Abstract
The present overview is intended to point the readers’ attention to the important subject of calcium orthophosphates. This type of materials is of special significance for human beings, because they represent the inorganic part of major normal (bones, teeth and antlers) and pathological (i.e., those appearing due to various diseases) calcified tissues of mammals. For example, atherosclerosis results in blood vessel blockage caused by a solid composite of cholesterol with calcium orthophosphates, while dental caries and osteoporosis mean a partial decalcification of teeth and bones, respectively, that results in replacement of a less soluble and harder biological apatite by more soluble and softer calcium hydrogenphosphates. Therefore, the processes of both normal and pathological calcifications are just an in vivo crystallization of calcium orthophosphates. Similarly, dental caries and osteoporosis might be considered an in vivo dissolution of calcium orthophosphates. Thus, calcium orthophosphates hold a great significance for humankind, and in this paper, an overview on the current knowledge on this subject is provided.
Keywords: calcium orthophosphates, hydroxyapatite, fluorapatite, bones, teeth, antlers, calcification, crystallization, biomimetics
Introduction
Due to their abundance in nature and presence in living organisms, calcium apatites[a] and other calcium orthophosphates remain the chemical compounds of a special interest in many fields of science, including geology, chemistry, biology and medicine. Due to big problems with access to the scientific literature published in the 19th century and before, a historical description of the subject appears to be very brief. Namely, according to the accessible literature,1 as early as the end of the 18th century, French chemist Joseph-Louis Proust (1754–1826) and German chemist Martin Klaproth (1743–1817) proposed that calcium apatite was the major inorganic component of bones. In the middle of the 19th century, attempts to establish the chemical composition of calcium apatites and other calcium orthophosphates were performed by J. Berzelius,2 R. Warington Jr.3 and R. Fresenius.4 The chemical formula of perfectly transparent crystals of natural fluorapatite (FA) as Ca5(PO4)3F was established in 1873,5 while the crystallographic faces of a natural calcium apatite were described in 1883.6 Furthermore, a paper on a behavior of an undisclosed calcium orthophosphate in organisms of carnivores was published in 1883.7 Further, the quantitative analysis of a calcium orthophosphate was performed in 1884,8 followed by remarks by C. Glaser in 1885.9 In the 1880s, occurrence of a calcium apatite10 and another calcium orthophosphate11-13 in a metallurgical slag was discovered. Chemical reactions between calcium orthophosphates and other chemicals were investigated in 1891.14 Research papers on bone repairing are known since at least 1892,15 while the earliest well-documented systematic studies of calcium orthophosphates were performed at the beginning of the 20th century by F.K. Cameron with coworkers16-20 and H. Bassett.21-24 The majority of the aforementioned researchers already operated with individual chemical compounds.
By definition, all calcium orthophosphates consist of three major chemical elements, calcium (oxidation state +2), phosphorus (oxidation state +5) and oxygen (reduction state -2), as a part of orthophosphate anions. These three chemical elements are present in abundance on the surface of our planet: oxygen is the most widespread chemical element of the Earth’s surface (~47 mass%), calcium occupies the fifth place (~3.3–3.4 mass%) and phosphorus (~0.08–0.12 mass%) is among the first 20 of the chemical elements most widespread on our planet.25 In addition, the chemical composition of many calcium orthophosphates includes hydrogen, as an acidic orthophosphate anion (for example, HPO42- or H2PO4-); hydroxide [for example, Ca10(PO4)6(OH)2] and/or incorporated water (for example, CaHPO4·2H2O). Diverse combinations of CaO and P2O5 (both in the presence of water and without it) provide a large variety of calcium phosphates, which are distinguished by the type of the phosphate anion: ortho-(PO43-), meta-(PO3-), pyro-(P2O74-) and poly-[(PO3)nn-]. In the case of multi-charged anions (orthophosphates and pyrophosphates), calcium phosphates are also differentiated by the number of hydrogen ions attached to the anion. Examples include mono-[Ca(H2PO4)2], di-(CaHPO4), tri-[Ca3(PO4)2] and tetra-(Ca2P2O7) calcium phosphates (here, prefixes “mono,” “di,” “tri” and “tetra” are related to the amount of hydrogen ions replaced by calcium).26-28 However, only calcium orthophosphates will be considered and discussed in this review. The names, standard abbreviations, chemical formulae and solubility values are listed in Table 1.29,30 Since all of them belong to calcium orthophosphates, strictly speaking, all abbreviations in Table 1 are incorrect; however, they are extensively used in the literature, and there is no need to modify them.
Table 1. Existing calcium orthophosphates and their major properties29,30.
| Ca/P molar ratio | Compound | Formula | Solubility at 25°C, -log(Ks) |
Solubility at 25°C, g/L |
pH stability range in aqueous solutions at 25°C |
|---|---|---|---|---|---|
| 0.5 |
Monocalcium phosphate monohydrate (MCPM) |
Ca(H2PO4)2·H2O |
1.14 |
~18 |
0.0–2.0 |
| 0.5 |
Monocalcium phosphate anhydrous (MCPA or MCP) |
Ca(H2PO4)2 |
1.14 |
~17 |
[c] |
| 1.0 |
Dicalcium phosphate dihydrate (DCPD), mineral brushite |
CaHPO4·2H2O |
6.59 |
~0.088 |
2.0–6.0 |
| 1.0 |
Dicalcium phosphate anhydrous (DCPA or DCP), mineral monetite |
CaHPO4 |
6.90 |
~0.048 |
[c] |
| 1.33 |
Octacalcium phosphate (OCP) |
Ca8(HPO4)2(PO4)4·5H2O |
96.6 |
~0.0081 |
5.5–7.0 |
| 1.5 |
α-Tricalcium phosphate (α-TCP) |
α-Ca3(PO4)2 |
25.5 |
~0.0025 |
[a] |
| 1.5 |
β-Tricalcium phosphate (β-TCP) |
β-Ca3(PO4)2 |
28.9 |
~0.0005 |
[a] |
| 1.2–2.2 |
Amorphous calcium phosphates (ACP) |
CaxHy(PO4)z·nH2O, n = 3–4.5; 15–20% H2O |
[b] |
[b] |
~5–12 [d] |
| 1.5–1.67 |
Calcium-deficient hydroxyapatite (CDHA or Ca-def HA)[e] |
Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0 < x < 1) |
~85 |
~0.0094 |
6.5–9.5 |
| 1.67 |
Hydroxyapatite (HA, HAp or OHAp) |
Ca10(PO4)6(OH)2 |
116.8 |
~0.0003 |
9.5–12 |
| 1.67 |
Fluorapatite (FA or FAp) |
Ca10(PO4)6F2 |
120.0 |
~0.0002 |
7–12 |
| 1.67 |
Oxyapatite (OA, OAp or OXA)[f] |
Ca10(PO4)6O |
~69 |
~0.087 |
[a] |
| 2.0 | Tetracalcium phosphate (TTCP or TetCP), mineral hilgenstockite | Ca4(PO4)2O | 38–44 | ~0.0007 | [a] |
[a] These compounds cannot be precipitated from aqueous solutions. [b] Cannot be measured precisely. However, the following values were found: 25.7 ± 0.1 (pH = 7.40), 29.9 ± 0.1 (pH = 6.00), 32.7 ± 0.1 (pH = 5.28).31 The comparative extent of dissolution in acidic buffer is: ACP > > α-TCP > > β-TCP > CDHA > > HA > FA.32[c] Stable at temperatures above 100°C. [d] Always metastable. [e] Occasionally, it is called “precipitated HA (PHA).” [f] Existence of OA remains questionable.
The atomic arrangement of calcium orthophosphates is built up around a network of orthophosphate (PO4) groups, which gives stability to the entire structure. The majority of calcium orthophosphates are sparingly soluble in water; however, all of them are easily soluble in acids but insoluble in alkaline solutions. All chemically pure calcium orthophosphates are crystals of white color and moderate hardness. However, natural minerals of calcium orthophosphates are always colored due to impurities, the most widespread of which are ions of Fe, Mn and rare earth elements.33,34 Biologically formed calcium orthophosphates are the major component of all mammalian calcified tissues,35 while the natural ones are the major raw material to produce phosphorus-containing fertilizers.36-39
Geological and Biological Occurrences
Geologically, natural calcium orthophosphates are found in different regions mostly as deposits of apatites (belong to igneous rocks), mainly as natural FA or phosphorites (a sedimentary rock).37-40 Some types of sedimentary rocks can be formed by weathering of igneous rocks into smaller particles.41 Other types of sedimentary rocks can be composed of minerals precipitated from the dissolution products of igneous rocks or minerals produced by biomineralization (Fig. 1).42 Thus, due to a sedimentary origin, both the general appearance and the chemical composition of natural phosphorites vary a great deal.43,44 It is common practice to consider francolite (or carbonate-hydroxyfluorapatite regarded as its synonym) as the basic phosphorite mineral.40,45-49 A cryptocrystalline (almost amorphous) variety of francolite (partly of a biological origin) is called collophane (synonyms: collophanit, collophanita, collophanite, grodnolite, kollophan).50-52 It occurs in natural phosphorites predominantly as fossil bones and phosphatized microbial pseudomorphs: phosphatic crusts of chasmolithic biofilms (or microstromatolites) and globular clusters with intra-particular porosities.53-56 Natural phosphorites (therefore, francolite and collophane as well) occur in various forms, such as nodules, crystals or masses. Occasionally, other types of natural calcium orthophosphates are found as minerals, for example, clinohydroxylapatite,57 staffelite (synonyms: staffelit, staffelita) belonging to carbonate-rich fluorapatites (chemical formula: Ca5[(F,O)(PO4,CO3)3])4,58 and DCPD.59 Furthermore, calcium orthophosphates were found in meteoric stones.60 The world deposits of natural calcium orthophosphates are estimated to exceed 150 billion tons, out of which approximately 85% belong to phosphorites and the remaining ~15% belong to apatites.40
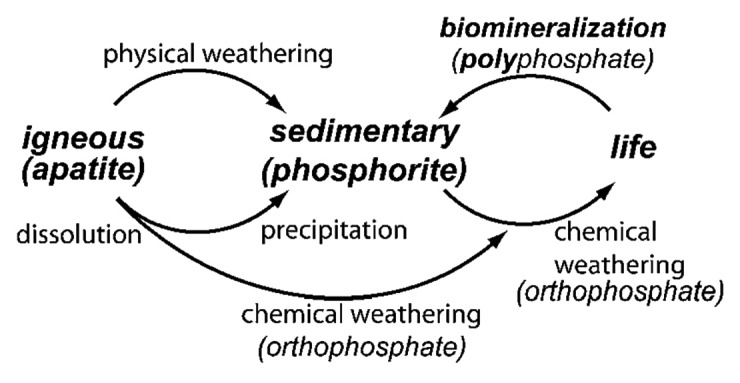
Figure 1. Simplified schematic of the phosphorus cycle from apatitic igneous rock to phosphorite sedimentary rock through chemical or physical weathering. Life forms accumulate soluble phosphorus species and can produce apatite through biomineralization. Reprinted from reference 42 with permission.
Natural calcium orthophosphates occur in most geological environments, usually as accessory minerals (< 5%). Concentrations sufficient for economic use (> 15%) are also available. The largest world deposits of natural apatites are located in Russia (the Khibiny and Kovdor massifs, Kola peninsula61,62), Brazil and Zambia, while the largest world deposits of natural phosphorites are located in Morocco, Russia, Kazakhstan, USA (Florida, Tennessee), China and Australia, as well as in the oceans.36-40 Most of natural calcium orthophosphates occur as small polycrystalline structures (spherulitic clusters). Larger crystals are rare.63 They usually have the crystal structure of apatites (hexagonal system, space group P63/m). Giant crystals, including “a solid but irregular mass of green crystalline apatite, 15 feet long and 9 feet wide”64 and a single euhedral crystal from the Aetna mine measuring 2.1 x 1.2 min with an estimated weight of 6 tons,65 were found. None of them are pure compounds; they always contain admixtures of other elements. For example, ions of calcium might be partially replaced by Sr, Ba, Mg, Mn, K, Na, Fe; ions of orthophosphate may be partly replaced by AsO43-, CO32- and VO4;2-30,33-66 ions of hydroxide, chloride, bromide, carbonate and oxide may, to a certain extent, substitute for fluoride in the crystal lattice of natural apatites.48 Furthermore, various organic radicals have been found in natural apatites.67,68 In principle, the crystal structure of apatites can incorporate half of the periodic table in its atomic arrangement. In medicine, this property might be used as an antidote for heavy metal intoxication.69 Ease of atomic substitution for apatite leaves this mineral open to a wide array of compositions. This might be related to the fact that the apatite structure type displays porous properties.70 The substitutions in apatites are usually in trace concentrations, but large concentrations and even complete solid solutions exist for certain substituents (e.g., F- and OH-). To make things even more complicated, some ions in the crystal structure may be missing, leaving crystallographic defects, which leads to formation of non-stoichiometric compounds. Figure 2 shows examples of polycrystalline and single-crystalline samples of natural FA.
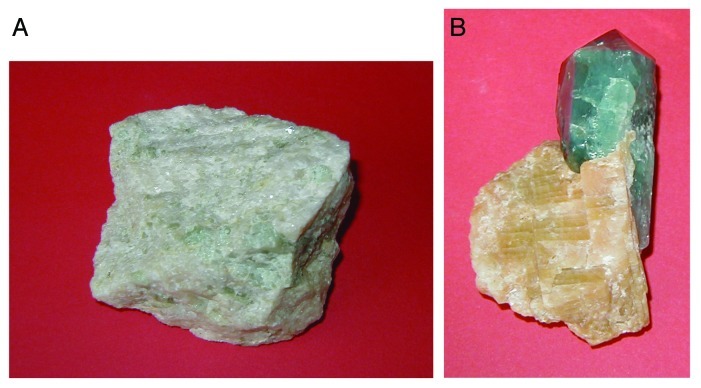
Figure 2. Polycrystalline (A) and single-crystalline (B) FA of a geological origin. The single crystal has a gray-green color due to incorporated ions of transition metals.
Manufacturing of elementary phosphorus (white and red),71,72 phosphoric acids,37,73-76 various phosphorus-containing chemicals and, especially, agricultural fertilizers (namely, superphosphate,77-79 ammonium orthophosphates80) are the major industrial applications of natural calcium orthophosphates. The annual consumption of a phosphate rock has approached ~150 million tons, and about 95 percent of this production is utilized in the fertilizer industry.81,82
In biological systems, many organisms, ranging from bacteria and isolated cells to invertebrates and vertebrates, synthesize calcium orthophosphates.42 Formation of calcium orthophosphates in primitive organisms is believed to enable the storage and regulation of essential elements, such as calcium, phosphorus and, possibly, magnesium. The morphology of precipitates in these organisms (small intracellular nodules of ACP often located in mitochondria) complies with the necessity for rapid mobilization and intracellular control of the concentration of these elements.83 In vertebrates, calcium orthophosphates occur as the principal inorganic constituent of normal (bones, teeth, fish enameloid, deer antlers and some species of shells) and pathological (dental and urinary calculus and stones, atherosclerotic lesions, etc.) calcifications.26,84-89 Except for small portions of the inner ear, all hard tissue of the human body is formed of calcium orthophosphates. Structurally, they occur mainly in the form of poorly crystalline, non-stoichiometric, calcium-deficient, Na-, Mg- and carbonate-containing HA [often called “biological apatite”90-94 (which might be abbreviated as BAp95,96), bioapatite97-100 or dahllite.[b],101 The main constituents of human bones are calcium orthophosphates (~60–70 wt%), collagen[c] (~20–30 wt%) and water (up to 10 wt%).32,88,97-99,101,102 Detailed information on the chemical composition of the most important human normal calcified tissues can be found in Table 2. One should note that the values mentioned in Table 2 are approximate; the main constituents can vary by one percent or more.106
Table 2. Comparative composition and structural parameters of inorganic phases of adult human calcified tissues. Due to the considerable variation found in biological samples, typical values are given in these cases26,32.
| Composition, wt% | Enamel | Dentine | Cementum | Bone | HA |
|---|---|---|---|---|---|
| Calcium[a] |
36.5 |
35.1 |
~35 |
34.8 |
39.6 |
| Phosphorus (as P)[a] |
17.7 |
16.9 |
~16 |
15.2 |
18.5 |
| Ca/P (molar ratio)[a] |
1.63 |
1.61 |
~1.65 |
1.71 |
1.67 |
| Sodium[a] |
0.5 |
0.6 |
[c] |
0.9 |
– |
| Magnesium[a] |
0.44 |
1.23 |
0.5–0.9 |
0.72 |
– |
| Potassium[a] |
0.08 |
0.05 |
[c] |
0.03 |
– |
| Carbonate (as CO32-)[b] |
3.5 |
5.6 |
[c] |
7.4 |
– |
| Fluoride[a] |
0.01 |
0.06 |
up to 0.9 |
0.03 |
– |
| Chloride[a] |
0.30 |
0.01 |
[c] |
0.13 |
– |
| Pyrophosphate (as P2O74-)[b] |
0.022 |
0.10 |
[c] |
0.07 |
– |
| Total inorganic[b] |
97 |
70 |
60 |
65 |
100 |
| Total organic[b] |
1.5 |
20 |
25 |
25 |
– |
| Water[b] |
1.5 |
10 |
15 |
10 |
– |
|
Crystallographic properties: Lattice parameters (± 0.003 Å) |
|
|
|
|
|
|
a-axis, Å |
9.441 |
9.421 |
[c] |
9.41 |
9.430 |
|
c-axis, Å |
6.880 |
6.887 |
[c] |
6.89 |
6.891 |
| Crystallinity index (HA = 100) |
70–75 |
33–37 |
~30 |
33–37 |
100 |
| Typical crystal sizes (nm)103–105 |
100 µm × 50 × 50 |
35 × 25 × 4 |
[c] |
50 × 25 × 4 |
200–600 |
| Ignition products (800°C) |
β-TCP + HA |
β-TCP+ HA |
β-TCP+ HA |
HA + CaO |
HA |
| Elastic modulus (GPa) |
80 |
23.8 ± 3.7 |
15.0 ± 3.6 |
0.34–13.8 |
10 |
| Tensile strength (MPa) | 10 | 100 | [c] | 150 | 100 |
[a] Ashed samples. [b] Unashed samples. [c] Numerical values were not found in the literature but they should be similar to those for dentine.
The Members of the Calcium Orthophosphate Family
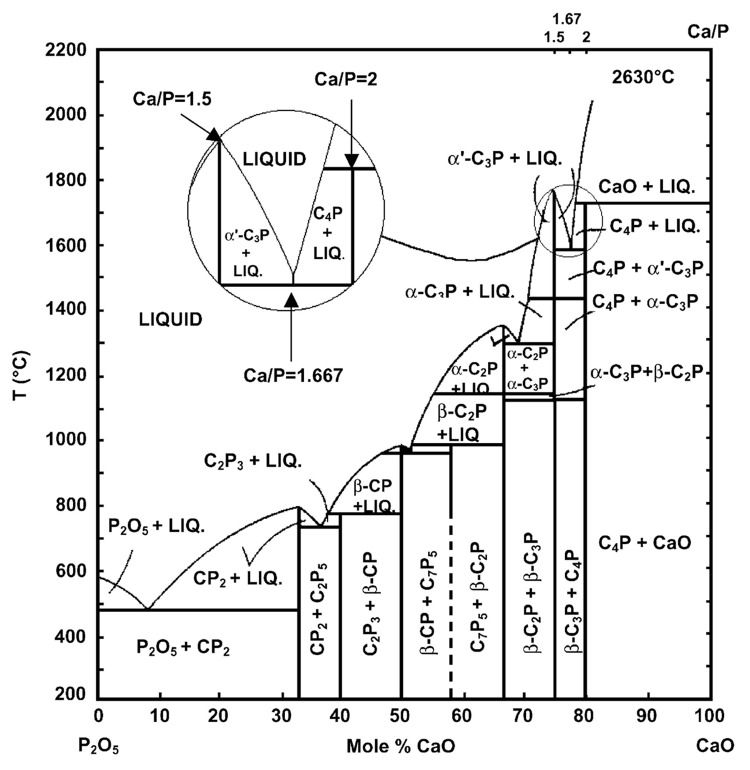
In the ternary aqueous system Ca(OH)2-H3PO4-H2O (or CaO-P2O5-H2O),107-109 there are 12 known non-ion-substituted calcium orthophosphates with the Ca/P molar ratio ranging between 0.5 and 2.0 (Table 1). An anhydrous phase diagram CaO-P2O5 at temperatures within 200–2,200°C is shown in Figure 3.110,111Table 3 comprises crystallographic data of the existing calcium orthophosphates.27,112-114 The most important parameters of calcium orthophosphates are the ionic Ca/P ratio, basicity/acidity and solubility. All these parameters strongly correlate with the solution pH. The lower the Ca/P molar ratio is, the more acidic and water-soluble the calcium orthophosphate is.26-28 One can see that the solubility ranges from high values for acidic compounds, such as MCPM, to very low values for basic compounds, such as apatites, which allows calcium orthophosphates to be dissolved, transported from one place to another and precipitated when necessary. Crystallization, dissolution and phase transformation processes of different calcium orthophosphates under various experimental conditions have been reviewed recently in reference 115.
Figure 3. Phase diagram of the system CaO-P2O5 (C = CaO, p = P2O5) at elevated temperatures. Here: C7P5 means 7CaO·5P2O5; other abbreviations should be written out in the same manner. Reprinted from references 110 and 111 with permission.
Table 3. Crystallographic data of calcium orthophosphates27,112,113.
| Compound | Space group | Unit cell parameters | Z[a] | Density, g cm−3 |
|---|---|---|---|---|
| MCPM |
triclinic P |
a = 5.6261(5), b = 11.889(2), c = 6.4731(8) Å, α = 98.633(6)°, β = 118.262(6)°, γ = 83.344(6)° |
2 |
2.23 |
| MCPA |
triclinic P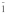 |
a = 7.5577(5), b = 8.2531(6), c = 5.5504(3) Å, α = 109.87(1)°, β = 93.68(1)°, γ = 109.15(1)° |
2 |
2.58 |
| DCPD |
monoclinic Ia |
a = 5.812(2), b = 15.180(3), c = 6.239(2) Å, β = 116.42(3)° |
4 |
2.32 |
| DCPA |
triclinic P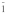 |
a = 6.910(1), b = 6.627(2), c = 6.998(2) Å, α = 96.34(2)°, β = 103.82(2)°, γ = 88.33(2)° |
4 |
2.89 |
| OCP |
triclinic P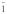 |
a = 19.692(4), b = 9.523(2), c = 6.835(2) Å, α = 90.15(2)°, β = 92.54(2)°, γ = 108.65(1)° |
1 |
2.61 |
| α-TCP |
monoclinic P21/a |
a = 12.887(2), b = 27.280(4), c = 15.219(2) Å, β = 126.20(1)° |
24 |
2.86 |
| β-TCP |
rhombohedral R3cH |
a = b = 10.4183(5), c = 37.3464(23) Å, γ = 120° |
21[b] |
3.08 |
| HA |
monoclinic P21/b or hexagonal P63/m |
a = 9.84214(8), b = 2a, c = 6.8814(7) Å, γ = 120° (monoclinic) a = b = 9.4302(5), c = 6.8911(2) Å, γ = 120° (hexagonal) |
4 2 |
3.16 |
| FA |
hexagonal P63/m |
a = b = 9.367, c = 6.884 Å, γ = 120° |
2 |
3.20 |
| OA |
hexagonal P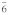 |
a = b = 9.432, c = 6.881 Å, α = 90.3°, β = 90.0°, γ = 119.9° |
1 |
~3.2 |
| TTCP | monoclinic P21 | a = 7.023(1), b = 11.986(4), c = 9.473(2) Å, β = 90.90(1)° | 4 | 3.05 |
[a] Number of formula units per unit cell. [b] Per the hexagonal unit cell.
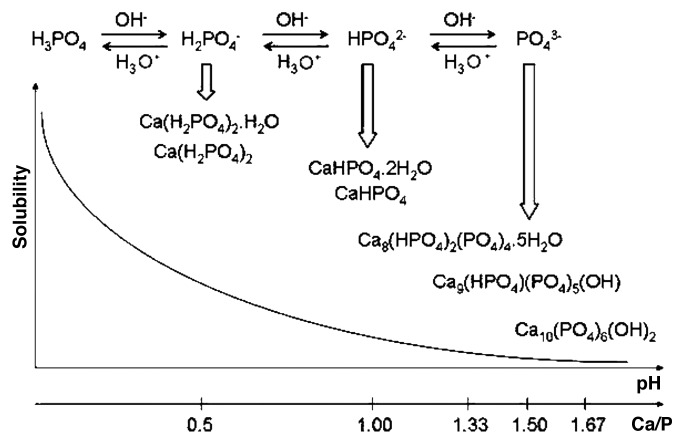
Due to the triprotic equilibrium that exists within orthophosphate-containing solutions, variations in pH alter the relative concentrations of the four polymorphs of orthophosphoric acid (Fig. 4)116 and, thus, both the chemical composition (Figure 5)117 and the amount of the calcium orthophosphates that are formed by a direct precipitation. The solubility isotherms of different calcium orthophosphates are shown in Figure 6.27,28,108,109,118-121 However, recently, the classic solubility data of calcium orthophosphates27,28,108,109,118-121 were mentioned to be inappropriate.122 According to the authors of the latter study, all previous solubility calculations were based on simplifications that are only crudely approximate. The problem lies in incongruent dissolution, leading to phase transformations and lack of the detailed solution equilibria. Using an absolute solid-titration approach, the true solubility isotherm of HA was found to lie substantially lower than previously reported. In addition, contrary to wide belief, DCPD appeared not to be the most stable phase below pH ~4.2, where CDHA is less soluble.122
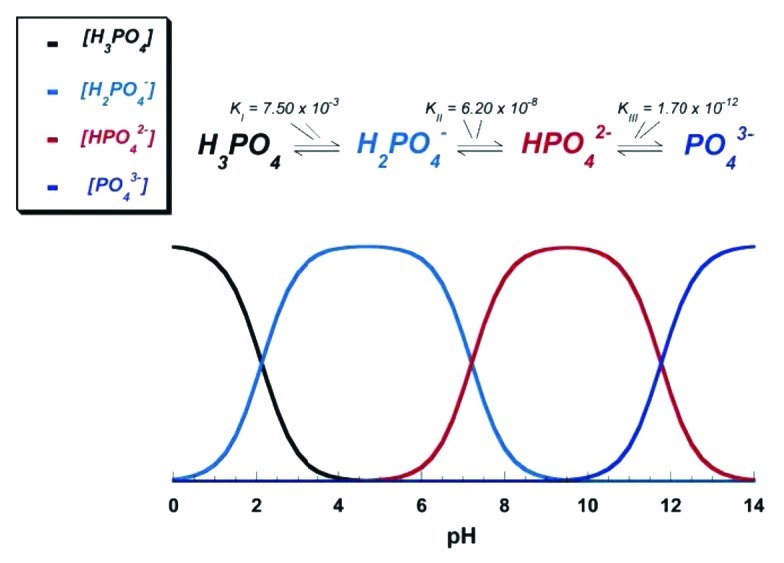
Figure 4. pH variation of ionic concentrations in triprotic equilibrium for phosphoric acid solutions. Reprinted from reference 116 with permission.
Figure 5. Various calcium orthophosphates obtained by neutralizing of orthophosphoric acid. Ca/P are reported in the figure. The solubility of calcium orthophosphates in water decreases drastically from left to right, HA being the most insoluble and stable phase. Reprinted from reference 117 with permission.
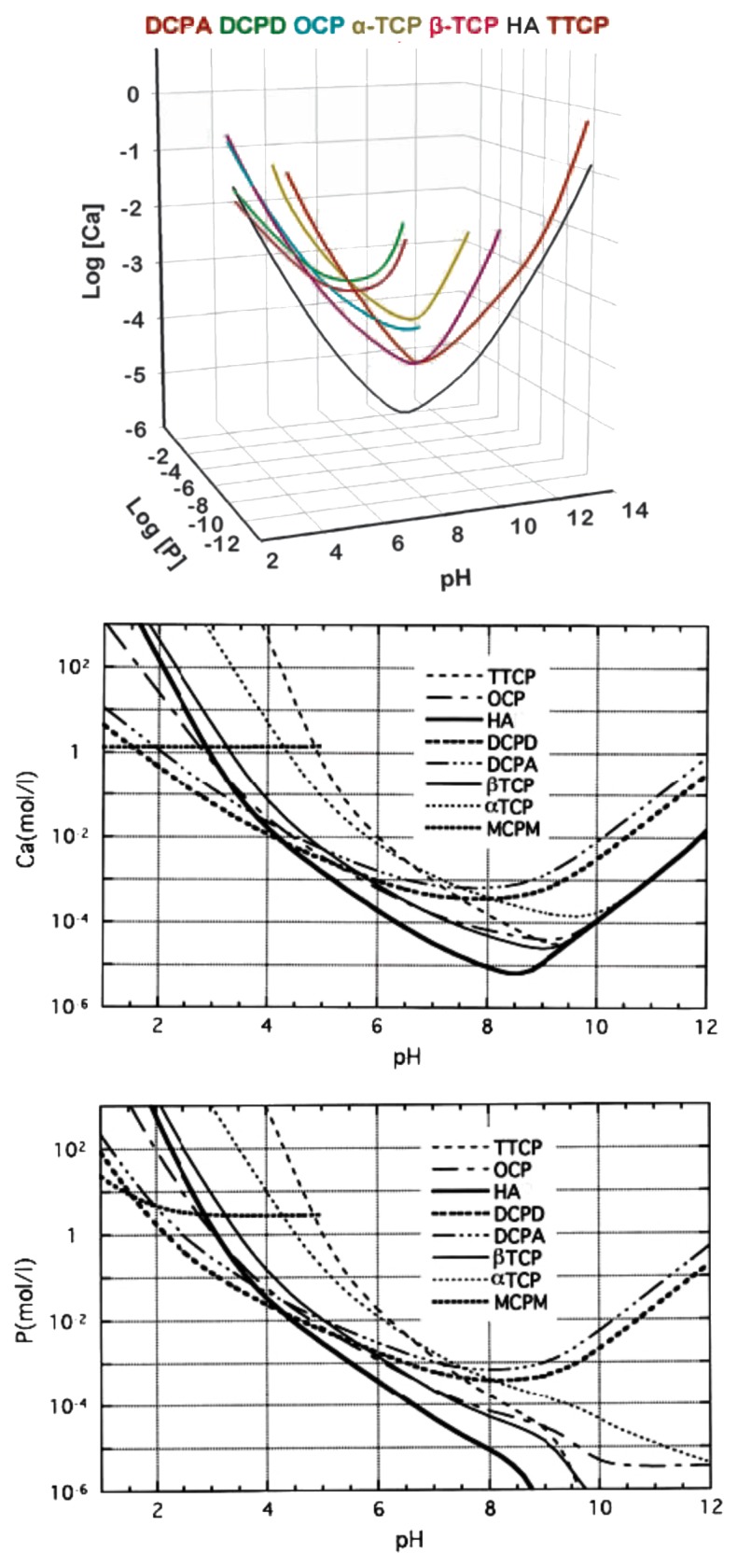
Figure 6. Top: a 3D version of the classical solubility phase diagrams for the ternary system Ca(OH)2-H3PO4-H2O. Reprinted from reference 118 with permission. Middle and bottom: solubility phase diagrams in two-dimensional graphs, showing two logarithms of the concentrations of (a) calcium and (b) orthophosphate ions as a function of the pH in solutions saturated with various salts. Reprinted from reference 119 with permission.
A brief description of all known calcium orthophosphates (Table 1) is given below.
MCPM
Monocalcium phosphate monohydrate [Ca(H2PO4)2·H2O; the IUPAC name is calcium dihydrogen orthophosphate monohydrate] is both the most acidic and the most water-soluble compound. It precipitates from highly acidic solutions that are normally used in the industry of phosphorus-containing fertilizer production (“triple superphosphate”).37 Besides, MCPM might be fabricated by a simple precipitation method using CaCO3 and H3PO4 in aqueous and acetone media at ambient temperature.123 At temperatures above ~100°C it releases a molecule of water and transforms into MCPA. Due to high acidity and solubility, MCPM is never found in biological calcifications. Moreover, pure MCPM is not biocompatible[d] with bones.124 However, in medicine, MCPM is used as a component of several self-hardening calcium orthophosphate cements.125-128 In addition, MCPM is used as a nutrient, acidulant and mineral supplement for dry baking powders, food, feed and some beverages.129,130 Coupled with NaHCO3, MCPM is used as a leavening agent for both dry baking powders and bakery dough. MCPM might be added to salt-curing preserves, pickled and marinated foods. According to the European classification of food additives, MCPM is marked as E341 additive. Occasionally, MCPM is added to toothpastes. MCPM might also be added to ceramics and glasses, while agriculture is the main consumer of a technical-grade MCPM, where it is used as a fertilizer.37,129
MCPA (or MCP)
Monocalcium phosphate anhydrous [Ca(H2PO4)2; the IUPAC name is calcium dihydrogen orthophosphate anhydrous] is the anhydrous form of MCPM. It crystallizes under the same conditions as MCPM but at temperatures above ~100°C (e.g., from highly concentrated mother liquors during fertilizer production). Like MCPM, MCPA never appears in calcified tissues, and it is not biocompatible due to its acidity. There is no current application of MCPA in medicine. Due to its similarity with MCPM, in many cases, MCPA might be used instead of MCPM;37,129 however, highly hydroscopic properties of MCPA reduce its commercial application.
DCPD
Dicalcium phosphate dihydrate (CaHPO4·2H2O; the IUPAC name is calcium hydrogen orthophosphate dihydrate; the mineral brushite131) can be easily crystallized from aqueous solutions at ~2.0 < pH < ~6.5. Interestingly, precipitation of DCPD by mixing a Ca(OH)2 suspension and a H3PO4 solution in the equimolar quantities was found to occur in five stages; HA being the first precipitated phase.132,133 Alternatively, DCPD might be prepared in gels.134,135 DCPD transforms into DCPA at temperatures above ~80°C, and this transformation is accompanied by ~11% decrease in volume136 and structural changes.137 The value for ⊗rG0 for DCPD → DCPA transformation is -1.032 kJ/mol.137 Briefly, DCPD crystals consist of CaPO4 chains arranged parallel to each other, while lattice water molecules are interlayered between them. Using surface X-ray diffraction, Arsic et al. determined the atomic structure of the {010} interface of DCPD with water.138,139 Since DCPD contains water layers as part of its crystal structure, special ordering properties at the interface are expected. This interface consists of two water bilayers with different ordering properties. The first is highly ordered and can be considered as part of the DCPD crystal structure. Surprisingly, the second water bilayer exhibits no in-plane order, but shows only layering in the perpendicular direction. It has been proposed that the low level of water ordering at the interface is correlated with the low solubility of DCPD in water.139 Recently, data on DCPD solubility have been updated by solid titration technique.140 The optical properties of DCPD are well described in reference 141, while many additional data on DCPD as well as a good picture of DCPD atomic structure are available in the literature.142
DCPD is of biological importance, because it is often found in pathological calcifications (dental calculi, crystalluria, chondrocalcinosis and urinary stones) and some carious lesions.26,84-86 It has been proposed as an intermediate in both bone mineralization and dissolution of enamel in acids (dental erosion).26,84,85 In medicine, DCPD is used in calcium orthophosphate cements126,143-146 and as an intermediate for tooth remineralization. DCPD is added to toothpaste both for caries protection (in this case, it is coupled with F-containing compounds such as NaF and/or Na2PO3F) and as a gentle polishing agent.147-151 Other applications include a flame retardant,152 a slow-release fertilizer, use in glass production as well as a calcium supplement in food, feed and cereals.129 The importance of DCPD as a constituent of infant’s food was discovered as early as in 1917.153 In the food industry, it serves as a texturizer, bakery improver and water retention additive. In the dairy industry, DCPD is used as a mineral supplement. If added to food products, DCPD should be marked as E341 according to the European classification of food additives. In addition, plate-like crystals of DCPD might be used as a non-toxic, anticorrosive and passivating pigment for some ground coat paints.
DCPA (or DCP)
Dicalcium phosphate anhydrous (CaHPO4; the IUPAC name is calcium hydrogen orthophosphate anhydrate, the mineral monetite154) is the anhydrous form of DCPD. It is less soluble than DCPD due to the absence of water inclusions. Like DCPD, DCPA can be crystallized from aqueous solutions but at temperatures ~100°C. Furthermore, it might be prepared at room temperature in gels,134 ethanol155 as well as in oil-in-water and water-in-oil systems.156 DCPA is physically stable and resisted hydration even when dispersed in water for over 7 mo in the temperature range of 4–50°C.157 A calcium-deficient DCPA was prepared recently. It might be sintered at ~300°C.158 Unlike DCPD, DCPA occurs in neither normal nor pathological calcifications. It is used in calcium orthophosphate cements.145,159-166 Besides, DCPA might be implanted.167 Other applications include use as a polishing agent, a source of calcium and phosphate in nutritional supplements (e.g., in prepared breakfast cereals, enriched flour and noodle products), a tabletting aid168 and a toothpaste component.129 In addition, it is used as a dough conditioner in the food industry.
OCP
Octacalcium phosphate [Ca8(HPO4)2(PO4)4·5H2O; the IUPAC name is tetracalcium hydrogen orthophosphate diorthophosphate pentahydrate; another name is octacalcium bis(hydrogenphosphate) tetrakis(phosphate) pentahydrate] is often found as an unstable transient intermediate during the precipitation of the thermodynamically more stable calcium orthophosphates (e.g., CDHA) in aqueous solutions. Its preparation technique might be found in references 169–174. A partially hydrolyzed form of OCP with Ca/P molar ratio of 1.37 might be prepared as well.174,175 The full hydrolysis of OCP into CDHA occurs within ~6 h.173 Furthermore, OCP might be non-stoichiometric and be either Ca-deficient (Ca/p = 1.26) or include excessive calcium (up to Ca/p = 1.48) in the structure.174 Ion-substituted OCP might be prepared as well.176 Crystals of OCP are typically small, extremely platy and almost invariably twinned.
