particles.15 Others claim that the wear particles that are produced when Si3N4 slides against Si3N4 in water often consists of silica (SiO2) and are mainly amorphous.14 Silicon nitride as a bulk material has been shown to be bioinert and having good biocompatibility.10,16,17 Guedes e Siliva et al. found small contents of Si4+ ions in extracts after solubility tests of Si3N4.17 Other investigations have shown that mesoporous silica degrades in Phosphate Buffered Saline (PBS),18 and that Si ions can be incorporated into bone tissue (or even stimulate new bone formation).19 Moreover, Boshitskaya et al.20 have shown that Si3N4-powders dissolve in blood serum, gastric juice and a synthetic biochemical media at pH 7.4. This suggests that the use of Si3N4 in hip implants may give less wear particles and those produced would be biocompatible and biodegradable. The less detrimental biological response may give a reduced number of revisions as an end result. However, whereas the wear and tribofilm formation of Si3N4 sliding against itself in water have been investigated,10,11,14,15,21 there is a lack of knowledge on the wear and tribofilm formation in PBS and bovine serum and also regarding the solubility of the wear particles.
The purpose of this work is to examine the friction, wear and chemical properties of silicon nitride as well as the solubility of silicon nitride particles in a simulated physiological fluid. In addition, the materials response of the material to blood coagulation and immune complement activation has been studied in vitro.
Results
Tribological testing and analysis
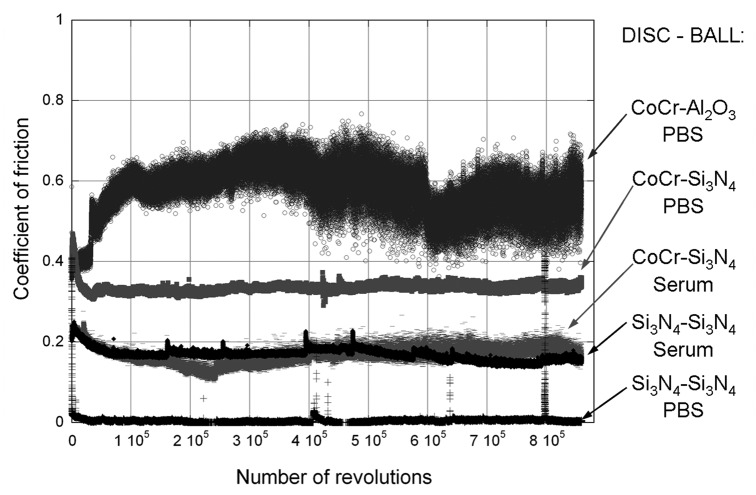
The friction curves varied dramatically between the different tests with respect to both the friction level and the fluctuations, see Figure 1. All the tests lubricated with PBS started with a coefficient of friction of approximately 0.4, whereas those lubricated with serum solution started at approximately 0.2. For the Si3N4-disc sliding against a Si3N4-ball in PBS the friction rapidly fell to around 0.01. The friction curve here shows a few peaks lasting around 7000 revolutions, which probably are connected to the refilling of water.
Figure 1. Coefficient of friction as a function of number of revolutions in the pin-on-disc test, with either PBS or a serum solution as lubricant.
The tests lubricated with the serum solution demonstrated much less friction difference between the different material combinations. Both combinations had mean value of µ = 0.17. However, the CoCr-Si3N4 combination demonstrated a slightly more fluctuating curve. The Si3N4- Si3N4 friction curve had a few peaks which all occurred in connection with the water refilling.
CoCr sliding against Al2O3 in PBS showed the highest friction. However, the CoCr disc that slid against Si3N4 showed most wear. This wear track had a cross-section area of 9600 µm2, Figure 2. While the other CoCr discs also had wear scar in the same order of magnitude, the Si3N4 disc that slid against Si3N4-ball in PBS had a significantly smaller wear track, with a width of 150 µm, depth of 30 nm and a cross-sectional area of 3 µm2. The Si3N4 disc that slid against a Si3N4-ball in the serum solution had a lager wear track, with a width of 600 µm, a depth of about 1.4 µm and a cross sectional area of 670 µm2.
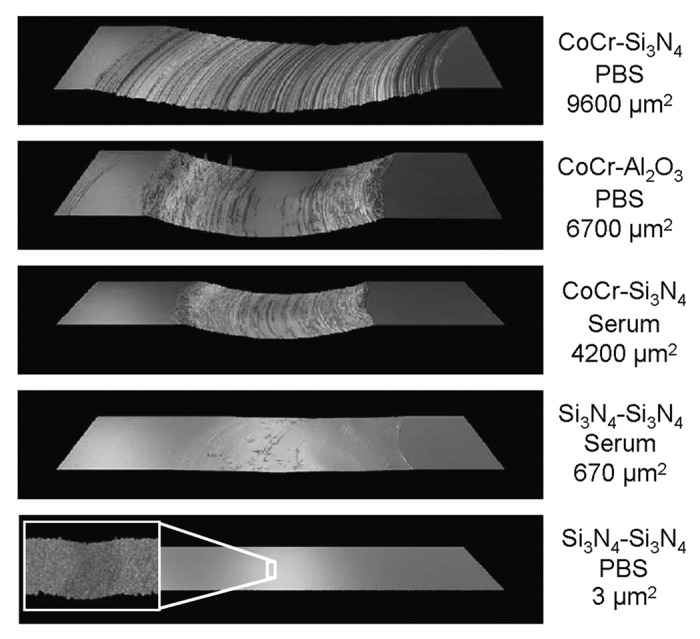
Figure 2. Optical profile images of worn discs and the calculated cross-section areas of the wear tracks. All the images have the same magnification. The width of the analyzed area is 1.1 mm, except for the enlarged area of the Si3N4 disc that slid against a Si3N4-ball in PBS.
