Abstract
Construction of biomaterials with the ability to guide cell function is a topic of high interest in biomaterial development. One approach is using components native to the ECM of the target tissue to generate in vitro a microenvironment that can also elicit specific responses in cells and tissues—an artificial ECM (aECM). The focus is on collagen as the basic material, which can be modified using a number of different glycoproteins, proteoglycans and glycosaminoglycans. Preparation, immobilization and the biochemical characteristics of such aECM are discussed, as well as the in vitro and in vivo response of cells and tissues, illustrating the potential of such matrices to direct cell fate.
Keywords: artificial extracellular matrix, bioactive coatings, collagen, immobilization, implants, surface functionalization
What is aECM?
Implant integration depends on the interaction of tissue cells with the implant surface. A prime goal of biomaterial development is consequently the development of a surface that can guide and direct these interactions.
One approach is based on the natural surroundings of the cells in vivo: the extracellular matrix (ECM). This physical network provides not only structure and support, but is also the biological information that plays an important role in guiding development, maintaining homeostasis and directing regeneration of cells in a tissue-specific manner. It consists of a large and very heterogeneous set of components that are assembled locally into an ordered, highly site-specific network: collagens, non-collageneous glycoproteins (GP), proteoglycans (PG) and glycosaminoglycans (GAG). Many of these ECM components confer cell adhesion and have been used to increase cell adhesion to biomaterial surfaces not optimally suited to it. Most adhesion receptors function as signaling molecules, and engagement of different receptors gives rise to a wide variety of intracellular signals that in turn influence proliferation, differentiation and apoptosis. Many structural components also associate with growth factors and cytokines, regulating their function: storing, activating or inactivating them, protecting them from degradation and generating gradients cells can follow.
An artificial ECM (aECM) tries to utilize these functionalities through reconstituting ECM components in vitro to construct a microenvironment that mimicks the ECMs in its ability to guide morphogenesis in tissue repair and engineering. Most ECM in vivo has the collagen fibril as a central building block, and consequently collagen, especially collagen type I, is most commonly used in constructing an aECM. But native ECM structure varies widely depending on the tissue and developmental stage, where collagen is modified in structure and function by glycoproteins, proteoglycans, and glycosaminoglycans. Through including some of these components in the aECM—depending on the target tissue and the purpose—aECM with different biochemical composition, fibrillar structure, and mechanical properties, as well as bioadhesive character, proteolytic susceptibility, and growth factor binding capacity can be constructed.1
Building aECM
Basis of aECM
The basis of aECM is in the majority of cases collagen, just as it is the basis of ECM in the body. Collagen-based aECM can be built using either suspensions of insoluble collagen fibers, or solutions of collagen monomers which are then allowed to form fibrils in vitro. Both can then be used in similar ways to coat scaffolds and implants. This review will focus on the use of fibrils generated in vitro from monomers, as this is based on the natural capacity of collagen to self-assemble, and thus bears a closer relation to the assembly and resultant structure of the fibrils in vivo especially if additional, non-collagenous components are used.
Theoretically all fibril-forming collagens (I, II, III, V, XI, XXIV and XXVII) could be used as described in the following if not otherwise indicated, but the overwhelming majority of the experiments were performed with collagen type I, as this is the primarily occurring collagen in the body and is easily extracted from tissues. There are some instances where recombinant collagen has been utilized,2 but the in vitro formation of fibrils from these monomers is often impaired as the post-translational processing is not comparable to that of tissue-derived collagens. Tissue-derived collagens, on the other hand, can be affected by the extraction method used. Collagen monomers are soluble in diluted acid, but most collagen in tissues is enzymatically crosslinked via the telopeptides. Using acid extraction thus intact collagen monomers can be attained, but only from the very small fraction of non-crosslinked collagen. Pepsin digestion breaks crosslinks by enzymatically degrading the non-helical telopeptides. This gives higher yields and also reduces the already low antigenicity of collagen further, but has consequences for the self-assembly of the monomers.
Formation of collagen fibrils
Allowing fibrils to form in vitro based on the natural self-assembly potential of collagen gives a certain amount of control over matrix properties by regulating composition and structure in analogy to the in vivo process. Such fibrils show the characteristical cross-striation pattern of in vivo fibrils,3 indicating a close resemblace. This may influence adherent cells, as the supramolecular organization of the collagen matrix is of importance in cellular reactions.4 By being present during fibril assembly and due to collagen-binding, non-collagenous components can be included in the collagen matrix by this method in a manner comparable to the in vivo process. This can also affect collagen organization into fibrils and thus structure and mechanical properties.
The self-association of collagen into fibrils is an entropy-driven process, but the assembly pattern is determined by the charge pattern on the monomer surface. Raising pH and temperature into the physiological range will induce monomer assembly. Fibril formation can be followed by the increase in turbidity. Nucleation, in which metastable nuclei consisting of pairs of 4D staggered molecules with a short N- and C-terminal overlap are formed, takes place in the initial lag phase where no changes in turbidity can be measured. Telopeptides are of importance in coordinating this step. It is followed by a rapid growth phase which is determined by longitudinal and lateral interactions of the monomers with the growing fibrils.5
In vitro fibrillogenesis has been studied for a considerable time. Most preparations of collagen fibrils are based on modifications of the method from Williams,6 who determined conditions under which the resultant fibril morphology was optimized with a clear D-periodic banding pattern (Figs. 1A and 2A). The process of fibril formation and the structure of the resultant fibrils are influenced by a large number of parameters: in vivo, these include the collagen type or types, the extent of procollagen processing, and the presence of non-collagenous components; in vitro, additional parameters to be considered are temperature as well as pH and buffer composition, which influence the electrostatic interactions of the monomers.7,8 The pH affects the formation of early subfibrils, with low pH possibly stabilizing intermediate states.9 A phosphate concentration of around 30 mM is necessary for producing well banded fibrils, but too much phosphate results fibrils with a changed morphology: increasingly DPS III and DPS IV forms occur (where microfibrils are packed antiparallel with different staggers), together with other, smaller filamentous aggregates.6,10 Ionic strength affects fibril size, probably by influencing superfibrillar bundling based on changes in electrostatic interactions.
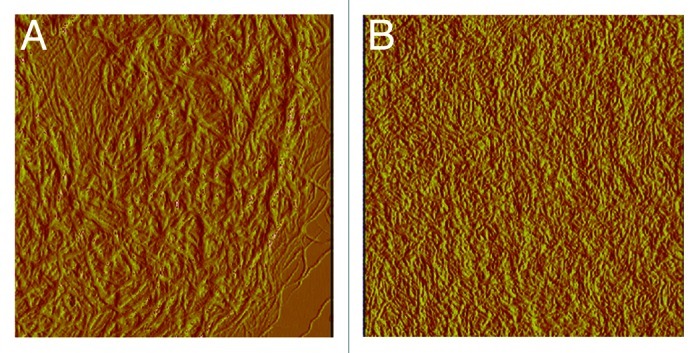
Figure 1. Type I collagen fibrils from rat tail prepared in vitro without (A) and with (B) chondroitin sulfate (10 mg/100 mg collagen). The CS added was chemically oversulfated to a sulfation degree of 3 sulfate groups per disaccharide. The resulting fibrils are finer than collagen fibrils without added CS. Images 5 × 5 µm, AFM tapping mode (the authors thank Dr. S. Heinemann for the AFM-investigation).