The triclinic structure of OCP displays apatitic layers (with atomic arrangements of calcium and orthophosphate ions similar to those of HA) separated by hydrated layers (with atomic arrangements of calcium and orthophosphate ions similar to those in DCPD).26-28,177,178 A similarity in crystal structure between OCP and HA179,180 is one reason that the epitaxial growth of these phases is observed. Morphologically, OCP crystallizes as {100} blades of triclinic pinacoidal symmetry elongated along the a-axis and bordered by the forms {010}, {001} and {011}. It is generally assumed that in solutions, the hydrated layer of the (100) face is the layer most likely exposed to solution. The water content of OCP crystals is about 20% that of DCPD, and this is partly responsible for its lower solubility. New data on OCP solubility have been published recently in reference 181.
OCP is of a great biological importance, because it is one of the stable components of human dental and urinary calculi.182-185 OCP was first proposed by W.E. Brown to participate as the initial phase in enamel mineral formation and bone formation through subsequent precipitation and stepwise hydrolysis of OCP.179,180,186 It plays an important role in in vivo formation of apatitic biominerals. A “central OCP inclusion” (also known as “central dark line”) is seen by transmission electron microscopy in many biological apatites and in some synthetically precipitated HA.187-191 Although OCP has not been observed in vascular calcifications, it has been strongly suggested as a precursor phase to biological apatite found in natural and prosthetic heart valves.192,193 In surgery, OCP is used for implantation into bone defects.194-200 For comprehensive information on OCP, the readers are referred to other reviews in references 174 and 184.
β-TCP
β-tricalcium phosphate [β-Ca3(PO4)2; the IUPAC name is tricalcium diorthophosphate β; other names are calcium orthophosphate tribasic β or tricalcium bis(orthophosphate) β] cannot be precipitated from aqueous solutions. It is a high temperature phase, which can be prepared at temperatures above 800°C by thermal decomposition of CDHA or by solid-state interaction of acidic calcium orthophosphates, e.g., DCPA, with a base, e.g., CaO. However, β-TCP can be obtained at a relatively low temperature (150°C) by precipitation in organic medium, such as ethylene glycol.201,202 Apart from the chemical preparation routes, ion-substituted β-TCP can be prepared by calcining of bones;203 such a type of β-TCP is occasionally called “bone ash.” In β-TCP, there are three types of crystallographically nonequivalent PO43- groups located at general points of the crystal, each type with different intratetrahedral bond lengths and angles. At temperatures above ~1,125°C, β-TCP is transformed into a high-temperature phase α-TCP. Being the stable phase at room temperature, β-TCP is less soluble in water than α-TCP (Table 1). Furthermore, the ideal β-TCP structure contains calcium ion vacancies that are too small to accommodate calcium ions but allow for the inclusion of magnesium ions, which thereby stabilize the structures.204,205 Both ion-substituted206-209 and organically modified210-212 forms of β-TCP can be synthesized as well. The maximum substitution of Mg2+ in β-TCP was found to correspond to the Ca2.61[Mg(1)0.28,Mg(2)0.11](PO4)2 stoichiometric equation.209 The modern structural data on β-TCP are available in references 213–215; those on Vicker’s and Knoop microhardness studies might be found if reference 216, while solubility data can be found in reference 217. Furthermore, the ability of β-TCP to store an electrical charge by electrical polarization was studied, and this material was found to have a suitable composition and structure for both ion conduction and charge storage.218
Pure β-TCP never occurs in biological calcifications. Only the Mg-substituted form, called whitlockite[e] [β-TCMP-β-tricalcium magnesium phosphate, β-(Ca,Mg)3(PO4)2], is found in dental calculi and urinary stones, dentineal caries, salivary stones, arthritic cartilage as well as in some soft tissue deposits.26,84-86,219-222 However, it has not been observed in enamel, dentine or bone. In biomedicine, β-TCP is used in calcium orthophosphate bone cements31,223-227 and other types of bone substitution bioceramics.203,228-235 Dental applications of β-TCP are also known.236 Pure β-TCP is added to some brands of toothpaste as a gentle polishing agent. Multivitamin complexes with calcium orthophosphate are widely available in the market, and β-TCP is used as the calcium phosphate there. In addition, β-TCP serves as a texturizer, bakery improver and anti-clumping agent for dry powdered food (flour, milk powder, dried cream, cocoa powder). Besides, β-TCP is added as a dietary or mineral supplement to food and feed, where it is marked as E341 according to the European classification of food additives. A prenatal development of rats during gestation was found to be sensitive to E341 (TCP) exposure.237 There is a good review on the toxicological properties of inorganic phosphates, where the interested readers are referred.238 Occasionally, β-TCP might be used as inert filler in pelleted drugs. Other applications comprise porcelains, pottery, enamel, use as a component for mordants and ackey as well as a polymer stabilizer.129 β-TCP of a technical grade (as either calcined natural phosphorites or bone dust) is used as a slow-release fertilizer for acidic soils.37
α-TCP
α-tricalcium phosphate [α-Ca3(PO4)2; the IUPAC name is tricalcium diorthophosphate α; other names are calcium orthophosphate tribasic α or tricalcium bis(orthophosphate) α] is usually prepared from β-TCP at heating above ~1,125°C,239 and it might be considered a high temperature phase of β-TCP. However, at the turn of the millennium, the previously forgotten data indicating that the presence of silicates stabilized α-TCP at lower temperatures of 800–1,000°C240 has been rediscovered again. Such type of α-TCP is called “silicon-stabilized α-TCP.”241-246
Although α-TCP and β-TCP have exactly the same chemical composition, they differ in their crystal structure (Table 3) and solubility (Table 1). In the absence of humidity, both polymorphs of TCP are stable at room temperatures; however, according to a density functional study, stability of β-TCP crystal lattice exceeds that of α-TCP.214 Therefore, of the two, α-TCP is more reactive in aqueous systems, has a higher specific energy, and in aqueous solutions, it can be hydrolyzed to CDHA.247-249 Milling was found to increase the α-TCP reactivity even more.250 Although, α-TCP never occurs in biological calcifications, in medicine, it is used as a component of calcium orthophosphate cements.126,143-146,161-163,251-254 On the other hand, the chemically pure α-TCP has received not much interest in the biomedical field.233 The disadvantage for using α-TCP is its quick resorption rate (faster than formation of a new bone), which limits its application in this area. However, the silicon-stabilized α-TCP (more precisely, a biphasic composite with HA) has been commercialized as a starting material to produce bioresorbable porous ceramic scaffolds to be used as artificial bone grafts.228,241-245 Upon implantation, α-TCP tends to convert to CDHA, which drastically reduces further degradation rate. Theoretical insights into bone grafting properties of the silicon-stabilized α-TCP might be found in reference 255. The structure of α-TCP is well-described in the literature,214,215,256 while the surface and adsorption properties are available in reference 257. Similar to β-TCP, α-TCP of a technical grade might be used in slow-release fertilizer for acidic soils.129
To conclude, one should briefly mention the existence of α'-TCP phase. However, it lacks a practical interest, because it only exists at temperatures above ~1,465 ± 5°C and reverts to α-TCP by cooling below the transition temperature.
ACP
Amorphous calcium phosphates (ACPs) represent a special class of calcium orthophosphate salts, having variable chemical but more or less identical glass-like physical properties, in which there are neither translational nor orientational long-range orders (LRO) of the atomic positions. Until recently,258 ACP has been considered as an individual chemical compound; however, this is just an amorphous state of other calcium orthophosphates. Therefore, in principle, all compounds mentioned in Table 1 might be somehow fabricated in an amorphous state, but, currently, only a few of them (e.g., an amorphous TCP) are known.258 Thus, strictly speaking, ACP should be excluded from Table 1.
Depending on the production temperatures, ACPs are divided into two major groups: low-temperature ACPs (prepared in aqueous solutions) and high-temperature ACPs. Low-temperature ACPs [described by the chemical formula CaxHy(PO4)z·nH2O, n = 3–4.5; 15–20% H2O] are often encountered as a transient precursor phase during precipitation of other calcium orthophosphates in aqueous systems. Usually, an ACP is the first phase precipitated from a supersaturated solution prepared by rapid mixing of solutions containing ions of calcium and orthophosphate;27,259-264 however, other production techniques are known. ACPs are thought to be formed at the beginning of the precipitation due to a lower surface energy than that of OCP and apatites.260 The amorphization degree of ACPs increases with the concentration increasing of Ca- and PO4-containing solutions as well as at a high solution pH and a low crystallization temperature. A continuous gentle agitation of as precipitated ACPs in the mother solution, especially at elevated temperatures, results in a slow recrystallization and formation of better crystalline calcium orthophosphates, such as CDHA.26,27 The lifetime of ACPs in aqueous solution was reported to be a function of the presence of additive molecules and ions, pH, ionic strength and temperature. Thus, ACPs may persist for appreciable periods and retain the amporphous state under some specific experimental conditions.265 The chemical composition of ACPs strongly depends on the solution pH and the concentrations of mixing solutions. For example, ACPs with Ca/P ratios in the range of 1.18 (precipitated at solution pH = 6.6) to 1.53 (precipitated at solution pH = 11.7)27,266 and even to 2.526,84,85 have been described. The presence of poly(ethylene glycol),267 ions of pyrophosphate, carbonate and/or magnesium in solution during the crystallization promotes formation of ACPs and slows down their further transformation into more crystalline calcium orthophosphates, while the presence of fluoride has the opposite effect.26-28,32,268 The solution-mediated transformation of an ACP to CDHA, which can be described by a “first-order” rate law, is a function only of the solution pH and depends upon the experimental conditions that regulate both the dissolution of ACP and the formation of early HA nuclei.269
High-temperature ACPs might be prepared using high-energy processing at elevated temperatures. This method is based on a rapid quenching of melted calcium orthophosphates occurring, e.g., during plasma spraying of HA.270-272 A plasma jet, possessing very high temperatures (5,000–20,000°C), partly decomposes HA. That results in formation of a complicated mixture of products, some of which would be ACPs. Obviously, all types of high-temperature ACPs are definitively anhydrous contrary to the precipitated ACPs. Unfortunately, no adequate chemical formula is available to describe the high-temperature ACPs.
In general, as all amorphous compounds are characterized by a lack of LRO, it is problematic to discuss the structure of ACPs (they are X-ray amorphous). Concerning a short-range order (SRO) in ACPs, it exists, just due to the nature of chemical bonds. Unfortunately, in many cases, the SRO in ACPs is uncertain, because it depends on many variables, such as Ca/P ratio, preparation conditions, storage, admixtures, etc. It is well known that freshly precipitated ACPs contain 10–20% by weight of tightly bound water, which is removed by vacuum drying at elevated temperature.273 Infrared spectra of ACPs show broad featureless phosphate absorption bands. Electron microscopy of freshly precipitated ACPs usually shows featureless nearly spherical particles with diameters in the range of 20 to 200 nm. However, there is a questionable opinion that ACPs might have an apatitic structure but with a crystal size so small that they are X-ray amorphous. This is supported by X-ray absorption spectroscopic data (EXAFS) on biogenic and synthetic samples.274-277 On the other hand, it was proposed that the basic structural unit of the precipitated ACPs is a 9.5 Å diameter, roughly spherical cluster of ions with the composition of Ca9(PO4)6 (Fig. 7).27,266,278,279 These clusters were found experimentally, first as nuclei during the crystallization of CDHA, and a model was developed to describe the crystallization of HA as a step-wise assembly of these units280 [see section 3.10. HA (or HAp or OHAp) below]. Biologically, ion-substituted ACPs (always containing ions of Na, Mg, carbonate and pyrophosphate) are found in soft-tissue pathological calcifications (e.g., heart valve calcifications of uremic patients).26,84-86
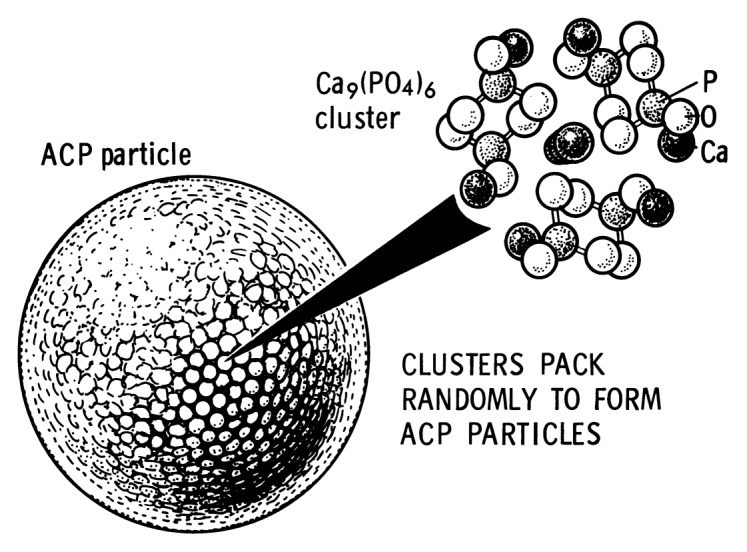
Figure 7. A model of ACP structure. Reprinted from reference 278 with permission.
In medicine, pure ACPs are used in calcium orthophosphate cements143-145 and as a filling material in dentistry.258 Bioactive composites of ACPs with polymers have properties suitable for use in dentistry281-284 and surgery.285-288 Due to a reasonable solubility and physiological pH of aqueous solutions, ACP appeared to be consumable by some microorganisms, and for this reason, it might be added as a mineral supplement to culture media. Non-biomedical applications of ACPs comprise their use as a component for mordants and ackey. In the food industry, ACPs are used for syrup clearing. Occasionally, they might be used as inert filler in pelleted drugs. In addition, ACPs are used in glass and pottery production and as a raw material for production of some organic phosphates. To get further details on ACPs, the readers are referred to special reviews in references 258, 279, 289 and 290.
CDHA (or Ca-def HA)
Calcium-deficient hydroxyapatite [Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0 < x < 1)] can be easily prepared by simultaneous addition of calcium- and orthophosphate-containing solutions into boiling water followed by boiling the suspension for several hours (an aging stage). That is why, in literature, it might be called as “precipitated HA (PHA).”291,292 Besides, it might be prepared by hydrolysis of α-TCP.247-249 Other preparation techniques of CDHA are known as well.293-295 During aging, initially precipitated ACPa are restructured and transformed into CDHA.[f] Therefore, there are many similarities in the structure, properties and application between the precipitated in alkaline solutions (pH > 8) ACPs and CDHA. Recent data indicated on presence of intermediate phases during further hydrolysis of CDHA to a more stable HA-like phase.296 CDHA crystals are poorly crystalline and of submicron dimensions. They have a very large specific surface area, typically 25–100 m2/g. On heating above ~700°C, dry CDHA with Ca/p = 1.5 will convert to β-TCP, and that with 1.5 < Ca/p < 1.67 will convert into a biphasic composite of HA and β-TCP (see the Biphasic, Triphasic and Multiphasic Calcium Orthophosphate Formulations section below).297-308 A reasonable solid-state mechanism of a high-temperature transformation of CDHA into BCP has been proposed.309,310
The variability in Ca/P molar ratio of CDHA has been explained through different models: surface adsorption, lattice substitution and intercrystalline mixtures of HA and OCP.311 Due to a lack of stoichiometry, CDHA usually contains other ions.83 The extent depends on the counter-ions of the chemicals used for preparation (e.g., Na+, Cl-). Direct determinations of the CDHA structures are still missing, and the unit cell parameters remain uncertain. However, unlike that in ACPs (see section 3.8. ACP above), a LRO exists in CDHA. The following lattice parameters were reported for formate (HCO2-) containing CDHA with Ca/p = 1.596 (ionic): a = 9.4729(20) and c = 6.8855(9) Å. A loss of Ca2+ ions happened exclusively from Ca(2) sites, while the PO4 tetrahedron volume and P-O bonds were ~4.4% and ~1.4% smaller, respectively, than those in HA.312
A systematic study of defect constellations in CDHA is available in literature.313 As a first approximation, CDHA may be considered as HA with some ions missing.314 The more calcium is deficient, the more disorder and imperfections are in CDHA structure.315 Furthermore, a direct correlation between Ca deficiency and the mechanical properties of the crystals was found: calcium deficiency lead to an 80% reduction in the hardness and elastic modulus and at least a 75% reduction in toughness in plate-shaped HA crystals.316 According to the chemical formula of CDHA (Table 1), there are vacancies of Ca2+ [mainly on Ca(2) sites] and OH- ions in crystal structure of this compound.312,314-319 However, due to Ca2+ vacancies in CDHA, the resulting negative charge might be compensated by protonation of both an OH- ion within the deficient calcium-triangle and a PO43- ion in the nearest neighborhood of the vacant calcium site. This results in the presence of some water in the CDHA structure: Ca10-x(HPO4)x(PO4)6-x(OH)2-x(H2O)x (0 < x < 1).313 According to this approach, there are no hydroxide vacancies in CDHA, just a portion of OH- ions are substituted by water molecules. Concerning possible vacancies of orthophosphate ions, nothing is known about their presence in CDHA. It is considered that a portion of PO43- ions is either protonated (as HPO42-) or substituted by other ions (e.g., CO32-).320 Theoretical investigations of the defect formation mechanism relevant to non-stoichiometry in CDHA are available in reference 321.
Unsubstituted CDHA (i.e., that containing ions of Ca2+, PO43-, HPO42- and OH- only) does not exist in biological systems. However, the ion substituted CDHA, Na+, K+, Mg2+, Sr2+ for Ca2+; CO32- for PO43- or HPO42-; F-, Cl-, CO32- for OH-, plus some water forms biological apatite, the main inorganic part of animal and human normal and pathological calcifications.26,83,84 Therefore, CDHA is a very promising compound for industrial manufacturing of artificial bone substitutes,322 including drug delivery applications.323 Non-biomedical applications of CDHA are similar to those of ACP and HA. Interestingly, CDHA was found to possess a catalytic activity to produce biogasoline.324
HA (or HAp, or OHAp)
Hydroxyapatite[g] [Ca5(PO4)3(OH), but is usually written as Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two molecules; the IUPAC name is pentacalcium hydroxide tris(orthophosphate)] is the second most stable and least soluble calcium orthophosphate after FA. Chemically pure HA crystallizes in the monoclinic space group P21/b.325 However, at temperatures above ~250°C, there is a monoclinic to hexagonal phase transition in HA (space group P63/m).27,113,266,326,327 The detailed description of the HA structure was first reported in 1964,328 and its interpretation in terms of aggregation of Ca9(PO4)6 clusters, the so-called Posner’s clusters, has been widely used since the publication of the article by Posner and Betts.273 The Ca9(PO4)6 clusters appeared to be energetically favored in comparison to alternative candidates, including Ca3(PO4)2 and Ca6(PO4)4 clusters.329 In hexagonal HA, the hydroxide ions are more disordered within each row when compared with the monoclinic form, pointing either upward or downward in the structure. This induces strains that are compensated for by substitutions or ion vacancies. Some impurities, like partial substitution of hydroxide by fluoride or chloride, stabilize the hexagonal structure of HA at ambient temperature. For this reason, hexagonal HA is seldom the stoichiometric phase, and it is very rare that single crystals of natural HA exhibit the hexagonal space group. The crystal structure of HA is well-described in references 27 and 112–114 the detailed analysis of the electronic structure, bonding, charge transfer, optical and elastic properties are also available,330-334 while the readers interested in Posner’s clusters are referred to other papers.329,335-337 A shell model was developed to study the lattice dynamics of HA,338 while a cluster growth model was created to illustrate its growth.280 Polarization characteristics339,340 and pyroelectrical properties341 of HA bioceramics have been investigated. First-principles calculations for the elastic properties of doped HA342 and vacancy formation in HA343 were performed. Computer simulations of the structures and properties of HA are well-described in recent feature articles.344,345
Several techniques might be utilized for HA preparation; they can be divided into solid-state reactions and wet methods,346 which include precipitation, hydrothermal synthesis and hydrolysis of other calcium orthophosphates. Even under the ideal stoichiometric conditions, the precipitates are generally non-stoichiometric, suggesting intermediate formation of precursor phases, such as ACP and CDHA. HA can be prepared in aqueous solutions by mixing exactly stoichiometric quantities of Ca- and PO4-containing solutions at pH > 9, followed by boiling for several days in CO2-free atmosphere (the aging or maturation stage), filtration, drying and, usually, sintering at about 1,000°C.347 As the first precipitates are rich in non-apatitic environments (see ACP and CDHA), the aging stage appears to be very important: the Ca/P molar ratio of 1.67 was attained in as little as 5 h after the completion of the reaction at 90°C.348 The surface of freshly precipitated HA is composed of a structured hydrated layer containing easily exchangeable mobile ionic species.349 Usually unsintered HA is poorly crystalline and often non-stoichiometric, resembling the aforementioned CDHA. However, well crystalline HA can be prepared from an aqueous solution.350 Microcrystalline samples of HA can also be prepared by solid-state reaction of other calcium phosphates (e.g., MCPM, DCPA, DCPD, OCP) with CaO, Ca(OH)2 or CaCO3 at temperatures above ~1,200°C in an atmosphere of equal volumes of water and nitrogen. HA can be prepared by hydrothermal synthesis.27,266,351,352 A water-free synthesis can be performed in ethanol from Ca(OEt)2 (Et = ethyl) and H3PO4.353,354 In addition, HA might be prepared by mechanochemical synthesis of a dry mixture of CaO and DCPD346,355 or from coral skeletal carbonate by hydrothermal exchange.356-358 Relatively large single crystals of HA might be prepared from those of chlorapatite359 or by a recently developed controlled homogeneous precipitation method.360 Smaller sized particles of HA might be prepared by a pyrosol technique, where an aerosol containing calcium and orthophosphate ions in the adequate ratio is transported to a furnace where the pyrolisis takes place.361 Synthesis of nano-sized HA has also been described in references 362 and 363, while the chronological development of nano-sized HA synthesis might be found in another paper.364 Two-dimensional nanocrystalline HA might be also synthesized.365 Space-grown and terrestrial HA crystals were found to differ in size: the former appeared to be at least 1–1.5 orders of magnitude bigger in length.366,367 Transparent HA ceramics is also known.368-371 The detailed information on HA synthesis is available in references 372–380. In addition, there are good reviews on HA solubility, crystal growth and intermediate phases of HA crystallization381 as well as on HA dissolution.382
Pure HA never occurs in biological systems. However, due to the chemical similarities to bone and teeth mineral (Table 2), HA is widely used as a coating on orthopedic (e.g., hip joint prosthesis) and dental implants.383-390 HA particles might be implanted as well.391 Due to a great similarity to biological apatite, HA has been used in liquid chromatography of nucleic acids, proteins and other biological compounds392-401 and for drug delivery purposes402-405 for a long time. Also, HA is added to some brands of toothpaste as a gentle polishing agent instead of calcium carbonate.406,407 Non-biomedical applications of HA include its use as an environmentally friendly filler for elastomers,408 a sorbent of poisonous chemical elements409,410 and a carrier for various catalysts.411-413 Furthermore, HA by itself might act as a catalyst for formaldehyde combustion at room temperature.414 To conclude this topic, one should mention other reviews devoted to HA and its biomedical applications.415-419
FA (or FAp)
Fluorapatite [Ca5(PO4)3F, usually written as Ca10(PO4)6F2 to denote that the crystal unit cell comprises two molecules; the IUPAC name is pentacalcium fluoride tris(orthophosphate) is the only ion-substituted calcium orthophosphate considered in this review. It is the hardest (5 according to the Mohs’ scale of mineral hardness), most stable and least soluble compound among all calcium orthophosphates (Table 1). Perhaps, such “extreme” properties of FA are related to the specific position of F- ions in the center of Ca(2) triangles of the crystal structure.113 Due to its properties, FA is the only calcium orthophosphate that naturally forms large deposits suitable for the commercial use36-39 (see also Fig. 2). Preparation techniques of the chemically pure FA are similar to the aforementioned ones for HA, but the synthesis must be performed in presence of the necessary amount of F- ions (usually, NaF or NH4F is added). Unlike that for HA (see CDHA), no data are available on existence of calcium-deficient FA. Under some special crystallization conditions (e.g., in presence of gelatin or citric acid), FA might form an unusual dumbbell-like fractal morphology that, finally, close into spheres (Fig. 8).420-426 A hierarchical structure for FA was proposed.427 The crystal structure of FA was studied for the first time in 1930428,429 and is well-described in references 27, 112–114 and 430. The detailed analysis of the electronic structure, bonding, charge transfer and optical properties is available as well.332 In addition, there are reviews on FA solubility381 and the dissolution mechanism.382
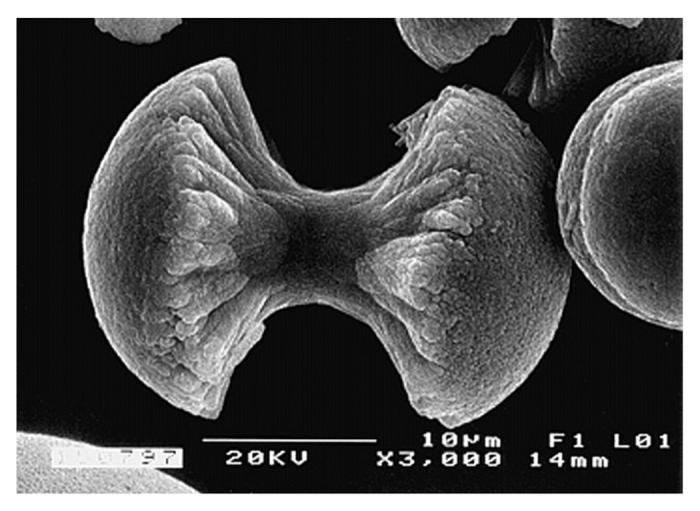
Figure 8. A biomimetically grown aggregate of FA that was crystallized in a gelatin matrix. Its shape can be explained and simulated by a fractal growth mechanism. Scale bar: 10 µm. Reprinted from reference 420 with permission.
FA easily forms solid solutions with HA with any desired F/OH molar ratio. Such compounds are called fluorhydroxyapatites (FHA) or hydroxyfluorapatites (HFA) and described with a chemical formula Ca10(PO4)6(OH)2-xFx, where 0 < x < 2. If the F/OH ratio is either uncertain or not important, the chemical formula of FHA and HFA is often written as Ca10(PO4)6(F,OH)2. The lattice parameters, crystal structure, solubility and other properties of FHA and HFA lay in between those for the chemically pure FA and HA.431-435
Similar to pure HA, pure FA never occurs in biological systems. Obviously, a lack of the necessary amount of toxic fluorides (the acute toxic dose of fluoride is ~5 mg/kg of body weight) in living organisms is the main reason of this fact (pure FA contains 3.7% mass F). Enameloid of shark teeth32,103,436-440 and some exoskeletons of mollusks441 seem to be the only exclusions, because they contain substantial amounts of FA. Among all normal calcified tissues of humans, the highest concentration of fluorides is found in bones and the lowest in dental enamel.[h] However, even in bones, the total amount of fluorides is not enough to form FA; it is generally considered that the inorganic part of bones consists of ion-substituted CDHA. Due to its low solubility, good chemical stability and the toxicity of high amounts of fluorides, chemically pure FA is rarely used as a bone substituting material.442 However, various FA-containing composites,443-445 FHA446,447 and porous FA bioceramics448 seem to be better candidates for biomedical applications. Furthermore, due to the ability to form FHA and/or HFA, minor amounts of fluorides might be intentionally added to calcium orthophosphate biomaterials.449-455 The effect of fluoride contents in FHA on both osteoblast behavior456,457 and leukemia cells proliferation458 has been described. Non-biomedical applications of FA include its application as a catalyst.459
OA (or OAp, or OXA)
Oxyapatite [Ca10(PO4)6O; the IUPAC name is decacalcium oxide hexakis(phosphate)] is the least studied calcium orthophosphate. To the best of my knowledge, pure OA has never been prepared; therefore, its properties are not well-established. Furthermore, still there are doubts that pure OA exists. However, a mixture of OA and HA (oxy-HA) might be prepared by dehydration of HA at temperatures exceeding ~900°C (e.g., during plasma-spray of HA) only in the absence of water vapor.27,28,460,461 It also might be crystallized in glass-ceramics.462 Computer modeling techniques have been employed to qualitatively and quantitatively investigate the dehydration of HA to OA.463 OA has the hexagonal space group symmetry P (174) of cesanite type,112 while the space group symmetry for partially dehydrated HA was found to change from hexagonal P63/m to triclinic P when more than ca. 35% of the structurally bound water had been removed.461 OA has no stability field in aqueous conditions;464 it is very reactive and transforms to HA in contact with water vapor.460 Due to the aforementioned problems with OA preparation, no information on biomedical applications of pure OA is available. Plasma-sprayed coatings of HA, in which OA might be present as an admixture phase, seem to be the only application.
TTCP (or TetCP)
Tetracalcium phosphate or tetracalcium orthophosphate monoxide [Ca4(PO4)2O; the IUPAC name is tetracalcium oxide bis(orthophosphate); the mineral hilgenstockite465] is the most basic calcium orthophosphate. However, its solubility in water is higher than that of HA (Table 1). TTCP cannot be precipitated from aqueous solutions. It can be prepared only by a solid-state reaction at temperatures above 1300°C, e.g., by heating homogenized equimolar quantities of DCPA and CaCO3 in dry air or in a flow of dry nitrogen.27,266,466,467 These reactions should be performed in a dry atmosphere in a vacuum or with rapid cooling (to prevent uptake of water and formation of HA). DCPA might easily be replaced by ammonium orthophosphates,468,469 while calcium carbonate might be replaced by calcium acetate.469 Furthermore, TTCP often appears as an unwanted byproduct in plasma-sprayed HA coatings, where it is formed as a result of the thermal decomposition of HA to a mixture of high-temperature phases of α-TCP, TTCP and CaO.470 TTCP is metastable: in both wet environment and aqueous solutions, it slowly hydrolyzes to HA and calcium hydroxide.27,266,471 Consequently, TTCP is never found in biological calcifications. In medicine, TTCP is widely used for preparation of various self-setting calcium orthophosphate cements;120,127,143,159,165,166,252,470,472 however, to the best of my knowledge, there is no commercial bone-substituting product consisting solely of TTCP. For the comprehensive information on TTCP, the readers are referred to a recent review in reference 470, while the structure,473 spectra474 and solubility217 of TTCP are well-described elsewhere.
There is an opinion,113,184 that all calcium orthophosphates listed in Table 1 might be classified into three major structural types: (1) the apatite type, Ca10(PO4)6X2, which includes HA, FA, OA, CDHA, OCP and TTCP; (2) the glaserite type, named after the mineral glaserite, K3Na(SO4)2, which includes all polymorphs of TCP and, perhaps, ACP and (3) the Ca-PO4 sheet-containing compounds, which include DCPD, DCPA, MCPM and MCPA. According to the authors, a closer examination of the structures revealed that all available calcium orthophosphates could be included into distorted glaserite type structures, but with varying degrees of distortion.113,184
Biphasic, triphasic and multiphasic calcium orthophosphate formulations
Calcium orthophosphates might form biphasic, triphasic and multiphasic (polyphasic) compositions, in which the individual components cannot be separated from each other.475 Presumably, the individual phases of such compositions are homogeneously “mixed” at well below submicron level (< 0.1 µm) and strongly integrated with each other. Nevertheless, the presence of all individual phases is easily seen by X-ray diffraction technique.
The main idea of the multiphasic concept is determined by a balance of more stable calcium orthophosphate phases (e.g., HA) and more soluble calcium orthophosphate phases (e.g., any type of TCP). The usual way to prepare biphasic, triphasic and multiphasic calcium orthophosphates consists of sintering non-stoichiometric calcium orthophosphates, such as ACP and CDHA, at temperatures above ~700°C. Furthermore, a thermal decomposition of the stoichiometric calcium orthophosphates at temperatures above ~1300°C might be used as well;476,477 however, this approach often results in the formation of complicated mixtures of various products including admixtures of CaO, calcium pyrophosphates, etc. Namely, transformation of HA into polyphasic calcium orthophosphates by annealing in a vacuum occurs as this: the outer part of HA is transformed into α-TCP and TTCP, while the α-TCP phase of the surface further transforms into CaO. Besides, in the boundary phase, HA is transformed into TTCP.476
Historically, Nery, Lynch and coworkers first used the term biphasic calcium phosphate (BCP) in 1986 to describe a bioceramic that consisted of a mixture of HA and β-TCP.226 Based on the results of X-ray diffraction analysis, these authors found that the “tricalcium phosphate” preparation material used in their early publication227 was in fact a mixture of ~20% HA and ~80% β-TCP. Currently, only biphasic and triphasic calcium orthophosphate formulations are known; perhaps more complicated formulations will be manufactured in the future. Furthermore, nowadays, only multiphasic and/or polyphasic compositions consisting of high-temperature phases of calcium orthophosphates, such as α-TCP, β-TCP, HA and, perhaps, high-temperature ACP, OA and TTCP, are known. No precise information on multiphasic compositions containing MCPM, MCPA, DCPD, DCPA, low-temperature ACP, OCP and CDHA has been found in the literature.475 Perhaps, such formulations will be produced in future.
All BCP formulations might be subdivided into two major groups: those consisting of calcium orthophosphates having either the same (e.g., α-TCP and β-TCP) or different (e.g., β-TCP and HA) molar Ca/P ratios. Among all known BCP formulations, BCP consisting of HA and β-TCP is both the most known and the best investigated.297-308 In 1986, LeGeros in the USA and Daculsi in France initiated the basic studies on preparation of this type of BCP and its in vitro properties. This material is soluble and gradually dissolves in the body, seeding new bone formation as it releases calcium and orthophosphate ions into the biological medium. Presently, commercial BCP products of different or similar HA/β-TCP ratios are manufactured in many parts of the world as bone-graft or bone substitute materials for orthopedic and dental applications under various trade marks and several manufacturers.307 A similar combination of α-TCP with HA forms BCP as well.241,242,244,478-481
Recently the concept of BCP has been extended by preparation and characterization of biphasic TCP (BTCP), consisting of α-TCP and β-TCP phases.482-486 The biphasic TCP is usually prepared by heating ACP precursors484-486 in which the α-TCP/β-TCP ratio can be controlled by aging time and pH value during synthesis of the amorphous precursor.485 Furthermore, triphasic formulations, consisting of HA, α-TCP and β-TCP487 or HA, α-TCP and TTCP476,477 have been prepared.
It is important to recognize that the major biomedical properties (such as bioactivity, bioresorbability, osteoconductivity and osteoinductivity) of the multiphasic and/or polyphasic compositions might be adjusted by changing the ratio among the calcium orthophosphate phases. When compared with both α- and β-TCP, HA is a more stable phase under the physiological conditions, as it has a lower solubility (Table 1) and, thus, slower resorption kinetics. Therefore, due to a higher biodegradability of the α- or β-TCP component, the reactivity of BCP increases with the TCP/HA ratio increasing. Thus, in vivo bioresorbability of BCP can be adjusted through the phase composition. Similar conclusions are also valid for both the biphasic TCP (in which α-TCP is a more soluble phase) and the triphasic (HA, α-TCP and β-TCP) formulation.
A phase transition from α-TCP into β-TCP in three types of BCPs (HA + TCP) was investigated, and the experimental results indicated that a sintering temperature for the complete phase transition from α-TCP into β-TCP increased with increasing HA content in BCP.488 Further details on biphasic, triphasic and multiphasic calcium orthophosphate formulations might be found in a very recent review in reference 475.
Ion-substituted calcium orthophosphates
Finally, one should very briefly mention the existence of carbonated apatite,489-495 chlorapatite496-498 as well as a great number of various ion-substituted calcium orthophosphates.83,499,500 Usually, they are of a non-stoichiometric nature, and there are too many of them to be mentioned in one review. Currently, this is a hot investigation topic; therefore, the readers are referred to other books and reviews in references 26–28, 32, 36, 38, 48, 266 and 416. In addition, there is a very good review, in which the structures of more than 75 chemically different apatites have been discussed in reference 112.