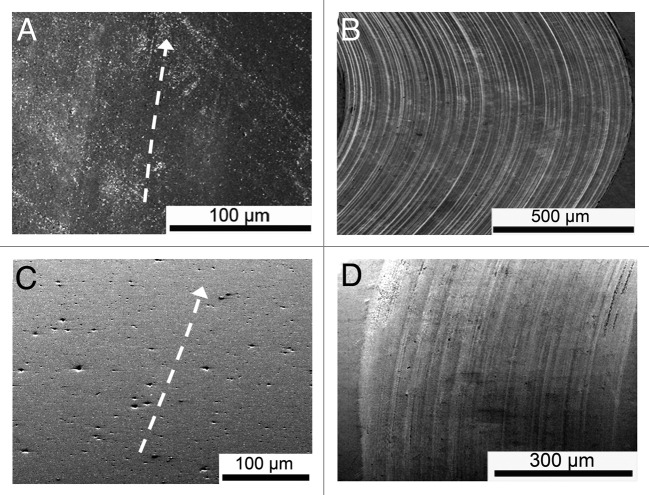
The Si3N4 discs did not show any clear wear marks irrespective of lubricant, whereas the wear marks on the CoCr discs appeared distinctly for both lubricants, Figure 3. The CoCr-disc tested in PBS had a more distinct scratch pattern when tested in bovine serum. The widths of the wear marks on the balls were about the same magnitude as the widths of the wear scars on the discs.
Figure 3. Surface appearance of the wear tracks in the SEM; (a) Si3N4-disc slid against Si3N4-ball in PBS, the arrow follows the wear track; (b) CoCr-disc slid against Si3N4-ball in PBS; (c) Si3N4-disc slid against Si3N4-ball in bovine serum, the arrow follows the wear track; (d) CoCr-disc slid against Si3N4-ball in bovine serum.
The original surface hardness for CoCr was lower than the hardness in the wear track, Table 1. The opposite trend was observed for Si3N4 both tested in PBS and in bovine serum. The hardness differences between the two unworn CoCr samples could be due to deformation hardening induced by small differences in the surface preparation process.
Table 1. Nano hardness values (and standard error) at indentation depth of 30 nm.
| Disc material | Unworn surface | Wear track (PBS) | Wear track (serum) |
|---|---|---|---|
| CoCr |
9.1 ± 0.5 GPa |
10.4 ± 0.3 GPa |
|
| CoCr |
8.1 ± 0.3 GPa |
|
8.5 ± 0.3 GPa |
| Si3N4 |
23.1 ± 0.6 GPa |
21.3 ± 0.9 GPa |
|
| Si3N4 | 23.8 ± 0.9 GPa | 22.0 ± 0.7 GPa |
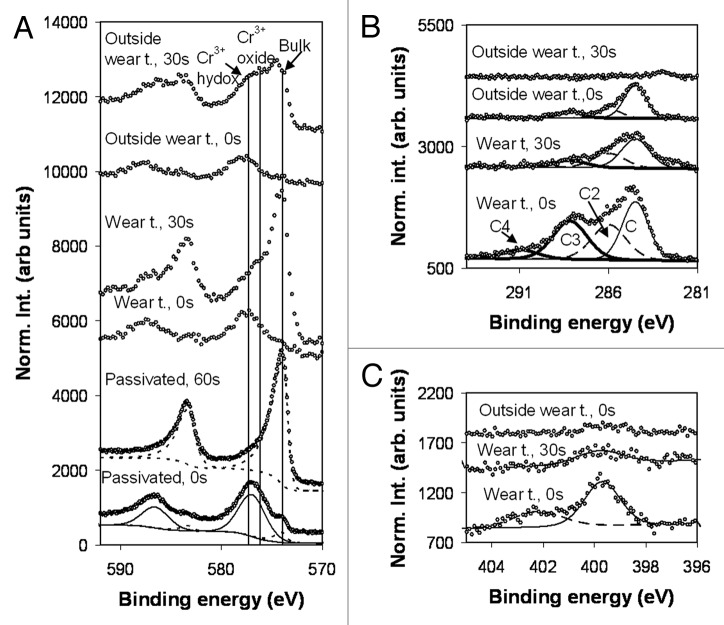
The XPS depth profiles of the CoCr samples showed that the atomic concentration has reached the bulk alloy values after about 2 min of sputtering for both the reference surface immersed in PBS and the unworn surface from the serum test, Figure 4A and B. Both surfaces consist mainly of Cr-oxide. The depth profile of the wear track from the serum test is quite different, Figure 4C. Here the affected layer of carbon and oxygen extends down to a sputter time of 30 min, i.e., 15 times longer than outside the wear track. This carbon and oxygen has been introduced into the bulk by the friction induced shear deformation of the surface layers.
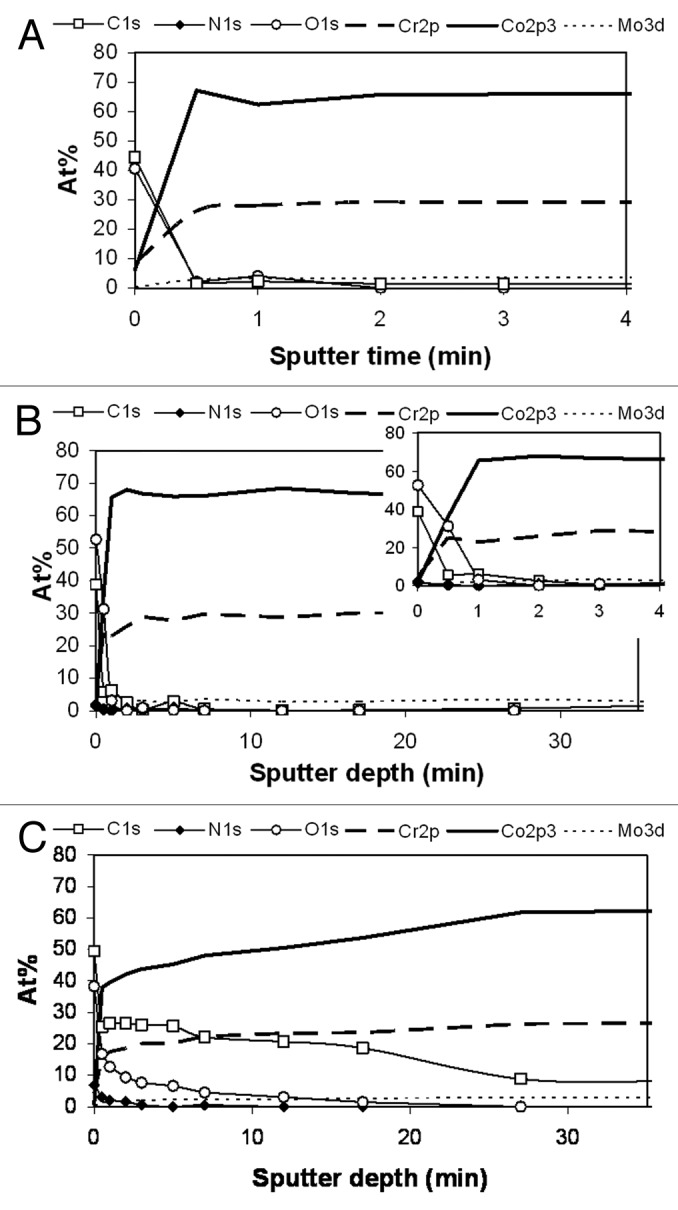
Figure 4. Depth distribution of different elements in the CoCr samples; (a) Surface immersed in PBS, (b) Surface exposed to bovine serum, outside the wear track; (c) Surface exposed to bovine serum in the wear track.