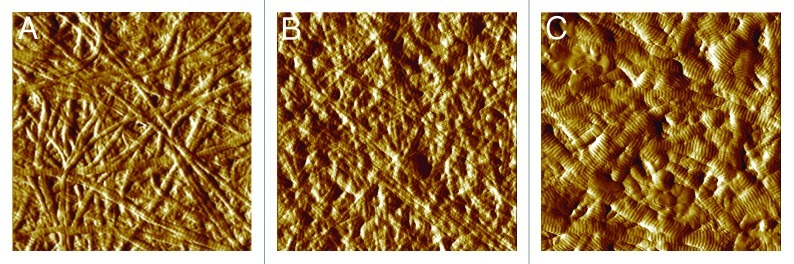
Figure 2. Type I collagen fibrils from bovine hide prepared in vitro. (A) Collagen without additives. Note that fibrils of bovine origin are generally thicker than those shown in Figure 1. (B) Collagen with 10 µg decorin per 100 μg collagen. Addition of decorin results in increasingly finer fibrils. (C) Collagen with 2 µg chondroitin sulfate A per 100 μg collagen. Lower amounts of CS with a lower sulfation degree (~0.9) result in fibrils that are larger than collagen without additives, compared with the higher amount and sulfation degree as shown in Figure 1. Images 5 x 5 µm, AFM tapping mode (the authors thank Dr. S. Heinemann for the AFM-measurement).
Another important factor is the collagen preparation. As mentioned above, acid-extracted collagen still has intact telopeptides, as opposed to pepsin-extracted collagen. These telopeptides play a role in initiating fibrillogenesis. For collagen with degraded telopeptides, self-assembly is slower, and the fibrils are slightly thicker and less stable. Selective removal of N-telopeptides results in so-called D-periodic symmetry (DPS) fibrils, in which molecules assemble in an antiparallel manner, while loss of the C-telopeptides results in relatively short cigar-shaped D-periodic fibrils.5
Generation of multi-component aECM
The modifications of collagen type I and II fibril architecture necessary in vivo are based on the addition of other ECM components. These can either be other collagens (the so-called minor collagens), glycoproteins or proteoglycans. The same principle can be applied during in vitro fibrillogenesis. As an example, collagen type I and III form heterotypic fibrils both in vivo11 and in vitro.12,13 In both cases this results in a decrease in fibril diameter of collagen I, probably due to a lessening of fibril bundling with collagen III inhibiting the interfibrillar interaction. Type V has a comparable effect where the N-terminal domains of type V extending outward through the gap zones.14 Non-collagenous ECM components with collagen-binding properties can also interact with the collagen monomers during fibrillogenesis, influencing matrix formation, composition, and morphology. Fibronectin (FN) for instance may accelerate fibril assembly, while the ionic strength of the buffer system determines the amount integrated into the fibrils.12,15 Also, collagen-laminin gels can seemingly be prepared over a wide range of different ratios (10:1 to 1:1).16,17 Another possibility to include such proteins in collagen matrices is through allowing them to bind to pre-formed fibrils. The interaction depends on the conditions under which the fibrils were formed: Collagen type I fibrils generated in buffers of high ionic strength bind about four times as much fibronectin as fibrils generated in buffers of low ionic strength, reflecting the importance of structural parameters.12 The collagen type also plays a role: collagen type III has a stronger interaction with FN than type I under all in vitro conditions, so that in heterotypic fibrils the amount of FN bound depends on the fraction of type III collagen in the fibrils. The effect may be due to several factors, such as the larger surface area of type III fibrils, changes in fibrillar architecture, or the different affinities of the collagen types for FN.12
Many ECM proteoglycans are strong regulators of fibrillar architecture; best known for this are the small leucine rich proteins (SLRPs), such as decorin, biglycan, fibromodulin, lumican, keratocan and osteoglycin. They consist of a protein core with one or more glycosaminoglycan (GAG) chains. In most cases they give rise to a delayed fibril assembly and a reduction in fibril diameter (Fig. 2A and B), but not always: lumican accelerates fibril formation, while biglycan does not affect fibril assembly. The effect is based on an interaction of the protein core with the collagen, though GAG variations can modulate it.18 The main role of the GAG chains is based on their extending outward from the bound core protein, thus inhibiting both the further association of monomers and the lateral fusion of fibrils.19 In vitro, PGs can only be included to a limited degree as they generally inhibit fibril formation. Decorin/collagen ratios of 1:10 already result in a strong decrease both in fibril diameter and the integration of collagen monomers into the fibrils.3 As with the glycoproteins, different collagen types have different affinities for PGs: collagen type II for instance binds more decorin and biglycan than type I and III.20
One of the most commonly used combinations for aECM is that of collagen with glycosaminoglycans. For this there is no true in vivo analogy, as GAGs, with the exception of hyaluronan (HA), are generally bound to a core protein, although there may be a role for sugars as demonstrated for deglycosilated proteoglycans.21 Despite this, the combination of collagen with GAG has some interesting properties.
Unlike the non-collagenous proteins, the interaction of GAGs with collagen is unspecific and based on the net negative charge of the sugars. Their effect on fibrillogenesis is heterogeneous: Heparin induces a dose-dependent increase in fibril diameter22 and results in cigar-shaped fibrils, while chondroitin sulfate (CS) (Fig. 2C) and heparan sulfate (HS) increase both mean fibril diameters and heterogeneity of diameter distribution.3 Collagen/CS gels also contain larger void spaces, and the viscous gel component (loss modulus) is reduced.23
Since the interaction between collagen and GAG is based on charge, the ionic strength is an important parameter in the formation of collagen/GAG aECM. Especially in buffers of low ionic strength, there is a very fast aggregation of collagen and sugars with significant consequences for fibril morphology (Fig. 2B), the degree of which depends on the individual collagen and GAG types,24 with an increase in sulfation of the GAG leading to a stronger interaction.25
To increase stability and reduce the speed of degradation in vitro, the collagen-based aECM can be crosslinked.26 Using EDC [1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride] is the most common method; the crosslinking efficiency depends on the molar ratio of EDC to GAG carboxylic groups.27 Other possibilities are (1) glutaraldehyde, though here there may be problems with cytotoxicity, (2) dehydrothermal crosslinking,28 where water content is reduced until interchain peptide bonds can form or (3) the use of transglutaminase, which forms ε(γ-glutamyl) lysine isopeptide bonds.29
Immobilizing aECM
In the biofunctionalization of surfaces with aECM the immobilization of the matrices is an important aspect. Basically two methods can be used, either through covalent crosslinking or through adsorption. Both have advantages and drawbacks: Covalent crosslinking results in a very stable coating, but the chemistry can be complicated and may result in changes that affect protein functions. Adsorptive immobilization on the other hand is a very simple process that can be conducted at physiological parameters, but the interaction is at least in parts reversible, and similar to crosslinking may lead to conformational changes of less stable proteins.