To conclude this topic, it is interesting to note that chemical elements not found in natural bones can be intentionally incorporated into calcium orthophosphate biomaterials to get special properties. For example, addition of Ag+,501-503 Zn2+,503,972 and Cu2+,503,972,973 has been used for imparting antimicrobial effect, while radioactive isotopes of 90Y,504,153Sm181,505-507Re505 have been incorporated into HA bioceramics and injected into knee joints to treat rheumatoid joint synovitis.504,505,507 More to the point, apatites were found to incorporate individual molecules, such as water, oxygen and carbon dioxide.83
Biological Hard Tissues of Calcium Orthophosphates
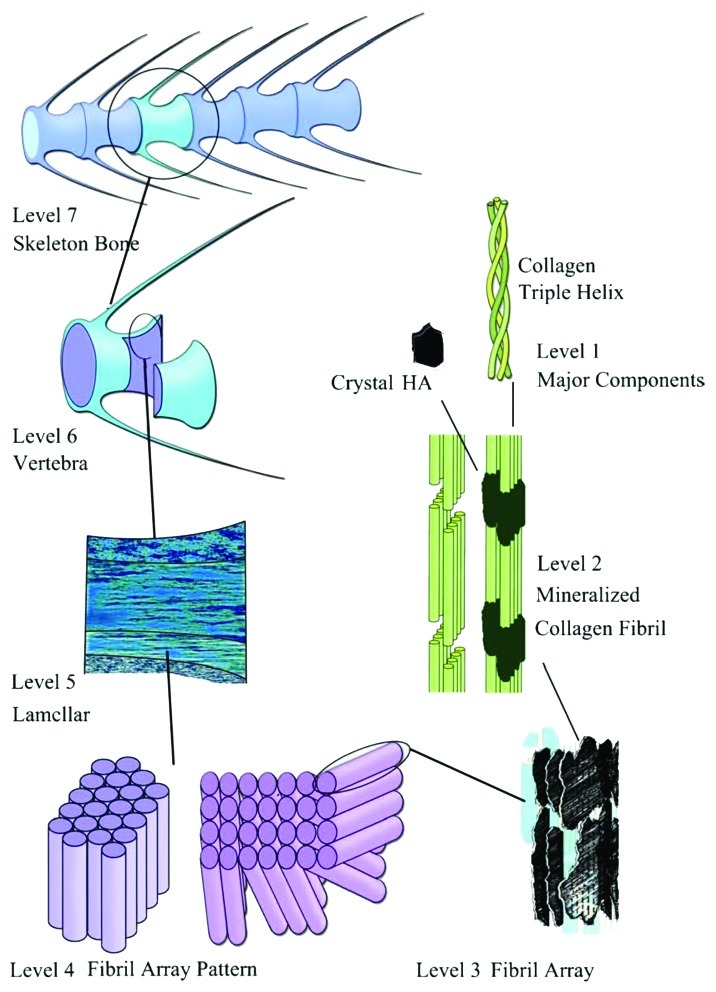
Biological mineralization (or biomineralization) is the process of in vivo formation of inorganic minerals (so-called, biominerals). One should stress, that the term “biomineral” refers not only to a mineral produced by organisms, but also to the fact that almost all of these mineralized products are composite materials comprised of both inorganic and bioorganic components. Furthermore, having formed in vivo under well-controlled conditions, the biomineral phases often have properties, such as shape, size, crystallinity, isotopic and trace element compositions, quite unlike their inorganically formed counterparts (please, compare Figs. 2, 8, 10 and 14). Thus, the term “biomineral” reflects all this complexity.103,438
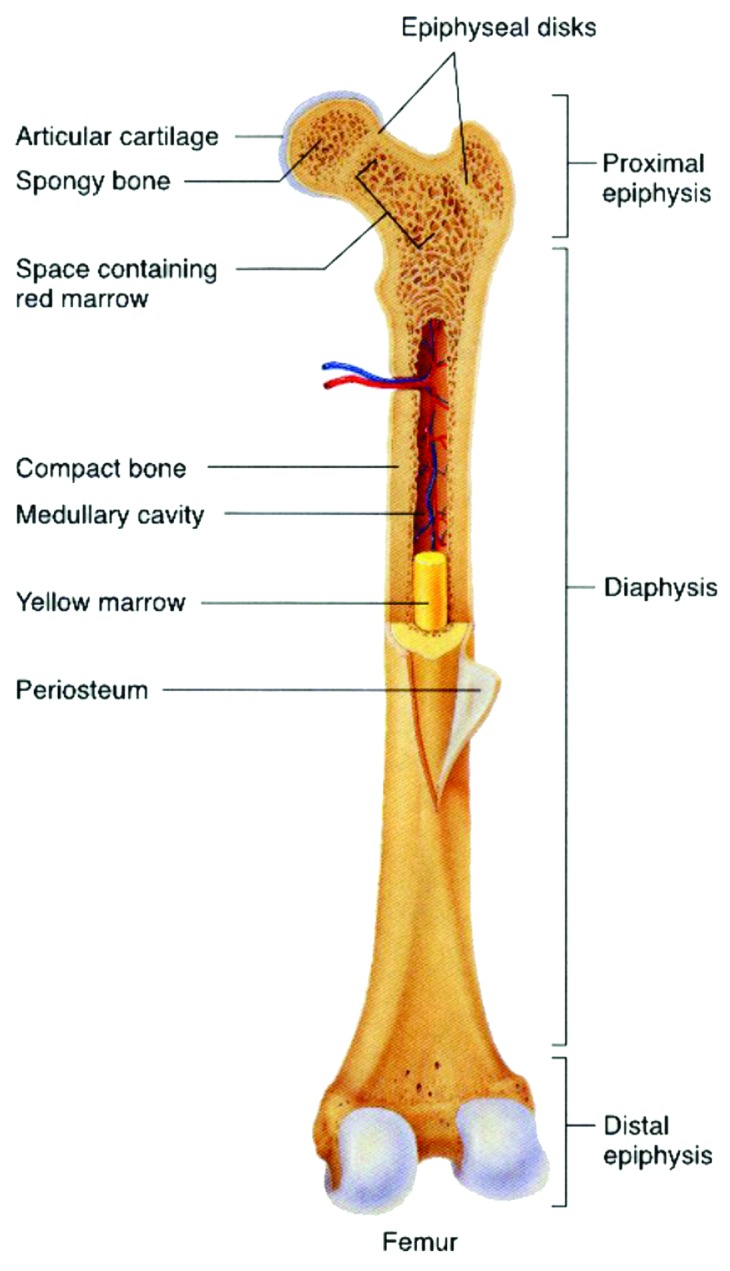
Figure 10. General structure of a mammalian bone. Other very good graphical sketches of the mammalian bone structure are available in references 88 and 508.

Figure 14. Scanning electron micrograph of the forming enamel of a continuously growing rat incisor showing ordered rods of calcium orthophosphates. Scale bar: 10 µm. Reprinted from reference 103 with permission.
As shown in Table 2 and discussed above, in the body of mammals, the vast majority of both normal and pathological calcifications consist of non-stoichiometric and ion-substituted calcium orthophosphates, mainly of apatitic structure.88,509 At the atomic scale, nano-sized crystals bone apatite exhibit a variety of substitutions and vacancies that make the Ca/P molar ratio distinct from the stoichiometric HA ratio of 1.67. Their chemical composition is complicated and varies in relatively wide ranges. This depends on what the animal has ingested.510 Occasionally, attempts are performed to compose chemical formulas of biological apatites. For example, the following formula Ca8.856Mg0.088Na0.292K0.010(PO4)5.312(HPO4)0.280(CO3)0.407(OH)0.702Cl0.078(CO3)0.050 was proposed to describe the chemical composition of the inorganic part of dental enamel.511
The impurities in biological apatite of bones and teeth introduce significant stresses into the crystal structure, which make it less stable and more reactive. Among all substituting ions, the presence of 4–8% of carbonates instead of orthophosphate anions (so called, B-type substitution26-28,493) and 0.5–1.5% of Mg is of special importance, because it leads to large lattice strain and significantly increases the solubility.509,511,512 Higher concentrations of magnesium and carbonates in bone or dentine compared with those in enamel (Table 2) may explain a higher solubility and a lower crystallinity (smaller crystal size) of bone or dentine compared with enamel.
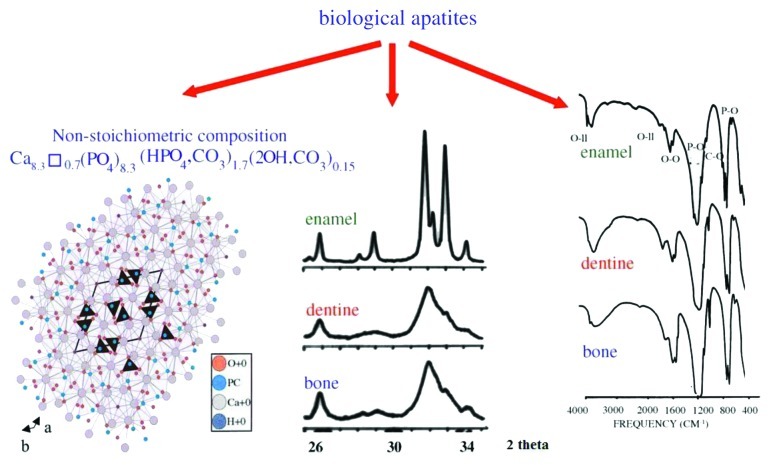
In addition, the crystals of biological apatite are always very small, which also increases its solubility when compared with that for the chemically pure HA and even CDHA.83 However, biologic apatites of enamel have considerably larger crystal size (about 2,000 nm) compared with that of either bone or dentine apatite, as indicated by the well-defined diffraction peaks in the X-ray diffraction profile of enamel apatite and much broader diffraction peaks of either bone or dentine apatites (Fig. 9, center). Small dimensions and a low crystallinity are two distinct features of biological apatites, which, combined with their non-stoichiometric composition, inner crystalline disorder and presence of other ions in the crystal lattice, allow explaining their special behavior. For example, the small crystal size means that a large percentage of the atoms are on the surface of the crystals, providing a large specific surface area for sorption of ions, proteins and drugs.508,512 The major physical properties of biological apatite are summarized in Figure 9. It is interesting to note, that the solubility and equilibrium phenomena of calcium orthophosphates related to the calcification process have been studied at least since 1925.513,514
Figure 9. Left: crystal structure of a biological apatite. Powder X-ray diffraction patterns (center) and infrared spectra (right) of human enamel, dentine and bone. Reprinted from reference 508 with permission.
Attempts to mimic the calcium orthophosphate nature of bones were first performed in 1913.515 This discovery was clarified afterwards, suggesting that the bone mineral could be carbonated apatite.516,517 Further optical and X-ray analysis of bones and other mineralized tissues matched analyses of two apatites: FA and dahllite.518 Additional historical data on this point are available in literature.42 Nowadays, according to Weiner and Wagner, “the term bone refers to a family of materials, all of which are built up of mineralized collagen fibrils.”104,519 For mammals, this family of materials includes dentine, the material that constitutes the inner layers of teeth, cementum, the thin layer that binds the roots of teeth to the jaw, deer antlers and some other materials.104,105 It is worth noting, that bones and teeth contain almost 99% of the total body calcium and about 85% of the total body phosphorus, which amounts to a combined mass of approximately 2 kg in an average person.520,521 In addition, it is important to recognize that calcium orthophosphates of bones are by no means inert; they play an important role in the metabolic functions of the body. The recent data on the physico-chemical and crystallographic study of biological apatite have been reviewed in reference 511. Besides, there is a comprehensive review on the application of surface science methods to study the properties of dental materials and related biomaterials.522
Bone
Bone, also called osseous tissue (Latin: os), is a type of hard endoskeletal connective tissue found in many vertebrate animals. All the bones of a single animal are, collectively, known as the skeleton. True bones are present in bony fish (osteichthyes) and all tetrapods. Bones support body structures, protect internal organs and, in conjunction with muscles, facilitate movement.523 In addition, bones are also involved in blood cell formation, calcium metabolism and act for mineral storage. From the material point of view, bone is a dynamic, highly vascularized tissue that is formed from a complicated biocomposite containing both inorganic (Table 2) and bioorganic (chiefly, collagen) compounds.509,524-530 Furthermore, there is a cellular phase that consists of three different types of cells: osteoblasts, osteoclasts and osteocytes. The inorganic to bioorganic ratio is approximately 75% to 25% by dry weight and about 65% to 35% by volume. This ratio not only differs among animals, among bones in the same animal and over time in the same animal, but also it exerts a major control on the material properties of bone, such as its toughness, ultimate strength and stiffness. In general, load-bearing ability of bones depends on not only architectural properties, such as cortical thickness and bone diameter, but also intrinsic, size-independent material properties, such as porosity, level of mineralization, crystal size and properties derived from the organic phase of bone.531 A higher mineral to collagen ratio typically yields stronger but more brittle, bones.532-534 For example, bone from the leg of a cow has a relatively high concentration of calcium orthophosphates (for support), whereas bone from the antler of a deer has a relatively high concentration of collagen (for flexibility).122 It is interesting to note, that bone exhibits several physical properties such as piezoelectricity535 and pyroelectricity.536
Stability of the mineral composition of bones has a very long history: calcium orthophosphates were found in dinosaur fossils.53,100,537-540 Therefore, organisms have had a great deal of time to exploit the feedback between composition and structure in apatite, on the one hand, and benefit from its biological functionality, on the other. Bone in modern animals is a relatively hard and lightweight porous composite material, formed mostly of biological apatite (i.e., poorly crystalline CDHA with ionic substitutions). It has relatively high compressive strength but poor tensile strength.541 While bone is essentially brittle, it has a degree of significant plasticity contributed by its organic components.
The distribution of the inorganic and bioorganic phases depends on a highly complex process that takes place during bone formation. Each of these components may be assembled in different proportions, creating two different architectural structures depending on the bone type and function. They are characterized by different structural features that strongly correlate with the mechanical performance of the tissue. These two types of bones are (1) the cortical bone (or compact bone), which is a dense structure and (2) the cancellous bone (also known as trabecular or spongious bone), which is less dense and less stiff than compact bone. Usually, bone is composed of a relatively dense outer layer of cortical bone covering an internal mesh-like structure (average porosity of 75–95%) of cancellous bone, the density of which is about 0.2 g/cm3, but it may vary at different points (Fig. 10). Cortical bone makes up a large portion of the skeletal mass; it has a high density (~1.80 g/cm3) and a low surface area. Cancellous bone has an open meshwork or honeycomb-like structure. It has a relatively high surface area but forms a smaller portion of the skeleton. Bone is a porous material, with the pore sizes range from 1 to 100 ∝m in normal cortical bones and 200 to 400 µm in trabecular bones. 55 to 70% of the pores in trabecular bones are interconnected. The porosity reduces the strength of bones but also reduces their weight.26,32,84,85,101-104,525-529,542-546
Bones can be either woven or lamellar. The fibers of woven bones are randomly aligned and, as a result, have a low strength. In contrast, lamellar bones have parallel fibers and are much stronger. Woven bones are put down rapidly during growth or repair,547 but as growth continues, they are often replaced by lamellar bones. The replacement process is called “secondary bone formation” and described in details in reference 548. In addition, bones might be long, short, flat and irregular. The sizes and shapes of bones reflect their function. Namely, broad and flat bones, such as scapulae, anchor large muscle masses, flat skull bones protect the brain, ribs protect the lungs, pelvis protects other internal organs, short tubular bones in the digits of hands and feet provide specific grasping functions, hollow and thick-walled tubular bones, such as femur or radius, support weight and long bones enable locomotion.549,550 Long bones are tubular in structure (e.g., the tibia). The central shaft of a long bone is called the diaphysis and has a medullar cavity filled with bone marrow (Fig. 10). Surrounding the medullar cavity is a thin layer of cancellous bone that also contains marrow. The extremities of the bone are called the epiphyses and are mostly cancellous bone covered by a relatively thin layer of compact bone. Short bones (e.g., finger bones) have a similar structure to long bones, except that they have no medullar cavity. Flat bones (e.g., the skull and ribs) consist of two layers of compact bone with a zone of cancellous bone sandwiched between them. Irregular bones (e.g., vertebrae) do not conform to any of the previous forms. Thus, bones are shaped in such a manner that strength is provided only where it is needed. All bones contain living cells embedded in a mineralized organic matrix that makes up the main bone material.549-551 The structure of bones is most easily understood by differentiating between seven levels of organization, because bones exhibit a strongly hierarchical structure (Fig. 11).103,104,416,509,524-529,535-540,542-545,553-558
Figure 11. The seven hierarchical levels of organization of the zebrafish skeleton bone. Level 1: Isolated crystals and part of a collagen fibril with the triple helix structure. Level 2: Mineralized collagen fibrils. Level 3: The array of mineralized collagen fibrils with a cross-striation periodicity of nearly 60–70 nm. Level 4: Two fibril array patterns of organization as found in the zebrafish skeleton bone. Level 5: The lamellar structure in one vertebra. Level 6: A vertebra. Level 7: Skeleton bone. Reprinted from reference 552 with permission. Other good graphical sketches of the hierarchical structure of bones are available in references 104, 553 and 554.
The mechanical properties of bones reconcile high stiffness and high elasticity in a manner that is not yet possible with synthetic materials.554 Cortical bone specimens have been found to have tensile strength in the range of 79–151 MPa in longitudinal direction and 51–56 MPa in transversal direction. Bone’s elasticity is also important for its function, giving the ability to the skeleton to withstand impact. Estimates of modulus of elasticity of bone samples are of the order of 17–20 GPa in the longitudinal direction and 6–13 GPa in the transversal direction.559 The elastic properties of bone were successfully modeled at the level of mineralized collagen fibrils via step-by-step homogenization from the staggered arrangement of collagen molecules up to an array of parallel mineralized fibrils.560 Recent investigations revealed that bone deformation was not homogeneous, but distributed between a tensile deformation of the fibrils and a shearing in the interfibrillar matrix between them.561,562 Readers who are interested in further details are addressed to a good review on the effects of the microscopic and nano-scale structure on bone fragility.563
The smallest level of the bone hierarchy consists of the molecular components: water, biological apatite, collagen and other proteins.509 The second smallest hierarchical level is formed by mineralization of collagen fibrils, which are of 80 to 100 nm thickness and a length of a few to tens of microns (Fig. 11). Thus, biocomposites of biological apatite and molecules of type I collagen are formed.88,104,524,530,564 Some evidence for direct physical bonding between the collagen fibers and apatite crystals in bone has been found.565 Eppell et al. used atomic force microscopy to measure the crystallites of mature cow bone.566 They are always platelet-like (elongated along the crystallographic c-axis) and very thin,87,567-569 with remarkably uniform thicknesses (determined in transmission electron microscopy) of 2–4 nm[i] (just a few unit cells thick, see Table 2). The nano-sized crystals of biological apatite exist in bones, not as discrete aggregates, but rather as a continuous phase, which is indirectly evidenced by a very good strength of bones. This results in a very large surface area facing extracellular fluids, which is critically important for the rapid exchange of ions with these fluids. The nano-sized crystals of biological apatite are inserted in a nearly parallel way into the collagen fibrils, while the latter are formed by self-assembly[j] of collagen triple helices104,524,552,570-572 using the self-organization mechanism.573,574 Recent data from electron diffraction studies revealed that the mineral plates of biological apatite are not quite as ordered as previously assumed.548 This imperfect arrangement of nearly parallel crystals has been supported by recent SAXS and transmission electron microscopy studies.575
The lowest level of hierarchical organization of bone has successfully been simulated by CDHA precipitation on peptide-amphiphile nanodimensional fibers.574 However, apatite platelets nucleating on the surface of peptide tubules are not similar to the nanostructure of bone, and they are only an example of surface-induced nucleation (and not accurately characterized either), while the nanostructure of bone consists of intra-fibrillar platelets intercalated within the collagen fibrils. Olszta and Gower were the first to truly duplicate the bone nanostructure.548 Unfortunately, the interface between collagen and crystals of biological apatite is still poorly understood; for the available details, the readers are referred to a review devoted to the structure and mechanical quality of the collagen/mineral nanodimensional biocomposite of bones.564 There is still no clear idea why the crystals of biological apatite are platelet-shaped even though dahllite has hexagonal crystal symmetry.103,104,525-529,535-540,542-546 One possible reason is that they grow via an OCP transition phase in which crystals are plate-shaped.104 Another explanation involves the presence of citrates, which strongly bound to the (10Ī0) surface of biological apatite because of space matching.576,577 Therefore, the crystal growth in the (10Ī0) direction becomes inhibited, while the citrate effect on other crystal surfaces of biological apatite appears to be very small, owing to poor space matching. Thus, after crystal growth, the (10Ī0) crystal face becomes predominant, resulting in the plate-like morphology of biological apatite.577
The processes of bone formation (ossification) and growth are very complicated ones, and it is difficult to describe them without making a deep invasion into biology. It has been studied for decades,547 but still there are missing points. Very briefly, ossification occurs through a direct or indirect process. In intramembranous (direct) ossification, direct transformation of mesenchymal cells from membranous tissue in osteoblasts occurs.199,548,578 In endochondral (or indirect) ossification, bones appear and grow as the result of calcification (or biomineralization) of connective tissues, mainly cartilage.509,548 The ossified tissue is invaginated by blood vessels, which bring ions of calcium and orthophosphate to be deposited in the ossifying tissue. The biomineralization process is controlled to some extent by cells, and the organic matrices made by those cells facilitate the deposition of crystals.551 There is an opinion, that, initially, the mineral crystals are formed in an environment rich in the so-called SIBLING (Small Integrin-Binding LIgand N-linked Glycoprotein) proteins. As bone crystals grow, there is greater association with proteins, such as osteocalcin, that regulate remodeling.579 Thus, in vivo formation of hard tissues always occurs by mineral reinforcement of the previously formed network of soft tissues.509,548-550,552
Cartilage is composed of cells (chondrocytes and their precursor forms known as chondroblasts), fibers (collagen and elastic fibers) and extracellular matrix proteins (proteoglycans, which are a special class of heavily glycosylated glycoproteins).580-582 The initial stage involves the synthesis and extracellular assembly of the collagen matrix framework of fibrils. At the second stage, the chondrocytes calcify the matrix before undergoing the programmed cell death (apoptosis). At this point, blood vessels penetrate this calcified matrix, bringing in osteoblasts (they are mononuclear cells primarily responsible for bone formation), which use the calcified cartilage matrix as a template to build bone, thus completing ossification.580-582
During ossification, the crystals of biological apatite grow with a specific crystalline orientation; the c-axes of the crystals are roughly parallel to the long axes of the collagen fibrils within which they are deposited.104,105,509,510,520-522,525-527,530,548 Earlier, it was believed that this process occurred via epitaxial growth mechanism.583 The same was suggested for dentine and enamel584,585 (see Teeth section below) as well as for more primitive living organisms. For example, in the shell of the fossil marine animal Lingula brachiopod unguis, which consists of a biological apatite, the crystal c-axes are oriented parallel to the β-chitin fibrils.441,586-589 Therefore, the orientation of biological apatite crystals parallel to the long axes of the organic framework could be a general feature of calcium orthophosphate biomineralization. However, the degree of biological apatite orientation appears to be a useful parameter to evaluate in vivo stress distribution, nano-scale microstructure and the related mechanical function, the regenerative process of the regenerated bone and to diagnose bone diseases such as osteoarthritis.590,591 It is interesting to note that, contrary to what might be expected in accordance with possible processes of dissolution, formation and remineralization of hard tissues, no changes in phase composition of mineral part, crystal sizes (length, width and thickness) and arrangement of crystals on collagen fibers were detected in abnormal (osteoporotic) human bones compared with the normal ones.592
Some animals, such as newts, are able to regenerate amputated limbs. This is, of course, of high interest in regenerative medicine. Bone regeneration in the forelimbs of mature newts was studied by noninvasive X-ray microtomography to image regenerating limbs from 37 to 85 d. The missing limb skeletal elements were restored in a proximal-to-distal direction, which reiterated the developmental patterning program. However, in contrast to this proximal-distal sequence, the portion of the humerus distal to the amputation site was found to fail to ossify in synchrony with the regenerating radius and ulna. This finding suggests that the replacement of cartilage with mineralized bone close to the amputation site is delayed with respect to other regenerating skeletal elements.593
Unlike other mineralized tissues, bone continuously undergoes a remodeling process, as it is resorbed by specialized cells called osteoclasts and formed by another type of cells called osteoblasts (so called “bone lining cells”) in a delicate equilibrium.509,548,551,594,595 The purpose of remodeling is the release of calcium and the repair of micro-damaged bones from everyday stress. Osteoblasts are mononuclear cells primarily responsible for bone formation. They contain alkaline phosphatase, which enzymatically produce orthophosphate anions needed for the mineralization. In addition, there is one more type of cells, called osteocytes, that originate from osteoblasts, which have migrated into, become trapped and surrounded by bone matrix that they themselves produce.509,525-528,548-551
If osteoblasts are bone-forming cells, osteoclasts are multinuclear, macrophage-like cells that can be described as bone-destroying cells, because they mature and migrate to discrete bone surfaces.551,594,595 Upon arrival, active enzymes, such as acid phosphatase, are secreted against the mineral substrate that causes dissolution. This process, called bone resorption, allows stored calcium to be released into systemic circulation and is an important process in regulating calcium balance.594,595 The iteration of remodeling events at the cellular level is influential on shaping and sculpting the skeleton both during growth and afterwards. That is why mature bones always consist of a very complex mesh of bone patches, each of which has both a slightly different structure and a different age.103-105,509-511,520-522,525-527,548 The interested readers are recommended a review on the interaction between biomaterials and osteoclasts.596
There is still no general agreement on the chemical mechanism of bone formation. It is clear that the inorganic part of bone consists of biological apatite, i.e., CDHA with ionic substitutions but without the detectable amounts of hydroxide.597-601 However, the recent results of solid-state nuclear magnetic resonance on fresh-frozen and ground whole bones of several mammalian species revealed that the bone crystal OH- was readily detectable; a rough estimate yielded an OH- content of human cortical bone of about 20% of the amount expected in stoichiometric HA.602 Various in vitro experiments on precipitation of CDHA and HA revealed that none of these compounds is directly precipitated from supersaturated aqueous solutions containing calcium and orthophosphate ions; some intermediate phases (precursors) are involved.26,84,85,187-193,259-263 Depending on both the solution pH and crystallization conditions, three calcium orthophosphates (DCPD, ACP and OCP) have been discussed as possible precursors of CDHA precipitation in vitro. Due to this reason, the same calcium orthophosphates are suggested as possible precursors of biological apatite formation in vivo.
The transient nature of the precursor phase of bone, if it exists at all, makes it very difficult to detect, especially in vivo.94 However, in 1966 W.E. Brown proposed that OCP was the initial precipitate that then acted as a template upon which biological apatite nucleates.186 This idea was extended in his further investigations.603-606 The principal support for this concept derived from the following: (1) the close structural similarity of OCP and HA;178,179 (2) formation of interlayered single crystals of OCP and HA (pseudomorphs of OCP); (3) the easier precipitation of OCP compared with HA; (4) the apparent plate- or lath-like habit of biological apatites that does not conform to hexagonal symmetry, but looks like a pseudomorph of triclinic OCP; (5) the presence of HPO42- in bone mineral, particularly in newly formed bones.511 Some evidences supporting this idea were found using high-resolution transmission electron microscopy: computer-simulated lattice images of the “central dark line” in mineralized tissues revealed that it consisted of OCP.187-191 Recently, Raman spectroscopic indication for an OCP precursor phase was found during intra-membranous bone formation.578 Other evidences of OCP to HA transformation, including a mechanistic model for the central dark line formation, might be found in literature.607
Simultaneously with Brown, the research group led by A.S. Posner proposed that ACP was the initially precipitated phase of bone and dentine mineral formation in vivo, thus explaining the non-stoichiometric Ca/P ratio in bones and teeth.608-610 This conclusion was drawn from the following facts: (1) when calcium orthophosphates are prepared by rapid precipitation from aqueous solutions containing ions of calcium and orthophosphate at pH > 8.5, the initial solid phase is amorphous; (2) mature bone mineral is composed of a mixture of ion-substituted ACP and poorly crystallized ion-substituted CDHA and (3) early bone mineral has a lower crystallinity than mature bone, and the observed improvement in crystallinity with the age of the bone mineral is a result of a progressive reduction in the ACP content.511,608-616 However, there are thermodynamic data proving that the transition of freshly precipitated ACP into CDHA involves intermediate formation of OCP.617,618 Recently, the discovery of a stable amorphous calcium carbonate in sea urchin spines619 reawakened the suggestion that a transient amorphous phase might also exist in bones.548,620-624 Even more recently, evidence of an abundant ACP phase in the continuously forming fin bones of zebrafish was found.625,626 The new bone mineral was found to be delivered and deposited as packages of nanodimensional spheres of ACP, which further transformed into platelets of crystalline apatite within the collagen matrix.626
Furthermore, to investigate how apatite crystals form inside collagen fibrils, researchers performed a time-resolved study starting from the earliest stages of mineral formation.627 After 24 h of mineralization, calcium orthophosphate particles were found outside the fibril, associated with the overlap region, in close proximity to the gap zone. Cryogenic energy-dispersive X-ray spectroscopy confirmed that these precipitates were composed of calcium orthophosphate, while a low-dose selected-area electron diffraction technique showed a diffuse band characteristic of ACP. After 48 h, apatite crystals started to develop within a bed of ACP, and after 72 h, elongated electron-dense crystals were abundant within the fibril, in many cases, still embedded within a less dense matrix. A low-dose selected-area electron diffraction technique demonstrated that the mineral phase consisted of both ACP and oriented apatite, the latter identical to bone apatite.627 This process is shown schematically in Figure 12.628 The modern points of view on the bone formation mechanisms have been summarized in a recent excellent review in reference 548, where the interested readers are referred.
Figure 12. A schematic illustration of in vivo mineralization of a collagen fibril: top layer-calcium orthophosphate clusters (green) form complexes with biopolymers (orange line), forming stable mineral droplets; second top layer-mineral droplets bind to a distinct region on the collagen fibers and enter the fibril; second bottom layer-once inside the collagen, the mineral in a liquid state diffuses through the interior of the fibril and solidifies into a disordered phase of ACP (black); bottom layer-finally, directed by the collagen, ACP is transformed into oriented crystals of biological apatite (yellow). Reprinted from reference 628 with permission.
The maturation mechanism of bone minerals is not well-established, mainly because of the difficulty involved in the nanostructural analyses of bone minerals.548,629 Only indirect evidence for the in vivo bone mineral maturation is available. For example, X-ray diffraction patterns of bones from animals of different ages show that the reflections become sharper with increasing age.99,630 This effect is more pronounced in the crystallographic a-axis [(310) reflections] as compared with the c-axis [(002) reflections].631,632 The most comprehensive report describing how normal human bone mineral changes in composition and crystal size as a function of age was based on X-ray diffraction analyses by Hanschin and Stern,633 who examined 117 homogenized iliac crest biopsies from patients aged 0–95 y. They found that the bone mineral crystal size and perfection increased during the first 25–30 y and then decreased thereafter, slightly increasing in the oldest individuals. The same 117 homogenized biopsy samples were analyzed by wavelength-dispersive X-ray fluorescence to quantify the carbonate substitution in biological apatite as a function of age. Although the changes observed in carbonate substitution were relatively slight (at most 10%), there was a general increase from 0 to 90 y that is distinct from the absence of a change in crystallinity after age 30 in these samples.553 In addition, other changes, like an increase of Ca2+ content and a decrease of HPO42-, occur in bone mineral with age.634-637 Both the crystal sizes and carbonate content were found to increase during aging in rats and cows.635,636 The increase in carbonate content with age has also been reported in still other studies.638-640 From a chemical point of view, these changes indicate to a slow transformation of poorly crystallized non-apatitic calcium orthophosphates into a better-crystallized ion-substituted carbonate-containing CDHA. While there are still many gaps in our knowledge, the researchers seem to be comfortable in stating that in all but the youngest bone and dentine, the only phase present is a highly disordered, highly substituted biological apatite.
In general, the biomineralization process (therefore, bone formation) can happen in two basic ways: either the mineral phase develops from the ambient environment, as it would from a supersaturated solution of the requisite ions, but requires the living system to nucleate and localize mineral deposition, or the mineral phase is developed under the direct regulatory control of the organism, so that the mineral deposits are not only localized but may be directed to form unique crystal habits not normally developed by a saturated solution of the requisite ions. In a very famous paper641 and two extended elaborations,103,642 the first type of biomineralization was called “biologically induced” mineralization and the second “(organic) matrix-mediated” biomineralization. In some papers, the former process is called “passive” and the latter one “active” biomineralization.35 Briefly, an “active process” means the assembly of nano-sized crystals of calcium orthophosphate into bones due to an activity of the suitable cells (e.g., osteoblasts), i.e., within a matrix vesicle. Such structures have been discovered by transmission electron microscopy for bone and teeth formation.643,644 A “passive” process does not require involvement of cells and means mineralization from supersaturated solutions with respect to the precipitation of biological apatite. In the latter case, thermodynamically, biomineralization might occur at any suitable nucleus. The collagen fibrils have a specific structure with a 67 nm periodicity and 35–40 nm gaps or holes between the ends of the collagen molecules, where bone mineral is incorporated in the mineralized fibril.103,104,519,530,549,550 Such a nucleation within these holes would lead to discrete crystals with a size related to the nucleating cavity in the collagen fibril (Fig. 11). It was proposed that a temporary absence of the specific inhibitors might regulate the process of bone formation.645-647
To conclude the bone subject, let me briefly mention the practical application of bones. In the Stone Age, bones were used to manufacture art, weapons, needles, catchers, amulets, pendants, headdresses, etc. Nowadays, cut and polished bones from a variety of animals are sometimes used as a starting material for jewelry and other crafts. Ground cattle bone is occasionally used as a fertilizer. Furthermore, in medicine, bones are used for bone graft substitutes, e.g., allografts from cadavers.
Teeth
Teeth (singular, tooth) are dense structures found in the jaws of many vertebrates. They have various structures to allow them to fulfill their different purposes. The primary function of teeth is to tear and chew food. For carnivores, teeth are also a weapon. Therefore, teeth have to withstand a range of physical and chemical processes, including compressive forces (up to ~700 N), abrasion and chemical attack due to acidic foods or products of bacterial metabolism.522 The roots of teeth are covered by gums. On the surface, teeth are covered by enamel up to ~2 mm thick at the cutting edges of the teeth, which helps to prevent cavities on the teeth. The biggest teeth of some gigantic animals (elephants, hippopotamuses, walruses, mammoths, narwhals, etc.) are known as tusks or ivory.
Similar to the various types of bones, there are various types of teeth. The shape of the teeth is related to the animal’s food as well as its evolutionary descent. For example, plants are hard to digest, so herbivores have many molars for chewing. Carnivores need canines to kill and tear, and since meat is easy to digest, they can swallow without the need for molars to chew the food well. Thus, the following types of teeth are known: molars (used for grinding up food), carnassials (used for slicing food), premolars (small molars), canines (used for tearing apart food) and incisors (used for cutting food). While humans only have two sets of teeth, some animals have many more; for example, sharks grow a new set of teeth every two weeks. Some other animals grow just one set during the life, while teeth of rodents grow and wear away continually through the animal gnawing, maintaining constant length.648,649
Similar to bones, the inorganic part of teeth also consists of biological apatite.650 The stability of the mineral composition of teeth also has a very long history; namely, calcium orthophosphates were found in fossil fish teeth.651 Recent investigations of biological apatite from fossil human and animal teeth revealed its similarity to the modem biological apatite.652
The structure of teeth appears to be even more complicated than that of bones (see Fig. 13). Unlike bones, teeth consist of at least two different materials: enamel, which is a hard outer layer consisting of calcium orthophosphates and dentine, which is a bone-like magnesium-rich tissue that forms the bulk of vertebrate teeth. In addition, there is a thin layer around the tooth roots called cementum. It is a thin layer of a bone-like calcified tissue that covers dentine at the roots of teeth and anchors them to the jaw.653-656 Finally, there is the core, called pulp (commonly called “the nerve”); it is a remnant of the embryologic organ for tooth development and contains nerves and blood vessels necessary for tooth function (Fig. 13).549,550,648,649 Both dentine and cementum are mineralized connective tissues with an organic matrix of collagenous proteins, while the inorganic component of them consists of biological apatite. As shown in Table 2, dentine, cementum and bone are quite similar, and for general purposes of material scientists, they can be regarded as being essentially the same material.103-105,511,520-522,525-529,535-540,542-544,564,568-570,634,635 Thus, most statements made in the previous section for bones are also valid for dentine and cementum; however, unlike bones, both dentine and cementum lack vascularization.[k]
Figure 13. A schematic drawing of a tooth. Other very good graphical sketches of the mammalian tooth structure, including the hierarchical levels, are available in references 509 and 554.
Dental enamel is the outermost layer of teeth. It is white and translucent, and its true color might be observed at the cutting edges of the teeth only. Enamel is highly mineralized and acellular, so it is not a living tissue. Nevertheless, it is sufficiently porous for diffusion and chemical reactions occur within its structure, particularly acidic dissolution (dental caries) and remineralization from saliva (possible healing of caries lesions). Enamel is the hardest substance in the body541 and forms a solid, tough and wear-resistant surface for malaxation. In the mature state, it contains up to 98% inorganic phase (Table 2). The crystals of biological apatite of enamel are much larger, as evidenced by the higher crystallinity (reflecting greater crystal size and perfection) demonstrated in their X-ray diffraction patterns, than those of bone and dentine. Besides, enamel apatite has fewer ionic substitutions than bone or dentine mineral and more closely approximates the stoichiometric HA.549 The organic phase of enamel does not contain collagen. Instead, enamel has two unique classes of proteins, called amelogenins and enamelins. While the role of these proteins is not yet fully understood, it is believed that both classes of proteins aid in enamel development by serving as a framework support.648,649,657 The large amount of minerals in enamel accounts not only for its strength, but also for its brittleness. Dentine, which is less mineralized and less brittle, compensates for enamel and is necessary as a support.648,649 Shark enameloid is an intermediate form bridging enamel and dentine. It has enamel-like crystals of fluoridated biological apatite associated with collagen fibrils.83,103,436-440 Due to the presence of fluorides, biological apatite of shark enameloid shows both higher crystal sizes and a more regular hexagonal symmetry compared with non-fluoridated biological apatite of bones and teeth.32 Similar correlation between the presence of fluorides and crystal dimensions was found for enamel.658
Like that for bones, seven levels of structural hierarchy have been also discovered in human enamel; moreover, the analysis of the enamel and bone hierarchical structure suggests similarities of the scale distribution at each level.509,559,659 On the mesoscale level, there are three main structural components: a rod, an interrod and aprismatic enamel. Among them, the enamel rod (formerly called an enamel prism) is the basic unit of enamel. It is a tightly packed mass of biological apatite in an organized pattern. Each rod traverses uninterrupted through the thickness of enamel. They number 5 to 12 million rods per crown. The rods increase in diameter (4 up to 8 microns) as they flare outward from the dentine-enamel junction (DEJ). Needle-like enamel rods might be tens of microns long (up to 100 ∝m) but sometimes only 50 nm wide and 30 nm thick (Fig. 14).648,649,660-667 They are quite different from the much smaller crystals of dentine and bone (Table 2), but all of them consist of biological apatite.422,668,669 In cross-section, an enamel rod is best compared with a keyhole, with the top or head, oriented toward the crown of the tooth and the bottom or tail, oriented toward the root of the tooth.