The Cr 2p spectra obtained from the passivated and bovine serum lubricated surfaces recorded in, and outside the wear track are shown in Figure 5A. The vertical lines mark the CoCr position of the following components in the 2p3/2 level: bulk metal, Cr3+ oxide (shifted 2.2 eV relative to the bulk22), and Cr3+ hydroxide (shifted 3.4 eV relative to the bulk22).
Figure 5. XPS spectra of CoCr samples at different times of sputtering (0, 30 and 60 sec); (a) Cr 2p peak recorded from the CoCr immersed in PBS (passivated); (b); C 1s-peak for CoCr in bovine serum, both in and outside wear track; (c) N 1s-peak for CoCr in bovine serum, both in and outside wear track.
It is in general difficult to separate the two ionic contributions from each other since they are internally broad and overlap. On the original surface of the PBS immersed sample, the two ionic contributions are fitted with one broad structure. After 60 sec of sputtering all structure related to the surface modification is removed and only the contribution from the bulk remains. The outermost part of the oxidized layer on the bovine lubricated surfaces is terminated by a Cr hydroxide. After 30 sec of sputtering the hydroxide decreases in intensity and the surface is now terminated by Cr3+ oxide with trace of hydroxide still left.
C 1s spectra from the bovine lubricated surfaces are displayed in Figure 5B. Spectra from the outermost surface obtained in and outside the wear track are decomposed into four and three peaks, respectively. The main peak at 284.5 (C1) can be associated to C–C and C–H bonds, the C2 peak shifted 1.5 eV is associated to C–O bonds, and the C3 component shifted 3.7 eV to N-C = O bonds.22,23 These structures are observed in the spectrum recorded in and outside the wear track of the original surfaces and after sputtering for 30 sec in the wear track. The C4 component shifted 6.4 eV relative to the main line is only observed in the spectrum from the wear track and is assigned to O = C-O bonds.24 The C4 structure shows that the normal peptide bonds have been partly oxidized in the wear track.
Figure 5C shows the N 1s spectra from the bovine lubricated CoCr surface. The main peak is situated at 399.9 eV. The peak on the high energy side shifted 2.5 eV to higher energies is only observed in the spectra from the wear track.
Si 2p spectra from Si3N4 samples lubricated with PBS solution and bovine serum are shown in Figure 6A. All spectra were recorded in un-sputtered condition and have similar appearance with one bulk related component (SiB) at 101.3 eV and one surface related component SiS shifted 1.3 eV. The SiS component is associated with SiO2/SiOx-OHy. The binding energy value for the SiB component is lower than the values reported in the literature (102 eV25,26) while the energy shift to the oxide component is in line with earlier reported values for the SiO2/SiOx-OHy.26,27
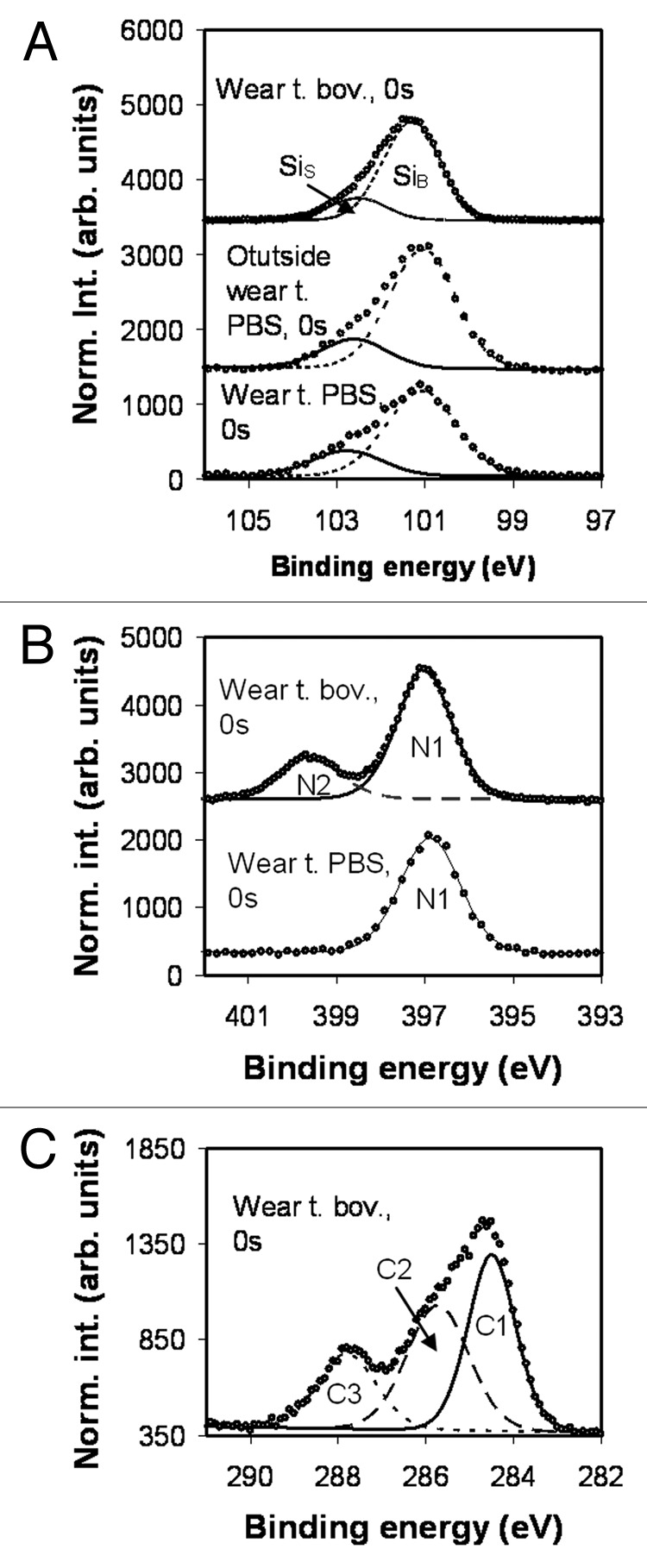
Figure 6. XPS spectra obtained from bovine and PBS lubricated Si3N4 surfaces; (a) Si2p peak; (b) N 1s peak; (c); C 1s peak.