Covalent immobilization
There are multiple methods of covalent immobilization. In all cases functional groups of the protein are part of the link. These can be lysine residues, which readily form stable amine bonds with supports bearing active esters, or amine groups. These form imines with aldehydes on the material surface, which can then either be reduced to a stable secondary amine linkage, or react with epoxides. Cystein residues can interact with α-, β-unsaturated carbonyls like maleimides and form stable thiolether bonds, and carbodiimide can convert acidic residues into active esters which react with amine-bearing supports.
Drawbacks are the generally random orientation of the immobilized biomolecule and the fact that a sizeable number may be inactive. This can be due either to sterical aspects, to changes in protein stability (especially when carboxy- or amino-bearing residues are used), or in protein activity (as for cystein residues). To overcome these limitations, more complex, biologically mediated methods are being developed (the reader is referred to the review in ref. 30).
Collagen can be covalently immobilized—some commonly used methods are low temperature glow discharge and crosslinking with glutaraldehyde31 carbodiimide crosslinking with coupling agent aminopropyl triethoxysilane and N, N′-disulphosuccinimidyl suberate as linker,32 or deposition on reactive maleic anhydride copolymers that mediate covalent attachment33—with the general result of an improved biocompatibility. Especially in the case of collagen, though, there is the question of whether a covalent linkage is indeed necessary, or if the alternative method of adsorptive immobilization is not sufficient.
Adsorptive immobilization
Entropic processes lead to an aggregation of proteins at the interface between solid and liquid phases. The interactions are determined by protein size, charge, hydrophobicity and structure, by surface parameters such as roughness, hydrophilicity and charge, and by the solvent and other solute components present. The mechanisms responsible are just as varied and include hydrophobic, electrostatic and van der Waals interactions as well as structural changes in the molecule, which may result in entropy or enthalpy changes, though hydrophobic ones are usually considered to be the most important.34
Adsorption is often considered to be a reversibel process, but this is only partially true and depends on the free energy of the adsorption: the higher this is, the less reversible the process will be. During the initial minutes the interaction between protein and surface is fast and indeed reversible, but then the surface induces conformational changes in the protein that increase the interaction and can lead to complete protein denaturation after unlimited adsorption time.35 These conformational changes contribute to the free energy and thus reduce desorption, but as these changes are comparatively slow the desorption rate can be described as a function of the contact time. Molecules with very stable conformations can be expected to have only negligible changes with little contribution to the free energy, thus forming no strong adsorptive interactions. An exception are large, fibrillar proteins: due to their high surface to volume ratio they have a much higher number of potential binding sites than globular proteins of a comparable size, and they can interact with the surface via several segments. Although the adsorption free energy of each segment may be small as no structural changes take place to contribute, engagement of many segments will increase the total adsorption free energy until the molecule does not readily desorb anymore.36 In this case there is no time dependency and adsorption is irreversible even after short times.
For this reason collagen fibrils can be immobilized using this method with concentrations between 40 to 60 µg/cm2, and are still stable against competitive adsorption of serum proteins.37 Solvents of a higher ionic strength tend to reduce immobilization by about 20%, which indicates the importance of electrostatic interactions. The effect is somewhat larger on collagen type III, but generally there are no significant differences to collagen I, and this may only be caused by the higher surface area resulting from the finer fibrils. Heterotypic fibrils again take intermediate positions.12
Including non-collagenous components usually had no influence on the adsorbed amount, excepting very thin fibrils as seen for high decorin concentrations.3 The adsorption of all types of collagen-based aECM is thus determined by the collagen part.
Characterizing aECM
Biochemical characteristics
This includes the stability of the matrices against desorption, the release of individual components (especially for multi-component aECM that depend on physiological, non-covalent interactions), as well as the bioavailability and functionality of the components (a large issue for crosslinked matrices). What will also be taken into account is the ability of matrices to interact with soluble mediators like growth factors and cytokines, as many non-collagenous aECM components have affinities for such factors.
Desorption of components
There is comparatively little information about the desorption behavior of the components of glycoprotein and proteoglycan containing matrices. For fibronectin there seem to be two binding sites of different affinities on collagen fibrils. There is no significant release of FN bound to the high affinity site over five days under physiological conditions. Any fibronectin bound after saturation of these high-affinit sites probably interacts via a more unspecific mechanism, and is released within the first two hours.12 Decorin shows a strong interaction with collagen (dissociation constant 2.3 × 10−10 M),38 and detectable desorption was small and limited to the first hour.3 This depended on the originally used amount, indicating that here, too, unspecific binding may play a role. It seems fair to assume that the mechanisms would be comparable for all collagen binding proteins.
The immobilization of larger amounts of non-collagenous proteins thus requires a crosslinking step as for collagen-elastin matrices with a ratio of 1:1. Without crosslinking 60% of the elastin are released during the first 24 h, but EDC crosslinking reduces this to 2% within 7 d.39
If GAGs are included in collagen matrices, there is a significant desorption due to the unspecific, low affinity interactions if the matrices are not crosslinked.3
Amount of immobilized components
The amount of GAG that associates with collagen fibrils during fibrillogenesis depends on the GAG, the collagen type and the ionic strength of the buffer. As an example, collagen II binds more CS than collagen type I, though fibril diameter is reduced instead of increased. The affinity for different chondroitin sulfates is in the following order: CS C > CS B > CS A,40 and is in all likelyhood due to the degree, and possibly the pattern, of sulfation. Sulfated GAGs are also associated to a much higher degree than unsulfated ones,23 underlining the importance of this factor.
If the GAG was included in the matrix by crosslinking it to preformed fibrils, the immobilized amount was a function of the available amino groups as well as on concentration of GAG and EDC41 and in the range of 8% (w/w) for CS and 6% (w/w) for HS.26,42
Interactions with growth factors
An interesting possible function of aECM is the inclusion of non-collagenous components that have the ability to bind growth factors and cytokines. Pure collagen matrices could be shown to interact with TGF-β with a slower release and a stabilizing effect on growth factor (GF) activity as compared with titanium, indicating a protective effect.43 The release of other GFs (bFGF, HGF and PDGF-BB) is also retarded, most effectively for bFGF.44 Including other ECM components like the collagen-binding domain I of the proteoglycan perlecan can increase this ability. Collagen/perlecan fibrils bound significantly more BMP-2 than collagen alone and sustained a better release with 7% vs. 47% of the initially bound amount after 3 d.45
Instead of whole proteoglycans, it often makes sense to use the GAG chains. These are not only more easily accessible and have a smaller effect on immune response and inflammation, but it has also been shown that many GAGs can specifically interact with GFs. Including heparin in collagen matrices impacts the release of VEGF.46 These matrices appear to have three differently bound subpopulations of VEGF. Fifteen to twenty-five percent of the total amount are non-bound and responsible for the burst release, 10–20% may be non-specifically adsorbed to the collagen matrix, while the rest is bound to the heparin molecules, probably released with them and responsible for the enhanced angiogenic potential of heparinized collagen.47 The activity of BMP was also shown to be improved by sulfated GAGs, possibly through retaining the growth factor in a soluble state.48
Behavior in vitro
The effect of ECM on cells has been studied extensively; in this review the focus is on those studies that are related to bone cells and tissues.