The arrangement of the crystals of biological apatite within each enamel rod is highly complex. Enamel crystals in the head of the enamel rod are oriented parallel to the long axis of the rod. When found in the tail of the enamel rod, the crystals’ orientation diverges slightly from the long axis.648,649 The arrangement of the enamel rods is understood more clearly than their internal structure. Enamel rods are found in rows along the tooth (Fig. 14) and, within each row, the long axis of the enamel rod is generally perpendicular to the underlying dentine.648,649,660-664 A recent AFM study indicated that CDHA crystals in enamel exhibited regular subdomains or subunits with distinct chemical properties related to topographical features and gave rise to patterned behavior in terms of the crystal surface itself and the manner in which it responded to low pH.670
The second structural component of the enamel matrix is the interrod (or interprismatic) enamel, which surrounds and packs between the rods. The difference between the rod and the interrod is the orientation of apatite crystals; the rod contains aligned crystallites, whereas the mineral in the interrod is less ordered. These structures coalesce to form the tough tissue of enamel, which can withstand high forces and resist damage by crack deflection. The third structure, aprismatic enamel, refers to the structures containing apatite crystals that show no mesoscale or macroscale alignment.509 Enamel is a selectively permeable membrane, allowing water and certain ions to pass via osmosis.648,649
The in vivo formation and development of teeth appears to be even more complicated when compared with the process described above for bone formation. The biological process by which teeth are formed from embryonic cells, grow and erupt into the mouth is very complex.551 For human teeth enamel, dentine and cementum must all be developed during the appropriate stages of fetal development. Primary (baby) teeth start to form between the sixth and eighth weeks in utero, while the permanent teeth begin to form in the twentieth week in utero.648,649 Recent data confirmed the necessity of calcium orthophosphates in the diet of pregnant and nursing mother to prevent early childhood dental caries.671
As teeth consist of at least two materials with different properties (enamel and dentine), the tooth bud (sometimes called “the tooth germ,” which is an aggregation of cells that eventually forms a tooth) is organized into three parts: the enamel organ, the dental papilla and the dental follicle. The enamel organ is composed of at least four other groups of cells (for the biological details see refs. 648 and 649). Altogether, these groups of cells give rise to ameloblasts, which secrete enamel matrix proteins. The protein gel adjacent to ameloblasts is supersaturated with calcium orthophosphates, which leads to the precipitation of biological apatite. Similarly, the dental papilla contains cells that develop into odontoblasts, which are dentine-forming cells. The dental follicle gives rise to three important entities: cementoblasts, osteoblasts and fibroblasts. Cementoblasts form the cementum of a tooth.654 Osteoblasts give rise to the alveolar bone around the roots of teeth (see bone formation above). Fibroblasts develop the periodontal ligaments that connect teeth to the alveolar bone through cementum.549-551,648,649
The first detectable crystals in enamel formation are flat thin ribbons662-664 that were reported to be OCP,542,672-674 β-(Ca,Mg)3(PO4)2,673 DCPD,597,600 or ACP.675 The formation process of enamel is different from that for bone or dentine: amelogenin being hydrophobic self-assembles into nano-sized spheres that guide the growth of the ribbon-like dental enamel crystals. During maturation of enamel, the mineral content increases from ~45 wt% initially up to ~98–99 wt%.597,648,649 The enamel crystal rods widen and thicken by additional growth597,600,676 with a simultaneous increase of the Ca/P molar ratio676 and a decrease in carbonate content,677-679 finally resulting in the most highly mineralized and hardest substance produced by vertebrates. It is interesting to note that in the radular teeth of chitons, ACP was found to be the first-formed calcium orthophosphate mineral, which, over a period of weeks, was transformed to dahllite.680
The crystal faces expressed in enamel are always (100) face and at the ends presumably (001),681,682 which are the ones usually found in HA. The centers of enamel crystals contain a linear structure known as the “central dark line” (this line was also observed in bone and dentine), which consists of OCP.187-191,607 As described above for bones, X-ray diffraction shows that the crystals of younger dentine are less crystalline than those of more mature dentine.634 Therefore, maturation of dentine also means a slow transformation (re-crystallization?) of biological calcium orthophosphates from ion-substituted ACP to a better-crystallized ion-substituted CDHA.
The development of individual enamel and dentine crystals was studied by high-resolution transmission electron microscopy.683-685 Both processes appear to be roughly comparable and were described in a four-step process. The first two steps include the initial nucleation and formation of nano-sized particles of biological apatite. They are followed by ribbon-like crystal formation, which, until recently, was considered the first step of biological crystal formation.683-685 These complicated processes, starting with the heterogeneous nucleation of inorganic calcium orthophosphates on an organic extracellular matrix, are controlled in both tissues by the organic matrix and are under cellular control.686 To complicate the process even further, regular and discrete domains of various charges or charge densities on the surface of apatite crystals derived from the maturation stage of enamel development were recently discovered by a combination of atomic and chemical force microscopy.687 Binding of organic molecules (e.g., amelogenin687) at physiological solution pH appears to occur on the charged surface domains of apatite. The modern visions on dental tissue research have been reviewed recently in reference 688.
As teeth consist of several materials, there are mutual junctions among them. For example, a dentine-enamel junction (DEJ) is the interface between dentine and enamel. It is a remnant of the onset of enamel formation, because enamel grows outwards from this junction.649,689,690 DEJ plays an important role in preventing crack propagation from enamel into dentine.691 The major steps of enamel crystal growth at the junction have been described above, but the mechanism of the junction formation is still debatable. Some authors claim that enamel crystals grow epitaxially on the preexisting dentine crystals because of a high continuity between enamel and dentine crystals.692-694 Others have shown that enamel crystals are formed at a given distance from the dentine surface672-674,695 and could either reach dentine crystals by a subsequent growth696 or remain distant.695,697 In addition, there are a cementum-enamel junction (CEJ),698 which is quite similar to DEJ, and a cementum-dentine junction (CDJ).653-655,699
Enamel formation or amelogenesis, is a highly regulated process involving precise genetic control as well as protein-protein interactions, protein-mineral interactions and interactions involving the cell membrane. Much is still unknown about the interactions among proteins present in enamel matrix and the final crystalline phase of biological apatite.509,700 At some point before a tooth erupts into the mouth, the ameloblasts are broken down. Consequently, enamel, unlike bones, has no way to regenerate itself using the process of “active mineralization” (see bone formation), because there is no biological process that repairs degraded or damaged enamel.648,649 In addition, certain bacteria in the mouth feed on the remains of foods, especially sugars. They produce lactic acid, which dissolves the biological apatite of enamel in a process known as enamel demineralization that takes place below the critical pH of about 5.5. A similar process, called enamel erosion, occurs when a person consumes acid-containing (citric, lactic, phosphoric, etc.) soft drinks.660,701-704 Evidence exist that there is a preferential loss of carbonates and Mg during acidic dissolution of mineral in dental caries. Luckily, saliva gradually neutralizes the acids that cause pH on teeth surface to rise above the critical pH. This might cause partial enamel remineralization, i.e., a return of the dissolved calcium orthophosphates to the enamel surface. Until recently, it was generally agreed, that if there was sufficient time between the intake of foods (generally, two to three hours) and if damage was very limited, teeth could repair themselves by the “passive mineralization” process.705 Data on increased remineralization of tooth enamel by milk containing added casein phosphopeptide-ACP nanodimensional complexes706 are in support of this hypothesis.
However, studies performed by using atomic force microscopy nano-indentation technique revealed that previously demineralized samples of dental enamel further exposed to remineralizing solutions did show a crystalline layer of calcium orthophosphates formed on their surface. Unfortunately, the re-precipitated deposits of calcium orthophosphates always consisted of loosely packed crystals and did not protect the underlying enamel from a subsequent acid attack. Furthermore, these surface deposits were completely removed by either a toothbrush or a short exposure to an erosive acidic solution.660,707-709 In this context, it should be emphasized that the term “remineralization,” which is often misused in the literature, should imply the process of mineral growth that goes hand in hand with a strengthening effect of the weakened enamel surface. Since no strengthening of an exposure to remineralizing solutions was observed, it might be considered that no “passive mineralization” was found (in spite of the real evidence of the re-precipitated surface deposits of calcium orthophosphates).660,708,709
An interesting hypothesis that nano-sized apatite crystallites occur in the oral cavity during extensive physiological wear of the hierarchical structured enamel surface due to dental abrasion and attrition has been published recently in reference 710. These nano-scaled apatite enamel crystallites might promote remineralization at the tooth surface. However, this idea should be verified experimentally. Thus, according to the current knowledge, the enamel self-repairing ability by a passive remineralization appears to be doubtful, while an active remineralization is impossible. Nevertheless, investigations in this field keep going.210-212,711-722 For example, ACP-containing orthodontic biocomposite resins might reduce the enamel decalcification found in patients with poor oral hygiene.722
A content of fluoride added to either toothpaste or mouthwash lowers the solubility of calcium orthophosphates (by formation of FHA on the surface) and therefore improves the acid-resistance of dental enamel.422,723-729 Furthermore, fluorides also reduce production of acids by bacteria in the mouth by reducing their ability to metabolize sugars. However, dental treatment by fluorides must be used with care, because an improper treatment results in formation of CaF2 globules deposited on the enamel surface.730
To conclude the subject of teeth, let me briefly mention the practical application of teeth. Due to relatively small dimensions of normal teeth, only tusks and ivory of giant animals are used. For example, both the Greek and Roman civilizations used large quantities of ivory to make high value works of art, precious religious objects and decorative boxes for costly objects. Ivory was often used to form the whites of the eyes of statues. Prior to introduction of plastics, it was used for billiard balls, piano keys, buttons and ornamental items. The examples of modern carved ivory objects are small statuary, netsukes, jewelry, flatware handles and furniture inlays.
Antlers
Deer antlers (Fig. 15) are unique biological structures, since their growth rate is without parallel in vertebrates, and because they are the only bony appendages in mammals capable of complete regeneration. This allows for basic research in bone biology without the interference of surgical procedures and their adverse effects in animals where samples are obtained. In addition, antlers also allow for the gathering of a large amount of samples from different populations to assess nutritional and ecological effects on bone composition and structure.732-735 They are costly sexual secondary characters of male deer and constitute 1 to 5% of their body weight.736 Recent studies suggest that antler regeneration is a stem cell-based process, and that these stem cells are located in the pedicle periosteum.731,737

Figure 15. Red deer stag at velvet shedding. The bare bone of the hard antlers is exposed. Reprinted from reference 731 with permission. A good cross-sectional image of a deer antler is available in reference 554.
Antlers are not true horns; they are a simple extension of bone, so they have a matrix of biological apatite similar to that of mammalian bones.738 Antlers are large and complex horn-like appendages of deer consisting of bony outgrowths from the head with no covering of keratin as found in true horns. Usually, they begin growing in March and reach maturity in August. In winter, antlers fall off; this is known as shedding. Similar to bones, antlers contain pores and can withstand applied stresses of over 300 MPa,739-743 which is even higher than that of bones (Table 2). Therefore, antlers are occasionally considered an almost unbreakable bone.534 Each antler grows from an attachment point on the skull called a pedicle. While an antler is growing, it is covered with highly vascular skin called velvet, which supplies oxygen and nutrients to the growing bone. Once the antler has achieved its proper size, the velvet starts to dry out, cracks and breaks off, while the antler’s bone dies. Fully developed antlers consist of dead bone only.744-753 It was found that food processing cannot supply the mineral needs required for antler growth and, thus, male deer must temporary resorb calcium orthophosphate minerals from their own skeleton for antler growth.754-756 Detailed studies revealed that daily food intake provided between 25 and 40% of calcium needed for antler mineralization, which resulted in a temporary skeleton demineralization.755,756 Interestingly, though, antlers may act as large hearing aids; moose with antlers have far more sensitive hearing than moose without.757
Antlers are a good model to study bone biology, because they are accessible, shed after mating season and cast every year.758 However, people seldom come across the antlers in the woods. Rabbits and rodents, such as mice and chipmunks, eat antlers (and bones of wild animals after they die) for calcium. Rodents and rabbits also gnaw bones and antlers to sharpen their incisors. Due to an extremely high growth rate, which can achieve 2–4 cm per day744 combined with a very fast biomineralization, these unique appendages might be a well-suited animal model for studying the disturbances of bone formation induced by additives (e.g., by excess of fluoride).746 Antler size and external characteristics were found to be influenced by nutrition, climatic variability and other factors. Thus, since antlers are periodically replaced, the analysis of naturally cast antlers offers the opportunity for a continuous and a noninvasive monitoring of the environmental pollution by these additives.746 Recently, the first attempt to evaluate a potential use of deer antlers as a bone regeneration biomaterial was performed.759
To conclude this part, let me briefly mention the practical application of antlers. Associated with aristocracy, antlers have adorned European castles and hunting lodges for centuries. Today, furnishings and accessories made from antlers are featured in fine homes throughout the world and are a reflection of grace and elegance.
Pathological Calcification of Calcium Orthophosphates
In the body of mammals, osteoblasts and odontoblasts fix ions of calcium and orthophosphate and then precipitate biological apatite onto an organic matrix. This is the process of physiological biomineralization, which is restricted to specific sites in skeletal tissues, including growth plate cartilage, bones, teeth and antlers.32,103 Normally, mammals are supposed to die with calcium orthophosphates located in bones and teeth (and antlers for male deer) only and nowhere else, because under normal conditions, soft tissues are not mineralized. Unfortunately, owing to aging, various diseases and certain pathological conditions, blood vessels, muscles, extracellular matrix of articular cartilaginous tissues of the joints and some internal organs are calcified as well. This process is called pathological calcification or ectopic (bio)mineralization and leads to morbidity and mortality.32,103,760 In general, any type of abnormal accumulation of calcium orthophosphates in the wrong place are accounted for by a disruption of systemic defense mechanism against calcification.761
To the best of my knowledge, the first paper on a negative influence of unwanted depositions of calcium orthophosphates in the body was published as early as in 1911.762 This finding was confirmed in later studies.763,764 Unwanted depositions always lead to various diseases, for instance, soft tissue calcification (in damaged joints, blood vessels, dysfunctional areas in the brain, diseased organs, scleroderma, prostate stones),220-222,765-770 kidney and urinary stones,26,771-774 dental pulp stones and dental calculus,182,183,185,219,775-778 salivary stones,779 gall stones, pineal gland calcification, atherosclerotic arteries and veins,86,780-783 coronary calcification,784 cardiac skeleton, damaged cardiac valves,785 calcification on artificial heart valves,786-790 carpal tunnel,791 cataracts,792 malacoplakia, calcified menisci,793,794 dermatomyositis795,796 and still other diseases.32 In addition, there is a metastatic calcification of nonosseous viable tissue occurring throughout the body,797,798 but it primarily affects the interstitial tissue of the blood vessels, kidney, lungs and gastric mucosa. A metastatic calcification is defined as a deposition of calcium orthophosphates in previously normal tissue due to an abnormal biochemistry with disturbances in the calcium or phosphorus metabolism.799 Common causes of the metastatic calcification include hyperparathyroidism, chronic renal disease, massive bone destruction in widespread bone metastases and increased intestinal calcium absorption. One author has mentioned “apatite diseases,” which are characterized by the appearance of needle-like crystals comparable to those of bone apatite in the fibrous connective tissue.800 All these cases are examples of calcinosis,801-803 which might be described as a formation of calcium orthophosphate deposits in any soft tissue. In dentistry, a calculus or a tartar refers to a hardened plaque on the teeth formed by the presence of saliva, debris and minerals.804 Its rough surface provides an ideal medium for bacterial growth, threatening the health of the gums and absorbing unaesthetic stains far more easily than natural teeth.26
Calcifying nanodimensional particles are the first calcium orthophosphate mineral containing particles isolated from human blood, and they were detected in numerous pathologic calcification related diseases.805 Interestingly, but contrary to the mineral phases of normal calcifications (bone, dentine, enamel, cementum, antlers), which consist of only one type of calcium orthophosphate (namely, biological apatite), the mineral phases of abnormal and/or pathological calcifications are found to occur as single or mixed phases of other types of calcium orthophosphates [ACP, DCPD, OCP, β-(Ca,Mg)3(PO4)2] and/or other phosphate and non-phosphate compounds (e.g., magnesium orthophosphates, calcium pyrophosphates, calcium oxalates, etc.) in addition to or in place of biological apatite (Table 4).26,28,32,85,142,219-222,298,775,806-810 However, precipitation of biological apatite in wrong places is also possible; this is so-called “HA deposition disease.”811-814
Table 4. Occurrence of calcium phosphates in biological systems (human)84.
| Calcium phosphate | Occurrence |
|---|---|
| biological apatite |
enamel, dentine, bone, dental calculi, stones, urinary stones, soft-tissue deposits |
| OCP |
dental calculi and urinary stones |
| DCPD |
dental calculi, crystalluria, chrondrocalcinosis, in some carious lesions |
| β-(Ca,Mg)3(PO4)2 |
dental calculi, salivary stones, arthritic cartilage, soft-tissue deposits |
| Ca2P2O7·2H2O |
pseudo-gout deposits in synovium fluids |
| ACP | heart calcifications in uremic patients, kidney stones |
Occurrence of non-apatite phases in the pathological calcifications may indicate that they were crystallized under the conditions different from homeostasis or crystallization of the apatite structures was inhibited and less stable phases crystallized instead, without further change to the more stable one. Furthermore, at the sites of pathological calcifications, the solution pH is often relatively low. Given that nucleation and crystal growth is not a highly regulated process in any pathological deposit, there is not likely just one fundamental formation mechanism for all possible calcification types. Furthermore, various bioorganic impurities in the local environment undoubtedly influence the crystallization process, resulting in a great variety of pathological deposits. Thus, it is a highly complex problem. In some cases, the chemical composition of an unwanted inorganic phase might depend on the age of the pathological calcification and its location. For example, DCPD is more frequently found in young (3 mo or younger) calculus; biological apatite is present in all ages of calculus, while β-(Ca,Mg)3(PO4)2 occurs more frequently in sub-gingival calculus. In mature calculus, the relative abundance of OCP, β-(Ca,Mg)3(PO4)2 and biological apatite also differ between the inner and outer layers.85 It is interesting to note that the mineral phases of animal calculus (e.g., from dog) was found to consist of calcium carbonate and biological apatite, while human calculi do not contain calcium carbonate.85,815
The nucleation process is the main step in both normal and pathological calcifications. In vitro experiments conducted by Grases and Llobera816 to simulate the formation of sedimentary urinary stones demonstrated that in the absence of organic matter, no calcium orthophosphates crystallized in cavities with scarce liquid renovation, but regular CDHA layers appeared on the wall around the cavity. Visible deposits of calcified organic materials (mixtures of organic matter and spherulites of CDHA) were formed when a glycoprotein (mucin) was present. In this case, the walls of the cavity as well as the glycoproteins had the capacity to act as heterogeneous nucleators of calcium orthophosphates. CDHA microcrystal nucleation on the surface of epithelial cells can be a critical step in the formation of kidney stones,817 and identical mechanisms can be thought for unwanted calcifications in other soft tissues of the body, such as cardiac valves or vascular ducts. Monolayers of CDHA crystals can bind to epithelial cells. A large amount of kidney stones contains CDHA as the crystallization nuclei.
In general, formation of crystals in pathological mineralizations follows the same principles as normal calcifications.818-820 Namely, local conditions for nucleation require a certain degree of local supersaturation induced by biochemical processes, which can be promoted by deficiency of inhibitors (like diphosphate, Mg2+ or even citrate ions) and/or the presence of matrix of a bioorganic material (such as cholesterol) or other crystals of different solids; those might act as heterogeneous nuclei. In addition, other regulators (activators and inhibitors) of physiological biomineralization have been identified and characterized.818-825 What’s more, the biological fluids (e.g., serum, saliva, synovial fluids) are normally supersaturated with respect to biological apatite precipitation;26,85,103 therefore, in principle, calcification is thermodynamically feasible in any part of the body. However, normally this is not the case. Therefore, in the healthy body, the appropriate inhibitory mechanisms must be at work to prevent a superfluous calcification of soft tissues. These inhibition mechanisms are a hot research topic in molecular medicine, but this subject is beyond the scope of current review. The interested readers are referred, for example, to a very interesting review on molecular recognition at the protein/HA interface.826 More to the point, molecular, endocrine and genetic mechanisms of arterial calcification have been reviewed in another paper.827
Very recently, an arachidic acid Langmuir monolayer system has been reported as a model for pathological mineralization of ion-substituted carbonated apatites from simulated body fluid.828 The authors have demonstrated that the surface-induced formation of carbonated apatites starts with aggregation of pre-nucleation clusters of yet-unknown calcium orthophosphates, leading to nucleation of ACP before further development of oriented apatite crystals. This process is schematically shown in Figure 16.628,828
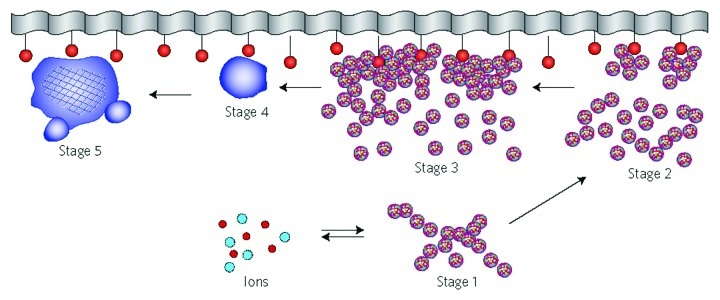
Figure 16. A schematic representation of the different stages of a surface-directed mineralization of calcium orthophosphates. In stage 1, aggregates of pre-nucleation clusters are in equilibrium with ions in solution. The clusters approach a surface with chemical functionality. In stage 2, pre-nucleation clusters aggregate near the surface, with loose aggregates still in solution. In stage 3, further aggregation causes densification near the surface. In stage 4, nucleation of spherical particles of ACP occurs at the surface only. In stage 5, crystallization occurs in the region of the ACP particles directed by the surface. Reprinted from references 628 and 828 with permission.
To conclude this part, it is worth remembering that calcium orthophosphates of biological origin are sparingly soluble in aqueous solutions. Removing them from the places of unwanted deposition would be the equivalent of bone demineralization; that is a challenge. Therefore, the majority of therapeutic approaches are directed at preventing the progression of pathological calcifications. Among them, a chelation therapy might be of some interest to chemists and materials researchers because it deals with chemical processes.829,830 The general principles of demineralization and decalcification [i.e., removing the mineral Ca-containing compounds (phosphates and carbonates) from the bioorganic matrix] have been extensively reviewed in references 831 and 832, where the interested readers are referred.
Biomimetic Crystallization of Calcium Orthophosphates
The term “biomimetics” (“the mimicry of life”) was coined by an American inventor, engineer and biophysicist Otto Herbert Schmitt (1913–1998) in the 1950s. Biomimetics (also known as bionics, biognosis and/or biomimicry) might be defined as application of the methods and systems found in nature to the study, design and construction of new engineering systems, materials, chemical compounds and modern technology. Another definition describes biomimetics as a micro-structural process that mimics or inspires the biological mechanism, in part or as a whole.833 This biological process generates highly ordered materials with a hybrid composition, a complex texture and ultrafine crystallites through a hierarchical self-assembly and begins by designing and synthesizing molecules that have an ability to self-assemble or self-organize spontaneously to higher order structures.
Historically, the biomimetic concept is very old (e.g., the Chinese wanted to make artificial silk ~3,000 y ago; Daedalus’ wings were one early design failure), but the implementation is gathering momentum only recently. The first papers with the term “biomimetics” in the title were published in 1972.834,835 In spite of the tremendous achievements of modern science and technology, nature’s ability to assemble inorganic compounds into hard tissues (shells, spicules, teeth, bones, antlers, skeletons, etc.) is still not achievable by the synthetic procedures. This is not surprising; designs found in nature are the result of millions of years of evolution and competition for survival. The models that failed are fossils; those that survived are the success.836 In the context of this review, biomimetics is considered the mimicking of natural manufacturing methods to generate artificial calcified tissues (grafts, implants, prostheses) that might be used as temporary or permanent replacements of the missing, lost, injured or damaged bones and teeth. It is important to notice that precipitation of calcium orthophosphates and calcium carbonates have been considered to correlate with bone formation at least since 1923.837
A key step in the biomimetic bone graft production is attributed to the crystal growth of apatite phase onto a collagen matrix. Therefore, the matter of choosing the correct experimental conditions and good mimicking solutions is of primary importance. The easiest way to perform the crystallization would be mixing of aqueous solutions containing the ions of calcium and orthophosphate.26-28 Unfortunately, such a type of crystallization provides precipitates with properties (chemical composition, Ca/P ratio, crystallinity level, particle size distribution, etc.) far different from those of biological apatite. This can be explained by the following paramount differences between the in vivo biological and in vitro chemical crystallization conditions:838 (1) In vitro crystallization normally occurs at permanently depleting concentrations of calcium and orthophosphate ions, while the concentrations of all ions and molecules are kept strictly constant during biological mineralization (the same is valid for the solution pH); (2) Chemical crystallization is a fast process (time scale of minutes to days), while the biological process is a slow one (time scale of weeks to years) and (3) Many inorganic, bioorganic, biological and polymeric compounds are present in biological liquids (blood plasma, serum, saliva). Each of these compounds might act as an inhibitor, promoter, nucleator or even as a template for the growth of biological apatite.508 In addition, each of them somehow influences the crystallization kinetics and might be either incorporated into the solid structure or co-precipitated with calcium orthophosphates. (4) Chemical crystallization is, by all means, a “passive” process, while the biological mineralization is strongly influenced by cells and occurs by the self-organization mechanisms.551,573,574 Still there are no good ways to overcome this difference.
The first and the second differences might be overcome by using the appropriate crystallization techniques. The details are available in reference 838, but, briefly, the first problem might be overcome by either a continuous flow of a supersaturated solution839,840 or using a constant-composition (CC) technique.193,841,842 The second difference might be surpassed by a restrained diffusion of calcium and orthophosphate ions from the opposite directions in, for example, a double-diffusion (DD) crystallization device or in viscous gels.420-422,424,425,843-846 The CC and DD techniques have been combined into a single constant-composition double-diffusion (CCDD) device, which currently seems to be the most advanced experimental tool to perform biomimetic crystallization.838,847-851 However, in no case should the CCDD device be considered the final construction; it still has much room for further improvement, e.g., by upgrading the design of the crystallization chamber.852 Other constructions, e.g., to study calcification of biological heart valve prostheses,853 are also possible. In addition, one should keep in mind that the potential of the standard CC technique has not reached its limit yet; for example, recently a good mimicking of the self-organized microstructure of tooth enamel has been achieved.854
The third major difference between the in vivo and in vitro crystallization conditions might be overcome by using the appropriate crystallization solutions.838 The presence of calcium and orthophosphate ions in some biological fluids has been known, at least, since 1921.855,856 Therefore, the best way would be to perform experiments using natural liquids (blood serum, saliva, lymph, etc.), but this is not easy due to great variability of the chemical and biochemical compositions of natural liquids and problems with their collection and storage. As stated before, using supersaturated aqueous solutions containing only the ions of calcium and orthophosphate appears to be unable to mimic the crystallization of biological apatite; therefore, more advanced solutions have been elaborated. To the best of my knowledge, Hanks’ balanced salt solution (HBSS)857 was the first successful simulating medium containing the ions of calcium and orthophosphate together with other inorganic ions and glucose. HBSS is commercially available and still used in biomimetic experiments;858-860 its chemical composition might be taken, e.g., from references 861 and 862. Other popular physiological solutions include α-modified Eagle’s[l] medium (α-MEM) and its variation, Dulbecco’s[m] modified Eagle’s medium (DMEM), which contain numerous bioorganic (alanine, aspartic acid, glycine, biotin, vitamin C, folic acid, riboflavin) and inorganic (CaCl2, KCl, NaCl, NaH2PO4) components,863-867 phosphate buffered saline (PBS) that contains only inorganic (CaCl2, MgCl2, KCl, KH2PO4, NaCl, NaH2PO4) components.868,869 Furthermore, artificial saliva,870-872 synthetic urine816,873 and simulated milk ultrafiltrate (SMUF)874-877 solutions are available. They contain both bioorganic (e.g., xantan gum or sodium carboxymethylcellulose, sorbitol, etc.) and inorganic (e.g., CaCl2, MgCl2, KCl, KH2PO4, NaCl, KH2PO4) compounds. Additional media used for mineralization studies are listed in Table 3 of reference 551. All these simulating solutions are commercially available.
However, the most popular biomimetic solution is a protein-free acellular simulated body fluid (SBF). It was introduced by Kokubo et al.878 and is occasionally called Kokubo’s SBF. It is a metastable aqueous solution with pH ~7.40, supersaturated with respect to the precipitation of OCP, β-TCP, CDHA and HA,879 containing only inorganic ions in concentrations nearly equal to those in human blood plasma. However, the standard SBF formulation, first, contains the tris/HCl buffer, and second, the concentration of hydrogencarbonate (4.2 mM) is only a fraction of that in blood plasma (27 mM).878 The problem of a low concentration of hydrogencarbonate ions has been overcome by first introducing a “synthetic body fluid”880-882 and later a revised SBF (rSBF).883,884 Due to the chemical similarity with human blood plasma, rSBF currently seems to be the best simulating solution. However, it contains Hepes buffer, loses CO2 in open vessels and does not contain any organic and/or biological molecules.883,884 Other types of SBF are also available,885-888 and the interested readers are referred to a leading opinion co-authored by the SBF inventor,889 where the entire history and the preparation techniques of various SBF formulations are well-described. Recently, another leading opinion on the suitability of SBF for the in vitro bioactivity tests was published.890 The authors demonstrated that (1) there is presently no enough scientific data to support the SBF suitability and (2) even though bioactivity tests with SBFs are valid, the way the tests are generally conducted leaves room for further improvements. Furthermore, the preparation protocol of SBF solutions was reconsidered, and a new procedure was suggested to improve the reproducibility of bioactivity tests.890 The application of SBF for the surface mineralization of various materials in vitro has been reviewed in reference 891, while the theoretical analysis of calcium orthophosphate precipitation (the driving force and the nucleation rate based on the classical crystallization theory) in SBF is also available.879 It is important to note that nanometer-sized prenucleation clusters in SBF solutions have been discovered;828 those clusters are believed to be the initial building blocks of crystallized calcium orthophosphates (e.g., CDHA280), while the crystallization process itself occurs via intermediate formation of ACP (Fig. 16).
Further attempts to improve the biomimetic properties of SBF and rSBF have been performed.889,890 Efforts were made to replace artificial buffers (tris/HCl, Hepes) while simultaneous;y increasing the concentration of hydrogencarbonates for SBF892-894 or avoiding losses of CO2 from open vessels for rSBF838,847-851 by means of permanent bubbling of gaseous CO2 through the solutions. Addition of the most important organic and biological compounds, like glucose,849 albumin,847,894 lactates895 and collagen896 is another direction for improving biomimetic properties of various types of SBF. Once a cow milk-based rSBF was prepared.897 Further improvements of all biomimetic solutions are to be made in future. Occasionally, condensed solutions of SBF (e.g., 1.5-fold, 2-fold,896,898,899 5-fold900,901 and even 10-fold902) are used to accelerate precipitation and increase the amount of precipitates. However, whenever possible this should be avoided, because the application of condensed solutions of SBF leads to changes in the chemical composition of the precipitates; namely, the concentration of carbonates increases, while the concentration of orthophosphates decreases.903
To conclude this part, one should note on difficulties in mimicking the calcification process that occurs in bones and teeth. A reasonable mechanism of the induction of CDHA nucleation and crystallization by carboxylate groups on the bioorganic matrices looks as this. At first, calcium and orthophosphate ions are combined with carboxylate groups. By using these as seeds, CDHA crystals then grow to generate interfaces that contain the most stable structure of the {100} faces. Such a crystallization mechanism explains why the c-axes of biological apatite are parallel to the organic matrices. Collagen fibers can be regarded as axis-like organic matrices: when CDHA is formed on the surface of collagen fibers parallel to the c-axes, the c-axes are oriented parallel to the fiber orientation.904 A step further would be to perform the precipitation from the simulating solutions on templates of biomineralization proteins for the control of crystal organization and properties. For example, there are successful attempts to crystallize calcium orthophosphates on collagen in order to obtain bone-like composites.545,905-914 Such collagen/calcium orthophosphate biocomposites are currently under investigation for clinical use. Other popular biomimetic matrixes to perform calcium orthophosphate crystallization comprise gelatin,420-425,915-917 chitosan,915,918,919 organic polyelectrolytes,920-923 metals and alloys,924-930 polymers,931 cellulose,932 self-assembled monolayers933 and many other materials. Such biomimetically prepared calcium orthophosphate precipitates are occasionally called “organoapatites.”509,934
Conclusions and Outlook
By the end of the 20th century, it became clear that calcium orthophosphate biomaterials and bioceramics by themselves could not give a complete response to the clinical needs for artificial implants. Biomaterials with more demanding properties were required. Namely, in 1998, Prof. Larry L. Hench published a forecast for the future of biomaterials development,935 where he noted that available that time bioactive materials (calcium orthophosphates, bioactive glasses and glass ceramics) had already improved prostheses lifetime, but, unfortunately, any type of prosthesis had mechanical limitations. As a solution, he proposed that biomaterial researchers would need to focus on tissue regeneration instead of tissue replacement. A working hypothesis was announced: “Long-term survivability of prosthesis will be increased by the use of biomaterials that enhance the regeneration of natural tissues.”935 One path to follow is the regeneration of bone using calcium orthophosphate scaffolds that mimic the structure of biological apatite, bond to bone, and in some cases, activate the genes within bone cells to stimulate new bone growth.936-938 Thus, more than 10 y ago Prof. Hench predicted a rapid development of tissue engineering field, where calcium orthophosphates play an auxiliary role. The history has shown that tissue engineering, indeed, is a very rapidly developed field of science and research.939
However, what can be said about calcium orthophosphates themselves? The major questions on chemistry, crystallization, ion-substitution, crystallography, thermodynamics and phase relationships for the chemically pure calcium orthophosphates were answered in the 20th century. Some important topics for DCPD and CDHA have been additionally investigated in the field of self-setting calcium orthophosphate formulations. Conversely, calcium orthophosphates of biological origin, including the control of their morphology and interaction of calcium orthophosphate bioceramics with various bioorganic compounds, are not well-investigated yet. The same is valid for the nanocrystalline and amorphous samples of calcium orthophosphates. Small amounts of bone-like apatite might be easily prepared by crystallization from SBF and rSBF, but what can be said about larger quantities? A standard method of increasing the concentration causes chemical changes in the precipitates.903 After a necessary technology is developed, one will have to think of scaffold preparation from this material, keeping in mind that any thermal treatment would destroy this material. A spark plasma sintering approach based on the use of pulsed current and enabling very fast heating and cooling rates seemed to be the first hint of achieving this goal.940 However, a rapid development of the self-setting calcium orthophosphate formulations, which can be easily doped by the necessary chemical elements, seems to be a better solution to this problem. Furthermore, the existence of OA remains to be questionable, and the bioactivity mechanism of calcium orthophosphates requires better understanding.
To date, although calcium orthophosphate biomaterials and bioceramics have been extensively studied for over 50 y, their ability to trigger bone formation is still incomparable with other biomaterials. Naturally, the biomaterials field is shifting toward biologically active systems in order to improve their performance and to expand their use.941 Because of this, tissue engineering is the strongest direction of current research, which, in the case of calcium orthophosphates, means fabrication of proper substrates and/or scaffolds to carry cells, hormones and biochemical factors to be further used in surgery and medicine. Presumably, a synthesis of various types of calcium orthophosphate-based biocomposites and hybrid biomaterials occupies the second important place. For example, even composites with carbon nanotubes already exist!942-944 The third important place is occupied by investigations devoted to the synthesis and characterization of various nano-sized particles and nanodimensional crystals of calcium orthophosphates as well as by synthesis of calcium orthophosphates with controlled particle geometry.508 In general, the geometry of crystal phases can be varied by controlling the precipitation conditions, such as temperature, solution pH, concentration of the reagents, hydrodynamics, presence of various admixtures, inhibitors or promoters, ultrasonication, etc. All these approaches might be useful in preparation of calcium orthophosphate fibers, whiskers, hollow microspheres, etc. In addition, a great attention is paid to manufacturing of the self-setting calcium orthophosphate formulations and multiphase[n] mixtures mimicking as closely as possible the mineral component of biological apatite. Work looking into ecological methods of synthesis of calcium orthophosphates might be of great importance as well.945 A deeper study of the fascinating growth rate of deer antlers and the ability of some animals, such as newts, to regenerate amputated limbs might provide new and unexpected approaches to the bone-healing concept, and this too will be important for further development of both biomimetics and biomineralization fields. Unfortunately, no currently available grafting biomaterials can substitute the bones’ mechanical function, illustrating the yet-unmet medical need that would entirely substitute and regenerate a damaged tissue or organ. In a close future, the foreseeable application of calcium orthophosphates will be as a component of the third generation biomaterials,935,938 where they will support cells and/or other biologically active substances (peptides, growth factors, hormones, drugs, etc.) to guide regeneration of hard tissues.946-956
To finalize this review, one should note that, in spite of a long history of calcium orthophosphate research and many important discoveries, many gaps still remain in our knowledge to be investigated in future.
Notes
[a] As a mineral species, apatite was first recognized by the father of German geology Abraham Gottlob Werner (1750–1817) in 1786 and named by him from the ancient Greek α≠α|ά⌉ (apatao)—“to mislead” or “to deceive,” because it had previously been mistaken for other minerals, such as beryl, tourmaline, chrysolite, amethyst, fluorite, etc. Currently, apatite is the name for a group of minerals with the same crystallographic structure and does not indicate one chemical composition. That is why, the term “calcium apatite” is used in this review.
[b] There are reports that dahllite belongs to the francolite group. Natural dahllite might be a rock-forming mineral.969 For example, it was found in some phosphorite concretions of Podolia.970,971 In addition, it was found in both massive and accretionary crustal phosphorites.49
[c] Collagens are fibrous, insoluble proteins found in the connective tissues, including skin, bone, ligaments and cartilage.
[d] Biocompatibility is the ability of a material to perform with an appropriate host response in a specific application.957 For further details on this topic, the interested readers are referred to reference 958.
[e] In 1941, to honor Mr. Herbert Percy Whitlock (1868–1948), an American mineralogist, the curator of the American Museum of Natural History, New York City, New York, USA, the term whitlockite was coined as a synonym for β-TCP identified by its X-ray diffraction pattern in phosphate rocks.775,959,960 Therefore, strictly speaking, β-TCMP should be called as a “magnesium whitlokite.” Its solubility is less than that of β-TCP.961 An iron-containing whitlockite with chemical formula Ca9(Mg,Fe2+)(PO4)6(PO3,OH) exists in nature: is a relatively rare natural mineral but is found in granitic pegmatite and has also been found in meteorites. It can form small, but distinct and well-formed crystals.962,963
[f] In some research papers, CDHA is defined as “precipitated HA.”964-966
[g] It is worth noting that hydroxylapatite would be a more accurate description (perhaps, hydroxideapatite would be even better because it relates to calcium hydroxide) while by both the medical and material communities it is usually called as hydroxyapatite.
[h] The amount of fluorides on the very surface of dental enamel might be increased by using fluoride-containing toothpastes and mouthwashes.723-726 Fluoride-containing toothpastes and mouthwashes are widely used in practice due to the well-known anti-cariogenic effect of fluorides that is related to the solubility decreasing.727,728
[i] Due to the nanoscopic dimensions, biological apatite is occasionally called “nano-apatite.”104
[j] Self-assembling is the autonomous organization of components into patterns or structures without human intervention. It is considered that self-assembling processes are common throughout nature and technology.967
[k] Strictly speaking, there are some differences among these biological materials. For example, the hardness of live dentine is less than that of enamel but is greater than that of bone or cementum.653 When pulp of the tooth dies or is removed by a dentist, the properties of dentine change: it becomes brittle, liable to fracture and looses a reparative capability.
[l] Named after Harry Eagle (1905–1992), an American physician and pathologist.
[m] Named after Renato Dulbecco (born February 22, 1914), an Italian-born US virologist, who shared the 1975 Nobel Prize in Physiology or Medicine for his work on reverse transcriptase.