The N 1s spectra are recorded from the wear track on samples that have been lubricated with either PBS solution or bovine serum, Figure 6. In the case of PBS solution the spectrum can be fitted with one component and in the case of bovine serum the spectrum is composed of two distinct components. During sputtering of the bovine lubricated surface the N2 component diminish after around 60 sec (not shown). The N1 component at a binding energy of 397 eV is associated to the bulk material and the N2 component shifted 2.6 eV to the peptide containing tribosurface. Also here the binding energy of the bulk component is somewhat lower than the values reported in the literature.28
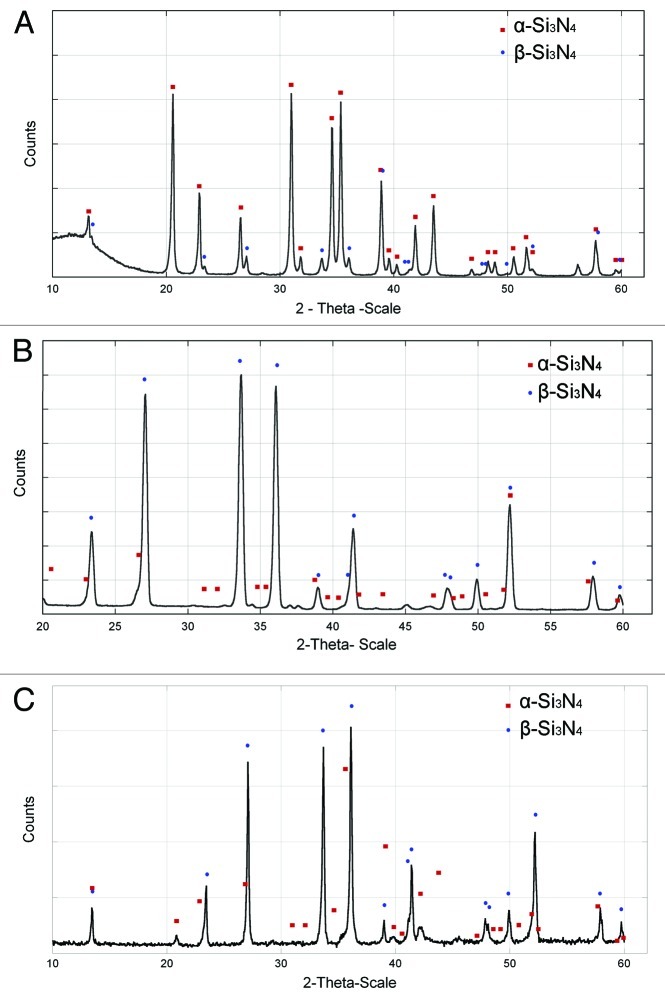
Figure 6C shows the C 1s spectrum from the wear track of the Si3N4 bovine lubricated surface. The C 1s peak can be fitted with three components labeled C1, C2, and C3. The interpretations of the different structures are the same as for those observed on the CoCr surface (i.e., C1, C–C and C–H, C2, C–O, and C3 N-C = O bonds). The X-ray diffractograms demonstrated that the disc and the ball were of β-Si3N4, Figure 7.
Figure 7. XRD of Si3N4; (a) powder; (b) disc; (c) ball.
Solubility test
The concentration of free Si in the filtered solutions indicates that some of the powder has been dissolved The concentration of Si in the filtered PBS solutions was about 75 mg/l for all pH variations and also approximately the same for the different incubation periods, see Figure 8. This would correspond to a dissolution of Si3N4 powder of 1.87%. All samples of PBS solutions without any addition of powder showed Si concentrations of less than 0.5 mg/l.
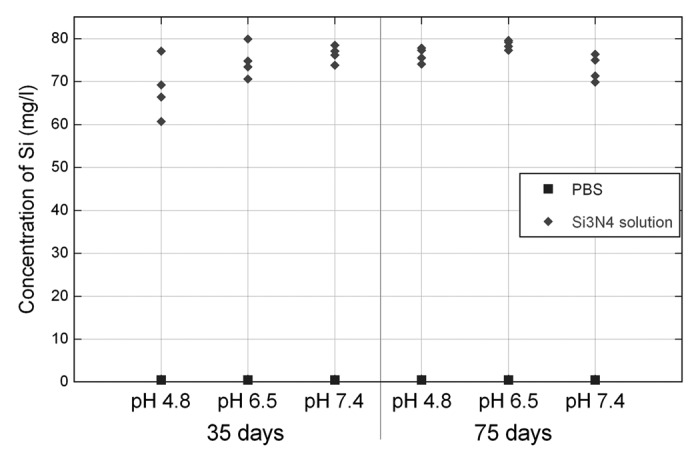
Figure 8. Concentration of free Si in PBS solutions of different pH after 35 and 75 d.