The surfaces of biomaterials can influence cell behavior through several mechanisms. With artificial ECM, biochemical interactions can be expected to be the most important ones, but morphological and mechanical parameters may also play a role. If the ECM is used as a coating (as opposed to a three-dimensional scaffold) mechanical aspects will hold less importance. Morphological aspects, on the other hand, will have to be taken into account, as fibril morphology can be severely affected by adding non-collagenous components, as described above. It is not easy, though, to distinguish the influence this may have on cells from the influence of biochemical changes, as composition and texture of the aECM depend on each other. That the effect of morphology is probably of a much lesser degree than the effect of the biochemical changes can be deduced from the fact that morphological changes of the collagen fibril induced by variations of the assembly buffer system have no significant effect on cell response.3
aECM with a specific interaction profile
All proteins of the ECM such as the collagens, proteoglycans and glycoproteins are able to specifically interact with cell receptors, which is to a large part the basis of their function. Collagen had a long history as a cell adhesion substrate, both as a coating (of monomers or fibrils) or as a 3D scaffold. On titanium it promotes adhesion of osteoblastic cells from fetal rat calvaria, though not the further differentiation of the osteogenic precursor stages included in this population,37,49 and prevents glyoxal-induced apoptosis.50 By including other ECM components, with different cell interaction profiles in the collagen matrix, it should be possible to achieve a broader effect spectrum through changing mechanical and biochemical properties of the fibrils. An increase in collagen type III in the matrix adsorbed to titanium for instance induced an increased collagen synthesis and a decrease in alkaline phosphatase (ALP) activity and calcium phosphate deposition in rat calvarial osteoblasts, while on collagen type I the situation was reversed.15 This effect may be based on the role collagen type III plays in the early phase of intramembraneous fracture healing while collagen I only appears later and is associated with matrix mineralization.11 Collagens I, III and V all promote attachment and spreading of fibroblasts if used as coating on rigid substrates,51 on pliable substrates, though, the collagen V and I have different effects, with I promoting and V impairing fibroblast spreading.52
Adding glycoproteins or proteoglycans instead of other collagen types is essentially of higher interest, as these multifunctional, multidomain proteins can convey a large number of ECM cell interactions. It is not very common, though; apart from fibronectin, mainly laminin and, to some extent elastin have been utilized. As they bind to a cell adhesion receptor set different from collagen, including them changes the mechanism of cell adhesion to the collagen matrix. Depending on whether collagen or collagen/FN is used, different integrin receptors are engaged and activated.15 This activates other signaling pathways, which in turn lead to other cell responses.
For laminin (or laminin derived peptides) in combination with collagen, a positive effect on neuronal cell growth has been shown.53 In human mesenchymal stem cells (hMSC) it activated an ERK dependent pathway and induced an osteogenic phenotype.54 Other ECM proteins have also been shown to influence hMSC. The cells bind to them with different integrin sets, respectively, and with varying affinities (FN > collagen I > collagen IV > vitronectin > laminin-1). Osteogenic differentiation was highest on vitronectin and collagen I, while almost none occurred on fibronectin.55 Of the two, vitronectin induced enhanced focal adhesion formation, activated FAK (focal adhesion kinase) and paxilling, and reduced the activity of ERK (extracellular signal regulated kinase) and PI3K (phosphoinositide 3 kinase) pathways. Collagen, on the other hand, reduced focal adhesion formation, reduced FAK and paxillin activation, and increased ERK and PI3K activation.56 Collagen and vitronectin are recognized by different integrin subsets (α2β1 for collagen, αvβ3 and αvβ5 for VN), which indicates the importance these interactions may have in regulating cell behavior.57
There are only comparatively few studies that deal with collagen/proteoglycan matrices and their effect on cells. The main influence of PGs is, unlike that of the glycoproteins, not based on the direct interaction with cell adhesion receptors, but rather with other cell surface receptors, and on their ability to bind growth factors and cytokines. Decorin and biglycan in a collagen matrix for instance are able to influence cell adhesion, as they accelerate and enhance the formation of focal adhesions in osteoblastic cells.3,20 They also promote proliferation of human osteoblastic cells. Interestingly this effect is species specific, as rat osteoblastic cells did not respond in this way. Also, biglycan inhibited collagen synthesis only in rat osteoblasts and had no effect on human cells.58 This illustrates that not only ECM composition should be considered carefully, but also the intended target cells.
The effect of growth factors can also be modulated by the matrix, either enhancing or reducing cellular responses. While on collagen TGFβ reduced cell proliferation and raised collagen synthesis, on collagen/biglycan there was a stronger reduction in proliferation and on collagen/decorin a stronger increase in collagen synthesis.20 BMP-2 in combination with collagen and perlecan (the domain I fragment) supported chondrogenic differentiation in mouse embryonic mesenchymal cells.45
aECM with an unspecific interaction profile
aECM that interact with their surroundings based on more unspecific mechanisms can also be created. A very common approach is based on including GAGs as components that bear a large negative charge, which determines their binding behavior. Tissue applications include skin, peripheral nerve, muscle, cartilage and bone. The effects of the matrices appear to be on cytoskeletal organization, protein expression and differentiation.
In application for bone, CS is often chosen as the GAG moiety as it is synthesized by both chondrocytes and bone cells. In bone CS can speed up the mineralization process and consequently bone repair, while in cartilage it is known to stimulate PG production, inhibit cartilage cytokine production and to induce apoptosis of articular chondrocytes. Like all GAGs it is anti-inflammatory and it inhibits extracellular proteases involved in the metabolism of connective tissues.59
Similar to proteoglycans, CS in collagen matrices promoted the formation of focal adhesions, probably due to the comparatively high density of negatively charged sulfate groups of the GAG. Negative charges have been shown to improve early cell adhesion.60,61 The mechanism may be based on an improved binding of Ca2+, which is required for the formation of focal adhesions.62 Charge may not be the only regulating feature, though, as there is also a dependence on CS type: CS-A and -B but not -C stimulated focal adhesion formation if combined with collagen II.40
For most cells, e.g., chondrocytes and endothelial cells, collagen/GAG matrices increased proliferation,63 an effect that can be potentiated by adding VEGF.41,46
In populations of osteoblastic cells derived from fetal rat calvaria, the interaction with glycosaminoglycans appears to promote the further osteoblastic differentiation3 even more than collagen without GAG.64 A comparable effect can be seen for hMSCs where expression of osteoblastic markers and calcium phosphate-deposition is increased even without the presence of differentiation additives.65,66
aECM that interact with growth factors
GAG containing matrices may also be able to interact with a number of soluble factors that play a role in osteogenic differentiation, as many of them require GAG side chains to facilitate their interaction to their cell surface receptors.67 If these chains are also present in the matrix, they can compete with the cell surface GAGs for GF binding, modulating their effects. These effects are dependent upon the GAG in the matrix. Heparin stimulated only mineralization and ALP activity, while CS-E additionally affected collagen deposition. Anti-BMP antibodies significantly reduced CS-E induced mineralization, which indicates a close interaction of BMP and the CS.68 Oversulfated CS can bind even more BMP-4 than the normally occurring types,69 stimulating osteoblastic differentiation in MC3T3 cells.68
If hyaluronic acid is the GAG included in the matrix, responses tend to be somewhat different. While small HA amounts (2.5% wt) promoted cell adhesion and growth, larger ones (over 5% wt) inhibited it.70 They also decreased the migration of hematopoetic stem cells.33 Chondogenesis in MSC was supported, but added TGF-β had no further effect,71 probably because no interaction as with the sulfated GAGs is possible.