[n] For multiphase compositions of various calcium orthophosphates, the problem of accurate phase quantification often arises. The problem is usually solved by the Rietveld refinement and the readers are referred to a recent paper on this subject.968
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/18790
References
- 1.Shepperd J. The early biological history of calcium phosphates. In: Epinette JA, Manley MT, (Eds.) Fifteen years of clinical experience with hydroxyapatite coatings in joint arthroplasty. Springer, France 2004; 3-8. [Google Scholar]
- 2.Berzelius J. Ueber basische phosphorsaure kalkerde. Ann Chem Pharmac. 1845;53:286–8. doi: 10.1002/jlac.18450530212. [DOI] [Google Scholar]
- 3.Warington R., Jr. Researches on the phosphates of calcium, and upon the solubility of tricalcic phosphate. J Chem Soc. 1866;19:296–318. doi: 10.1039/js8661900296. [DOI] [Google Scholar]
- 4.Fresenius R. Ueber die Bestimmung der Phosphorsäure im Phosphorit nebst Mittheilung der Analysen des Phosphorits und Staffelits aus dem Lahnthal. Z Anal Chem. 1867;6:403–9. doi: 10.1007/BF01347651. [DOI] [Google Scholar]
- 5.Church AH. New analyses of certain mineral arseniates and phosphates. 1. Apatite; 2. Arseniosiderite; 3. Childrenite; 4. Ehlite; 5. Tyrolite; 6. Wavellite. J Chem Soc. 1873;26:101–11. doi: 10.1039/js8732600101. [DOI] [Google Scholar]
- 6.LWJ On a fine specimen of apatite from Tyrol, lately in the possession of Mr. Samuel Henson. Nature. 1883;27:608–9. doi: 10.1038/027608a0. [DOI] [Google Scholar]
- 7.Tereg A. Das Verhalten der Calciumphosphate im Organismus der Fleischfresser. Pflüger, Archiv für die Gesammte Physiologie des Menschen und der Thiere. Pflügers Archiv Eur J Physiology. 1883;32:122–70. doi: 10.1007/BF01628854. [DOI] [Google Scholar]
- 8.Mohr C. Ueber die quantitative Bestimmung der zurückgegangenen Phosphorsäure und der Phosphorsäure im Dicalciumphosphat. Z Anal Chem. 1884;23:487–91. doi: 10.1007/BF01360583. [DOI] [Google Scholar]
- 9.Glaser C. Bemerkungen zu der Abhandlung des Herrn Carl Mohr über die quantitative Bestimmung der zurückgegangenen Phosphorsäure und der Phosphorsäure im Dicalciumphosphat. Z Anal Chem. 1885;24:180. doi: 10.1007/BF01366673. [DOI] [Google Scholar]
- 10.Hutchings WM. Occurrence of apatite in slag. Nature. 1887;36:460. doi: 10.1038/036460a0. [DOI] [Google Scholar]
- 11.Hilgenstock G. Eine neue Verbindung von P2O5 und CaO. Stahl und Eisen. 1883;3:498. [Google Scholar]
- 12.Hilgenstock G. Das vierbasische Kalkphosphat und die Basicitätsstufe des Silicats in der Thomas-Schlacxke. Stahl und Eisen. 1887;7:557–60. [Google Scholar]
- 13.Scheibler C. Ueber die Herstellung reicher Kalkphosphate in Verbindung mit einer Verbesserung des Thomasprocesses. Ber Dtsch Chem Ges. 1886;19:1883–93. doi: 10.1002/cber.18860190258. [DOI] [Google Scholar]
- 14.Georgievics GV. Über das Verhalten des Tricalciumphosphats gegen Kohlensäure und Eisenhydroxyd. Monatsh Chem. 1891;12:566–81. doi: 10.1007/BF01538628. [DOI] [Google Scholar]
- 15.Dreesmann H. Ueber Knochenplombierung. Beitr Klin Chir. 1892;9:804–10. [Google Scholar]
- 16.Cameron FK. The action of water upon the phosphates of calcium. J Am Chem Soc. 1904;26:1454–63. doi: 10.1021/ja02001a007. [DOI] [Google Scholar]
- 17.Cameron FK, Seidell A. The phosphates of calcium. I. J Am Chem Soc. 1905;27:1503–12. doi: 10.1021/ja01990a005. [DOI] [Google Scholar]
- 18.Cameron FK, Bell JM. The phosphates of calcium. II. J Am Chem Soc. 1905;27:1512–4. doi: 10.1021/ja01990a006. [DOI] [Google Scholar]
- 19.Cameron FK, Bell JM. The phosphates of calcium, III; Superphosphate. J Am Chem Soc. 1906;28:1222–9. doi: 10.1021/ja01975a016. [DOI] [Google Scholar]
- 20.Cameron FK, Bell JM. The phosphates of calcium. IV. J Am Chem Soc. 1910;32:869–73. doi: 10.1021/ja01925a003. [DOI] [Google Scholar]
- 21.Bassett H., Jr. Beiträge zum Studium der Calciumphosphate I. Die Hydrate der Calcium-Hydroorthophosphate. Z Anorg Chem. 1907;53:34–48. doi: 10.1002/zaac.19070530104. [DOI] [Google Scholar]
- 22.Bassett H., Jr. Beiträge zum Studium der Calciumphosphate II. Die Einwirkung von Ammoniakgas auf Calcium-Hydroorthophosphate Z Anorg Chem. 1907;53:49–62. [Google Scholar]
- 23.Bassett H., Jr. Beiträge zum Studium der Calciumphosphate III. Das System CaO-P2O5-H2O. Z Anorg Chem. 1908;59:1–55. doi: 10.1002/zaac.19080590102. [DOI] [Google Scholar]
- 24.Bassett H., Jr. The phosphates of calcium. Part IV. The basic phosphates. J Chem Soc. 1917;111:620–42. [Google Scholar]
- 25.Lide DR. The CRC handbook of chemistry and physics. CRC Press, Boca Raton, Florida 2005; 86:2544. [Google Scholar]
- 26.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monographs in Oral Science. Karger, Basel 1991; 15:201. [PubMed] [Google Scholar]
- 27.Elliott JC. Structure and chemistry of the apatites and other calcium orthophosphates. Studies in inorganic chemistry; Elsevier: Amsterdam, Netherlands 1994; 18:389. [Google Scholar]
- 28.Amjad Z, ed. Calcium phosphates in biological and industrial systems. Kluwer Academic Publishers: Boston MA USA 1997; 529. [Google Scholar]
- 29.Dorozhkin SV. Calcium orthophosphates. J Mater Sci. 2007;42:1061–95. doi: 10.1007/s10853-006-1467-8. [DOI] [Google Scholar]
- 30.Dorozhkin SV. Calcium orthophosphates in nature, biology and medicine. Materials. 2009;2:399–498. doi: 10.3390/ma2020399. [DOI] [Google Scholar]
- 31.Ohura K, Bohner M, Hardouin P, Lemaître J, Pasquier G, Flautre B. Resorption of, and bone formation from, new beta-tricalcium phosphate-monocalcium phosphate cements: an in vivo study. J Biomed Mater Res. 1996;30:193–200. doi: 10.1002/(SICI)1097-4636(199602)30:2<193::AID-JBM9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Daculsi G, Bouler JM, LeGeros RZ. Adaptive crystal formation in normal and pathological calcifications in synthetic calcium phosphate and related biomaterials. Int Rev Cytol. 1997;172:129–91. doi: 10.1016/S0074-7696(08)62360-8. [DOI] [PubMed] [Google Scholar]
- 33.Cantelar E, Lifante G, Calderón T, Meléndrez R, Millán A, Alvarez MA, et al. Optical characterisation of rare earths in natural fluorapatite. J Alloy Comp. 2001;323:851–4. doi: 10.1016/S0925-8388(01)01159-8. [DOI] [Google Scholar]
- 34.Ribeiro HB, Guedes KJ, Pinheiro MVB, Greulich-Weber S, Krambrock K. About the blue and green colours in natural fluorapatite. Phys Status Solidi, C Conf Crit Rev. 2005;2:720–3. doi: 10.1002/pssc.200460274. [DOI] [Google Scholar]
- 35.Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed Engl. 2002;41:3130–46. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.McConnell D. Apatite: its crystal chemistry, mineralogy, utilization, and geologic and biologic occurrences. Applied Mineralogy. Springer-Verlag: Vienna and New York USA 1973; 5:111. [Google Scholar]
- 37.Becker P. Phosphates and phosphoric acid: raw materials technology and economics of the wet process. Fertilizer science and technology series. Marcel Dekker: New York USA 1989; 2:760. [Google Scholar]
- 38.Smith DK. Calcium phosphate apatites in nature. In: Hydroxyapatite and related materials. Brown PW, Constantz B, Eds. CRC Press Inc.: Boca Raton FL USA 1994; 29-44. [Google Scholar]
- 39.Angelov AI, Levin BV, Chernenko YD. Phosphate ore. A reference book. (in Russian). Nedra Busyness Centre: Moscow 2000; 120. [Google Scholar]
- 40.Cook PJ, Shergold JH, Davidson DF, eds. Phosphate deposits of the world: phosphate rock resources. Cambridge University Press: Cambridge MA USA 2005; 2:600. [Google Scholar]
- 41.Zhang JZ, Guo L, Fischer CJ. Abundance and chemical speciation of phosphorus in sediments of the Mackenzie river delta, the Chukchi sea and the Bering sea: importance of detrital apatite. Aquat Geochem. 2010;16:353–71. doi: 10.1007/s10498-009-9081-4. [DOI] [Google Scholar]
- 42.Omelon SJ, Grynpas MD. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem Rev. 2008;108:4694–715. doi: 10.1021/cr0782527. [DOI] [PubMed] [Google Scholar]
- 43.Jarvis I. Phosphorite geochemistry: state-of-the-art and environmental concerns. Eclogae Geol Helv. 1994;87:643–700. [Google Scholar]
- 44.Glenn CR. Phosphorus and phosphorites: sedimentology and environments of formation. Eclogae Geol Helv. 1994;87:747–88. [Google Scholar]
- 45.McClellan GH. Mineralogy of carbonate fluorapatites (Francolites) J Geological Soc. 1980;137:675–81. doi: 10.1144/gsjgs.137.6.0675. [DOI] [Google Scholar]
- 46.Mcarthur JM. Francolite geochemistry-compositional controls during formation, diagenesis, metamorphism and weathering. Geochim Cosmochim Acta. 1985;49:23–35. doi: 10.1016/0016-7037(85)90188-7. [DOI] [Google Scholar]
- 47.Schuffert JD, Kastner M, Emanuele G, Jahnke RA. Carbonate-ion substitution in francolite: a new equation. Geochim Cosmochim Acta. 1990;54:2323–8. doi: 10.1016/0016-7037(90)90058-S. [DOI] [Google Scholar]
- 48.Pan Y, Fleet ME. Compositions of the apatite-group minerals: substitution mechanisms and controlling factors. In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews in Mineralogy and Geochemistry. Hughes JM, Kohn M, Rakovan J, Eds. Mineralogical Society of America: Washington DC USA 2002; 48:13-49. [Google Scholar]
- 49.Zanin YN. The classification of calcium phosphates of phosphorites. Lithol Miner Resour. 2004;39:281–2. doi: 10.1023/B:LIMI.0000027613.56744.c8. [DOI] [Google Scholar]
- 50.Rogers AF. Collophane, a much neglected mineral. Am J Sci. 1922;3:269–76. doi: 10.2475/ajs.s5-3.16.269. [DOI] [Google Scholar]
- 51.Kozlovsky YG, Yarygin VI, Shokarev MM. Colloid calcium phosphate (collophan) in the composition of urinary calculi. Urologiya i Nefrologiya. 1977;42:27–32. [PubMed] [Google Scholar]
- 52.http://www.mindat.org/min-10072.html (accessed in September 2010).
- 53.Elorza J, Astibia H, Murelaga X, Pereda-Suberbiola X. Francolite as a diagenetic mineral in dinosaur and other upper cretaceous reptile bones (Lano, Iberian peninsula): microstructural, petrological and geochemical features. Cretac Res. 1999;20:169–87. doi: 10.1006/cres.1999.0144. [DOI] [Google Scholar]
- 54.Hubert B, Álvaro JJ, Chen JY. Microbially mediated phosphatization in the Neoproterozoic Doushantuo Lagerstätte, South China. Bull Soc Geol Fr. 2005;176:355–61. doi: 10.2113/176.4.355. [DOI] [Google Scholar]
- 55.Xiao S, Zhang Y, Knoll AH. Three-dimensional preservation of algae and animal embryos in a neoproterozoic phosphorite. Nature. 1998;391:553–8. doi: 10.1038/35318. [DOI] [Google Scholar]
- 56.Xiao S, Yuan X, Knoll AH. Eumetazoan fossils in terminal proterozoic phosphorites? Proc Natl Acad Sci U S A. 2000;97:13684–9. doi: 10.1073/pnas.250491697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chakhmouradian AR, Medici L. Clinohydroxylapatite: a new apatite-group mineral from northwestern Ontario (Canada), and new data on the extent of Na-S substitution in natural apatites. Eur J Mineral. 2006;18:105–12. doi: 10.1127/0935-1221/2006/0018-0105. [DOI] [Google Scholar]
- 58.http://www.mindat.org/gallery.php?min=9293 (accessed in October 2010).
- 59.Klein C. Brushite from the island of Mona (between Haiti and Puerto Rico). Sitzber K Preuss Aka 1901; 720-5. [Google Scholar]
- 60.Merrill GP. On the calcium phosphate in meteoric stones. Am J Sci. 1917;43:322–4. doi: 10.2475/ajs.s4-43.256.322. [DOI] [Google Scholar]
- 61.Tyrrell GW. Apatite, nepheline and rare-earth mining in the Kola Peninsula. Nature. 1938;141:354–5. doi: 10.1038/141354a0. [DOI] [Google Scholar]
- 62.Kogarko LN. Problems of the genesis of giant apatite and rare metal deposits of the Kola Peninsula, Russia. Geology of Ore Deposits. 1999;41:351–66. [Google Scholar]
- 63.Ford AK. A remarkable crystal of apatite from Mt. Apatite, Auburn, Maine. Am J Sci. 1917;44:245–6. doi: 10.2475/ajs.s4-44.261.245. [DOI] [Google Scholar]
- 64.Hogarth DD. The discovery of apatite on the Lievre River, Quebec. Mineral Rec. 1974;5:178–82. [Google Scholar]
- 65.van Velthuizen J. Giant fluorapatite crystals: a question of locality. Mineral Rec. 1992;23:459–63. [Google Scholar]
- 66.Trueman NA. Substitutions for phosphate ions in apatite. Nature. 1966;210:937–8. doi: 10.1038/210937a0. [DOI] [Google Scholar]
- 67.Gilinskaya LG. Organic radicals in natural apatites according to EPR data: potential genetic and paleoclimatic indicators. J Struct Chem. 2010;51:471–81. doi: 10.1007/s10947-010-0069-0. [DOI] [Google Scholar]
- 68.Gilinskaya LG. A stable perinaphthenyl radical in natural apatites. J Struct Chem. 2010;51:761–4. doi: 10.1007/s10947-010-0112-1. [DOI] [Google Scholar]
- 69.Sánchez-Salcedo S, Vila M, Izquierdo-Barba I, Cicuéndez M, Vallet-Regí M. Biopolymer-coated hydroxyapatite foams: a new antidote for heavy metal intoxication. J Mater Chem. 2010;20:6956–61. doi: 10.1039/c0jm01260b. [DOI] [Google Scholar]
- 70.White T, Ferraris C, Kim J, Madhavi S. Apatite—an adaptive framework structure. In: Micro- and mesoporous mineral phases. Series: Reviews in Mineralogy and Geochemistry. Ferraris G, Merlino S, Eds. Mineralogical Society of America: Washington DC USA 2005; 57:307-401. [Google Scholar]
- 71.Jacob KD, Reynolds DS. Reduction of tricalcium phosphate by carbon. Ind Eng Chem. 1928;20:1204–10. doi: 10.1021/ie50227a027. [DOI] [Google Scholar]
- 72.Emsley J. The shocking history of phosphorus: a biography of the devil’s element. Pan Books, Macmillan UK 2001; 336. [Google Scholar]
- 73.van der Sluis S, Meszaros Y, Marchee WGJ, Wesselingh HA, van Rosmalen GM. The digestion of phosphate ore in phosphoric acid. Ind Eng Chem Res. 1987;26:2501–5. doi: 10.1021/ie00072a020. [DOI] [Google Scholar]
- 74.Dorozhkin SV. Fundamentals of the wet-process phosphoric acid production. 1. Kinetics and mechanism of the phosphate rock dissolution. Ind Eng Chem Res. 1996;35:4328–35. doi: 10.1021/ie960092u. [DOI] [Google Scholar]
- 75.Dorozhkin SV. Fundamentals of the wet-process phosphoric acid production. 2. kinetics and mechanism of CaSO4·0.5H2O surface crystallization and coating formation. Ind Eng Chem Res. 1997;36:467–73. doi: 10.1021/ie960219f. [DOI] [Google Scholar]
- 76.Dorozhkin SV. Ecological principles of wet-process phosphoric acid technology. J Chem Technol Biotechnol. 1998;71:227–33. doi: 10.1002/(SICI)1097-4660(199803)71:3<227::AID-JCTB801>3.0.CO;2-E. [DOI] [Google Scholar]
- 77.Copson RL, Newton RH, Lindsay JD. Superphosphate manufacture—mixing phosphate rook with concentrated phosphoric acid. Ind Eng Chem. 1936;28:923–7. doi: 10.1021/ie50320a012. [DOI] [Google Scholar]
- 78.Newton RH, Copson RL. Superphosphate manufacture—composition of superphosphate made from phosphate rock and concentrated phosphoric acid. Ind Eng Chem. 1936;28:1182–6. doi: 10.1021/ie50322a014. [DOI] [Google Scholar]
- 79.Rossete ALRM, Carneiro JMT, Bendassolli JA, Tavares CRO, Sant’Ana Filho CR. Production of single superphosphate labeled with 34S. Scientia Agricola. 2008;65:91–4. doi: 10.1590/S0103-90162008000100013. [DOI] [Google Scholar]
- 80.Magda A, Pode V, Niculescu M, Muntean C, Bandur G, Iovi A. Studies on process of obtaining the fertilizers based on ammonium phosphates with addition of boric acid. Rev Chim Bucharest. 2008;59:1340–4. [Google Scholar]
- 81.Abouzeid AZM. Upgrading of phosphate ores—a review. Powder Handling and Processing. 2007;19:92–109. [Google Scholar]
- 82.Abouzeid AZM. Physical and thermal treatment of phosphate ores—an overview. Int J Miner Process. 2008;85:59–84. doi: 10.1016/j.minpro.2007.09.001. [DOI] [Google Scholar]
- 83.Rey C, Combes C, Drouet C, Sfihi H. Chemical diversity of apatites. Adv Sci Technol. 2006;49:27–36. doi: 10.4028/www.scientific.net/AST.49.27. [DOI] [Google Scholar]
- 84.O’Neill WC. The fallacy of the calcium-phosphorus product. Kidney Int. 2007;72:792–6. doi: 10.1038/sj.ki.5002412. [DOI] [PubMed] [Google Scholar]
- 85.LeGeros RZ. Formation and transformation of calcium phosphates: relevance to vascular calcification. Z Kardiol. 2001;90(Suppl 3):116–24. doi: 10.1007/s003920170032. [DOI] [PubMed] [Google Scholar]
- 86.Becker A, Epple M, Müller KM, Schmitz I. A comparative study of clinically well-characterized human atherosclerotic plaques with histological, chemical, and ultrastructural methods. J Inorg Biochem. 2004;98:2032–8. doi: 10.1016/j.jinorgbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Wopenka B, Pasteris JD. A mineralogical perspective on the apatite in bone. Mater Sci Eng C. 2005;25:131–43. doi: 10.1016/j.msec.2005.01.008. [DOI] [Google Scholar]
- 88.Pasteris JD, Wopenka B, Valsami-Jones E. Bone and tooth mineralization: why apatite? Elements. 2008;4:97–104. doi: 10.2113/GSELEMENTS.4.2.97. [DOI] [Google Scholar]
- 89.Sun Y, Hanley EN., Jr. Calcium-containing crystals and osteoarthritis. Curr Opin Orthop. 2007;18:472–8. doi: 10.1097/BCO.0b013e32825e1d95. [DOI] [Google Scholar]
- 90.Daculsi G, LeGeros RZ, Mitre D. Crystal dissolution of biological and ceramic apatites. Calcif Tissue Int. 1989;45:95–103. doi: 10.1007/BF02561408. [DOI] [PubMed] [Google Scholar]
- 91.Lee-Thorp JA, van der Merwe NJ. Aspects of the chemistry of modern and fossil biological apatites. J Archaeol Sci. 1991;18:343–54. doi: 10.1016/0305-4403(91)90070-6. [DOI] [Google Scholar]
- 92.Bigi A, Foresti E, Gregorini R, Ripamonti A, Roveri N, Shah JS. The role of magnesium on the structure of biological apatites. Calcif Tissue Int. 1992;50:439–44. doi: 10.1007/BF00296775. [DOI] [PubMed] [Google Scholar]
- 93.Nakano T, Kaibara K, Tabata Y, Nagata N, Enomoto S, Marukawa E, et al. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone. 2002;31:479–87. doi: 10.1016/S8756-3282(02)00850-5. [DOI] [PubMed] [Google Scholar]
- 94.Grynpas MD, Omelon S. Transient precursor strategy or very small biological apatite crystals? Bone. 2007;41:162–4. doi: 10.1016/j.bone.2007.04.176. [DOI] [PubMed] [Google Scholar]
- 95.Lee JW, Sasaki K, Ferrara JD, Akiyama K, Sasaki T, Nakano T. Evaluation of preferential alignment of biological apatite (BAp) crystallites in bone using transmission X-ray diffraction optics. J Jpn Ins Metal. 2009;73:786–93. doi: 10.2320/jinstmet.73.786. [DOI] [Google Scholar]
- 96.Ishimoto T, Sakamoto T, Nakano T. Orientation of biological apatite in rat calvaria analyzed by microbeam X-ray diffractometer. Mater Sci Forum. 2010;638:576–81. doi: 10.4028/www.scientific.net/MSF.638-642.576. [DOI] [Google Scholar]
- 97.Danil’chenko SN, Kulik AN, Bugai AN, Pavlenko PA, Kalinichenko TG, Ul’yanchich NV, et al. Thermally activated diffusion of magnesium from bioapatite crystals. J Appl Spectrosc. 2005;72:899–905. [Google Scholar]
- 98.Passey BH, Robinson TF, Ayliffe LK, Cerling TE, Sphonheimer M, Dearing MD, et al. Carbon isotopic fractionation between diet, breath and bioapatite in different mammals. J Arch Sci. 2005;32:1459–70. doi: 10.1016/j.jas.2005.03.015. [DOI] [Google Scholar]
- 99.Meneghini C, Dalconi MC, Nuzzo S, Mobilio S, Wenk RH. Rietveld refinement on x-ray diffraction patterns of bioapatite in human fetal bones. Biophys J. 2003;84:2021–9. doi: 10.1016/S0006-3495(03)75010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eagle RA, Schauble EA, Tripati AK, Tütken T, Hulbert RC, Eiler JM. Body temperatures of modern and extinct vertebrates from (13)C-(18)O bond abundances in bioapatite. Proc Natl Acad Sci U S A. 2010;107:10377–82. doi: 10.1073/pnas.0911115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skinner HCW. Biominerals. Mineral Mag. 2005;69:621–41. doi: 10.1180/0026461056950275. [DOI] [Google Scholar]
- 102.Daculsi G. Physicochemical and ultrastructural analysis of bone bioactive interface. Biomater Tissue Int. 1992;10:296–304. [Google Scholar]
- 103.Lowenstam HA, Weiner S. On biomineralization. New York: Oxford University Press 1989; 324. [Google Scholar]
- 104.Weiner S, Wagner HD. Material bone: structure-mechanical function relations. Annu Rev Mater Sci. 1998;28:271–98. doi: 10.1146/annurev.matsci.28.1.271. [DOI] [Google Scholar]
- 105.Limeback H. Molecular mechanisms in dental hard tissue mineralization. Curr Opin Dent. 1991;1:826–35. [PubMed] [Google Scholar]
- 106.Driessens FCM, Verbeeck RMH. Biominerals. CRC Press: Boca Raton FL USA 1990; 440. [Google Scholar]
- 107.Clark NA. The system P2O5-CaO-H2O and the recrystallization of monocalcium phosphate. J Phys Chem. 1931;35:1232–8. doi: 10.1021/j150323a005. [DOI] [Google Scholar]
- 108.Brown PW. Phase relationships in the ternary system CaO-P2O5-H2O at 25°C. J Am Ceram Soc. 1992;75:17–22. doi: 10.1111/j.1151-2916.1992.tb05435.x. [DOI] [Google Scholar]
- 109.Martin RI, Brown PW. Phase equilibria among acid calcium phosphates. J Am Ceram Soc. 1997;80:1263–6. doi: 10.1111/j.1151-2916.1997.tb02973.x. [DOI] [Google Scholar]
- 110.Kreidler ER, Hummel FA. Phase relationships in the system SrO-P2O5 and the influence of water vapor on the formation of Sr4P2O9. Inorg Chem. 1967;6:884–91. doi: 10.1021/ic50051a007. [DOI] [Google Scholar]
- 111.Carayon MT, Lacout JL. Study of the Ca/P atomic ratio of the amorphous phase in plasma-sprayed hydroxyapatite coatings. J Solid State Chem. 2003;172:339–50. doi: 10.1016/S0022-4596(02)00085-3. [DOI] [Google Scholar]
- 112.White TJ, ZhiLi D. Structural derivation and crystal chemistry of apatites. Acta Crystallogr B. 2003;59:1–16. doi: 10.1107/S0108768102019894. [DOI] [PubMed] [Google Scholar]
- 113.Mathew M, Takagi S. Structures of biological minerals in dental research. J Res Natl Inst Stand Technol. 2001;106:1035–44. doi: 10.6028/jres.106.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hughes JM, Rakovan J. The crystal structure of apatite, Ca5(PO4)3(F,OH,Cl). In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews in Mineralogy and Geochemistry. Hughes JM, Kohn M, Rakovan J, Eds. Mineralogical Society of America: Washington DC, USA 2002; 48:1-12. [Google Scholar]
- 115.Wang L, Nancollas GH. Calcium orthophosphates: crystallization and dissolution. Chem Rev. 2008;108:4628–69. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lynn AK, Bonfield W. A novel method for the simultaneous, titrant-free control of pH and calcium phosphate mass yield. Acc Chem Res. 2005;38:202–7. doi: 10.1021/ar040234d. [DOI] [PubMed] [Google Scholar]
- 117.León B, Jansen JJ, eds. Thin calcium phosphate coatings for medical implants. Springer: New York USA 2009; 326. [Google Scholar]
- 118.Chow LC. Next generation calcium phosphate-based biomaterials. Dent Mater J. 2009;28:1–10. doi: 10.4012/dmj.28.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ishikawa K. Bone substitute fabrication based on dissolution-precipitation reactions. Materials. 2010;3:1138–55. doi: 10.3390/ma3021138. [DOI] [Google Scholar]
- 120.Fernández E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. Part I: solution chemistry. J Mater Sci Mater Med. 1999;10:169–76. doi: 10.1023/A:1008937507714. [DOI] [PubMed] [Google Scholar]
- 121.McDowell H, Gregory TM, Brown WE. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25 and 37°C. J Res Natl Bur Stand Sect A Phys Chem 1977; 81:273-81.
- 122.Pan HB, Darvell BW. Calcium phosphate solubility: the need for re-evaluation. Cryst Growth Des. 2009;9:639–45. doi: 10.1021/cg801118v. [DOI] [Google Scholar]
- 123.Boonchom B. Parallelogram-like microparticles of calcium dihydrogen phosphate monohydrate (Ca(H2PO4)2·H2O) obtained by a rapid precipitation route in aqueous and acetone media. J Alloy Comp. 2009;482:199–202. doi: 10.1016/j.jallcom.2009.03.157. [DOI] [Google Scholar]
- 124.Köster K, Heide H, König R. Resorbierbare Calciumphosphatkeramik im Tierexperiment unter Belastung. Langenbecks Arch Chir. 1977;343:173–81. doi: 10.1007/BF01267989. [DOI] [PubMed] [Google Scholar]
- 125.Bermúdez O, Boltong MG, Driessens FCM, Planell JA. Optimization of a calcium orthophosphate cement formulation occurring in the combination of monocalcium phosphate monohydrate with calcium oxide. J Mater Sci Mater Med. 1994;5:67–71. doi: 10.1007/BF00121693. [DOI] [Google Scholar]
- 126.Bermúdez O, Boltong MG, Driessens FCM, Planell JA. Development of some calcium phosphate cements from combinations of α-TCP, MCPM and CaO. J Mater Sci Mater Med. 1994;5:160–3. doi: 10.1007/BF00053337. [DOI] [Google Scholar]
- 127.Driessens FCM, Boltong MG, Bermúdez O, Planell JA, Ginebra MP, Fernández E. Effective formulations for the preparation of calcium phosphate bone cements. J Mater Sci Mater Med. 1994;5:164–70. doi: 10.1007/BF00053338. [DOI] [Google Scholar]
- 128.Huan Z, Chang J. Novel bioactive composite bone cements based on the beta-tricalcium phosphate-monocalcium phosphate monohydrate composite cement system. Acta Biomater. 2009;5:1253–64. doi: 10.1016/j.actbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 129.Budavari S, O’Neil MJ, Smith A, Heckelman PE, Kinneary JF, eds. The Merck Index: an encyclopedia of chemicals, drugs and biologicals. Chapman & Hall: USA 1996; 12:1741. [Google Scholar]
- 130.Stein HH, Kadzere CT, Kim SW, Miller PS. Influence of dietary phosphorus concentration on the digestibility of phosphorus in monocalcium phosphate by growing pigs. J Anim Sci. 2008;86:1861–7. doi: 10.2527/jas.2008-0867. [DOI] [PubMed] [Google Scholar]
- 131.To honor Prof. George Jarvis Brush (1831–1912), an American mineralogist, Yale University, New Haven, Connecticut USA.
- 132.Ferreira A, Oliveira C, Rocha F. The different phases in the precipitation of dicalcium phosphate dihydrate. J Cryst Growth. 2003;252:599–611. doi: 10.1016/S0022-0248(03)00899-6. [DOI] [Google Scholar]
- 133.Oliveira C, Ferreira A, Rocha F. Dicalcium phosphate dihydrate precipitation: characterization and crystal growth. Chem Eng Res Des. 2007;85:1655–61. [Google Scholar]
- 134.Sivkumar GR, Girija EK, Kalkura SN, Subramanian C. Crystallization and characterization of calcium phosphates: brushite and monetite. Cryst Res Technol. 1997;33:197–205. doi: 10.1002/(SICI)1521-4079(1998)33:2<197::AID-CRAT197>3.0.CO;2-K. [DOI] [Google Scholar]
- 135.Madhurambal G, Subha R, Mojumdar SC. Crystallization and thermal characterization of calcium hydrogen phosphate dihydrate crystals. J Therm Anal Calorim. 2009;96:73–6. doi: 10.1007/s10973-008-9841-1. [DOI] [Google Scholar]
- 136.MacDowell H, Brown WE, Sutter JR. Solubility study of calcium hydrogenphosphate. Ion pair formation. Inorg Chem. 1971;10:1638–43. doi: 10.1021/ic50102a020. [DOI] [Google Scholar]
- 137.Landin M, Rowe RC, York P. Structural changes during the dehydration of dicalcium phosphate dihydrate. Eur J Pharm Sci. 1994;2:245–52. doi: 10.1016/0928-0987(94)90029-9. [DOI] [Google Scholar]
- 138.Curry NA, Jones DW. Crystal structure of brushite, calcium hydrogen orthophosphate dihydrate: a neutron-diffraction investigation. J Chem Soc A Inorg Phys Theoret Chem 1971; 3725-9. [Google Scholar]
- 139.Arsic J, Kaminski D, Poodt P, Vlieg E. Liquid ordering at the brushite-{010}-water interface. Phys Rev B. 2004;69:245406–10. doi: 10.1103/PhysRevB.69.245406. [DOI] [Google Scholar]
- 140.Pan HB, Darvell BW. Solubility of dicalcium phosphate dihydrate by solid titration. Caries Res. 2009;43:254–60. doi: 10.1159/000217857. [DOI] [PubMed] [Google Scholar]
- 141.Lundager-Madsen HE. Optical properties of synthetic crystals of brushite (CaHPO4·2H2O) J Cryst Growth. 2008;310:617–23. doi: 10.1016/j.jcrysgro.2007.11.110. [DOI] [Google Scholar]
- 142.Qiu SR, Orme CA. Dynamics of biomineral formation at the near-molecular level. Chem Rev. 2008;108:4784–822. doi: 10.1021/cr800322u. [DOI] [PubMed] [Google Scholar]
- 143.Kurashina K, Kurita H, Hirano M, Kotani A, Klein CP, de Groot K. In vivo study of calcium phosphate cements: implantation of an alpha-tricalcium phosphate/dicalcium phosphate dibasic/tetracalcium phosphate monoxide cement paste. Biomaterials. 1997;18:539–43. doi: 10.1016/S0142-9612(96)00162-7. [DOI] [PubMed] [Google Scholar]
- 144.Driessens FCM, Planell JA, Boltong MG, Khairoun I, Ginebra MP. Osteotransductive bone cements. Proc Inst Mech Eng H. 1998;212:427–35. doi: 10.1243/0954411981534196. [DOI] [PubMed] [Google Scholar]
- 145.Takagi S, Chow LC, Ishikawa K. Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials. 1998;19:1593–9. doi: 10.1016/S0142-9612(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto H, Niwa S, Hori M, Hattori T, Sawai K, Aoki S, et al. Mechanical strength of calcium phosphate cement in vivo and in vitro. Biomaterials. 1998;19:1587–91. doi: 10.1016/S0142-9612(97)00121-X. [DOI] [PubMed] [Google Scholar]
- 147.Crall JJ, Bjerga JM. Effects of DCPD/APF application and prolonged exposure to fluoride on caries-like lesion formation in vitro. J Oral Pathol. 1987;16:488–91. doi: 10.1111/j.1600-0714.1987.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 148.Wefel JS, Harless JD. The use of saturated DCPD in remineralization of artificial caries lesions in vitro. J Dent Res. 1987;66:1640–3. doi: 10.1177/00220345870660110701. [DOI] [PubMed] [Google Scholar]
- 149.Hoppenbrouwers PM, Groenendijk E, Tewarie NR, Driessens FCM. Improvement of the caries resistance of human dental roots by a two-step conversion of the root mineral into fluoridated hydroxylapatite. J Dent Res. 1988;67:1254–6. doi: 10.1177/00220345880670100101. [DOI] [PubMed] [Google Scholar]
- 150.Gaffar A, Blake-Haskins J, Mellberg J. In vivo studies with a dicalcium phosphate dihydrate/MFP system for caries prevention. Int Dent J. 1993;43(Suppl 1):81–8. [PubMed] [Google Scholar]
- 151.Sullivan RJ, Charig A, Blake-Haskins J, Zhang YP, Miller SM, Strannick M, et al. In vivo detection of calcium from dicalcium phosphate dihydrate dentifrices in demineralized human enamel and plaque. Adv Dent Res. 1997;11:380–7. doi: 10.1177/08959374970110040201. [DOI] [PubMed] [Google Scholar]
- 152.Mostashari SM, Haddadi H, Hashempoor Z. Effect of deposited calcium hydrogen phosphate dihydrate on the flame retardancy imparted to cotton fabric. Asian J Chem. 2006;18:2388–90. [Google Scholar]
- 153.Powditch HI, Bosworth AW. Studies on infant feeding. IX. The availability of dicalcium phosphate when present as a constituent of infant’s food. Boston Med Surg J. 1917;177:864–7. doi: 10.1056/NEJM191712201772502. [DOI] [Google Scholar]
- 154.For Moneta (now Monito) Island (archipelago of Puerto Rico), which contains a notable occurrence.