Blood plasma incubations
Surface mass densities on Si3N4, CoCr and Ti references resulting from exposure to human blood plasma and subsequent antibody incubations are plotted in Figure 9 for antibodies of factor C3c, HMWK and Fib. Results from adsorption experiments on ZrO2 surfaces, a material commonly assumed to be compatible with soft as well as hard tissues, are included in the plot.29 Some variations of the adsorbed amounts of plasma (lower part of bars) among the samples can be observed, mainly due to a variation in surface roughness. The lower values of adsorbed plasma on ZrO2 can be explained by a shorter incubation time. The results from exposure to antibody solutions probes the composition of the layer adsorbed from plasma.
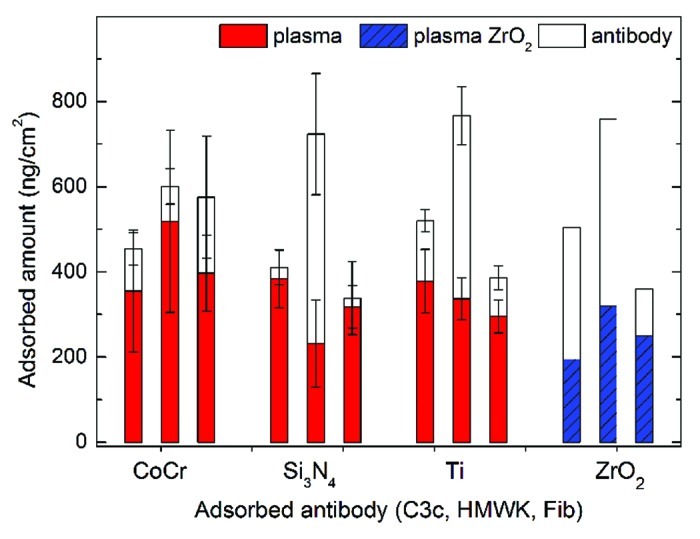
Figure 9. Adsorbed amounts of blood plasma and antibodies onto surfaces of CoCr, Si3N4 and reference surfaces of titanium. The lower part of the bar represents adsorbed plasma and the upper part represents adsorbed antibody. Results from adsorption experiments on zirconia surfaces29 are also included.
Discussion
The tribological tests performed in this study showed that the coefficient of friction does not directly correlate to the wear of the components. The highest friction was achieved with CoCr disc against an Al2O3 ball in PBS. However, this disc showed lower wear than the CoCr disc slid against the Si3N4 ball in PBS, which had a lower coefficient of friction, Figures 1 and 2. Further, in the bovine serum solution both material combinations exhibited similar friction, while the CoCr disc was substantially more worn than the Si3N4 disc. The similarity in friction could be explained by the presence of the same type of tribofilm: the XPS results showed that both the CoCr and Si3N4 discs have tribosurfaces consisting of metal oxide/hydroxide mixed with fragments from the serum proteins. The amount of protein fragments is highest in the wear track even if the areas outside the wear track also show traces of these fragments. The hardness measurements of the Si3N4 discs indicated that the surfaces became slightly softer with the deposited layer. The thin deposited layers are probably much softer than the measured values where the contribution form the bulk is significant. It was further noticed that the CoCr discs were hardened by wear. The CoCr disc tested in bovine serum exhibited less wear, had a smoother wear track and less hardness increase than the CoCr disc tested in PBS.
The XPS data from the C 1s and N 1s show that samples lubricated with bovine serum contain traces of peptide fragments, which stem from the proteins in the serum. The absorption of proteins occurs both in and outside the wear track, even if the thickness of the peptide containing layer is thicker in the wear track than outside. In the ceramic on metal contact there is, besides wear, a component of mechanical mixing that occurs in the sub surface layer. This effect is clearest distinguished on the CoCr-Si3N4 system lubricated with bovine serum were the carbon profile extend into the material in a manner that can described as mechanical mixing (Fig. 4C). For the CoCr-CoCr system it has been shown that the mechanical mixing can be described as a formation of a nano-crystalline layer by rotation of clusters of atoms. In this process denatured proteins from the solution is incorporated into the metal.30 This is in line with the data shown in Figure 4C. The underlying reason for the binding energy being lower for the Si and N bulk levels compared with other studies could be found in the shielding of the final state. It is likely that the bulk material in the present measurement contains higher number of defects/doping states that act as free charges that can shield the final state, which may result in a lower binding energy.
The tribofilm present on the Si3N4 disc tested in PBS contributed to an extremely low coefficient of friction. However, that tribofilm was found to be very sensitive and after it had been broken, it took approximately 600 revolutions until it was reformed and the coefficient of friction reached a value of 0.02. The occasional high friction periods could be connected to the refilling of deionized water. The friction sensor was never touched but the tribofilm might have been disturbed due to fluctuation and foaming of the PBS liquid. These high friction periods were not just instant peaks, the highest lasted for about 1000 revolutions. The peaks show that this tribofilm is very sensitive to external disturbance. These results are in agreement with Xu and Kato,14 who suggested that the silica tribofilm is dispersed when it reaches a certain thickness and replaced by a new film which forms on the surface.
The XRD measurement shows that the tested discs and balls contain predominantly β-Si3N4. As previously mentioned, β-Si3N4 is advantageous due to its higher wear resistance and higher toughness than the α-Si3N4 phase. However, the α-Si3N4 phase is harder than β-Si3N4.13,31 According to Zhu and Sakka13 the α-phase is less chemically stable since the α-phase transforms into the β-phase at high temperatures when a liquid phase is present.
The solubility tests showed that Si3N4-powder, which was mainly of α-phase, was soluble in PBS at 37°C, which is in accordance with Boshitskaya et al.20 These authors have shown that Si3N4-powders dissolve in blood serum, gastric juice and a synthetic biochemical media at pH 7.4. The solubility tests in the present investigation showed no difference in the amount of Si-ions between 35 and 75 d or between the different pH values. This may be due to a saturation of the solutions at an early stage, in accordance with a previous study.32 Future studies will focus on lower concentrations of Si3N4-powder as well as powders of different phases, as sintered silicon nitride commonly consists mainly of β-phase. However, if the silicon nitride is to be realized as a coating on a metal substrate, it may be of an amorphous nature, and such particles will hence also be of interest. Ultimately, wear particles from tribological tests of the final material will need to be tested in vivo.