Behavior in vivo
Many experiments have been conducted using collagen, either as a scaffold or as a coating for implant materials. As with the studies on cell response, the focus here will be on these studies that utilized collagen and other ECM molecules as implant coatings in bone. A very common approach for bone relevant materials is the use of mineral phases, but this moves into the specific subtopic of biomineralisation and will, despite their indisputabel relevance for bone applications, not be considered here.
One caveat concerning the experiments discussed here has to be mentioned: Complex aECM consisting of more than one component have only very seldomly been used for in vivo experiments. For this reason many of the results presented below deal with single component coatings only.
aECM with a specific interaction profile
Used most often by far is collagen type I. Other collagen types such as III have shown effects on osteoblastic cells, and in a goat model collagen type III appeared to be more effective in less dense bone after 5 and 12 weeks.72 After 6 months (mandible of minipigs), though, no differences could be seen between I and III anymore; both coatings had given rise to significantly higher bone contact compared with the uncoated, sandblasted control.73
Another common application of collagen is the use as a carrier for growth factors. GFs are potent regulators of tissue repair, but still have to be used in very high amounts to achieve the desired effects. The amounts that can be adsorptively immobilized to collagen coatings are much smaller than this, but despite promising cell culture results often have no significant effect in vivo.74 Including growth factor binding ECM proteins in the collagen matrix may be of use in this respect.
Glycoprotein and proteoglycans have only very rarely been used in animal experiments. Between collagen and collagen/decorin matrices adsorbed on titanium and implanted in the mandible of minipigs no significant differences could be detected.75 Experiments in rats, on the other hand, indicated that different ECM components can elicit different tissue responses. Coated onto porous ePTFE discs and implanted into the adipose tissue, fibronectin gave rise to an extensive inflammatory response with limited angiogenesis and neovascularisation, collagen IV to a significant peri-implant angiogenic response but little neovascularisation, and laminin-1 to both peri-implant angiogenesis and a coordinate neovascularization of the porous interstices of the biomaterial.76 The mechanism of the response to FN seems to be based on stimulation of the cytokine response which increases monocyte/macrophage recruitment and differentiation into giant cells. Collagen IV, on the other hand, functions as an angiostatic factor. It supports blood vessel maturation in the peri-implant region, but not endothelial cell migration and invascularisation. Laminin, lastly, seems to have the widest effect, inducing both angiogenic factors and supporting early as well as late angiogenic events.76
Structural aspects—that is not only which components are included in a coating, but also the way they are presented to the cells—may also be of importance: collagen/solubilized elastin scaffolds induce angiogenesis and show no calcification in rats, as opposed to collagen/fibrillar elastin.39
aECM with an unspecific interaction profile
For collagen/ glycosaminoglycan matrices there is again a larger body of in vivo data. These matrices are usually crosslinked to limit the otherwise inevitable desorption,26 but independent of this, their effect on bone tissue appears to be in the early stages of healing.
One of the main effects of GAG seems to be on inflammation and foreign body response. If either CS or HS are included in a collagen matrix, there is only a transient inflammatory response and a reduced foreign body reaction in rats,26 and macrophage activity was reduced significantly.77 GAGs also may play a role in inducing and promoting angiogenesis: collagen with heparin or HS showed improved angiogenesis over collagen alone.26,41 The vascularisation was improved only in the periphery and probably transiently,42 but adding VEGF could further increased the angiogenic potential.41 With bFGF the scaffolds were vascularized through the whole matrix and remained so over the entire implantation period of 10 weeks, considerably promoting the generation of new tissue.42 Interestingly, bFGF in combination with collagen alone had similar effects (e.g., transient and peripheral vascularization) to the collagen/HS matrix without growth factor. This seems to indicate that HS acts synergistically with bFGF.
Including GAGs also led to positive results in the osseous integration of implants, although this is probably due to a different mechanism. Collagen coatings with CS on titanium give a higher bone volume and bone implant contact than collagen alone in the minipig mandible,78 with the main effect taking place in the early healing phase.75 There may be qualitative differences, too, compared with pure collagen coatings, as the extraction torque of collagen/CS implants in the sheep tibia was higher than for collagen and resembled that of hydroxyl apatite coatings, despite the fact that after two months the differences in bone apposition were no longer significant.79
The improved bone apposition for collagen and collagen/CS coatings may be due to the quicker appearance of relevant cell types compared with uncoated, grid blasted implants. Also, osteoblast activity was higher on both coatings.77 This indicates an increase in bone remodelling in the early stages of healing, which leads to an improved bone implant contact after four weeks: 89% for collagen/CS, 76% for collagen and 62% for uncoated implants.80
Surface topography may also play a not insignificant role: In the foxhound mandible, for instance, the bone implant contact of both machined and acid-etched titanium samples was improved through a collagen/CS coating, but the effect was much more obvious for the machined surfaces.81
aECM that interact with growth factors
The ability of GAGs to bind growth factors is another interesting aspect. Both BMP-2 and BMP-4 are known to induce bone formation when released from a three dimensional collagen carrier, but unphysiologically high amounts (often in the µg range) have to be used. Collagen/heparin matrices have the ability to enhace the BMP effect on osteoblastic differentiation, probably through protecting the growth factors from degradation, and possibly by inhibiting BMP antagonists.82
With two-dimensional coatings the situation is somewhat different, as only much lower amounts can be immobilized due to the reduced surface area. As a consequence either no BMP effect could be seen,81 or, surprisingly, even a detrimental one. Bone implant contact in the minipig mandible was 40% for collagen/CS, but if a small amount of BMP-4 was included it was reduced to 27%.83,84 The reason for this is unclear, but is serves to illustrate the fact that much still needs to be uncovered to fully understand how the ECM and soluble factors interact with each other as well as with cells and proteins of the host tissue in the process of healing.
Outlook
Up to now, only a few of the components that make up the ECM in vivo have been utilized in the construction of aECM, which is often due to difficulties in attaining them in sufficient quantities. So far not used components or combinations of components thus still offer much potential in constructing defined microenvironments. Other promising approaches are the incorporation of engineered proteins and protein fragments, or GAGs with modified sulfation patterns. Both could enhance specific functionalities over the naturally occurring levels. In GAGs the sulfation impacts the interaction with growth factors,69 so that modified GAGs might be used either in GF carriers to immobilize higher amounts of growth factor, or can perhaps even be sufficiently engineered to exploit their ability to act synergistically with soluble factors.
The effect of mechanical cues on cells may also depend more on matrix composition than commonly assumed, as the adhesion receptors, especially the integrins, are probably involved in the whole plethora of mechanotransduction phenomenons.85
A better understanding of the complex mechanism behind the observed effects of the extracellular matrix will be instrumental for significant progress in this field, and the developments in many areas of relevant research such as molecular medicine, cell-matrix interactions or glycomics, to name only a few, hold much promise for the future.