- 155.Tas AC. Monetite (CaHPO4) synthesis in ethanol at room temperature. J Am Ceram Soc. 2009;92:2907–12. doi: 10.1111/j.1551-2916.2009.03351.x. [DOI] [Google Scholar]
- 156.Chen GG, Luo GS, Yang LM, Xu JH, Sun Y, Wang JD. Synthesis and size control of CaHPO4 particles in a two-liquid phase micro-mixing process. J Cryst Growth. 2005;279:501–7. doi: 10.1016/j.jcrysgro.2005.02.069. [DOI] [Google Scholar]
- 157.Miyazaki T, Sivaprakasam K, Tantry J, Suryanarayanan R. Physical characterization of dibasic calcium phosphate dihydrate and anhydrate. J Pharm Sci. 2009;98:905–16. doi: 10.1002/jps.21443. [DOI] [PubMed] [Google Scholar]
- 158.Eshtiagh-Hosseini H, Houssaindokht MR, Chahkandhi M, Youssefi A. Preparation of anhydrous dicalcium phosphate, DCPA, through sol-gel process, identification and phase transformation evaluation. J Non-Cryst Solids. 2008;354:3854–7. doi: 10.1016/j.jnoncrysol.2008.04.016. [DOI] [Google Scholar]
- 159.Fukase Y, Eanes ED, Takagi S, Chow LC, Brown WE. Setting reactions and compressive strengths of calcium phosphate cements. J Dent Res. 1990;69:1852–6. doi: 10.1177/00220345900690121201. [DOI] [PubMed] [Google Scholar]
- 160.TenHuisen KS, Brown PW. The formation of hydroxyapatite-ionomer cements at 38 ° C. J Dent Res. 1994;73:598–606. doi: 10.1177/00220345940730030501. [DOI] [PubMed] [Google Scholar]
- 161.Fernández E, Ginebra MP, Boltong MG, Driessens FCM, Ginebra J, De Maeyer EA, et al. Kinetic study of the setting reaction of a calcium phosphate bone cement. J Biomed Mater Res. 1996;32:367–74. doi: 10.1002/(SICI)1097-4636(199611)32:3<367::AID-JBM9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 162.Fernández E, Gil FJ, Best SM, Ginebra MP, Driessens FCM, Planell JA. The cement setting reaction in the CaHPO4-alpha-Ca3(PO4)2 system: an X-ray diffraction study. J Biomed Mater Res. 1998;42:403–6. doi: 10.1002/(SICI)1097-4636(19981205)42:3<403::AID-JBM8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 163.Fernández E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM. Production and characterization of new calcium phosphate bone cements in the CaHPO4-alpha-Ca3(PO4)2 system: pH, workability and setting times. J Mater Sci Mater Med. 1999;10:223–30. doi: 10.1023/A:1008958112257. [DOI] [PubMed] [Google Scholar]
- 164.Desai TR, Bhaduri SB, Tas AC. A self-setting, monetite (CaHPO4) cement for skeletal repair. Ceram Eng Sci Proc. 2006;27:61–9. [Google Scholar]
- 165.Sturgeon JL, Brown PW. Effects of carbonate on hydroxyapatite formed from CaHPO(4) and Ca(4)(PO(4))(2)O. J Mater Sci Mater Med. 2009;20:1787–94. doi: 10.1007/s10856-009-3752-y. [DOI] [PubMed] [Google Scholar]
- 166.Chen G, Li W, Yu X, Sun K. Study of the cohesion of TTCP/DCPA phosphate cement through evolution of cohesion time and remaining percentage. J Mater Sci. 2009;44:828–34. doi: 10.1007/s10853-008-3126-8. [DOI] [Google Scholar]
- 167.Tamimi F, Torres J, Bassett D, Barralet J, Cabarcos EL. Resorption of monetite granules in alveolar bone defects in human patients. Biomaterials. 2010;31:2762–9. doi: 10.1016/j.biomaterials.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 168.Takami K, Machimura H, Takado K, Inagaki M, Kawashima Y. Novel preparation of free flowing spherically granulated dibasic calcium phosphate anhydrous for direct tabletting. Chem Pharm Bull (Tokyo) 1996;44:868–70. doi: 10.1248/cpb.44.868. [DOI] [Google Scholar]
- 169.LeGeros RZ. Preparation of octacalcium phosphate (OCP): a direct fast method. Calcif Tissue Int. 1985;37:194–7. doi: 10.1007/BF02554841. [DOI] [PubMed] [Google Scholar]
- 170.Bigi A, Boanini E, Borghi M, Cojazzi G, Panzavolta S, Roveri N. Synthesis and hydrolysis of octacalcium phosphate: effect of sodium polyacrylate. J Inorg Biochem. 1999;75:145–51. doi: 10.1016/S0162-0134(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 171.Nakahira A, Aoki S, Sakamoto K, Yamaguchi S. Synthesis and evaluation of various layered octacalcium phosphates by wet-chemical processing. J Mater Sci Mater Med. 2001;12:793–800. doi: 10.1023/A:1017968818168. [DOI] [PubMed] [Google Scholar]
- 172.Shelton RM, Liu Y, Cooper PR, Gbureck U, German MJ, Barralet JE. Bone marrow cell gene expression and tissue construct assembly using octacalcium phosphate microscaffolds. Biomaterials. 2006;27:2874–81. doi: 10.1016/j.biomaterials.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 173.Arellano-Jiménez MJ, García-García R, Reyes-Gasga J. Synthesis and hydrolysis of octacalcium phosphate and its characterization by electron microscopy and X-ray diffraction. J Phys Chem Solids. 2009;70:390–5. doi: 10.1016/j.jpcs.2008.11.001. [DOI] [Google Scholar]
- 174.Suzuki O. Octacalcium phosphate: osteoconductivity and crystal chemistry. Acta Biomater. 2010;6:3379–87. doi: 10.1016/j.actbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 175.Miyatake N, Kishimoto KN, Anada T, Imaizumi H, Itoi E, Suzuki O. Effect of partial hydrolysis of octacalcium phosphate on its osteoconductive characteristics. Biomaterials. 2009;30:1005–14. doi: 10.1016/j.biomaterials.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 176.Boanini E, Gazzano M, Rubini K, Bigi A. Collapsed octacalcium phosphate stabilized by ionic substitutions. Cryst Growth Des. 2010;10:3612–7. doi: 10.1021/cg100494f. [DOI] [Google Scholar]
- 177.Brown WE, Mathew M, Tung MS. Crystal chemistry of octacalcium phosphate. Prog. Cryst. Growth Charact. 1981;4:59–87. doi: 10.1016/0146-3535(81)90048-4. [DOI] [Google Scholar]
- 178.Mathew M, Brown WE, Schroeder L, Dickens B. Crystal structure of octacalcium bis(hydrogenphosphate) tetrakis(phosphate) pentahydrate, Ca8(HPO4)2(PO4)4·5H2O. J Crystallogr Spectrosc Res. 1988;18:235–50. doi: 10.1007/BF01194315. [DOI] [Google Scholar]
- 179.Brown WE. Octacalcium phosphate and hydroxyapatite: crystal structure of octacalcium phosphate. Nature. 1962;196:1048–50. doi: 10.1038/1961048b0. [DOI] [Google Scholar]
- 180.Brown WE, Smith JP, Lehr JR, Frazier AW. Octacalcium phosphate and hydroxyapatite: crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature. 1962;196:1050–5. doi: 10.1038/1961050a0. [DOI] [Google Scholar]
- 181.Pan HB, Darvell BW. Solid titration of octacalcium phosphate. Caries Res. 2009;43:322–30. doi: 10.1159/000226231. [DOI] [PubMed] [Google Scholar]
- 182.Le Geros RZ. Variations in the crystalline components of human dental calculus. I. Crystallographic and spectroscopic methods of analysis. J Dent Res. 1974;53:45–50. doi: 10.1177/00220345740530012801. [DOI] [PubMed] [Google Scholar]
- 183.Schroeder HE. Formation and inhibition of dental calculus. J Periodontol. 1969;40:643–6. doi: 10.1902/jop.1969.40.11.643. [DOI] [PubMed] [Google Scholar]
- 184.Chow LC, Eanes ED, eds. Octacalcium phosphate. Monographs in Oral Science. Karger: Basel, Switzerland 2001; 18:167. [Google Scholar]
- 185.Kakei M, Sakae T, Yoshikawa M. Electron microscopy of octacalcium phosphate in the dental calculus. J Electron Microsc (Tokyo) 2009;58:393–8. doi: 10.1093/jmicro/dfp034. [DOI] [PubMed] [Google Scholar]
- 186.Brown WE. Crystal growth of bone mineral. Clin Orthop Relat Res. 1966;44:205–20. [PubMed] [Google Scholar]
- 187.Nelson DGA, Wood GJ, Barry JC, Featherstone JDB. The structure of (100) defects in carbonated apatite crystallites: a high resolution electron microscope study. Ultramicroscopy. 1986;19:253–65. doi: 10.1016/0304-3991(86)90213-5. [DOI] [PubMed] [Google Scholar]
- 188.Iijima M, Nelson DGA, Pan Y, Kreinbrink AT, Adachi M, Goto T, et al. Fluoride analysis of apatite crystals with a central planar OCP inclusion: concerning the role of F- ions on apatite/OCP/apatite structure formation. Calcif Tissue Int. 1996;59:377–84. doi: 10.1007/s002239900143. [DOI] [PubMed] [Google Scholar]
- 189.Bodier-Houllé P, Steuer P, Voegel JC, Cuisinier FJG. First experimental evidence for human dentine crystal formation involving conversion of octacalcium phosphate to hydroxyapatite. Acta Crystallogr D Biol Crystallogr. 1998;54:1377–81. doi: 10.1107/S0907444998005769. [DOI] [PubMed] [Google Scholar]
- 190.Aoba T, Komatsu H, Shimazu Y, Yagishita H, Taya Y. Enamel mineralization and an initial crystalline phase. Connect Tissue Res. 1998;38:129–37, discussion 139-45. doi: 10.3109/03008209809017029. [DOI] [PubMed] [Google Scholar]
- 191.Rodríguez-Hernández AG, Fernández ME, Carbajal-de-la-Torre G, García-García R, Reyes-Gasga J. Electron microscopy analysis of the central dark line defect of the human tooth enamel. Mater Res Soc Symp Proc 2005; 839:157-62. [Google Scholar]
- 192.Tomazic BB, Brown WE, Schoen FJ. Physicochemical properties of calcific deposits isolated from porcine bioprosthetic heart valves removed from patients following 2-13 years function. J Biomed Mater Res. 1994;28:35–47. doi: 10.1002/jbm.820280106. [DOI] [PubMed] [Google Scholar]
- 193.Nancollas GH, Wu W. Biomineralization mechanisms: a kinetics and interfacial energy approach. J Cryst Growth. 2000;211:137–42. doi: 10.1016/S0022-0248(99)00816-7. [DOI] [Google Scholar]
- 194.Kamakura S, Sasano Y, Homma H, Suzuki O, Kagayama M, Motegi K. Implantation of octacalcium phosphate (OCP) in rat skull defects enhances bone repair. J Dent Res. 1999;78:1682–7. doi: 10.1177/00220345990780110401. [DOI] [PubMed] [Google Scholar]
- 195.Kamakura S, Sasano Y, Homma H, Suzuki O, Kagayama M, Motegi K. Implantation of octacalcium phosphate nucleates isolated bone formation in rat skull defects. Oral Dis. 2001;7:259–65. doi: 10.1034/j.1601-0825.2001.70410.x. [DOI] [PubMed] [Google Scholar]
- 196.Sargolzaei-Aval F, Sobhani A, Arab MR, Sarani SA, Heydari MH. The efficacy of implant of octacalcium phosphate in combination with bone matrix gelatin (BMG) on bone regeneration in skull defects in rat. Iran J Med Sci. 2004;29:124–9. [Google Scholar]
- 197.Suzuki O, Kamakura S, Katagiri T, Nakamura M, Zhao B, Honda Y, et al. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials. 2006;27:2671–81. doi: 10.1016/j.biomaterials.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 198.Suzuki O, Imaizumi H, Kamakura S, Katagiri T. Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr Med Chem. 2008;15:305–13. doi: 10.2174/092986708783497283. [DOI] [PubMed] [Google Scholar]
- 199.Kikawa T, Kashimoto O, Imaizumi H, Kokubun S, Suzuki O. Intramembranous bone tissue response to biodegradable octacalcium phosphate implant. Acta Biomater. 2009;5:1756–66. doi: 10.1016/j.actbio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 200.Murakami Y, Honda Y, Anada T, Shimauchi H, Suzuki O. Comparative study on bone regeneration by synthetic octacalcium phosphate with various granule sizes. Acta Biomater. 2010;6:1542–8. doi: 10.1016/j.actbio.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 201.Tao J, Jiang W, Zhai H, Pan H, Xu X, Tang R. Structural components and anisotropic dissolution behaviors in one hexagonal single crystal of β-tricalcium phosphate. Cryst Growth Des. 2008;8:2227–34. doi: 10.1021/cg700808h. [DOI] [Google Scholar]
- 202.Tao J, Pan H, Zhai H, Wang J, Li L, Wu J, et al. Controls of tricalcium phosphate single-crystal formation from its amorphous precursor by interfacial energy. Cryst Growth Des. 2009;9:3154–60. doi: 10.1021/cg801130w. [DOI] [Google Scholar]
- 203.Hou XJ, Mao KY, Chen DF. Bone formation performance of beta-tricalcium phosphate sintered bone. J Clin Rehabil Tiss Eng Res. 2008;12:9627–30. [Google Scholar]
- 204.Dickens B, Schroeder LW, Brown WE. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. I. The crystal structure of pure β-Ca3(PO4)2. J Solid State Chem. 1974;10:232–48. doi: 10.1016/0022-4596(74)90030-9. [DOI] [Google Scholar]
- 205.Schroeder LW, Dickens B, Brown WE. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. II. Refinement of Mg-containing β-Ca3(PO4)2. J Solid State Chem. 1977;22:253–62. doi: 10.1016/0022-4596(77)90002-0. [DOI] [Google Scholar]
- 206.Ito A, LeGeros RZ. Magnesium- and zinc-substituted beta-tricalcium phosphates as potential bone substitute biomaterials. Key Eng Mater. 2008;377:85–98. doi: 10.4028/www.scientific.net/KEM.377.85. [DOI] [Google Scholar]
- 207.Kannan S, Goetz-Neunhoeffer F, Neubauer J, Ferreira JMF. Synthesis and structure refinement of zinc-doped β-tricalcium phosphate powders. J Am Ceram Soc. 2009;92:1592–5. doi: 10.1111/j.1551-2916.2009.03093.x. [DOI] [Google Scholar]
- 208.Kannan S, Goetz-Neunhoeffer F, Neubauer J, Pina S, Torres PMC, Ferreira JMF. Synthesis and structural characterization of strontium- and magnesium-co-substituted beta-tricalcium phosphate. Acta Biomater. 2010;6:571–6. doi: 10.1016/j.actbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 209.Araújo JC, Sader MS, Moreira EL, Moraes VCA, LeGeros RZ, Soares GA. Maximum substitution of magnesium for calcium sites in Mg-β-TCP structure determined by X-ray powder diffraction with the Rietveld refinement. Mater Chem Phys. 2009;118:337–40. doi: 10.1016/j.matchemphys.2009.07.064. [DOI] [Google Scholar]
- 210.Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically modified calcium phosphate. J Mater Sci. 2009;44:346–9. doi: 10.1007/s10853-008-3068-1. [DOI] [Google Scholar]
- 211.Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Preparation, characterization and in vitro efficacy of an acid-modified beta-TCP material for dental hard-tissue remineralization. Acta Biomater. 2010;6:969–78. doi: 10.1016/j.actbio.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 212.Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Surfactant-modified beta-TCP: structure, properties, and in vitro remineralization of subsurface enamel lesions. J Mater Sci Mater Med. 2010;21:2009–20. doi: 10.1007/s10856-010-4064-y. [DOI] [PubMed] [Google Scholar]
- 213.Yashima M, Sakai A, Kamiyama T, Hoshikawa A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J Solid State Chem. 2003;175:272–7. doi: 10.1016/S0022-4596(03)00279-2. [DOI] [Google Scholar]
- 214.Yin X, Stott MJ, Rubio A. α- and β-tricalcium phosphate: a density functional study. Phys Rev B. 2003;68:205205–12. doi: 10.1103/PhysRevB.68.205205. [DOI] [Google Scholar]
- 215.Liang L, Rulis P, Ching WY. Mechanical properties, electronic structure and bonding of alpha- and beta-tricalcium phosphates with surface characterization. Acta Biomater. 2010;6:3763–71. doi: 10.1016/j.actbio.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 216.Kumar AR, Kalainathan S. Microhardness studies on calcium hydrogen phosphate (brushite) crystals. Mater Res Bull. 2010;45:1664–7. doi: 10.1016/j.materresbull.2010.07.002. [DOI] [Google Scholar]
- 217.Pan HB, Darvell BW. Solubility of TTCP and beta-TCP by solid titration. Arch Oral Biol. 2009;54:671–7. doi: 10.1016/j.archoralbio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 218.Wang W, Itoh S, Yamamoto N, Okawa A, Nagai A, Yamashita K. Electrical polarization of β-tricalcium phosphate ceramics. J Am Ceram Soc. 2010;93:2175–7. doi: 10.1111/j.1551-2916.2010.03710.x. [DOI] [Google Scholar]
- 219.Kodaka T, Debari K, Higashi S. Magnesium-containing crystals in human dental calculus. J Electron Microsc (Tokyo) 1988;37:73–80. [PubMed] [Google Scholar]
- 220.Reid JD, Andersen ME. Medial calcification (whitlockite) in the aorta. Atherosclerosis. 1993;101:213–24. doi: 10.1016/0021-9150(93)90118-E. [DOI] [PubMed] [Google Scholar]
- 221.Scotchford CA, Ali SY. Magnesium whitlockite deposition in articular cartilage: a study of 80 specimens from 70 patients. Ann Rheum Dis. 1995;54:339–44. doi: 10.1136/ard.54.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.P’ng CH, Boadle R, Horton M, Bilous M, Bonar F. Magnesium whitlockite of the aorta. Pathology. 2008;40:539–40. doi: 10.1080/00313020802198044. [DOI] [PubMed] [Google Scholar]
- 223.Mirtchi AA, Lemaître J, Munting E. Calcium phosphate cements: study of the beta-tricalcium phosphate--dicalcium phosphate--calcite cements. Biomaterials. 1990;11:83–8. doi: 10.1016/0142-9612(90)90121-6. [DOI] [PubMed] [Google Scholar]
- 224.Mirtchi AA, Lemaître J, Munting E. Calcium phosphate cements: effect of fluorides on the setting and hardening of beta-tricalcium phosphate-dicalcium phosphate-calcite cements. Biomaterials. 1991;12:505–10. doi: 10.1016/0142-9612(91)90150-9. [DOI] [PubMed] [Google Scholar]
- 225.Lemaître J, Munting E, Mirtchi AA. Setting, hardening and resorption of calcium phosphate hydraulic cements. Rev Stomatol Chir Maxillofac. 1992;93:163–5. [PubMed] [Google Scholar]
- 226.Ellinger RF, Nery EB, Lynch KL. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int J Periodontics Restorative Dent. 1986;6:22–33. [PubMed] [Google Scholar]
- 227.Nery EB, Lynch KL, Hirthe WM, Mueller KH. Bioceramic implants in surgically produced infrabony defects. J Periodontol. 1975;46:328–47. doi: 10.1902/jop.1975.46.6.328. [DOI] [PubMed] [Google Scholar]
- 228.Metsger DS, Driskell TD, Paulsrud JR. Tricalcium phosphate ceramic--a resorbable bone implant: review and current status. J Am Dent Assoc. 1982;105:1035–8. doi: 10.14219/jada.archive.1982.0408. [DOI] [PubMed] [Google Scholar]
- 229.Galois L, Mainard D, Delagoutte JP. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop. 2002;26:109–15. doi: 10.1007/s00264-001-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ogose A, Hotta T, Kawashima H, Kondo N, Gu W, Kamura T, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res B Appl Biomater. 2005;72:94–101. doi: 10.1002/jbm.b.30136. [DOI] [PubMed] [Google Scholar]
- 231.Horch HH, Sader R, Pautke C, Neff A, Deppe H, Kolk A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb) for bone regeneration in the reconstructive surgery of the jaws. Int J Oral Maxillofac Surg. 2006;35:708–13. doi: 10.1016/j.ijom.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 232.Ogose A, Kondo N, Umezu H, Hotta T, Kawashima H, Tokunaga K, et al. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion) in human bones. Biomaterials. 2006;27:1542–9. doi: 10.1016/j.biomaterials.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 233.Kamitakahara M, Ohtsuki C, Miyazaki T. Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J Biomater Appl. 2008;23:197–212. doi: 10.1177/0885328208096798. [DOI] [PubMed] [Google Scholar]
- 234.Liu Y, Pei GX, Shan J, Ren GH. New porous beta-tricalcium phosphate as a scaffold for bone tissue engineering. J Clin Rehabil Tiss Eng Res. 2008;12:4563–7. [Google Scholar]
- 235.Epstein NE. Beta tricalcium phosphate: observation of use in 100 posterolateral lumbar instrumented fusions. Spine J. 2009;9:630–8. doi: 10.1016/j.spinee.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 236.Shayegan A, Petein M, Vanden Abbeele A. The use of beta-tricalcium phosphate, white MTA, white Portland cement and calcium hydroxide for direct pulp capping of primary pig teeth. Dent Traumatol. 2009;25:413–9. doi: 10.1111/j.1600-9657.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 237.Güngörmüş C, Kılıç A, Akay MT, Kolankaya D. The effects of maternal exposure to food additive E341 (tricalcium phosphate) on foetal development of rats. Environ Toxicol Pharmacol. 2010;29:111–6. doi: 10.1016/j.etap.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 238.Weiner ML, Salminen WF, Larson PR, Barter RA, Kranetz JL, Simon GS. Toxicological review of inorganic phosphates. Food Chem Toxicol. 2001;39:759–86. doi: 10.1016/S0278-6915(01)00028-X. [DOI] [PubMed] [Google Scholar]
- 239.Jokic B, Jankovic-Castvan I, Veljovic DJ, Bucevac D, Obradovic-Djuricic K, Petrovic R, et al. Synthesis and settings behavior of α-TCP from calcium deficient hyroxyapatite obtained by hydrothermal method. J Optoelectron Adv Mater. 2007;9:1904–10. [Google Scholar]
- 240.Nurse RW, Welch JB, Gun W. High-temperature phase equilibria in the system dicalcium silicate-tricalcium phosphate. J Chem Soc. 1959:1077–83. doi: 10.1039/jr9590001077. [DOI] [Google Scholar]
- 241.Langstaff SD, Sayer M, Smith TJN, Pugh SM, Hesp SAM, Thompson WT. Resorbable bioceramics based on stabilized calcium phosphates. Part I: rational design, sample preparation and material characterization. Biomaterials. 1999;20:1727–41. doi: 10.1016/S0142-9612(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 242.Langstaff SD, Sayer M, Smith TJN, Pugh SM. Resorbable bioceramics based on stabilized calcium phosphates. Part II: evaluation of biological response. Biomaterials. 2001;22:135–50. doi: 10.1016/S0142-9612(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 243.Sayer M, Stratilatov AD, Reid JW, Calderin L, Stott MJ, Yin X, et al. Structure and composition of silicon-stabilized tricalcium phosphate. Biomaterials. 2003;24:369–82. doi: 10.1016/S0142-9612(02)00327-7. [DOI] [PubMed] [Google Scholar]
- 244.Reid JW, Pietak AM, Sayer M, Dunfield D, Smith TJN. Phase formation and evolution in the silicon substituted tricalcium phosphate/apatite system. Biomaterials. 2005;26:2887–97. doi: 10.1016/j.biomaterials.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 245.Reid JW, Tuck L, Sayer M, Fargo K, Hendry JA. Synthesis and characterization of single-phase silicon-substituted alpha-tricalcium phosphate. Biomaterials. 2006;27:2916–25. doi: 10.1016/j.biomaterials.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 246.Astala R, Calderin L, Yin X, Stott MJ. Ab initio simulation of Si-doped hydroxyapatite. Chem Mater. 2006;18:413–22. doi: 10.1021/cm051989x. [DOI] [Google Scholar]
- 247.TenHuisen KS, Brown PW. Formation of calcium-deficient hydroxyapatite from alpha-tricalcium phosphate. Biomaterials. 1998;19:2209–17. doi: 10.1016/S0142-9612(98)00131-8. [DOI] [PubMed] [Google Scholar]
- 248.Durucan C, Brown PW. alpha-Tricalcium phosphate hydrolysis to hydroxyapatite at and near physiological temperature. J Mater Sci Mater Med. 2000;11:365–71. doi: 10.1023/A:1008934024440. [DOI] [PubMed] [Google Scholar]
- 249.Durucan C, Brown PW. Kinetic model for α-tricalcium phosphate hydrolysis. J Am Ceram Soc. 2002;85:2013–8. doi: 10.1111/j.1151-2916.2002.tb00397.x. [DOI] [Google Scholar]
- 250.Camiré CL, Gbureck U, Hirsiger W, Bohner M. Correlating crystallinity and reactivity in an alpha-tricalcium phosphate. Biomaterials. 2005;26:2787–94. doi: 10.1016/j.biomaterials.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 251.Constantz BR, Ison IC, Fulmer MT, Poser RD, Smith ST, VanWagoner M, et al. Skeletal repair by in situ formation of the mineral phase of bone. Science. 1995;267:1796–9. doi: 10.1126/science.7892603. [DOI] [PubMed] [Google Scholar]
- 252.Tagaya M, Goto H, Iinuma M, Wakamatsu N, Tamura Y, Doi Y. Development of self-setting Te-Cp/alpha-TCP cement for pulpotomy. Dent Mater J. 2005;24:555–61. doi: 10.4012/dmj.24.555. [DOI] [PubMed] [Google Scholar]
- 253.Oda M, Takeuchi A, Lin X, Matsuya S, Ishikawa K. Effects of liquid phase on basic properties of alpha-tricalcium phosphate-based apatite cement. Dent Mater J. 2008;27:672–7. doi: 10.4012/dmj.27.672. [DOI] [PubMed] [Google Scholar]
- 254.Camiré CL, Nevsten P, Lidgren L, McCarthy I. The effect of crystallinity on strength development of alpha-TCP bone substitutes. J Biomed Mater Res B Appl Biomater. 2006;79:159–65. doi: 10.1002/jbm.b.30526. [DOI] [PubMed] [Google Scholar]
- 255.Yin X, Stott MJ. Theoretical insights into bone grafting Si-stabilized α-tricalcium phosphate. J Chem Phys. 2005;122:24709–18. doi: 10.1063/1.1829995. [DOI] [PubMed] [Google Scholar]
- 256.Mathew M, Schroeder LW, Dickens B, Brown WE. The crystal structure of α-Ca3(PO4)2. Acta Crystallogr B. 1977;33:1325–33. doi: 10.1107/S0567740877006037. [DOI] [Google Scholar]
- 257.Yin X, Stott MJ. Surface and adsorption properties of alpha-tricalcium phosphate. J Chem Phys. 2006;124:124701–10. doi: 10.1063/1.2178800. [DOI] [PubMed] [Google Scholar]
- 258.Dorozhkin SV. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010;6:4457–75. doi: 10.1016/j.actbio.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 259.Termine JD, Eanes ED. Comparative chemistry of amorphous and apatitic calcium phosphate preparations. Calcif Tissue Res. 1972;10:171–97. doi: 10.1007/BF02012548. [DOI] [PubMed] [Google Scholar]
- 260.Eanes ED, Termine JD, Nylen MU. An electron microscopic study of the formation of amorphous calcium phosphate and its transformation to crystalline apatite. Calcif Tissue Res. 1973;12:143–58. doi: 10.1007/BF02013730. [DOI] [PubMed] [Google Scholar]
- 261.Meyer JL, Eanes ED. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif Tissue Res. 1978;25:59–68. doi: 10.1007/BF02010752. [DOI] [PubMed] [Google Scholar]
- 262.Meyer JL, Eanes ED. A thermodynamic analysis of the secondary transition in the spontaneous precipitation of calcium phosphate. Calcif Tissue Res. 1978;25:209–16. doi: 10.1007/BF02010771. [DOI] [PubMed] [Google Scholar]
- 263.Wuthier RE, Rice GS, Wallace JE, Jr., Weaver RL, LeGeros RZ, Eanes ED. In vitro precipitation of calcium phosphate under intracellular conditions: formation of brushite from an amorphous precursor in the absence of ATP. Calcif Tissue Int. 1985;37:401–10. doi: 10.1007/BF02553710. [DOI] [PubMed] [Google Scholar]
- 264.Sinyaev VA, Shustikova ES, Levchenko LV, Sedunov AA. Synthesis and dehydration of amorphous calcium phosphate. Inorg Mater. 2001;37:619–22. doi: 10.1023/A:1017572502092. [DOI] [Google Scholar]
- 265.Termine JD, Peckauskas RA, Posner AS. Calcium phosphate formation in vitro. II. Effects of environment on amorphous-crystalline transformation. Arch Biochem Biophys. 1970;140:318–25. doi: 10.1016/0003-9861(70)90072-X. [DOI] [PubMed] [Google Scholar]
- 266.Elliott JC. Recent studies of apatites and other calcium orthophosphates. In: Les matériaux en phosphate de calcium. Aspects fondamentaux./Calcium phosphate materials. Fundamentals. Brès E, Hardouin P, Eds. Sauramps Medical: Montpellier, France 1998; 25-66. [Google Scholar]
- 267.Li Y, Weng W. In vitro synthesis and characterization of amorphous calcium phosphates with various Ca/P atomic ratios. J Mater Sci Mater Med. 2007;18:2303–8. doi: 10.1007/s10856-007-3132-4. [DOI] [PubMed] [Google Scholar]
- 268.Tadic D, Peters F, Epple M. Continuous synthesis of amorphous carbonated apatites. Biomaterials. 2002;23:2553–9. doi: 10.1016/S0142-9612(01)00390-8. [DOI] [PubMed] [Google Scholar]
- 269.Boskey AL, Posner AS. Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-dependent, solution-mediated, solid-solid conversion. J Phys Chem. 1973;77:2313–7. doi: 10.1021/j100638a011. [DOI] [Google Scholar]
- 270.Keller L, Dollase WA. X-ray determination of crystalline hydroxyapatite to amorphous calcium-phosphate ratio in plasma sprayed coatings. J Biomed Mater Res. 2000;49:244–9. doi: 10.1002/(SICI)1097-4636(200002)49:2<244::AID-JBM13>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 271.Carayon MT, Lacout JL. Study of the Ca/P atomic ratio of the amorphous phase in plasma-sprayed hydroxyapatite coatings. J Solid State Chem. 2003;172:339–50. doi: 10.1016/S0022-4596(02)00085-3. [DOI] [Google Scholar]
- 272.Kumar R, Cheang P, Khor KA. Phase composition and heat of crystallization of amorphous calcium phosphate in ultra-fine radio frequency suspension plasma sprayed hydroxyapatite powders. Acta Mater. 2004;52:1171–81. doi: 10.1016/j.actamat.2003.11.016. [DOI] [Google Scholar]
- 273.Posner AS, Betts F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc Chem Res. 1975;8:273–81. doi: 10.1021/ar50092a003. [DOI] [Google Scholar]
- 274.Harries JE, Hukins DWL, Hasnain SS. Analysis of the EXAFS spectrum of hydroxyapatite. J Phys C Solid State Phys. 1986;19:6859–72. doi: 10.1088/0022-3719/19/34/022. [DOI] [Google Scholar]
- 275.Harries JE, Hukins DWL, Holt C, Hasnain SS. Conversion of amorphous calcium phosphate into hydroxyapatite investigated by EXAFS spectroscopy. J Cryst Growth. 1987;84:563–70. doi: 10.1016/0022-0248(87)90046-7. [DOI] [Google Scholar]
- 276.Taylor MG, Simkiss K, Simmons J, Wu LNY, Wuthier RE. Structural studies of a phosphatidyl serine-amorphous calcium phosphate complex. Cell Mol Life Sci. 1998;54:196–202. doi: 10.1007/s000180050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Peters F, Schwarz K, Epple M. The structure of bone studied with synchrotron X-ray diffraction, X-ray absorption spectroscopy and thermal analysis. Thermochim Acta. 2000;361:131–8. doi: 10.1016/S0040-6031(00)00554-2. [DOI] [Google Scholar]
- 278.Posner AS, Betts F, Blumenthal NC. Formation and structure of synthetic and bone hydroxyapatite. Progr Cryst Growth Char. 1980;3:49–64. doi: 10.1016/0146-3535(80)90011-8. [DOI] [Google Scholar]
- 279.Boskey AL. Amorphous calcium phosphate: the contention of bone. J Dent Res. 1997;76:1433–6. doi: 10.1177/00220345970760080501. [DOI] [PubMed] [Google Scholar]
- 280.Onuma K, Ito A. Cluster growth model for hydroxyapatite. Chem Mater. 1998;10:3346–51. doi: 10.1021/cm980062c. [DOI] [Google Scholar]
- 281.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75:1679–86. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 282.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/S0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 283.Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stand Technol. 2003;108:167–82. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53:381–91. doi: 10.1002/1097-4636(2000)53:4<381::AID-JBM12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 285.Schiller C, Siedler M, Peters F, Epple M. Functionally graded materials of biodegradable polyesters and bone-like calcium phosphates for bone replacement. Ceram Transact. 2001;114:97–108. [Google Scholar]
- 286.Linhart W, Peters F, Lehmann W, Schwarz K, Schilling AF, Amling M, et al. Biologically and chemically optimized composites of carbonated apatite and polyglycolide as bone substitution materials. J Biomed Mater Res. 2001;54:162–71. doi: 10.1002/1097-4636(200102)54:2<162::AID-JBM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 287.Tadic D, Beckmann F, Schwarz K, Epple M. A novel method to produce hydroxyapatite objects with interconnecting porosity that avoids sintering. Biomaterials. 2004;25:3335–40. doi: 10.1016/j.biomaterials.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 288.Tadic D, Epple M. Amorphous calcium phosphates as bone substitution materials. Eur J Trauma. 2002;28:136–7. [Google Scholar]
- 289.Eanes ED. Amorphous calcium phosphate. In: Octacalcium phosphate. Monographs in Oral Science. Chow LC, Eanes ED, Eds. Karger: Basel, Switzerland 2001; 18:130-47. [DOI] [PubMed] [Google Scholar]
- 290.Combes C, Rey C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomater. 2010;6:3362–78. doi: 10.1016/j.actbio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 291.Sinha A, Nayar S, Agrawal A, Bhattacharyya D, Ramachandrarao P. Synthesis of nanosized and microporous precipitated hydroxyapatite in synthetic polymers and biopolymers. J Am Ceram Soc. 2003;86:357–9. doi: 10.1111/j.1151-2916.2003.tb00024.x. [DOI] [Google Scholar]
- 292.Mayer I, Jacobsohn O, Niazov T, Werckmann J, Iliescu M, Richard-Plouet M, et al. Manganese in precipitated hydroxyapatites. Eur J Inorg Chem. 2003:1445–51. doi: 10.1002/ejic.200390188. [DOI] [Google Scholar]
- 293.Vallet-Regí M, Rodríguez-Lorenzo LM, Salinas AJ. Synthesis and characterisation of calcium deficient apatite. Solid State Ion. 1997;101-103:1279–85. doi: 10.1016/S0167-2738(97)00213-0. [DOI] [Google Scholar]
- 294.Siddharthan A, Seshadri SK, Sampath Kumar TS. Microwave accelerated synthesis of nanosized calcium deficient hydroxyapatite. J Mater Sci Mater Med. 2004;15:1279–84. doi: 10.1007/s10856-004-5735-3. [DOI] [PubMed] [Google Scholar]
- 295.Hutchens SA, Benson RS, Evans BR, O’Neill HM, Rawn CJ. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials. 2006;27:4661–70. doi: 10.1016/j.biomaterials.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 296.Brès EF, Duhoo T, Leroy N, Lemaitre J. Evidence of a transient phase during the hydrolysis of calcium-deficient hydroxyapatite. Zeitschrift fuer Metallkunde/Mater. Res Adv Tech. 2005;96:503–6. [Google Scholar]
- 297.Lecomte A, Gautier H, Bouler JM, Gouyette A, Pegon Y, Daculsi G, et al. Biphasic calcium phosphate: a comparative study of interconnected porosity in two ceramics. J Biomed Mater Res B Appl Biomater. 2008;84:1–6. doi: 10.1002/jbm.b.30569. [DOI] [PubMed] [Google Scholar]
- 298.Tancret F, Bouler JM, Chamousset J, Minois LM. Modelling the mechanical properties of microporous and macroporous biphasic calcium phosphate bioceramics. J Eur Ceram Soc. 2006;26:3647–56. doi: 10.1016/j.jeurceramsoc.2005.12.015. [DOI] [Google Scholar]
- 299.Bouler JM, Trécant M, Delécrin J, Royer J, Passuti N, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of five synthesis parameters on compressive strength. J Biomed Mater Res. 1996;32:603–9. doi: 10.1002/(SICI)1097-4636(199612)32:4<603::AID-JBM13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 300.Wang J, Chen W, Li Y, Fan S, Weng J, Zhang X. Biological evaluation of biphasic calcium phosphate ceramic vertebral laminae. Biomaterials. 1998;19:1387–92. doi: 10.1016/S0142-9612(98)00014-3. [DOI] [PubMed] [Google Scholar]
- 301.Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials. 1998;19:1473–8. doi: 10.1016/S0142-9612(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 302.Daculsi G, Weiss P, Bouler JM, Gauthier O, Millot F, Aguado E. Biphasic calcium phosphate/hydrosoluble polymer composites: a new concept for bone and dental substitution biomaterials. Bone. 1999;25(Suppl):59S–61S. doi: 10.1016/S8756-3282(99)00135-0. [DOI] [PubMed] [Google Scholar]
- 303.LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003;14:201–9. doi: 10.1023/A:1022872421333. [DOI] [PubMed] [Google Scholar]
- 304.Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003;14:195–200. doi: 10.1023/A:1022842404495. [DOI] [PubMed] [Google Scholar]
- 305.Alam I, Asahina I, Ohmamiuda K, Enomoto S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res. 2001;54:129–38. doi: 10.1002/1097-4636(200101)54:1<129::AID-JBM16>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 306.Daculsi G. Biphasic calcium phosphate granules concept for injectable and mouldable bone substitute. Adv Sci Technol. 2006;49:9–13. doi: 10.4028/www.scientific.net/AST.49.9. [DOI] [Google Scholar]
- 307.Daculsi G, Baroth S, LeGeros RZ. 20 years of biphasic calcium phosphate bioceramics development and applications. Ceram Eng Sci Proc. 2010;30:45–58. [Google Scholar]