Large amounts of HMWK adsorbed on the Si3N4 surfaces indicate that the intrinsic pathway of coagulation is activated,33 probably due to the negative charge of the surfaces. Negatively charged surfaces tend to denature proteins at the surface to a lower extent. Low binding of complement (C3c) was found on Si3N4, as compared with both Ti and ZrO2, indicating less immune activation. The adsorption results on CoCr surfaces are comparable to results of Wälivaara.34 The small amounts of HMWK and the large amounts of Fib indicate a hydrophobic surface. The high levels of adsorption of Fib indicate binding and activation of platelets and thereby clotting.35 The small amounts of Fib on Si3N4 indicate the opposite, i.e., less activation of platelets and less clotting.
The results from this study indicate that Si3N4 fulfil the initial profile set-out to find a wear resistant low-friction material that also has the potential to give resorbable wear particles in vivo. Future work will focus on developing a deposition method for coatings on metals with the same properties as the bulk material, as well as confirming the solubility of produced wear particles.
Materials and Methods
Tribological testing and analysis
The tribological tests were performed with ball-on-disc equipment, where a stationary ball was pressed against a rotating disc and the friction force was continuously measured.36 The balls had a diameter of 6 mm, the sliding speed was 0.05 m/s, the radius of the rotation was 3.2 mm and the normal load was 1 N. The sliding speed and the radius of the rotation are in accordance with the ASTM standard test.37 However, due to the small ball radius and the limitation of the lowest load for the equipment, the maximum Hertzian contact pressure is about 0.8 GPa. This pressure corresponds to a normal load of 400 kN for a Si3N4 head of 28 mm in diameter against a cup of Si3N4 with a radial clearance of 100 µm. The tests were performed for approximately 900 000 revolutions. The tests were performed with commercial balls of Si3N4 or Al2O3 (Spekuma), sliding against discs of commercial sintered silicon nitride (Keranova AB) or commercial CoCr (ASTM F75, Sandvik AB). CoCr and Al2O3 were included in the tests as references since both materials are commonly used in hip joint implants. The discs were mounted in a steel cup. The tests were performed in PBS (Sigma-Aldrich) or in bovine serum diluted with deionized water according to the ASTM standard.37 The cup was refilled with deionized water once or twice a day to make up for evaporation losses. Before the tests, the discs were polished to a surface roughness (Ra) of 5–15 nm and subsequently cleaned in an ultrasonic bath with acetone and then ethanol. The balls had a surface roughness of approximately 50 nm. As a reference, a CoCr sample was immersed in PBS for 90 d. The surface appearance of the wear tracks of the discs was analyzed using Scanning Electron Microscopy (SEM), the surface roughness and volume loss was studied using an optical profiler based on white light interference (OP). The chemical composition of the wear tracks and unworn reference surfaces was analyzed with XPS (X-ray Photoelectronic Spectroscopy, PHI Quantum 200). XRD (X-ray diffraction, Siemens P5000) was used in order to analyze the phase composition of the Si3N4 discs and powder.
The superficial hardness of the unworn discs and the wear tracks was measured with an ultra nano hardness tester (UNHT, CSM) using a Berkovich indenter. The hardness was measured at an indentation depth of 30 nm. The indents were analyzed in accordance with the Oliver-Pharr method.38
Solubility test
Since actual wear particles of silicon nitride were inaccessible due to the very low wear in the tribological tests, a commercial silicon nitride powder (P95H, Akzo Nobel) was used for a preliminary solubility study. This powder was reported to contain 98.5+% Si3N4, with a minimum of 90% alfa-Si3N4, d10 = 0.23 µm, d50 = 0.77 µm, d90 = 4.0 µm, corresponding to BET surface area 9 ± 3 m2/g. The average grain size of the powder was confirmed to be approximately 1 µm (measured with SEM and optical microscopy). The powder was mixed with PBS using three different pH values: 4.8, 6.5 and 7.4. It has been shown that the pH value in the vicinity of the implant can vary between 4.4–7.7.39 PBS has a natural pH of 7.4 and hydrochloric acid was added to decrease the value. The mixture was stored in plastic tubes with plastic lids. The tubes were placed on a rocking platform shaker and stored at 37°C during 35 and 75 d respectively, (Table 2). 100 mg of silicon nitride powder was mixed with 15 ml PBS. After the storage time, the mixture was filtered through a 0.2 µm PTFE membrane using a syringe. Dynamic light scattering analysis (Malvern Zetasizer NanoZS, Malvern Instruments, Nordic AB) did not detect any particles smaller than 200 nm (diameter in the original powder. Subsequently to the filtration, ICP-MS (Inductively Coupled Plasma Mass Spectrometry) was used to determine the silicon ion content of the solutions.
Table 2. Solubility test: number of samples per pH and time point.
| |
100 mg Si3N4-powder |
PBS without powder |
||||
|---|---|---|---|---|---|---|
| pH |
4.8 |
6.5 |
7.4 |
4.8 |
6.5 |
7.4 |
| Time (days) | ||||||
| 35 |
4 |
4 |
4 |
1 |
1 |
1 |
| 75 | 4 | 4 | 4 | 1 | 1 | 1 |
Blood plasma incubations
The adsorption experiments with blood plasma and antibodies were performed and measured in situ using a null ellipsometer. The experiment started with incubation in human blood plasma for 20 min and after measurement of adsorbed protein thicknesses the samples were incubated in three antibodies [of complement 3c (C3c), high molecular weight kininogen (HMWK) and fibrinogen (Fib)] for 30 min. Details on the experimental setup can be found elsewhere.40
Conclusions
Friction, wear and chemical properties of Si3N4 as a potential joint implant material have been studied. Pin-on-disc wear tests have been performed with Si3N4 and CoCr discs sliding against Si3N4 or Al2O3 balls in PBS and bovine serum solutions. Subsequently the surface appearance, wear volume and chemical compositions of the wear were analyzed. Furthermore, a preliminary study on the solubility of Si3N4-powder in PBS was analyzed. In addition, responses to immune complement activation and blood coagulation have been reported. The following main conclusions were drawn: (1) α-Si3N4-powder dissolves in PBS. Further studies are needed to confirm the solubility of other phases as well as the in vivo solubility of wear particles from the final material. (2) Antibody adsorbtion indicated that the Si3N4-surface has relatively low immune activation and tends to denature proteins to a low extent at the surface which might be an indication of an improved acceptance of the body. (3) Si3N4 sliding against Si3N4 showed low wear rate in both bovine serum and PBS. (4) Tribofilms were built-up on the Si3N4 surfaces in both PBS and in bovine serum which contributed to a decrease in friction. (5) The build-up of tribofilm and the type of tribofilm strongly affects the coefficient of friction. However, the material combination is of high importance for the total amount of wear. Si3N4 against Si3N4 was shown to be a material combination with a good performance for hip joints, possibly realized as a coating on CoCr.