Glossary
Abbreviations:
- aECM
artificial extracellular matrix
- ECM
extracellular matrix
- GP
glycoprotein
- PG
proteoglycan
- GAG
glycosaminoglycan
- DPS
D-periodic symmetry
- SLRPs
small leucine rich proteins
- FN
fibronectin
- HA
hyaluronan
- CS
chondroitin sulfate
- HS
heparan sulfate
- EDC
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
- TGF-β
transforming growth factor–β
- GF
growth factor
- bFGF
basic fibroblast growth factor
- HGF
hepatocyte growth factor
- PDGF-BB
patlelet deribed growth factor-BB
- BMP
bone morphogenetic factor
- VEGF
vascular endothelial growth factor
- ALP
alkaline phosphatase
- hMSC
human mesenchymal stem cells
- ERK
extracellular signal regulated kinase
- FAK
focal adhesion kinase
- PI3K
phosphoinositide 3 kinase
- VN
vitronectin
- ePTFE
expanded polytetrafluoroethylene
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/20921
References
- 1.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551–8. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, et al. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18:103–19. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bierbaum S, Douglas T, Hanke T, Scharnweber D, Tippelt S, Monsees TK, et al. Collageneous matrix coatings on titanium implants modified with decorin and chondroitin sulfate: characterization and influence on osteoblastic cells. J Biomed Mater Res A. 2006;77:551–62. doi: 10.1002/jbm.a.30572. [DOI] [PubMed] [Google Scholar]
- 4.Mercier I, Lechaire JP, Desmouliere A, Gaill F, Aumailley M. Interactions of human skin fibroblasts with monomeric or fibrillar collagens induce different organization of the cytoskeleton. Exp Cell Res. 1996;225:245–56. doi: 10.1006/excr.1996.0174. [DOI] [PubMed] [Google Scholar]
- 5.Hulmes DJS. Collagen Diversity, Synthesis and Assembly. New York: Springer Science+Business Media, 2008. [Google Scholar]
- 6.Williams BR, Gelman RA, Poppke DC, Piez KA. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem. 1978;253:6578–85. [PubMed] [Google Scholar]
- 7.Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54:222–34. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Freudenberg U, Behrens SH, Welzel PB, Müller M, Grimmer M, Salchert K, et al. Electrostatic interactions modulate the conformation of collagen I. Biophys J. 2007;92:2108–19. doi: 10.1529/biophysj.106.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris JR, Reiber A. Influence of saline and pH on collagen type I fibrillogenesis in vitro: fibril polymorphism and colloidal gold labelling. Micron. 2007;38:513–21. doi: 10.1016/j.micron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Mertz EL, Leikin S. Interactions of inorganic phosphate and sulfate anions with collagen. Biochemistry. 2004;43:14901–12. doi: 10.1021/bi048788b. [DOI] [PubMed] [Google Scholar]
- 11.Kurdy NM, Bowles S, Marsh DR, Davies A, France M. Serology of collagen types I and III in normal healing of tibial shaft fractures. J Orthop Trauma. 1998;12:122–6. doi: 10.1097/00005131-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bierbaum S, Beutner R, Hanke T, Scharnweber D, Hempel U, Worch H. Modification of Ti6Al4V surfaces using collagen I, III, and fibronectin. I. Biochemical and morphological characteristics of the adsorbed matrix. J Biomed Mater Res A. 2003;67:421–30. doi: 10.1002/jbm.a.10080. [DOI] [PubMed] [Google Scholar]
- 13.Stuart K, Panitch A. Characterization of gels composed of blends of collagen I, collagen III, and chondroitin sulfate. Biomacromolecules. 2009;10:25–31. doi: 10.1021/bm800888u. [DOI] [PubMed] [Google Scholar]
- 14.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–37. doi: 10.1016/S0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 15.Bierbaum S, Hempel U, Geissler U, Hanke T, Scharnweber D, Wenzel KW, et al. Modification of Ti6AL4V surfaces using collagen I, III, and fibronectin. II. Influence on osteoblast responses. J Biomed Mater Res A. 2003;67:431–8. doi: 10.1002/jbm.a.10084. [DOI] [PubMed] [Google Scholar]
- 16.Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J Tissue Eng Regen Med. 2009;3:208–17. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- 17.Deister C, Aljabari S, Schmidt CE. Effects of collagen 1, fibronectin, laminin and hyaluronic acid concentration in multi-component gels on neurite extension. J Biomater Sci Polym Ed. 2007;18:983–97. doi: 10.1163/156856207781494377. [DOI] [PubMed] [Google Scholar]
- 18.Milan AM, Sugars RV, Embery G, Waddington RJ. Modulation of collagen fibrillogenesis by dentinal proteoglycans. Calcif Tissue Int. 2005;76:127–35. doi: 10.1007/s00223-004-0033-0. [DOI] [PubMed] [Google Scholar]
- 19.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 1996;271:31767–70. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 20.Douglas T, Hempel U, Mietrach C, Heinemann S, Scharnweber D, Worch H. Fibrils of different collagen types containing immobilised proteoglycans (PGs) as coatings: characterisation and influence on osteoblast behaviour. Biomol Eng. 2007;24:455–8. doi: 10.1016/j.bioeng.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Waddington RJ, Roberts HC, Sugars RV, Schönherr E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cell Mater. 2003;6:12–21, discussion 21. doi: 10.22203/ecm.v006a02. [discussion] [DOI] [PubMed] [Google Scholar]
- 22.McPherson JM, Sawamura SJ, Condell RA, Rhee W, Wallace DG. The effects of heparin on the physicochemical properties of reconstituted collagen. Coll Relat Res. 1988;8:65–82. doi: 10.1016/S0174-173X(88)80036-0. [DOI] [PubMed] [Google Scholar]
- 23.Stuart K, Panitch A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers. 2008;89:841–51. doi: 10.1002/bip.21024. [DOI] [PubMed] [Google Scholar]
- 24.Smith GN, Jr., Brandt KD. Interaction of cartilage collagens with heparin. Coll Relat Res. 1987;7:315–21. doi: 10.1016/S0174-173X(87)80024-9. [DOI] [PubMed] [Google Scholar]
- 25.Lilja S, Barrach HJ. Normally sulphated and highly sulphated glycosaminoglycans (GAG) affecting fibrillogenesis of type I and type II collagen in vitro. Exp Pathol. 1983;23:173–81. doi: 10.1016/S0232-1513(83)80055-3. [DOI] [PubMed] [Google Scholar]
- 26.Pieper JS, van Wachem PB, Brouwer LA, Hafmans T, Veerkamp JH, van Kuppevelt TH, van Luyn MJA Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 2000;21:1689–99. doi: 10.1016/S0142-9612(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 27.Pieper JS, Hafmans T, Veerkamp JH, van Kuppevelt TH. Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials. 2000;21:581–93. doi: 10.1016/S0142-9612(99)00222-7. [DOI] [PubMed] [Google Scholar]