- 308.Lobo SE, Arinzeh TL. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials. 2010;3:815–26. doi: 10.3390/ma3020815. [DOI] [Google Scholar]
- 309.Dorozhkina EI, Dorozhkin SV. Mechanism of the solid-state transformation of a calcium-deficient hydroxyapatite (CDHA) into biphasic calcium phosphate (BCP) at elevated temperatures. Chem Mater. 2002;14:4267–72. doi: 10.1021/cm0203060. [DOI] [Google Scholar]
- 310.Dorozhkin SV. Mechanism of solid-state conversion of non-stoichiometric hydroxyapatite to diphase calcium phosphate. Russ Chem Bull (Int. Ed.) 2003; 52:2369-75. [Google Scholar]
- 311.Rodríguez-Lorenzo LM. Studies on calcium deficient apatites structure by means of MAS-NMR spectroscopy. J Mater Sci Mater Med. 2005;16:393–8. doi: 10.1007/s10856-005-6977-4. [DOI] [PubMed] [Google Scholar]
- 312.Wilson RM, Elliott JC, Dowker SEP. Formate incorporation in the structure of Ca-deficient apatite: Rietveld structure refinement. J Solid State Chem. 2003;174:132–40. doi: 10.1016/S0022-4596(03)00188-9. [DOI] [Google Scholar]
- 313.Zahn D, Hochrein O. On the composition and atomic arrangement of calcium-deficient hydroxyapatite: an ab-initio analysis. J Solid State Chem. 2008;181:1712–6. doi: 10.1016/j.jssc.2008.03.035. [DOI] [Google Scholar]
- 314.Brown PW, Martin RI. An analysis of hydroxyapatite surface layer formation. J Phys Chem B. 1999;103:1671–5. doi: 10.1021/jp982554i. [DOI] [Google Scholar]
- 315.Honghui Z, Hui L, Linghong G. Molecular and crystal structure characterization of calcium-deficient apatite. Key Eng Mater. 2007;330-332:119–22. doi: 10.4028/www.scientific.net/KEM.330-332.119. [DOI] [Google Scholar]
- 316.Viswanath B, Shastry VV, Ramamurty U, Ravishankar N. Effect of calcium deficiency on the mechanical properties of hydroxyapatite crystals. Acta Mater. 2010;58:4841–8. doi: 10.1016/j.actamat.2010.05.019. [DOI] [Google Scholar]
- 317.Mortier A, Lemaître J, Rodrique L, Rouxhet PG. Synthesis and thermal behavior of well-crystallized calcium-deficient phosphate apatite. J Solid State Chem. 1989;78:215–9. doi: 10.1016/0022-4596(89)90099-6. [DOI] [Google Scholar]
- 318.Jeanjean J, McGrellis S, Rouchaud JC, Fedoroff M, Rondeau A, Perocheau S, et al. A crystallographic study of the sorption of cadmium on calcium hydroxyapatites: incidence of cationic vacancies. J Solid State Chem. 1996;126:195–201. doi: 10.1006/jssc.1996.0329. [DOI] [Google Scholar]
- 319.Wilson RM, Elliott JC, Dowker SEP, Rodriguez-Lorenzo LM. Rietveld refinements and spectroscopic studies of the structure of Ca-deficient apatite. Biomaterials. 2005;26:1317–27. doi: 10.1016/j.biomaterials.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 320.Ivanova TI, Frank-Kamenetskaya OV, Kol’tsov AB, Ugolkov VL. Crystal structure of calcium-deficient carbonated hydroxyapatite thermal decomposition. J Solid State Chem. 2001;160:340–9. doi: 10.1006/jssc.2000.9238. [DOI] [Google Scholar]
- 321.Matsunaga K. Theoretical investigation of the defect formation mechanism relevant to nonstoichiometry in hydroxyapatite. Phys Rev B. 2008;77:104106–20. doi: 10.1103/PhysRevB.77.104106. [DOI] [Google Scholar]
- 322.Bourgeois B, Laboux O, Obadia L, Gauthier O, Betti E, Aguado E, et al. Calcium-deficient apatite: a first in vivo study concerning bone ingrowth. J Biomed Mater Res A. 2003;65:402–8. doi: 10.1002/jbm.a.10518. [DOI] [PubMed] [Google Scholar]
- 323.Liu TY, Chen SY, Liu DM, Liou SC. On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J Control Release. 2005;107:112–21. doi: 10.1016/j.jconrel.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 324.Tsuchida T, Yoshioka T, Sakuma S, Takeguchi T, Ueda W. Synthesis of biogasoline from ethanol over hydroxyapatite catalyst. Ind Eng Chem Res. 2008;47:1443–52. doi: 10.1021/ie0711731. [DOI] [Google Scholar]
- 325.Elliott JC, Mackie PE, Young RA. Monoclinic hydroxyapatite. Science. 1973;180:1055–7. doi: 10.1126/science.180.4090.1055. [DOI] [PubMed] [Google Scholar]
- 326.Rangavittal N, Landa-Cánovas AR, González-Calbet JM, Vallet-Regí M. Structural study and stability of hydroxyapatite and beta-tricalcium phosphate: two important bioceramics. J Biomed Mater Res. 2000;51:660–8. doi: 10.1002/1097-4636(20000915)51:4<660::AID-JBM14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 327.Kim JY, Fenton RR, Hunter BA, Kennedy BJ. Powder diffraction studies of synthetic calcium and lead apatites. Aust J Chem. 2000;53:679–86. doi: 10.1071/CH00060. [DOI] [Google Scholar]
- 328.Kay MI, Young RA, Posner AS. Crystal structure of hydroxyapatite. Nature. 1964;204:1050–2. doi: 10.1038/2041050a0. [DOI] [PubMed] [Google Scholar]
- 329.Treboux G, Layrolle P, Kanzaki N, Onuma K, Ito A. Existence of Posner’s cluster in vacuum. J Phys Chem A. 2000;104:5111–4. doi: 10.1021/jp994399t. [DOI] [Google Scholar]
- 330.Gilmore RS, Katz JL. Elastic properties of apatites. J Mater Sci. 1982;17:1131–41. doi: 10.1007/BF00543533. [DOI] [Google Scholar]
- 331.Calderin L, Stott MJ, Rubio A. Electronic and crystallographic structure of apatites. Phys Rev B. 2003;67:134106–12. doi: 10.1103/PhysRevB.67.134106. [DOI] [Google Scholar]
- 332.Rulis P, Ouyang L, Ching WY. Electronic structure and bonding in calcium apatite crystals: hydroxyapatite, fluorapatite, chlorapatite and bromapatite. Phys Rev B. 2004;70:155104–12. doi: 10.1103/PhysRevB.70.155104. [DOI] [Google Scholar]
- 333.Snyders R, Music D, Sigumonrong D, Schelnberger B, Jensen J, Schneider JM. Experimental and ab initio study of the mechanical properties of hydroxyapatite. Appl Phys Lett. 2007;90:193902–5. doi: 10.1063/1.2738386. [DOI] [Google Scholar]
- 334.Ching WY, Rulis P, Misra A. Ab initio elastic properties and tensile strength of crystalline hydroxyapatite. Acta Biomater. 2009;5:3067–75. doi: 10.1016/j.actbio.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 335.Treboux G, Layrolle P, Kanzaki N, Onuma K, Ito A. Symmetry of Posner’s cluster. J Am Chem Soc. 2000;122:8323–4. doi: 10.1021/ja994286n. [DOI] [Google Scholar]
- 336.Yin X, Stott MJ. Biological calcium phosphates and Posner’s cluster. J Chem Phys. 2003;118:3717–23. doi: 10.1063/1.1539093. [DOI] [Google Scholar]
- 337.Kanzaki N, Treboux G, Onuma K, Tsutsumi S, Ito A. Calcium phosphate clusters. Biomaterials. 2001;22:2921–9. doi: 10.1016/S0142-9612(01)00039-4. [DOI] [PubMed] [Google Scholar]
- 338.Calderin L, Dunfield D, Stott MJ. Shell-model study of the lattice dynamics of hydroxyapatite. Phys Rev B. 2005;72:224304–17. doi: 10.1103/PhysRevB.72.224304. [DOI] [Google Scholar]
- 339.Tanaka Y, Iwasaki T, Nakamura M, Nagai A, Katayama K, Yamashita K. Polarization and microstructural effects of ceramic hydroxyapatite electrets. J Appl Phys. 2010;107:014107–17. doi: 10.1063/1.3265429. [DOI] [Google Scholar]
- 340.Tanaka Y, Iwasaki T, Katayama K, Hojo J, Yamashita K. Effect of ionic polarization on crystal structure of hydroxyapatite ceramic with hydroxide nonstoichiometry. J Jpn Soc Powder and Powder Metall. 2010;57:520–8. doi: 10.2497/jjspm.57.520. [DOI] [Google Scholar]
- 341.Tofail SAM, Baldisserri C, Haverty D, McMonagle JB, Erhart J. Pyroelectric surface charge in hydroxyapatite ceramics. J Appl Phys. 2009;106:106104. doi: 10.1063/1.3262628. [DOI] [Google Scholar]
- 342.Kawabata K, Yamamoto T. First-principles calculations of the elastic properties of hydroxyapatite doped with divalent ions. J Ceram Soc Jpn. 2010;118:548–9. doi: 10.2109/jcersj2.118.548. [DOI] [Google Scholar]
- 343.Matsunaga K, Kuwabara A. First-principles study of vacancy formation in hydroxyapatite. Phys Rev B. 2007;75:14102–11. doi: 10.1103/PhysRevB.75.014102. [DOI] [Google Scholar]
- 344.de Leeuw NH. Computer simulations of structures and properties of the biomaterial hydroxyapatite. J Mater Chem. 2010;20:5376–89. doi: 10.1039/b921400c. [DOI] [Google Scholar]
- 345.Corno M, Rimola A, Bolis V, Ugliengo P. Hydroxyapatite as a key biomaterial: quantum-mechanical simulation of its surfaces in interaction with biomolecules. Phys Chem Chem Phys. 2010;12:6309–29. doi: 10.1039/c002146f. [DOI] [PubMed] [Google Scholar]
- 346.El Briak-Benabdeslam H, Ginebra MP, Vert M, Boudeville P, Briak-Ben El Wet or dry mechanochemical synthesis of calcium phosphates? Influence of the water content on DCPD-CaO reaction kinetics. Acta Biomater. 2008;4:378–86. doi: 10.1016/j.actbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 347.LeGeros RZ, LeGeros JP. Dense hydroxyapatite. In: An introduction to bioceramics. Hench LL, Wilson J, Eds. World Scientific: London UK 1993; 139-80. [Google Scholar]
- 348.Rodriguez-Lorenzo LM, Vallet-Regí M. Controlled crystallization of calcium phosphate apatites. Chem Mater. 2000;12:2460–5. doi: 10.1021/cm001033g. [DOI] [Google Scholar]
- 349.Cazalbou S, Combes C, Eichert D, Rey C. Adaptive physico-chemistry of bio-related calcium phosphates. J Mater Chem. 2004;14:2148–53. doi: 10.1039/b401318b. [DOI] [Google Scholar]
- 350.Markovic M, Fowler BO, Tung MS. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J Res Natl Inst Stand Technol. 2004;109:553–68. doi: 10.6028/jres.109.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ioku K, Kawachi G, Sasaki S, Fujimori H, Goto S. Hydrothermal preparation of tailored hydroxyapatite. J Mater Sci. 2006;41:1341–4. doi: 10.1007/s10853-006-7338-5. [DOI] [Google Scholar]
- 352.Ito N, Kamitakahara M, Murakami S, Watanabe N, Ioku K. Hydrothermal synthesis and characterization of hydroxyapatite from octacalcium phosphate. J Ceram Soc Jpn. 2010;118:762–6. doi: 10.2109/jcersj2.118.762. [DOI] [Google Scholar]
- 353.Layrolle P, Lebugle A. Characterization and reactivity of nanosized calcium phosphates prepared in anhydrous ethanol. Chem Mater. 1994;6:1996–2004. doi: 10.1021/cm00047a019. [DOI] [Google Scholar]
- 354.Layrolle P, Lebugle A. Synthesis in pure ethanol and characterization of nanosized calcium phosphate fluoroapatite. Chem Mater. 1996;8:134–44. doi: 10.1021/cm950326k. [DOI] [Google Scholar]
- 355.Yeong B, Junmin X, Wang J. Mechanochemical synthesis of hydroxyapatite from calcium oxide and brushite. J Am Ceram Soc. 2001;84:465–7. doi: 10.1111/j.1151-2916.2001.tb00681.x. [DOI] [Google Scholar]
- 356.Roy DM, Linnehan SK. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature. 1974;247:220–2. doi: 10.1038/247220a0. [DOI] [PubMed] [Google Scholar]
- 357.Ben-Nissan B. Natural bioceramics: from coral to bone and beyond. Curr Opin Solid State Mater Sci. 2003;7:283–8. doi: 10.1016/j.cossms.2003.10.001. [DOI] [Google Scholar]
- 358.Ben-Nissan B, Milev A, Vago R. Morphology of sol-gel derived nano-coated coralline hydroxyapatite. Biomaterials. 2004;25:4971–5. doi: 10.1016/j.biomaterials.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 359.Elliott JC, Young RA. Conversion of single crystals of chlorapatite into single crystals of hydroxyapatite. Nature. 1967;214:904–6. doi: 10.1038/214904b0. [DOI] [Google Scholar]
- 360.Tao J, Jiang W, Pan H, Xu X, Tang R. Preparation of large-sized hydroxyapatite single crystals using homogeneous releasing controls. J Cryst Growth. 2007;308:151–8. doi: 10.1016/j.jcrysgro.2007.08.009. [DOI] [Google Scholar]
- 361.Vallet-Regí M, Gutierrez Rios MT, Alonso MP, de Frutos MI, Nicolopoulos S. Hydroxyapatite particles synthesized by pyrolysis of an aerosol. J Solid State Chem. 1994;112:58–64. doi: 10.1006/jssc.1994.1264. [DOI] [Google Scholar]
- 362.Montero ML, Sáenz A, Rodríguez JG, Arenas J, Castaño VM. Electrochemical synthesis of nanosized hydroxyapatite. J Mater Sci. 2006;41:2141–4. doi: 10.1007/s10853-006-5231-x. [DOI] [Google Scholar]
- 363.Kumar AR, Kalainathan S. Growth and characterization of nano-crystalline hydroxyapatite at physiological conditions. Cryst Res Technol. 2008;43:640–4. doi: 10.1002/crat.200711094. [DOI] [Google Scholar]
- 364.Kalita SJ, Bhardwaj A, Bhatt HA. Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater Sci Eng C. 2007;27:441–9. doi: 10.1016/j.msec.2006.05.018. [DOI] [Google Scholar]
- 365.Melikhov IV, Komarov VF, Severin AV, Bozhevolnov VE, Rudin VN. Two-dimensional crystalline hydroxyapatite. Dokl Phys Chem. 2000;373:125–8. [Google Scholar]
- 366.Suvorova EI, Buffat PA. Electron diffraction and HRTEM characterization of calcium phosphate precipitation from aqueous solutions under biomineralization conditions. Eur Cell Mater. 2001;1:27–42. doi: 10.22203/ecm.v001a04. [DOI] [PubMed] [Google Scholar]
- 367.Suvorova EI, Buffat PA. Model of the mechanism of Ca loss by bones under microgravity and earth conditions. J Biomed Mater Res. 2002;63:424–32. doi: 10.1002/jbm.10256. [DOI] [PubMed] [Google Scholar]
- 368.Uematsu K, Takagi M, Honda T, Uchida N, Saito K. Transparent hydroxyapatite prepared by hot isostatic pressing of filter cake. J Am Ceram Soc. 1989;72:1476–8. doi: 10.1111/j.1151-2916.1989.tb07680.x. [DOI] [Google Scholar]
- 369.Takikawa K, Akao M. Fabrication of transparent hydroxyapatite and application to bone marrow derived cell/hydroxyapatite interaction observation in vivo. J Mater Sci Mater Med. 1996;7:439–45. doi: 10.1007/BF00122014. [DOI] [Google Scholar]
- 370.Watanabe Y, Ikoma T, Monkawa A, Suetsugu Y, Yamada H, Tanaka J, et al. Fabrication of transparent hydroxyapatite sintered body with high crystal orientation by pulse electric current sintering. J Am Ceram Soc. 2005;88:243–5. doi: 10.1111/j.1551-2916.2004.00041.x. [DOI] [Google Scholar]
- 371.Kotobuki N, Ioku K, Kawagoe D, Fujimori H, Goto S, Ohgushi H. Observation of osteogenic differentiation cascade of living mesenchymal stem cells on transparent hydroxyapatite ceramics. Biomaterials. 2005;26:779–85. doi: 10.1016/j.biomaterials.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 372.Perloff A, Posner AS. Preparation of pure hydroxyapatite crystals. Science. 1956;124:583–4. doi: 10.1126/science.124.3222.583-a. [DOI] [PubMed] [Google Scholar]
- 373.LeGeros RZ, LeGeros JP, Daculsi G, Kijkowska R. Calcium phosphate biomaterials: preparation, properties and biodegradation. In: Encyclopedic handbook of biomaterials and bioengineering. Part A: Materials. Wise DL, Trantolo DJ, Altobelli DE, Yaszemski MJ, Gresser JD, Schwartz ER, Eds., Marcel Dekker: New York USA 1995; 2:1429-63. [Google Scholar]
- 374.Narasaraju TSB, Phebe DE. Some physico-chemical aspects of hydroxylapatite. J Mater Sci. 1996;31:1–21. doi: 10.1007/BF00355120. [DOI] [Google Scholar]
- 375.Orlovskii VP, Barinov SM. Hydroxyapatite and hydroxyapatite-matrix materials: a survey. Russ J Inorg Chem. 2001;46:129–49. [Google Scholar]
- 376.Riman RE, Suchanek WL, Byrappa K, Chen CW, Shuk P, Oakes CS. Solution synthesis of hydroxyapatite designer particulates. Solid State Ion. 2002;151:393–402. doi: 10.1016/S0167-2738(02)00545-3. [DOI] [Google Scholar]
- 377.Orlovskii VP, Komlev VS, Barinov SM. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg Mater. 2002;38:973–84. doi: 10.1023/A:1020585800572. [DOI] [Google Scholar]
- 378.Pena J, Vallet-Regí M. Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. J Eur Ceram Soc. 2003;23:1687–96. doi: 10.1016/S0955-2219(02)00369-2. [DOI] [Google Scholar]
- 379.Koutsopoulos S. Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods. J Biomed Mater Res. 2002;62:600–12. doi: 10.1002/jbm.10280. [DOI] [PubMed] [Google Scholar]
- 380.Norton J, Malik KR, Darr JA, Rehman IU. Recent developments in processing and surface modification of hydroxyapatite. Adv Appl Ceramics. 2006;105:113–39. doi: 10.1179/174367606X102278. [DOI] [Google Scholar]
- 381.Rakovan J. Growth and surface properties of apatite. In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews in Mineralogy and Geochemistry. Hughes JM, Kohn M, Rakovan J, Eds. Mineralogical Society of America: Washington DC, USA 2002; 48:51-86. [Google Scholar]
- 382.Dorozhkin SV. A review on the dissolution models of calcium apatites. Prog Cryst Growth Charact. 2002;44:45–61. doi: 10.1016/S0960-8974(02)00004-9. [DOI] [Google Scholar]
- 383.Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13:94–117. doi: 10.1557/JMR.1998.0015. [DOI] [Google Scholar]
- 384.Willmann G. Coating of implants with hydroxyapatite-material connections between bone and metal. Adv Eng Mater. 1999;1:95–105. doi: 10.1002/(SICI)1527-2648(199910)1:2<95::AID-ADEM95>3.0.CO;2-P. [DOI] [Google Scholar]
- 385.Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001;58:570–92. doi: 10.1002/jbm.1056. [DOI] [PubMed] [Google Scholar]
- 386.Ong JL, Chan DCN. Hydroxyapatites and their use as coatings in dental implants: a review. Crit Rev Biomed Eng. 1999;28:667–707. doi: 10.1615/critrevbiomedeng.v28.i56.10. [DOI] [PubMed] [Google Scholar]
- 387.Geesink RG. Osteoconductive coatings for total joint arthroplasty. Clin Orthop Relat Res. 2002;395:53–65. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 388.Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74:1487–510. doi: 10.1111/j.1151-2916.1991.tb07132.x. [DOI] [Google Scholar]
- 389.Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–28. doi: 10.1111/j.1151-2916.1998.tb02540.x. [DOI] [Google Scholar]
- 390.Dey A, Mukhopadhyay AK, Gangadharan S, Sinha MK, Basu D. Characterization of microplasma sprayed hydroxyapatite coating. J. Thermal Spray Technol. 2009;18:578–92. doi: 10.1007/s11666-009-9386-2. [DOI] [Google Scholar]
- 391.Mangano C, Piattelli A, Perrotti V, Iezzi G. Dense hydroxyapatite inserted into postextraction sockets: a histologic and histomorphometric 20-year case report. J Periodontol. 2008;79:929–33. doi: 10.1902/jop.2008.070245. [DOI] [PubMed] [Google Scholar]
- 392.Dickinson W. Chromatography of insulin on calcium phosphate columns. Nature. 1956;178:994–5. doi: 10.1038/178994b0. [DOI] [PubMed] [Google Scholar]
- 393.Bernardi G. Chromatography of nucleic acids on hydroxyapatite. Nature. 1965;206:779–83. doi: 10.1038/206779a0. [DOI] [PubMed] [Google Scholar]
- 394.Xia Z, Duan X, Locklin RM, Quijano M, Dobson RL, Triffitt JT, et al. Evaluation of the relative mineral-binding affinities of clinically-relevant bisphosphonates by using hydroxyapatite-column chromatography and adsorption isotherms combined with mass spectrometric analysis. Bone. 2008;42:90–1. doi: 10.1016/j.bone.2007.12.172. [DOI] [Google Scholar]
- 395.Brand M, Rampalli S, Chaturvedi CP, Dilworth FJ. Analysis of epigenetic modifications of chromatin at specific gene loci by native chromatin immunoprecipitation of nucleosomes isolated using hydroxyapatite chromatography. Nat Protoc. 2008;3:398–409. doi: 10.1038/nprot.2008.8. [DOI] [PubMed] [Google Scholar]
- 396.Yoshitake T, Kobayashi S, Ogawa T, Okuyama T. Hydroxyapatite chromatography of guanidine denatured proteins: 1. Guanidine containing phosphate buffer system. Chromatography. 2006;27:19–26. [Google Scholar]
- 397.Smith GP, Gingrich TR. Hydroxyapatite chromatography of phage-display virions. Biotechniques. 2005;39:879–84. doi: 10.2144/000112032. [DOI] [PubMed] [Google Scholar]
- 398.Jungbauer A, Hahn R, Deinhofer K, Luo P. Performance and characterization of a nanophased porous hydroxyapatite for protein chromatography. Biotechnol Bioeng. 2004;87:364–75. doi: 10.1002/bit.20121. [DOI] [PubMed] [Google Scholar]
- 399.Doonan S. Chromatography on hydroxyapatite. Methods Mol Biol. 2004;244:191–4. doi: 10.1385/1-59259-655-x:191. [DOI] [PubMed] [Google Scholar]
- 400.Cummings LJ, Snyder MA, Brisack K. Protein chromatography on hydroxyapatite columns. Methods Enzymol. 2009;463:387–404. doi: 10.1016/S0076-6879(09)63024-X. [DOI] [PubMed] [Google Scholar]
- 401.Gagnon P. Monoclonal antibody purification with hydroxyapatite. N Biotechnol. 2009;25:287–93. doi: 10.1016/j.nbt.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 402.Palazzo B, Sidoti MC, Roveri N, Tampieri A, Sandri M, Bertolazzi L, et al. Controlled drug delivery from porous hydroxyapatite grafts: an experimental and theoretical approach. Mater Sci Eng C. 2005;25:207–13. doi: 10.1016/j.msec.2005.01.011. [DOI] [Google Scholar]
- 403.Palazzo B, Iafisco M, Laforgia M, Margiotta N, Natile G, Bianchi CL, et al. Biomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv Funct Mater. 2007;17:2180–8. doi: 10.1002/adfm.200600361. [DOI] [Google Scholar]
- 404.Tang SH, Jin AM, Lü RF, Wang XD, Wang YF, Yu B, et al. Preparation and in vivo release experiment of nano-hydroxyapatite/gentamicin drug delivery system. J Clin Rehabil Tiss Eng Res. 2007;11:3573–6. [Google Scholar]
- 405.Ye F, Guo H, Zhang H, He X. Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 2010;6:2212–8. doi: 10.1016/j.actbio.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 406.Niwa M, Sato T, Li W, Aoki H, Aoki H, Daisaku T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J Mater Sci Mater Med. 2001;12:277–81. doi: 10.1023/A:1008927502523. [DOI] [PubMed] [Google Scholar]
- 407.Kim BI, Jeong SH, Jang SO, Kim KN, Kwon HK, Park YD. Tooth whitening effect of toothpastes containing nano-hydroxyapatite. Key Eng Mater. 2006;309-311:541–4. doi: 10.4028/www.scientific.net/KEM.309-311.541. [DOI] [Google Scholar]
- 408.Pietrasik J, Szustakiewicz K, Zaborski M, Haberko K. Hydroxyapatite: an environmentally friendly filler for elastomers. Mol Cryst Liq Cryst (Phila Pa) 2008;483:172–8. doi: 10.1080/15421400801904880. [DOI] [Google Scholar]
- 409.Bailliez S, Nzihou A, Bèche E, Flamant G. Removal of lead (Pb) by hydroxyapatite sorbent. Process Saf Environ Prot. 2004;82:175–80. doi: 10.1205/095758204322972816. [DOI] [Google Scholar]
- 410.Corami A, Mignardi S, Ferrini V. Cadmium removal from single- and multi-metal (Cd + Pb + Zn + Cu) solutions by sorption on hydroxyapatite. J Colloid Interface Sci. 2008;317:402–8. doi: 10.1016/j.jcis.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 411.Phonthammachai N, Ziyi Z, Jun G, Fan HY, White TJ. Synthesis of high performance hydroxyapatite-gold catalysts for CO oxidation. Gold Bull. 2008;41:42–50. doi: 10.1007/BF03215622. [DOI] [Google Scholar]
- 412.Chen W, Huang ZL, Liu Y, He QJ. Preparation and characterization of a novel solid base catalyst hydroxyapatite loaded with strontium. Catal Commun. 2008;9:516–21. doi: 10.1016/j.catcom.2007.02.011. [DOI] [Google Scholar]
- 413.Domínguez MI, Romero-Sarria F, Centeno MA, Odriozola JA. Gold/hydroxyapatite catalysts. Synthesis, characterization and catalytic activity to CO oxidation. Appl Catal B. 2009;87:245–51. doi: 10.1016/j.apcatb.2008.09.016. [DOI] [Google Scholar]
- 414.Xu J, White T, Li P, He C, Han YF. Hydroxyapatite foam as a catalyst for formaldehyde combustion at room temperature. J Am Chem Soc. 2010;132:13172–3. doi: 10.1021/ja1058923. [DOI] [PubMed] [Google Scholar]
- 415.de Groot K, Wolke JGC, Jansen JA. Calcium phosphate coatings for medical implants. Proc Inst Mech Eng H. 1998;212:137–47. doi: 10.1243/0954411981533917. [DOI] [PubMed] [Google Scholar]
- 416.Gross KA, Berndt CC. Biomedical application of apatites. In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews in Mineralogy and Geochemistry. Hughes JM, Kohn M, Rakovan J, Eds. Mineralogical Society of America: Washington DC, USA 2002; 48:631-72. [Google Scholar]
- 417.Ferraz MP, Monteiro FJ, Manuel CM. Hydroxyapatite nanoparticles: a review of preparation methodologies. J Appl Biomater. Biomech. 2004;2:74–80. [PubMed] [Google Scholar]
- 418.Damien E, Revell PA. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J Appl Biomater Biomech. 2004;2:65–73. [PubMed] [Google Scholar]
- 419.Aoki H. Science and medical applications of hydroxyapatite. JAAS: Tokyo, Japan 1991; 245. [Google Scholar]
- 420.Busch S, Dolhaine H, Duchesne A, Heinz S, Hochrein O, Laeri F, et al. Biomimetic morphogenesis of fluorapatite-gelatin composites: fractal growth, the question of intrinsic electric fields, core/shell assemblies, hollow spheres and reorganization of denatured collagen. Eur J Inorg Chem. 1999:1643–53. doi: 10.1002/(SICI)1099-0682(199910)1999:10<1643::AID-EJIC1643>3.0.CO;2-J. [DOI] [Google Scholar]
- 421.Kniep R, Busch S. Biomimetic growth and self-assembly of fluorapatite aggregates by diffusion into denatured collagen matrices. Angew Chem Int Ed Engl. 1996;35:2624–6. doi: 10.1002/anie.199626241. [DOI] [Google Scholar]
- 422.Godiebel C, Simon P, Buder J, Tlatlik H, Kniep R. Phase formation and morphology of calcium phosphate-gelatine-composites grown by double diffusion technique: the influence of fluoride. J Mater Chem. 2004;14:2225–30. doi: 10.1039/b403503h. [DOI] [Google Scholar]
- 423.Prymak O, Sokolova V, Peitsch T, Epple M. The crystallization of fluoroapatite dumbbells from supersaturated aqueous solution. Cryst Growth Des. 2006;6:498–506. doi: 10.1021/cg050428f. [DOI] [Google Scholar]
- 424.Tlatlik H, Simon P, Kawska A, Zahn D, Kniep R. Biomimetic fluorapatite-gelatine nanocomposites: pre-structuring of gelatine matrices by ion impregnation and its effect on form development. Angew Chem Int Ed Engl. 2006;45:1905–10. doi: 10.1002/anie.200503610. [DOI] [PubMed] [Google Scholar]
- 425.Simon P, Zahn D, Lichte H, Kniep R. Intrinsic electric dipole fields and the induction of hierarchical form developments in fluorapatite-gelatine nanocomposites: a general principle for morphogenesis of biominerals? Angew Chem Int Ed Engl. 2006;45:1911–5. doi: 10.1002/anie.200504465. [DOI] [PubMed] [Google Scholar]
- 426.Wu YJ, Tseng YH, Chan JCC. Morphology control of fluorapatite crystallites by citrate ions. Cryst Growth Des. 2010;10:4240–2. doi: 10.1021/cg100859m. [DOI] [Google Scholar]
- 427.Dorozhkin SV. A hierarchical structure for apatite crystals. J Mater Sci Mater Med. 2007;18:363–6. doi: 10.1007/s10856-006-0701-x. [DOI] [PubMed] [Google Scholar]
- 428.Mehmel M. On the structure of apatite. I. Z Kristallogr. 1930;75:323–31. [Google Scholar]
- 429.Naray-Szabo S. The structure of apatite (CaF)Ca4(PO4)3. Z Kristallogr. 1930;75:387–98. [Google Scholar]
- 430.Sudarsanan K, Mackie PE, Young RA. Comparison of synthetic and mineral fluorapatite, Ca5(PO4)3F, in crystallographic detail. Mater Res Bull. 1972;7:1331–7. doi: 10.1016/0025-5408(72)90113-4. [DOI] [Google Scholar]
- 431.Barinov SM, Shvorneva LI, Ferro D, Fadeeva IV, Tumanov SV. Solid solution formation at the sintering of hydroxyapatite-fluorapatite ceramics. Sci Technol Adv Mater. 2004;5:537–41. doi: 10.1016/j.stam.2004.02.012. [DOI] [Google Scholar]
- 432.Nikcevic I, Jokanovic V, Mitric M, Nedic Z, Makovec D, Uskokovic D. Mechanochemical synthesis of nanostructured fluorapatite/fluorhydroxyapatite and carbonated fluorapatite/fluorhydroxyapatite. J Solid State Chem. 2004;177:2565–74. doi: 10.1016/j.jssc.2004.03.024. [DOI] [Google Scholar]
- 433.Cheng K, Weng W, Qu H, Du P, Shen G, Han G, et al. Sol-gel preparation and in vitro test of fluorapatite/hydroxyapatite films. J Biomed Mater Res B Appl Biomater. 2004;69:33–7. doi: 10.1002/jbm.b.20027. [DOI] [PubMed] [Google Scholar]
- 434.Rodríguez-Lorenzo LM, Hart JN, Gross KA. Influence of fluorine in the synthesis of apatites. Synthesis of solid solutions of hydroxy-fluorapatite. Biomaterials. 2003;24:3777–85. doi: 10.1016/S0142-9612(03)00259-X. [DOI] [PubMed] [Google Scholar]
- 435.Wei M, Evans JH, Bostrom T, Grøndahl L. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. J Mater Sci Mater Med. 2003;14:311–20. doi: 10.1023/A:1022975730730. [DOI] [PubMed] [Google Scholar]
- 436.Daculsi G, Kerebel LM. Ultrastructural study and comparative analysis of fluoride content of enameloid in sea-water and fresh-water sharks. Arch Oral Biol. 1980;25:145–51. doi: 10.1016/0003-9969(80)90013-8. [DOI] [PubMed] [Google Scholar]
- 437.Daculsi G, Kerebel LM, Kerebel B. Effects of fluoride on human enamel and selachian enameloid in vitro: a high-resolution TEM and electron diffraction study. Calcif Tissue Int. 1981;33:9–13. doi: 10.1007/BF02409406. [DOI] [PubMed] [Google Scholar]
- 438.Weiner S, Dove PM. An overview of biomineralization processes and the problem of the vital effect. In: Biomineralization. Series: Reviews in Mineralogy and Geochemistry. Dove PM, de Yoreo JJ, Weiner S, Eds. Mineralogical Society of America: Washington DC, USA 2003; 54:1-29. [Google Scholar]
- 439.Dahm S, Risnes S. A comparative infrared spectroscopic study of hydroxide and carbonate absorption bands in spectra of shark enameloid, shark dentin, and a geological apatite. Calcif Tissue Int. 1999;65:459–65. doi: 10.1007/s002239900733. [DOI] [PubMed] [Google Scholar]
- 440.Carr A, Kemp A, Tibbetts I, Truss R, Drennan J. Microstructure of pharyngeal tooth enameloid in the parrotfish Scarus rivulatus (Pisces: Scaridae) J Microsc. 2006;221:8–16. doi: 10.1111/j.1365-2818.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 441.Lévêque I, Cusack M, Davis SA, Mann S. Promotion of fluorapatite crystallization by soluble-matrix proteins from Lingula anatina shells. Angew Chem Int Ed Engl. 2004;43:885–8. doi: 10.1002/anie.200353115. [DOI] [PubMed] [Google Scholar]
- 442.Heling I, Heindel R, Merin B. Calcium-fluorapatite. A new material for bone implants. J Oral Implantol. 1981;9:548–55. [PubMed] [Google Scholar]
- 443.Busch S, Schwarz U, Kniep R. Morphogenesis and structure of human teeth in relation to biomimetically grown fluorapatite-gelatine composites. Chem Mater. 2001;13:3260–71. doi: 10.1021/cm0110728. [DOI] [Google Scholar]
- 444.Kniep R, Simon P. Fluorapatite-gelatine-nanocomposites: self-organized morphogenesis, real structure and relations to natural hard materials. Top Curr Chem. 2006;270:73–125. doi: 10.1007/128_053. [DOI] [Google Scholar]
- 445.Bogdanov BI, Pashev PS, Hristov JH, Markovska IG. Bioactive fluorapatite-containing glass ceramics. Ceram Int. 2009;35:1651–5. doi: 10.1016/j.ceramint.2008.07.021. [DOI] [Google Scholar]
- 446.Savarino L, Fini M, Ciapetti G, Cenni E, Granchi D, Baldini N, et al. Biologic effects of surface roughness and fluorhydroxyapatite coating on osteointegration in external fixation systems: an in vivo experimental study. J Biomed Mater Res A. 2003;66:652–61. doi: 10.1002/jbm.a.10018. [DOI] [PubMed] [Google Scholar]
- 447.Vitkovic M, Noaman MSM, Palou MT, Jantová S. Potential applications of fluorhydroxyapatite as biomaterials in medicine. Cent Eur J Chem. 2009;7:246–51. doi: 10.2478/s11532-009-0010-6. [DOI] [Google Scholar]
- 448.Chaari K, Ayed FB, Bouaziz J, Bouzouita K. Elaboration and characterization of fluorapatite ceramic with controlled porosity. Mater Chem Phys. 2009;113:219–26. doi: 10.1016/j.matchemphys.2008.07.079. [DOI] [Google Scholar]
- 449.Bibby JK, Bubb NL, Wood DJ, Mummery PM. Fluorapatite-mullite glass sputter coated Ti6Al4V for biomedical applications. J Mater Sci Mater Med. 2005;16:379–85. doi: 10.1007/s10856-005-6975-6. [DOI] [PubMed] [Google Scholar]
- 450.Yoon BH, Kim HW, Lee SH, Bae CJ, Koh YH, Kong YM, et al. Stability and cellular responses to fluorapatite-collagen composites. Biomaterials. 2005;26:2957–63. doi: 10.1016/j.biomaterials.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 451.Gineste L, Gineste M, Ranz X, Ellefterion A, Guilhem A, Rouquet N, et al. Degradation of hydroxylapatite, fluorapatite, and fluorhydroxyapatite coatings of dental implants in dogs. J Biomed Mater Res. 1999;48:224–34. doi: 10.1002/(SICI)1097-4636(1999)48:3<224::AID-JBM5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 452.Bhadang KA, Gross KA. Influence of fluorapatite on the properties of thermally sprayed hydroxyapatite coatings. Biomaterials. 2004;25:4935–45. doi: 10.1016/j.biomaterials.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 453.Gross KA, Bhadang KA. Sintered hydroxyfluorapatites. Part III: sintering and resultant mechanical properties of sintered blends of hydroxyapatite and fluorapatite. Biomaterials. 2004;25:1395–405. doi: 10.1016/j.biomaterials.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 454.Barinov SM, Rustichelli F, Orlovskii VP, Lodini A, Oscarsson S, Firstov SA, et al. Influence of fluorapatite minor additions on behavior of hydroxyapatite ceramics. J Mater Sci Mater Med. 2004;15:291–6. doi: 10.1023/B:JMSM.0000015490.32304.f4. [DOI] [PubMed] [Google Scholar]
- 455.Agathopoulos S, Tulyaganov DU, Marques PAAP, Ferro MC, Fernandes MH, Correia RN. The fluorapatite-anorthite system in biomedicine. Biomaterials. 2003;24:1317–31. doi: 10.1016/S0142-9612(02)00468-4. [DOI] [PubMed] [Google Scholar]
- 456.Qu H, Wei M. The effect of fluoride contents in fluoridated hydroxyapatite on osteoblast behavior. Acta Biomater. 2006;2:113–9. doi: 10.1016/j.actbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 457.Bhadang KA, Holding CA, Thissen H, McLean KM, Forsythe JS, Haynes DR. Biological responses of human osteoblasts and osteoclasts to flame-sprayed coatings of hydroxyapatite and fluorapatite blends. Acta Biomater. 2010;6:1575–83. doi: 10.1016/j.actbio.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 458.Theiszova M, Jantova S, Letasiova S, Palou M, Cipak L. Cytotoxicity of hydroxyapatite, fluorapatite and fluor-hydroxyapatite: a comparative in vitro study. Neoplasma. 2008;55:312–6. [PubMed] [Google Scholar]
- 459.An L, Zhang L, Zou J. Fluorapatite: an effective solid base catalyst for Michael addition of indole/pyrrole to nitroalkenes under solventless condition. Chin J Chem. 2009;27:2223–8. doi: 10.1002/cjoc.200990373. [DOI] [Google Scholar]
- 460.Gross KA, Berndt CC, Dinnebier R, Stephens P. Oxyapatite in hydroxyapatite coatings. J Mater Sci Mater Med. 1998;33:3985–91. [Google Scholar]
- 461.Alberius-Henning P, Adolfsson E, Grins J, Fitch A. Triclinic oxy-hydroxyapatite. J Mater Sci. 2001;36:663–8. doi: 10.1023/A:1004876622105. [DOI] [Google Scholar]
- 462.van’t Hoen C, Rheinberger V, Höland W, Apel E. Crystallization of oxyapatite in glass-ceramics. J Eur Ceram Soc. 2007;27:1579–84. doi: 10.1016/j.jeurceramsoc.2006.04.095. [DOI] [Google Scholar]
- 463.de Leeuw NH, Bowe JR, Rabone JAL. A computational investigation of stoichiometric and calcium-deficient oxy- and hydroxy-apatites. Faraday Discuss. 2007;134:195–214, discussion 215-33, 415-9. doi: 10.1039/b602012g. [DOI] [PubMed] [Google Scholar]
- 464.Duff EJ. Orthophosphates—VII. Thermodynamical considerations concerning the stability of oxyapatite, Ca10O(PO4)6, in aqueous media. J Inorg Nucl Chem. 1972;34:853–7. doi: 10.1016/0022-1902(72)80059-9. [DOI] [Google Scholar]
- 465.To honor Gustav Hilgenstock (1844–1913), a German metallurgist, who first discovered it in Thomas slags nearly 120 years ago [12 and 13].