Acknowledgments
The authors are grateful for the financial support from Strategic Materials Research Centre on Materials Science for Nanoscale Surface Engineering (MS2E) and Swedish Foundation for Strategic Research. Åsa Kassman Rudolphi and Frida Riddar are acknowledged for valuable discussions of the manuscript. Teresa Zardán Gómez de la Torre is acknowledged for the dynamic light scattering analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/20710
References
- 1.Garrett WE, Jr., Swiontkowski MF, Weinstein JN, Callaghan J, Rosier RN, Berry DJ, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88:660–7. doi: 10.2106/JBJS.E.01208. [DOI] [PubMed] [Google Scholar]
- 2.Sargeant A, Goswami T. Hip implants: Paper V. Physiological effects. Mater Des. 2006;27:287–307. doi: 10.1016/j.matdes.2004.10.028. [DOI] [Google Scholar]
- 3.Landgraeber S, von Knoch M, Löer F, Wegner A, Tsokos M, Hussmann B, et al. Extrinsic and intrinsic pathways of apoptosis in aseptic loosening after total hip replacement. Biomaterials. 2008;29:3444–50. doi: 10.1016/j.biomaterials.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Sargeant A, Goswami T. Hip implants - Paper VI - Ion concentrations. Mater Des. 2007;28:155–71. doi: 10.1016/j.matdes.2005.05.018. [DOI] [Google Scholar]
- 5.Xiong D, Ge S. Friction and wear properties of UHMWPE/Al2O3 ceramic under different lubricating conditions. Wear. 2001;250:242–5. doi: 10.1016/S0043-1648(01)00647-0. [DOI] [Google Scholar]
- 6.Derbyshire B, Fisher J, Dowson D, Hardaker C, Brummitt K. Comparative study of the wear of UHMWPE with zirconia ceramic and stainless steel femoral heads in artificial hip joints. Med Eng Phys. 1994;16:229–36. doi: 10.1016/1350-4533(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 7.Aspenberg P, Anttila A, Konttinen YT, Lappalainen R, Goodman SB, Nordsletten L, et al. Benign response to particles of diamond and SiC: bone chamber studies of new joint replacement coating materials in rabbits. Biomaterials. 1996;17:807–12. doi: 10.1016/0142-9612(96)81418-9. [DOI] [PubMed] [Google Scholar]
- 8.Bizot P, Sedel L. Alumina bearings in hip replacement: Theoretical and practical aspects. Oper Tech Orthop. 2001;11:263–9. doi: 10.1016/S1048-6666(01)80040-9. [DOI] [Google Scholar]
- 9.Willmann G, Früh HJ, Pfaff HG. Wear characteristics of sliding pairs of zirconia (Y-TZP) for hip endoprostheses. Biomaterials. 1996;17:2157–62. doi: 10.1016/0142-9612(96)00042-7. [DOI] [PubMed] [Google Scholar]
- 10.Bal BS, Khandkar A, Lakshminarayanan R, Clarke I, Hoffman AA, Rahaman MN. Fabrication and testing of silicon nitride bearings in total hip arthroplasty: winner of the 2007 “HAP” PAUL Award. J Arthroplasty. 2009;24:110–6. doi: 10.1016/j.arth.2008.01.300. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Titze M, Cappi B, Wirtz DC, Telle R, Fischer H. Improved mechanical long-term reliability of hip resurfacing prostheses by using silicon nitride. J Mater Sci Mater Med. 2010;21:3049–57. doi: 10.1007/s10856-010-4144-z. [DOI] [PubMed] [Google Scholar]
- 12.Cappi B, Neuss S, Salber J, Telle R, Knüchel R, Fischer H. Cytocompatibility of high strength non-oxide ceramics. J Biomed Mater Res A. 2010;93:67–76. doi: 10.1002/jbm.a.32527. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Sakka Y. Textrued silicon nitride: processing and anisotropic properties. Sci Technol Adv Mater. 2008;9:033001. doi: 10.1088/1468-6996/9/3/033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Kato K. Formation of tribochemical layer of ceramics sliding in water and its role for low friction. Wear. 2000;245:61–75. doi: 10.1016/S0043-1648(00)00466-X. [DOI] [Google Scholar]
- 15.Tomizawa H, Fischer TE. Friction and Wear of Silicon Nitride and Silicon Carbide in Water: Hydrodynamic Lubrication at Low Sliding Speed Obtained by Tribochemical Wear. ASLE Transactions. 1986;30:41–6. doi: 10.1080/05698198708981728. [DOI] [Google Scholar]
- 16.Neumann A, Unkel C, Werry C, Herborn CU, Maier HR, Ragoss C, et al. Prototype of a silicon nitride ceramic-based miniplate osteofixation system for the midface. Otolaryngol Head Neck Surg. 2006;134:923–30. doi: 10.1016/j.otohns.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Guedes e Silva CC, Higa OZ, Bressiani JC. Cytotoxic evaluation of silicon nitride-based ceramics. Mater Sci Eng C. 2004;24:643–6. doi: 10.1016/j.msec.2004.08.007. [DOI] [Google Scholar]
- 18.He Q, Shi J, Zhu M, Chen Y, Chen F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 2010;131:314–20. doi: 10.1016/j.micromeso.2010.01.009. [DOI] [Google Scholar]