- 28.Tierney CM, Haugh MG, Liedl J, Mulcahy F, Hayes B, O’Brien FJ. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J Mech Behav Biomed Mater. 2009;2:202–9. doi: 10.1016/j.jmbbm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Damodaran G, Collighan R, Griffin M, Navsaria H, Pandit A. Tailored laminin-332 alpha3 sequence is tethered through an enzymatic linker to a collagen scaffold to promote cellular adhesion. Acta Biomater. 2009;5:2441–50. doi: 10.1016/j.actbio.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Wong LS, Khan F, Micklefield J. Selective covalent protein immobilization: strategies and applications. Chem Rev. 2009;109:4025–53. doi: 10.1021/cr8004668. [DOI] [PubMed] [Google Scholar]
- 31.Chang WJ, Ou KL, Lee SY, Chen JY, Abiko Y, Lin CT, et al. Type I collagen grafting on titanium surfaces using low-temperature glow discharge. Dent Mater J. 2008;27:340–6. doi: 10.4012/dmj.27.340. [DOI] [PubMed] [Google Scholar]
- 32.Müller R, Abke J, Schnell E, Macionczyk F, Gbureck U, Mehrl R, et al. Surface engineering of stainless steel materials by covalent collagen immobilization to improve implant biocompatibility. Biomaterials. 2005;26:6962–72. doi: 10.1016/j.biomaterials.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Salchert K, Oswald J, Streller U, Grimmer M, Herold N, Werner C. Fibrillar collagen assembled in the presence of glycosaminoglycans to constitute bioartificial stem cell niches in vitro. J Mater Sci Mater Med. 2005;16:581–5. doi: 10.1007/s10856-005-0535-y. [DOI] [PubMed] [Google Scholar]
- 34.Norde W, Lyklema J. Why proteins prefer interfaces. J Biomater Sci Polym Ed. 1991;2:183–202. doi: 10.1080/09205063.1991.9756659. [DOI] [PubMed] [Google Scholar]
- 35.Soderquist ME, Gershman H, Anderson JM, Walton AG. Adhesion of cells to random copolypeptide films. J Biomed Mater Res. 1979;13:865–86. doi: 10.1002/jbm.820130606. [DOI] [PubMed] [Google Scholar]
- 36.Norde W. Adsorption of proteins from solution at the solid-liquid interface. Adv Colloid Interface Sci. 1986;25:267–340. doi: 10.1016/0001-8686(86)80012-4. [DOI] [PubMed] [Google Scholar]
- 37.Geissler U, Hempel U, Wolf C, Scharnweber D, Worch H, Wenzel K. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J Biomed Mater Res. 2000;51:752–60. doi: 10.1002/1097-4636(20000915)51:4<752::AID-JBM25>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Nareyeck G, Seidler DG, Troyer D, Rauterberg J, Kresse H, Schönherr E. Differential interactions of decorin and decorin mutants with type I and type VI collagens. Eur J Biochem. 2004;271:3389–98. doi: 10.1111/j.1432-1033.2004.04273.x. [DOI] [PubMed] [Google Scholar]
- 39.Daamen WF, Nillesen ST, Wismans RG, Reinhardt DP, Hafmans T, Veerkamp JH, et al. A biomaterial composed of collagen and solubilized elastin enhances angiogenesis and elastic fiber formation without calcification. Tissue Eng Part A. 2008;14:349–60. doi: 10.1089/tea.2007.0076. [DOI] [PubMed] [Google Scholar]
- 40.Douglas T, Heinemann S, Mietrach C, Hempel U, Bierbaum S, Scharnweber D, et al. Interactions of collagen types I and II with chondroitin sulfates A-C and their effect on osteoblast adhesion. Biomacromolecules. 2007;8:1085–92. doi: 10.1021/bm0609644. [DOI] [PubMed] [Google Scholar]
- 41.Steffens GC, Yao C, Prével P, Markowicz M, Schenck P, Noah EM, et al. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng. 2004;10:1502–9. doi: 10.1089/ten.2004.10.1502. [DOI] [PubMed] [Google Scholar]
- 42.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, Veerkamp JH, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–94. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 43.Fischer U, Hempel U, Becker D, Bierbaum S, Scharnweber D, Worch H, et al. Transforming growth factor beta1 immobilized adsorptively on Ti6Al4V and collagen type I coated Ti6Al4V maintains its biological activity. Biomaterials. 2003;24:2631–41. doi: 10.1016/S0142-9612(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 44.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, et al. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–20. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Yang W, Gomes RR, Brown AJ, Burdett AR, Alicknavitch M, Farach-Carson MC, et al. Chondrogenic differentiation on perlecan domain I, collagen II, and bone morphogenetic protein-2-based matrices. Tissue Eng. 2006;12:2009–24. doi: 10.1089/ten.2006.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf-Brandstetter C, Lode A, Hanke T, Scharnweber D, Worch H. Influence of modified extracellular matrices on TI6AL4V implants on binding and release of VEGF. J Biomed Mater Res A. 2006;79:882–94. doi: 10.1002/jbm.a.30826. [DOI] [PubMed] [Google Scholar]
- 47.Yao C, Roderfeld M, Rath T, Roeb E, Bernhagen J, Steffens G. The impact of proteinase-induced matrix degradation on the release of VEGF from heparinized collagen matrices. Biomaterials. 2006;27:1608–16. doi: 10.1016/j.biomaterials.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 48.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, et al. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–35. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 49.Becker D, Geissler U, Hempel U, Bierbaum S, Scharnweber D, Worch H, et al. Proliferation and differentiation of rat calvarial osteoblasts on type I collagen-coated titanium alloy. J Biomed Mater Res. 2002;59:516–27. doi: 10.1002/jbm.1265. [DOI] [PubMed] [Google Scholar]
- 50.Tippelt S, Ma C, Witt M, Bierbaum S, Funk RH. Collagen type I prevents glyoxal-induced apoptosis in osteoblastic cells cultured on titanium alloy. Cells Tissues Organs. 2004;177:29–36. doi: 10.1159/000078425. [DOI] [PubMed] [Google Scholar]
- 51.Kerkvliet EH, Jansen IC, Schoenmaker T, Beertsen W, Everts V. Collagen type I, III and V differently modulate synthesis and activation of matrix metalloproteinases by cultured rabbit periosteal fibroblasts. Matrix Biol. 2003;22:217–27. doi: 10.1016/S0945-053X(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 52.Breuls RG, Klumpers DD, Everts V, Smit TH. Collagen type V modulates fibroblast behavior dependent on substrate stiffness. Biochem Biophys Res Commun. 2009;380:425–9. doi: 10.1016/j.bbrc.2009.01.110. [DOI] [PubMed] [Google Scholar]
- 53.Yao L, Damodaran G, Nikolskaya N, Gorman AM, Windebank A, Pandit A. The effect of laminin peptide gradient in enzymatically cross-linked collagen scaffolds on neurite growth. J Biomed Mater Res A. 2009;92:484–92. doi: 10.1002/jbm.a.32359. [DOI] [PubMed] [Google Scholar]
- 54.Salasznyk RM, Klees RF, Boskey A, Plopper GE. Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5. J Cell Biochem. 2007;100:499–514. doi: 10.1002/jcb.21074. [DOI] [PubMed] [Google Scholar]
- 55.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kundu AK, Putnam AJ. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:347–57. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 57.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–81. doi: 10.1016/S8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 58.Douglas T, Heinemann S, Hempel U, Mietrach C, Knieb C, Bierbaum S, et al. Characterization of collagen II fibrils containing biglycan and their effect as a coating on osteoblast adhesion and proliferation. J Mater Sci Mater Med. 2008;19:1653–60. doi: 10.1007/s10856-007-3250-z. [DOI] [PubMed] [Google Scholar]
- 59.Bali JP, Cousse H, Neuzil E. Biochemical basis of the pharmacologic action of chondroitin sulfates on the osteoarticular system. Semin Arthritis Rheum. 2001;31:58–68. doi: 10.1053/sarh.2000.24874. [DOI] [PubMed] [Google Scholar]
- 60.Erskine L, McCaig CD. Integrated interactions between chondroitin sulphate proteoglycans and weak dc electric fields regulate nerve growth cone guidance in vitro. J Cell Sci. 1997;110:1957–65. doi: 10.1242/jcs.110.16.1957. [DOI] [PubMed] [Google Scholar]
- 61.Ohgaki M, Kizuki T, Katsura M, Yamashita K. Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res. 2001;57:366–73. doi: 10.1002/1097-4636(20011205)57:3<366::AID-JBM1179>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 62.D’Souza SE, Haas TA, Piotrowicz RS, Byers-Ward V, McGrath DE, Soule HR, et al. Ligand and cation binding are dual functions of a discrete segment of the integrin beta 3 subunit: cation displacement is involved in ligand binding. Cell. 1994;79:659–67. doi: 10.1016/0092-8674(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 63.Cao H, Xu SY. EDC/NHS-crosslinked type II collagen-chondroitin sulfate scaffold: characterization and in vitro evaluation. J Mater Sci Mater Med. 2008;19:567–75. doi: 10.1007/s10856-007-3281-5. [DOI] [PubMed] [Google Scholar]
- 64.Roehlecke C, Witt M, Kasper M, Schulze E, Wolf C, Hofer A, et al. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs. 2001;168:178–87. doi: 10.1159/000047833. [DOI] [PubMed] [Google Scholar]
- 65.Wollenweber M, Domaschke H, Hanke T, Boxberger S, Schmack G, Gliesche K, et al. Mimicked bioartificial matrix containing chondroitin sulphate on a textile scaffold of poly(3-hydroxybutyrate) alters the differentiation of adult human mesenchymal stem cells. Tissue Eng. 2006;12:345–59. doi: 10.1089/ten.2006.12.345. [DOI] [PubMed] [Google Scholar]
- 66.Rentsch B, Hofmann A, Breier A, Rentsch C, Scharnweber D. Embroidered and surface modified polycaprolactone-co-lactide scaffolds as bone substitute: in vitro characterization. Ann Biomed Eng. 2009;37:2118–28. doi: 10.1007/s10439-009-9731-0. [DOI] [PubMed] [Google Scholar]
- 67.Manton KJ, Leong DF, Cool SM, Nurcombe V. Disruption of heparan and chondroitin sulfate signaling enhances mesenchymal stem cell-derived osteogenic differentiation via bone morphogenetic protein signaling pathways. Stem Cells. 2007;25:2845–54. doi: 10.1634/stemcells.2007-0065. [DOI] [PubMed] [Google Scholar]
- 68.Miyazaki T, Miyauchi S, Tawada A, Anada T, Matsuzaka S, Suzuki O. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. J Cell Physiol. 2008;217:769–77. doi: 10.1002/jcp.21557. [DOI] [PubMed] [Google Scholar]
- 69.Hintze V, Moeller S, Schnabelrauch M, Bierbaum S, Viola M, Worch H, et al. Modifications of hyaluronan influence the interaction with human bone morphogenetic protein-4 (hBMP-4) Biomacromolecules. 2009;10:3290–7. doi: 10.1021/bm9008827. [DOI] [PubMed] [Google Scholar]
- 70.Koller J, Bakos D, Sadlonová II. Biocompatibility Studies of a New Biosynthetic Dermal Substitute Based on Collagen/Hyaluronan Conjugate. Cell Tissue Bank. 2000;1:75–80. doi: 10.1023/A:1010181925236. [DOI] [PubMed] [Google Scholar]
- 71.Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431–8. doi: 10.1016/j.tice.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Bernhardt R, van den Dolder J, Bierbaum S, Beutner R, Scharnweber D, Jansen J, et al. Osteoconductive modifications of Ti-implants in a goat defect model: characterization of bone growth with SR muCT and histology. Biomaterials. 2005;26:3009–19. doi: 10.1016/j.biomaterials.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Stadlinger B, Pilling E, Huhle M, Khavkin E, Bierbaum S, Scharnweber D, et al. Suitability of differently designed matrix-based implant surface coatings: an animal study on bone formation. J Biomed Mater Res B Appl Biomater. 2008;87:516–24. doi: 10.1002/jbm.b.31138. [DOI] [PubMed] [Google Scholar]
- 74.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Roessler S, Sewing A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin Oral Implants Res. 2005;16:563–9. doi: 10.1111/j.1600-0501.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 75.Stadlinger B, Bierbaum S, Grimmer S, Schulz MC, Kuhlisch E, Scharnweber D, et al. Increased bone formation around coated implants. J Clin Periodontol. 2009;36:698–704. doi: 10.1111/j.1600-051X.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- 76.Williams SK, Kleinert LB, Hagen KM, Clapper DL. Covalent modification of porous implants using extracellular matrix proteins to accelerate neovascularization. J Biomed Mater Res A. 2006;78:59–65. doi: 10.1002/jbm.a.30659. [DOI] [PubMed] [Google Scholar]
- 77.Rammelt S, Heck C, Bernhardt R, Bierbaum S, Scharnweber D, Goebbels J, et al. In vivo effects of coating loaded and unloaded Ti implants with collagen, chondroitin sulfate, and hydroxyapatite in the sheep tibia. J Orthop Res. 2007;25:1052–61. doi: 10.1002/jor.20403. [DOI] [PubMed] [Google Scholar]
- 78.Stadlinger B, Pilling E, Mai R, Bierbaum S, Berhardt R, Scharnweber D, et al. Effect of biological implant surface coatings on bone formation, applying collagen, proteoglycans, glycosaminoglycans and growth factors. J Mater Sci Mater Med. 2008;19:1043–9. doi: 10.1007/s10856-007-3077-7. [DOI] [PubMed] [Google Scholar]
- 79.Ferguson SJ, Langhoff JD, Voelter K, von Rechenberg B, Scharnweber D, Bierbaum S, et al. Biomechanical comparison of different surface modifications for dental implants. Int J Oral Maxillofac Implants. 2008;23:1037–46. [PubMed] [Google Scholar]
- 80.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27:5561–71. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 81.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Sewing A. Effect of modifications of dual acid-etched implant surfaces on peri-implant bone formation. Part I: organic coatings. Clin Oral Implants Res. 2009;20:31–7. doi: 10.1111/j.1600-0501.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, et al. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281:23246–53. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 83.Stadlinger B, Pilling E, Huhle M, Mai R, Bierbaum S, Scharnweber D, et al. Evaluation of osseointegration of dental implants coated with collagen, chondroitin sulphate and BMP-4: an animal study. Int J Oral Maxillofac Surg. 2008;37:54–9. doi: 10.1016/j.ijom.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 84.Stadlinger B, Pilling E, Huhle M, Mai R, Bierbaum S, Bernhardt R, et al. Influence of extracellular matrix coatings on implant stability and osseointegration: an animal study. J Biomed Mater Res B Appl Biomater. 2007;83:222–31. doi: 10.1002/jbm.b.30787. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–6. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