- 19.Boanini E, Gazzano M, Bigi A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6:1882–94. doi: 10.1016/j.actbio.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Boshitskaya NV, Bartnitskaya TS, Makarenko GN, Lavrenkko VA, Danilenko NM, Tel'nikova NP. Theory, synthesis technology, properties of powders and fibers, Chemical stability of silicon nitride powders in biochemical media. Powder Metallurgy and Metal Ceramics. 1996;35:497–500. [Google Scholar]
- 21.Kusaka J, Takashima K, Yamane D, Ikeuchi K. Fundamental study for all-ceramic artificial hip joint. Wear. 1999;225-229:734–42. doi: 10.1016/S0043-1648(98)00386-X. [DOI] [Google Scholar]
- 22.Hodgson AWE, Kurz S, Virtanen S, Fervel V, Olsson COA, Mischler S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim Acta. 2004;49:2167–78. doi: 10.1016/j.electacta.2003.12.043. [DOI] [Google Scholar]
- 23.Vanea E, Simon V. XPS study of protein adsorption onto nanocrystalline aluminosilicate microparticles. Appl Surf Sci. 2011;257:2346–52. doi: 10.1016/j.apsusc.2010.09.101. [DOI] [Google Scholar]
- 24.Zhang Z, Yoo R, Wells M, Beebe TP, Jr., Biran R, Tresco P. Neurite outgrowth on well-characterized surfaces: preparation and characterization of chemically and spatially controlled fibronectin and RGD substrates with good bioactivity. Biomaterials. 2005;26:47–61. doi: 10.1016/j.biomaterials.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Aarnink WAM, Weishaupt A, van Silfhout A. Angle-resolved X-ray photoelectron spectroscopy (ARXPS) and a modified Levenberg-Marquardt fit procedure: a new combination for modeling thin layers. Appl Surf Sci. 1990;45:37–48. doi: 10.1016/0169-4332(90)90018-U. [DOI] [Google Scholar]
- 26.Ingo GM, Zacchetti N, della Sala D, Coluzza C. X-ray photoelectron spectroscopy investigation on the chemical structure of amorphous silicon nitride (a-SiN[sub x]) J Vac Sci Technol A. 1989;7:3048–55. doi: 10.1116/1.576314. [DOI] [Google Scholar]
- 27.Taylor JA, Lancaster GM, Ignatiev A, Rabalais JW. Interactions of ion beams with surfaces. Reactions of nitrogen with silicon and its oxides. J Chem Phys. 1978;68:1776–84. doi: 10.1063/1.435869. [DOI] [Google Scholar]
- 28.Taylor JA. Further examination of the Si KLL Auger line in silicon nitride thin films. Appl Surf Sci. 1981;7:168–84. doi: 10.1016/0378-5963(81)90068-4. [DOI] [Google Scholar]
- 29.Tengvall P, Askendal A. Ellipsometric in vitro studies on blood plasma and serum adsorption to zirconium. J Biomed Mater Res. 2001;57:285–90. doi: 10.1002/1097-4636(200111)57:2<285::AID-JBM1169>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Pourzal R, Theissmann R, Williams S, Gleising B, Fisher J, Fischer A. Subsurface changes of a MoM hip implant below different contact zones. J Mech Behav Biomed Mater. 2009;2:186–91. doi: 10.1016/j.jmbbm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Gomes JR, Oliveira FJ, Silva RF, Osendi MI, Miranzo P. Effect of [alpha]-/[beta] Si3N4-phase ratio and microstructure on the tribological behaviour up to 700°C. Wear. 2000;239:59–68. doi: 10.1016/S0043-1648(99)00367-1. [DOI] [Google Scholar]
- 32.Laarz E, Zhmud BV, Bergström L. Dissolution and Deagglomeration of Silicon Nitride in Aqueous Medium. J Am Ceram Soc. 2000;83:2394–400. doi: 10.1111/j.1151-2916.2000.tb01567.x. [DOI] [Google Scholar]
- 33.Wälivaara B, Askendal A, Lundström I, Tengvall P, Tengvall P. Blood protein interactions with titanium surfaces. J Biomater Sci Polym Ed. 1996;8:41–8. doi: 10.1163/156856297X00560. [DOI] [PubMed] [Google Scholar]
- 34.Wälivaara B, Askendal A, Elwing H, Lundström I, Tengvall P. Antisera binding onto metals immersed in human plasma in vitro. J Biomed Mater Res. 1992;26:1205–16. doi: 10.1002/jbm.820260910. [DOI] [PubMed] [Google Scholar]
- 35.Tsai W-B, Grunkemeier JM, Horbett TA. Human plasma fibrinogen adsorption and platelet adhesion to polystyrene. J Biomed Mater Res. 1999;44:130–9. doi: 10.1002/(SICI)1097-4636(199902)44:2<130::AID-JBM2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Czichos H, Becker S, Lexow J. International multilaboratory sliding wear tests with ceramics and steel. Wear. 1989;135:171–91. doi: 10.1016/0043-1648(89)90104-X. [DOI] [Google Scholar]
- 37.ASTM Standards. Standard test for Wear Testing of Polymeric Materials Used in Total Joint Prostheses. ASTM International 2003; F 732-00.
- 38.Oliver WC, Pharr GM. An improved technique for determing hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–83. doi: 10.1557/JMR.1992.1564. [DOI] [Google Scholar]
- 39.Konttinen YT, Takagi M, Mandelin J, Lassus J, Salo J, Ainola M, et al. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. J Bone Miner Res. 2001;16:1780–6. doi: 10.1359/jbmr.2001.16.10.1780. [DOI] [PubMed] [Google Scholar]
- 40.Berlind T, Tengvall P, Hultman L, Arwin H. Protein adsorption on thin films of carbon and carbon nitride monitored with in situ ellipsometry. Acta Biomater. 2011;7:1369–78. doi: 10.1016/j.actbio.2010.10.024. [DOI] [PubMed] [Google Scholar]