- 466.Kai D, Fan H, Li D, Zhu X, Zhang X. Preparation of tetracalcium phosphate and the effect on the properties of calcium phosphate cement. Mater Sci Forum. 2009;610-613:1356–9. doi: 10.4028/www.scientific.net/MSF.610-613.1356. [DOI] [Google Scholar]
- 467.Brown WE, Epstein EF. Crystallography of tetracalcium phosphate. J Res Nat Bur Stand A. 1965;69:547–51. doi: 10.6028/jres.069A.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Romeo HE, Fanovich MA. Synthesis of tetracalcium phosphate from mechanochemically activated reactants and assessment as a component of bone cements. J Mater Sci Mater Med. 2008;19:2751–60. doi: 10.1007/s10856-008-3403-8. [DOI] [PubMed] [Google Scholar]
- 469.Jalota S, Tas AC, Bhaduri SB. Synthesis of HA-seeded TTCP (Ca4(PO4)2O) powders at 1,230°C from Ca(CH3COO)2·H2O and NH4H2PO4. J Am Ceram Soc. 2005;88:3353–60. doi: 10.1111/j.1551-2916.2005.00623.x. [DOI] [Google Scholar]
- 470.Moseke C, Gbureck U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010;6:3815–23. doi: 10.1016/j.actbio.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 471.Martin I, Brown PW. Hydration of tetracalcium phosphate. Adv Chem Res. 1993;5:115–25. [Google Scholar]
- 472.Fernández E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. Part II: precipitate formation during setting reactions. J Mater Sci Mater Med. 1999;10:177–83. doi: 10.1023/A:1008989525461. [DOI] [PubMed] [Google Scholar]
- 473.Dickens B, Brown WE, Kruger GJ, Stewart JM. Ca4(PO4)2O, tetracalcium diphosphate monoxide, crystal structures and relationships to Ca5(PO4)3OH and K3Na(SO4)2. Acta Crystallogr B. 1973;29:2046–56. doi: 10.1107/S0567740873006102. [DOI] [Google Scholar]
- 474.Jillavenatesa A, Condrate RA., Sr. The infrared and Raman spectra of tetracalcium phosphate (Ca4(PO4)2O) Spectrosc Lett. 1997;30:1561–70. doi: 10.1080/00387019708006744. [DOI] [Google Scholar]
- 475.Dorozhkin SV. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012 doi: 10.1016/j.actbio.2011.09.003. In press. [DOI] [PubMed] [Google Scholar]
- 476.Nakano T, Kaibara K, Umakoshi Y, Imazato S, Ogata K, Ehara A, et al. Change in microstructure and solubility improvement of HAp ceramics by heat-treatment in a vacuum. Mater Trans. 2002;43:3105–11. doi: 10.2320/matertrans.43.3105. [DOI] [Google Scholar]
- 477.Kiba W, Imazato S, Takahashi Y, Yoshioka S, Ebisu S, Nakano T. Efficacy of polyphasic calcium phosphates as a direct pulp capping material. J Dent. 2010;38:828–37. doi: 10.1016/j.jdent.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 478.Pan L, Li Y, Weng W, Cheng K, Song C, Du P, et al. Preparation of submicron biphasic α-TCP/HA powders. Key Eng Mater. 2006;309-311:219–22. doi: 10.4028/www.scientific.net/KEM.309-311.219. [DOI] [Google Scholar]
- 479.Kui C. Slip casting derived α-TCP/HA biphasic ceramics. Key Eng Mater. 2007;330-332:51–4. doi: 10.4028/www.scientific.net/KEM.330-332.51. [DOI] [Google Scholar]
- 480.Sanchez-Sálcedo S, Arcos D, Vallet-Regí M. Upgrading calcium phosphate scaffolds for tissue engineering applications. Key Eng Mater. 2008;377:19–42. doi: 10.4028/www.scientific.net/KEM.377.19. [DOI] [Google Scholar]
- 481.Li Y, Kong F, Weng W. Preparation and characterization of novel biphasic calcium phosphate powders (alpha-TCP/HA) derived from carbonated amorphous calcium phosphates. J Biomed Mater Res B Appl Biomater. 2009;89:508–17. doi: 10.1002/jbm.b.31242. [DOI] [PubMed] [Google Scholar]
- 482.Oishi M, Ohtsuki C, Kitamura M, Kamitakahara M, Ogata S, Miyazaki T, et al. Fabrication and chemical durability of porous bodies consisting of biphasic tricalcium phosphates. Phosphorus Res Bull. 2004;17:95–100. [Google Scholar]
- 483.Kamitakahara M, Ohtsuki C, Oishi M, Ogata S, Miyazaki T, Tanihara M. Preparation of porous biphasic tricalcium phosphate and its in vivo behavior. Key Eng Mater. 2005;284-286:281–4. doi: 10.4028/www.scientific.net/KEM.284-286.281. [DOI] [Google Scholar]
- 484.Wang R, Weng W, Deng X, Cheng K, Liu X, Du P, et al. Dissolution behavior of submicron biphasic tricalcium phosphate powders. Key Eng Mater. 2006;309-311:223–6. doi: 10.4028/www.scientific.net/KEM.309-311.223. [DOI] [Google Scholar]
- 485.Li Y, Weng W, Tam KC. Novel highly biodegradable biphasic tricalcium phosphates composed of alpha-tricalcium phosphate and beta-tricalcium phosphate. Acta Biomater. 2007;3:251–4. doi: 10.1016/j.actbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 486.Li Y, Li D, Weng W. In vitro dissolution behavior of biphasic tricalcium phosphate composite powders composed of α-tricalcium phosphate and β-tricalcium phosphate. Key Eng Mater. 2008;368-372:1206–8. doi: 10.4028/www.scientific.net/KEM.368-372.1206. [DOI] [Google Scholar]
- 487.Vani R, Girija EK, Elayaraja K, Prakash Parthiban S, Kesavamoorthy R, Narayana Kalkura S. Hydrothermal synthesis of porous triphasic hydroxyapatite/(alpha and beta) tricalcium phosphate. J Mater Sci Mater Med. 2009;20(Suppl 1):S43–8. doi: 10.1007/s10856-008-3480-8. [DOI] [PubMed] [Google Scholar]
- 488.Huang Y, Huang W, Sun L, Wang Q, He A, Han CC. Phase transition from α-TCP into β-TCP in TCP/HA composites. Int J Appl Ceram Technol. 2010;7:184–8. doi: 10.1111/j.1744-7402.2009.02384.x. [DOI] [Google Scholar]
- 489.McConnell D, Posner AS. Carbonate in Apatites. Science. 1961;134:213–5. doi: 10.1126/science.134.3473.213. [DOI] [PubMed] [Google Scholar]
- 490.Zapanta-LeGeros R. Effect of carbonate on the lattice parameters of apatite. Nature. 1965;206:403–4. doi: 10.1038/206403a0. [DOI] [PubMed] [Google Scholar]
- 491.Legeros RZ, Trautz OR, Legeros JP, Klein E, Shirra WP. Apatite crystallites: effects of carbonate on morphology. Science. 1967;155:1409–11. doi: 10.1126/science.155.3768.1409. [DOI] [PubMed] [Google Scholar]
- 492.Astala R, Stott MJ. First principles investigation of mineral component of bone: CO3 substitutions in hydroxyapatite. Chem Mater. 2005;17:4125–33. doi: 10.1021/cm050523b. [DOI] [Google Scholar]
- 493.Lafon JP, Champion E, Bernache-Assollant D. Processing of AB-type carbonated hydroxyapatite Ca10-x(PO4)(6-x)(CO3)(x)(OH)(2-x-2y)(CO3)(y) ceramics with controlled composition. J Eur Ceram Soc. 2008;28:139–47. doi: 10.1016/j.jeurceramsoc.2007.06.009. [DOI] [Google Scholar]
- 494.Tonegawa T, Ikoma T, Yoshioka T, Hanagata N, Tanaka J. Crystal structure refinement of A-type carbonate apatite by X-ray powder diffraction. J Mater Sci. 2010;45:2419–26. doi: 10.1007/s10853-010-4209-x. [DOI] [Google Scholar]
- 495.Yahia FBH, Jemal M. Synthesis, structural analysis and thermochemistry of B-type carbonate apatites. Thermochim Acta. 2010;50:22–32. doi: 10.1016/j.tca.2010.03.017. [DOI] [Google Scholar]
- 496.Kannan S, Rebelo A, Lemos AF, Barba A, Ferreira JMF. Synthesis and mechanical behaviour of chlorapatite and chlorapatite/β-TCP composites. J Eur Ceram Soc. 2007;27:2287–94. doi: 10.1016/j.jeurceramsoc.2006.07.004. [DOI] [Google Scholar]
- 497.Teshima K, Yubuta K, Ooi S, Suzuki T, Shishido T, Oishi S. Environmentally friendly growth of calcium chlorapatite whiskers from a sodium chloride flux. Cryst Growth Des. 2006;6:2538–42. doi: 10.1021/cg0603671. [DOI] [Google Scholar]
- 498.García-Tuñón E, Franco J, Dacuña B, Zaragoza G, Guitián F. Chlorapatite conversion to hydroxyapatite under high temperature hydrothermal conditions. Mater Sci Forum. 2010;636-637:9–14. doi: 10.4028/www.scientific.net/MSF.636-637.9. [DOI] [Google Scholar]
- 499.Kannan S, Goetz-Neunhoeffer F, Neubauer J, Ferreira JMF. Ionic substitutions in biphasic hydroxyapatite and β-tricalcium phosphate mixtures: structural analysis by Rietveld refinement. J Am Ceram Soc. 2008;91:1–12. doi: 10.1111/j.1551-2916.2007.02117.x. [DOI] [Google Scholar]
- 500.Boanini E, Gazzano M, Bigi A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6:1882–94. doi: 10.1016/j.actbio.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 501.Pushpakanth S, Srinivasan B, Sastry TP, Mandal AB. Biocompatible and antibacterial properties of silver-doped hydroxyapatite. J Biomed Nanotechnol. 2008;4:62–6. [Google Scholar]
- 502.Zhang Y, Yin QS, Zhang Y, Xia H, Ai FZ, Jiao YP, et al. Determination of antibacterial properties and cytocompatibility of silver-loaded coral hydroxyapatite. J Mater Sci Mater Med. 2010;21:2453–62. doi: 10.1007/s10856-010-4101-x. [DOI] [PubMed] [Google Scholar]
- 503.Kim TN, Feng QL, Kim JO, Wu J, Wang H, Chen GC, et al. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J Mater Sci Mater Med. 1998;9:129–34. doi: 10.1023/A:1008811501734. [DOI] [PubMed] [Google Scholar]
- 504.Thomas S, Assi P, Marycel B, Correa M, Liberato W, Brito V. Yttrium 90-hydroxyapatite, a new radioisotope for chronic synovitis in hemophilia. Haemophilia. 2008;14:77. [Google Scholar]
- 505.Chinol M, Vallabhajosula S, Goldsmith SJ, Klein MJ, Deutsch KF, Chinen LK, et al. Chemistry and biological behavior of samarium-153 and rhenium-186-labeled hydroxyapatite particles: potential radiopharmaceuticals for radiation synovectomy. J Nucl Med. 1993;34:1536–42. [PubMed] [Google Scholar]
- 506.Argüelles MG, Berlanga ISL, Torres EA. Preparation of 153Sm-particles for radiosynovectomy. J Radioanal Nucl Chem. 1999;240:509–11. doi: 10.1007/BF02349404. [DOI] [Google Scholar]
- 507.O’Duffy EK, Clunie GP, Lui D, Edwards JC, Ell PJ. Double blind glucocorticoid controlled trial of samarium-153 particulate hydroxyapatite radiation synovectomy for chronic knee synovitis. Ann Rheum Dis. 1999;58:554–8. doi: 10.1136/ard.58.9.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Vallet-Regí M, González-Calbet JM. Calcium phosphates as substitution of bone tissues. Prog Solid State Chem. 2004;32:1–31. doi: 10.1016/j.progsolidstchem.2004.07.001. [DOI] [Google Scholar]
- 509.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108:4754–83. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Grynpas MD, Hancock RG, Greenwood C, Turnquist J, Kessler MJ. The effects of diet, age, and sex on the mineral content of primate bones. Calcif Tissue Int. 1993;52:399–405. doi: 10.1007/BF00310206. [DOI] [PubMed] [Google Scholar]
- 511.Elliott JC. Calcium phosphate biominerals. In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews in Mineralogy and Geochemistry. Hughes JM, Kohn M, Rakovan J, Eds. Mineralogical Society of America: Washington DC, USA 2002; 48:13-49. [Google Scholar]
- 512.Boskey AL. Assessment of bone mineral and matrix using backscatter electron imaging and FTIR imaging. Curr Osteoporos Rep. 2006;4:71–5. doi: 10.1007/s11914-006-0005-6. [DOI] [PubMed] [Google Scholar]
- 513.Holt LE, la Mer VK, Chown HB. Studies in calcification. I. The solubility product of secondary and tertiary calcium phosphate under various conditions. J Biol Chem. 1925;64:509–65. [Google Scholar]
- 514.Holt LE, la Mer VK, Chown HB. Studies in calcification. II. Delayed equilibrium between the calcium phosphates and its biological significance. J Biol Chem. 1925;64:567–78. [Google Scholar]
- 515.Gassmann T. The preparation of a complex salt corresponding to apatite-typhus and its relations to the constitution of bones. HSZ Physiol Chem. 1913;83:403–8. [Google Scholar]
- 516.de Jong WF. La substance minérale dans les os. Recl Trav Chim Pays Bas. 1926;45:445–9. doi: 10.1002/recl.19260450613. [DOI] [Google Scholar]
- 517.Bredig MA. The apatite structure of inorganic bone and tooth substance. HS Z Physiol Chem. 1933;216:239–43. doi: 10.1515/bchm2.1933.216.5-6.239. [DOI] [Google Scholar]
- 518.Taylor NW, Sheard C. Microscopic and X-ray investigations on the calcification of tissue. J Biol Chem. 1929;81:479–93. [Google Scholar]
- 519.Weiner S, Traub W, Wagner HD. Lamellar bone: structure-function relations. J Struct Biol. 1999;126:241–55. doi: 10.1006/jsbi.1999.4107. [DOI] [PubMed] [Google Scholar]
- 520.Matkovic V. Calcium metabolism and calcium requirements during skeletal modeling and consolidation of bone mass. Am J Clin Nutr. 1991;54(Suppl):245S–60S. doi: 10.1093/ajcn/54.1.245S. [DOI] [PubMed] [Google Scholar]
- 521.Power ML, Heaney RP, Kalkwarf HJ, Pitkin RM, Repke JT, Tsang RC, et al. The role of calcium in health and disease. Am J Obstet Gynecol. 1999;181:1560–9. doi: 10.1016/S0002-9378(99)70404-7. [DOI] [PubMed] [Google Scholar]
- 522.Jones FH. Teeth and bones: application of surface science to dental materials and related biomaterials. Surf Sci Rep. 2001;42:75–205. doi: 10.1016/S0167-5729(00)00011-X. [DOI] [Google Scholar]
- 523.Loveridge N. Bone: more than a stick. J Anim Sci. 1999;77(Suppl 2):190–6. doi: 10.2527/1999.77suppl_2190x. [DOI] [PubMed] [Google Scholar]
- 524.Nightingale JP, Lewis D. Pole figures of the orientation of apatite in bones. Nature. 1971;232:334–5. doi: 10.1038/232334a0. [DOI] [PubMed] [Google Scholar]
- 525.Currey JD. Bones: structure and mechanics. Princeton Univercity Press: Princeton USA 2002; 456. [Google Scholar]
- 526.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. doi: 10.1016/S1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 527.Mann S. Biomimetic materials chemistry. VCH, Weinheim, Germany 1996; 400. [Google Scholar]
- 528.Hancox NM. Biology of bone. Cambridge University Press: Cambridge, MA USA 1972; 199. [Google Scholar]
- 529.Martin RB. Bone as a ceramic composite material. Mater Sci Forum. 1999;7:5–16. doi: 10.4028/www.scientific.net/MSF.293.5. [DOI] [Google Scholar]
- 530.Tzaphlidou M. Bone architecture: collagen structure and calcium/phosphorus maps. J Biol Phys. 2008;34:39–49. doi: 10.1007/s10867-008-9115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Davison KS, Siminoski K, Adachi JD, Hanley DA, Goltzman D, Hodsman AB, et al. Bone strength: the whole is greater than the sum of its parts. Semin Arthritis Rheum. 2006;36:22–31. doi: 10.1016/j.semarthrit.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 532.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-K. [DOI] [PubMed] [Google Scholar]
- 533.Currey JD. Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J Biomech. 2004;37:549–56. doi: 10.1016/j.jbiomech.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 534.Currey JD, Brear K, Zioupos P. Notch sensitivity of mammalian mineralized tissues in impact. Proc Biol Sci. 2004;271:517–22. doi: 10.1098/rspb.2003.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Anderson JC, Eriksson C. Piezoelectric properties of dry and wet bone. Nature. 1970;227:491–2. doi: 10.1038/227491a0. [DOI] [PubMed] [Google Scholar]
- 536.Lang SB. Pyroelectric effect in bone and tendon. Nature. 1966;212:704–5. doi: 10.1038/212704a0. [DOI] [Google Scholar]
- 537.Haynes V. Radiocarbon: analysis of inorganic carbon of fossil bone and enamel. Science. 1968;161:687–8. doi: 10.1126/science.161.3842.687. [DOI] [PubMed] [Google Scholar]
- 538.Rensberger JM, Watabe M. Fine structure of bone in dinosaurs, birds and mammals. Nature. 2000;406:619–22. doi: 10.1038/35020550. [DOI] [PubMed] [Google Scholar]
- 539.Kolodny Y, Luz B, Sander M, Clemens WA. Dinosaur bones: fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils. Palaeo. 1996;126:161–71. doi: 10.1016/S0031-0182(96)00112-5. [DOI] [Google Scholar]
- 540.Trueman CN, Tuross N. Trace elements in recent and fossil bone apatite. In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews in Mineralogy and Geochemistry. Hughes JM, Kohn M, Rakovan J, Eds. Mineralogical Society of America: Washington DC, USA 2002; 48:489-522. [Google Scholar]
- 541.Currey JD, Brear K. Hardness, Young’s modulus and yield stress in mammalian mineralized tissues. J Mater Sci Mater Med. 1990;1:14–20. doi: 10.1007/BF00705348. [DOI] [Google Scholar]
- 542.Brown WE, Chow LC. Chemical properties of bone mineral. Annu Rev Mater Sci. 1976;6:213–36. doi: 10.1146/annurev.ms.06.080176.001241. [DOI] [Google Scholar]
- 543.Lakes R. Materials with structural hierarchy. Nature. 1993;361:511–5. doi: 10.1038/361511a0. [DOI] [Google Scholar]
- 544.Meyers MA, Lin AYM, Seki Y, Chen PY, Kad BK, Bodde S. Structural biological composites: an overview. JOM. 2006;58:36–43. doi: 10.1007/s11837-006-0138-1. [DOI] [Google Scholar]
- 545.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 546.Rey C, Combes C, Drouet C, Glimcher MJ. Bone mineral: update on chemical composition and structure. Osteoporos Int. 2009;20:1013–21. doi: 10.1007/s00198-009-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Watt JC. The deposition of calcium phosphate and calcium carbonate in bone and in areas of calcification. Arch Surg Chicago. 1925;10:983–90. doi: 10.1001/archsurg.1925.01120120171007. [DOI] [Google Scholar]
- 548.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim YY, Kaufman MJ, et al. Bone structure and formation: a new perspective. Mater Sci Eng Rep. 2007;58:77–116. doi: 10.1016/j.mser.2007.05.001. [DOI] [Google Scholar]
- 549.Boskey AL. Mineralization of bones and teeth. Elements. 2007;3:385–91. doi: 10.2113/GSELEMENTS.3.6.385. [DOI] [Google Scholar]
- 550.Glimcher MJ. Bone: nature of the calcium phosphate crystals and cellular, structural and physical chemical mechanisms in their formation. In: Medical Mineralogy and Geochemistry, Series: Reviews in Mineralogy and Geochemistry. Sahai N, Schoonen MAA, Eds. Mineralogical Society of America: Washington DC, USA 2006; 64:223-82. [Google Scholar]
- 551.Boskey AL, Roy R. Cell culture systems for studies of bone and tooth mineralization. Chem Rev. 2008;108:4716–33. doi: 10.1021/cr0782473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Cui FZ, Li Y, Ge J. Self-assembly of mineralized collagen composites. Mater Sci Eng Rep. 2007;57:1–27. doi: 10.1016/j.mser.2007.04.001. [DOI] [Google Scholar]
- 553.Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89:1333–48. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Meyers MA, Chen PY, Lin AYM, Seki Y. Biological materials: structure and mechanical properties. Prog Mater Sci. 2008;53:1–206. doi: 10.1016/j.pmatsci.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 555.McKee MD, Addison WN, Kaartinen MT. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs. 2005;181:176–88. doi: 10.1159/000091379. [DOI] [PubMed] [Google Scholar]
- 556.Currey JD. Materials science. Hierarchies in biomineral structures. Science. 2005;309:253–4. doi: 10.1126/science.1113954. [DOI] [PubMed] [Google Scholar]
- 557.Fantner GE, Rabinovych O, Schitter G, Thurner P, Kindt JH, Finch MM, et al. Hierarchical interconnections in the nano-composite material bone: fibrillar cross-links resist fracture on several length scales. Compos Sci Technol. 2006;66:1202–8. doi: 10.1016/j.compscitech.2005.10.005. [DOI] [Google Scholar]
- 558.Fratzl P, Weinkamer R. Nature’s hierarchical materials. Prog Mater Sci. 2007;52:1263–334. doi: 10.1016/j.pmatsci.2007.06.001. [DOI] [Google Scholar]
- 559.Athanasiou KA, Zhu C, Lanctot DR, Agrawal CM, Wang X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000;6:361–81. doi: 10.1089/107632700418083. [DOI] [PubMed] [Google Scholar]
- 560.Nikolov S, Raabe D. Hierarchical modeling of the elastic properties of bone at submicron scales: the role of extrafibrillar mineralization. Biophys J. 2008;94:4220–32. doi: 10.1529/biophysj.107.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Gupta HS, Wagermaier W, Zickler GA, Raz-Ben Aroush D, Funari SS, Roschger P, et al. Nanoscale deformation mechanisms in bone. Nano Lett. 2005;5:2108–11. doi: 10.1021/nl051584b. [DOI] [PubMed] [Google Scholar]
- 562.Peterlik H, Roschger P, Klaushofer K, Fratzl P. From brittle to ductile fracture of bone. Nat Mater. 2006;5:52–5. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- 563.Ruppel ME, Miller LM, Burr DB. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos Int. 2008;19:1251–65. doi: 10.1007/s00198-008-0579-1. [DOI] [PubMed] [Google Scholar]
- 564.Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–23. doi: 10.1039/b402005g. [DOI] [Google Scholar]
- 565.Marino AA, Becker RO. Evidence for direct physical bonding between the collagen fibres and apatite cystals in bone. Nature. 1967;213:697–8. doi: 10.1038/213697a0. [DOI] [PubMed] [Google Scholar]
- 566.Eppell SJ, Tong W, Katz JL, Kuhn L, Glimcher MJ. Shape and size of isolated bone mineralites measured using atomic force microscopy. J Orthop Res. 2001;19:1027–34. doi: 10.1016/S0736-0266(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 567.Clark SM, Iball J. Orientation of apatite crystals in bone. Nature. 1954;174:399–400. doi: 10.1038/174399a0. [DOI] [PubMed] [Google Scholar]
- 568.Rubin MA, Jasiuk I, Taylor J, Rubin J, Ganey T, Apkarian RP. TEM analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone. 2003;33:270–82. doi: 10.1016/S8756-3282(03)00194-7. [DOI] [PubMed] [Google Scholar]
- 569.Su X, Sun K, Cui FZ, Landis WJ. Organization of apatite crystals in human woven bone. Bone. 2003;32:150–62. doi: 10.1016/S8756-3282(02)00945-6. [DOI] [PubMed] [Google Scholar]
- 570.Fratzl P, Fratzl-Zelman N, Klaushofer K, Vogl G, Koller K. Nucleation and growth of mineral crystals in bone studied by small-angle X-ray scattering. Calcif Tissue Int. 1991;48:407–13. doi: 10.1007/BF02556454. [DOI] [PubMed] [Google Scholar]
- 571.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech. 1996;33:192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 572.Rosen VB, Hobbs LW, Spector M. The ultrastructure of anorganic bovine bone and selected synthetic hyroxyapatites used as bone graft substitute materials. Biomaterials. 2002;23:921–8. doi: 10.1016/S0142-9612(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 573.Sato K. Inorganic-organic interfacial interactions in hydroxyapatite mineralization processes. Top Curr Chem. 2006;270:127–53. doi: 10.1007/128_075. [DOI] [Google Scholar]
- 574.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–8. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 575.Burger C, Zhou HW, Wang H, Sics I, Hsiao BS, Chu B, et al. Lateral packing of mineral crystals in bone collagen fibrils. Biophys J. 2008;95:1985–92. doi: 10.1529/biophysj.107.128355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci U S A. 2010;107:22425–9. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Xie B, Nancollas GH. How to control the size and morphology of apatite nanocrystals in bone. Proc Natl Acad Sci U S A. 2010;107:22369–70. doi: 10.1073/pnas.1017493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Crane NJ, Popescu V, Morris MD, Steenhuis P, Ignelzi MA., Jr. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone. 2006;39:434–42. doi: 10.1016/j.bone.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 579.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008;108:4670–93. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Hall BK, ed. Cartilage: structure, function and biochemistry. Academic Press: New York USA 1983; 385. [Google Scholar]
- 581.Robins SP, Bilezikian JP, Seibel MJ. Dynamics of bone and cartilage metabolism. Academic Press: New York USA 1999; 672. [Google Scholar]
- 582.Hall BK, ed. Bones and cartilage: developmental skeletal biology. Academic Press: New York USA 2005; 792. [Google Scholar]
- 583.Marino AA, Becker RO. Evidence for epitaxy in the formation of collagen and apatite. Nature. 1970;226:652–3. doi: 10.1038/226652a0. [DOI] [PubMed] [Google Scholar]
- 584.Jodaikin A, Weiner S, Talmon Y, Grossman E, Traub W. Mineral-organic matrix relations in tooth enamel. Int J Biol Macromol. 1988;10:349–52. doi: 10.1016/0141-8130(88)90027-X. [DOI] [Google Scholar]
- 585.Fincham AG, Moradian-Oldak J, Diekwisch TGH, Lyaruu DM, Wright JT, Bringas P, Jr., et al. Evidence for amelogenin “nanospheres” as functional components of secretory-stage enamel matrix. J Struct Biol. 1995;115:50–9. doi: 10.1006/jsbi.1995.1029. [DOI] [PubMed] [Google Scholar]
- 586.LeGeros RZ, Pan CM, Suga S, Watabe N. Crystallo-chemical properties of apatite in atremate brachiopod shells. Calcif Tissue Int. 1985;37:98–100. doi: 10.1007/BF02557687. [DOI] [PubMed] [Google Scholar]
- 587.Iijima M, Moriwaki Y. Orientation of apatite and organic matrix in Lingula unguis shell. Calcif Tissue Int. 1990;47:237–42. doi: 10.1007/BF02555925. [DOI] [PubMed] [Google Scholar]
- 588.Williams A, Cusack M, Buckman JO, Stachel T. Siliceous tablets in the larval shells of apatitic discinid brachiopods. Science. 1998;279:2094–6. doi: 10.1126/science.279.5359.2094. [DOI] [PubMed] [Google Scholar]
- 589.Rohanizadeh R, LeGeros RZ. Mineral phase in linguloid brachiopod shell: Lingula adamsi. Lethaia. 2007;40:61–8. doi: 10.1111/j.1502-3931.2006.00006.x. [DOI] [Google Scholar]
- 590.Nakano T, Ishimoto T, Lee JW, Umakoshi Y. Preferential orientation of biological apatite crystallite in original, regenerated and diseased cortical bones. J Ceram Soc Jpn. 2008;116:313–5. doi: 10.2109/jcersj2.116.313. [DOI] [Google Scholar]
- 591.Nakano T, Ishimoto T, Umakoshi Y, Tabata Y. Variation in bone quality during regenerative process. Mater Sci Forum. 2007;539-543:675–80. doi: 10.4028/www.scientific.net/MSF.539-543.675. [DOI] [Google Scholar]
- 592.Suvorova EI, Petrenko PP, Buffat PA. Scanning and transmission electron microscopy for evaluation of order/disorder in bone structure. Scanning. 2007;29:162–70. doi: 10.1002/sca.20058. [DOI] [PubMed] [Google Scholar]
- 593.Stock SR, Blackburn D, Gradassi M, Simon HG. Bone formation during forelimb regeneration: a microtomography (microCT) analysis. Dev Dyn. 2003;226:410–7. doi: 10.1002/dvdy.10241. [DOI] [PubMed] [Google Scholar]
- 594.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 595.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 596.Schilling AF, Filke S, Brink S, Korbmacher H, Amling M, Rueger JM. Osteoclasts and biomaterials. Eur J Trauma. 2006;32:107–13. doi: 10.1007/s00068-006-6043-1. [DOI] [Google Scholar]
- 597.Bonar LC, Shimizu M, Roberts JE, Griffin RG, Glimcher MJ. Structural and composition studies on the mineral of newly formed dental enamel: a chemical, x-ray diffraction, and 31P and proton nuclear magnetic resonance study. J Bone Miner Res. 1991;6:1167–76. doi: 10.1002/jbmr.5650061105. [DOI] [PubMed] [Google Scholar]
- 598.Biltz RM, Pellegrino ED. The hydroxyl content of calcified tissue mineral. Calcif Tissue Res. 1971;7:259–63. doi: 10.1007/BF02062614. [DOI] [PubMed] [Google Scholar]
- 599.Rey C, Renugopalakrishnan V, Collins B, Glimcher MJ. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif Tissue Int. 1991;49:251–8. doi: 10.1007/BF02556214. [DOI] [PubMed] [Google Scholar]
- 600.Rey C, Miquel JL, Facchini L, Legrand AP, Glimcher MJ. Hydroxyl groups in bone mineral. Bone. 1995;16:583–6. doi: 10.1016/8756-3282(95)00101-I. [DOI] [PubMed] [Google Scholar]
- 601.Loong CK, Rey C, Kuhn LT, Combes C, Wu Y, Chen SH, et al. Evidence of hydroxyl-ion deficiency in bone apatites: an inelastic neutron-scattering study. Bone. 2000;26:599–602. doi: 10.1016/S8756-3282(00)00273-8. [DOI] [PubMed] [Google Scholar]
- 602.Cho G, Wu Y, Ackerman JL. Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science. 2003;300:1123–7. doi: 10.1126/science.1078470. [DOI] [PubMed] [Google Scholar]
- 603.Tung MS, Brown WE. An intermediate state in hydrolysis of amorphous calcium phosphate. Calcif Tissue Int. 1983;35:783–90. doi: 10.1007/BF02405124. [DOI] [PubMed] [Google Scholar]
- 604.Tung MS, Brown WE. The role of octacalcium phosphate in subcutaneous heterotopic calcification. Calcif Tissue Int. 1985;37:329–31. doi: 10.1007/BF02554883. [DOI] [PubMed] [Google Scholar]
- 605.Brown WE, Eidelman N, Tomazic BB. Octacalcium phosphate as a precursor in biomineral formation. Adv Dent Res. 1987;1:306–13. doi: 10.1177/08959374870010022201. [DOI] [PubMed] [Google Scholar]
- 606.Siew C, Gruninger SE, Chow LC, Brown WE. Procedure for the study of acidic calcium phosphate precursor phases in enamel mineral formation. Calcif Tissue Int. 1992;50:144–8. doi: 10.1007/BF00298792. [DOI] [PubMed] [Google Scholar]
- 607.Tseng YH, Mou CY, Chan JCC. Solid-state NMR study of the transformation of octacalcium phosphate to hydroxyapatite: a mechanistic model for central dark line formation. J Am Chem Soc. 2006;128:6909–18. doi: 10.1021/ja060336u. [DOI] [PubMed] [Google Scholar]
- 608.Eanes ED, Gillessen IH, Posner AS. Intermediate states in the precipitation of hydroxyapatite. Nature. 1965;208:365–7. doi: 10.1038/208365a0. [DOI] [PubMed] [Google Scholar]
- 609.Termine JD, Posner AS. Infrared analysis of rat bone: age dependency of amorphous and crystalline mineral fractions. Science. 1966;153:1523–5. doi: 10.1126/science.153.3743.1523. [DOI] [PubMed] [Google Scholar]
- 610.Termine JD, Posner AS. Infra-red determinaion of the percentage of crystallinity in apatitic calcium phosphates. Nature. 1966;211:268–70. doi: 10.1038/211268a0. [DOI] [PubMed] [Google Scholar]
- 611.Harper RA, Posner AS. Measurement of non-crystalline calcium phosphate in bone mineral. Proc Soc Exp Biol Med. 1966;122:137–42. doi: 10.3181/00379727-122-31073. [DOI] [PubMed] [Google Scholar]
- 612.Posner AS. Crystal chemistry of bone mineral. Physiol Rev. 1969;49:760–92. doi: 10.1152/physrev.1969.49.4.760. [DOI] [PubMed] [Google Scholar]
- 613.Posner AS. Bone mineral on the molecular level. Fed Proc. 1973;32:1933–7. [PubMed] [Google Scholar]
- 614.Boskey AL, Posner AS. Formation of hydroxyapatite at low supersaturation. J Phys Chem. 1976;80:40–4. doi: 10.1021/j100542a009. [DOI] [Google Scholar]
- 615.Posner AS. “The chemistry of bone mineral”. Bull Hosp Joint Dis. 1978;39:126–44. [PubMed] [Google Scholar]
- 616.Glimcher MJ, Bonar LC, Grynpas MD, Landis WJ, Roufosse AH. Recent studies of bone mineral: is the amorphous calcium phosphate theory valid? J Cryst Growth. 1981;53:100–19. doi: 10.1016/0022-0248(81)90058-0. [DOI] [Google Scholar]
- 617.Meyer JL, Eanes ED. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif Tissue Res. 1978;25:59–68. doi: 10.1007/BF02010752. [DOI] [PubMed] [Google Scholar]
- 618.Meyer JL, Eanes ED. A thermodynamic analysis of the secondary transition in the spontaneous precipitation of calcium phosphate. Calcif Tissue Res. 1978;25:209–16. doi: 10.1007/BF02010771. [DOI] [PubMed] [Google Scholar]
- 619.Politi Y, Arad T, Klein E, Weiner S, Addadi L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science. 2004;306:1161–4. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- 620.Weiner S, Sagi I, Addadi L. Structural biology. Choosing the crystallization path less traveled. Science. 2005;309:1027–8. doi: 10.1126/science.1114920. [DOI] [PubMed] [Google Scholar]
- 621.Weiner S. Transient precursor strategy in mineral formation of bone. Bone. 2006;39:431–3. doi: 10.1016/j.bone.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 622.Pekounov Y, Petrov OE. Bone resembling apatite by amorphous-to-crystalline transition driven self-organisation. J Mater Sci Mater Med. 2008;19:753–9. doi: 10.1007/s10856-007-3085-7. [DOI] [PubMed] [Google Scholar]
- 623.Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;108:4551–627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Weiner S, Mahamid J, Politi Y, Ma Y, Addadi L. Overview of the amorphous precursor phase strategy in biomineralization. Front Mater Sci China 2009; 3:104 8.
- 625.Mahamid J, Sharir A, Addadi L, Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc Natl Acad Sci U S A. 2008;105:12748–53. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li C, Siegel S, et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci U S A. 2010;107:6316–21. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, et al. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 2010;9:1004–9. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Cölfen H. Biomineralization: A crystal-clear view. Nat Mater. 2010;9:960–1. doi: 10.1038/nmat2911. [DOI] [PubMed] [Google Scholar]
- 629.Sahar ND, Hong SI, Kohn DH. Micro- and nano-structural analyses of damage in bone. Micron. 2005;36:617–29. doi: 10.1016/j.micron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 630.Bilezikian JP, Raisz LG, Martin TJ. Principles of Bone Biology. Academic Press: New York USA 2008; 3:1900. [Google Scholar]
- 631.Burnell JM, Teubner EJ, Miller AG. Normal maturational changes in bone matrix, mineral, and crystal size in the rat. Calcif Tissue Int. 1980;31:13–9. doi: 10.1007/BF02407162. [DOI] [PubMed] [Google Scholar]
- 632.Weiner S, Traub W. Organization of hydroxyapatite crystals within collagen fibrils. FEBS Lett. 1986;206:262–6. doi: 10.1016/0014-5793(86)80993-0. [DOI] [PubMed] [Google Scholar]
- 633.Hanschin RG, Stern WB. X-ray diffraction studies on the lattice perfection of human bone apatite (Crista iliaca) Bone. 1995;16(Suppl):355S–63S. [PubMed] [Google Scholar]
- 634.Pellegrino ED, Biltz RM. Mineralization in the chick embryo. I. Monohydrogen phosphate and carbonate relationships during maturation of the bone crystal complex. Calcif Tissue Res. 1972;10:128–35. doi: 10.1007/BF02012542. [DOI] [PubMed] [Google Scholar]
- 635.Legros R, Balmain N, Bonel G. Age-related changes in mineral of rat and bovine cortical bone. Calcif Tissue Int. 1987;41:137–44. doi: 10.1007/BF02563793. [DOI] [PubMed] [Google Scholar]
- 636.Rey C, Hina A, Tofighi A, Glimcher MJ. Maturation of poorly crystalline apatites: chemical and structural aspect in vivo and in vitro behavior. Cell Mater. 1995;5:345–56. [Google Scholar]
- 637.Verdelis K, Lukashova L, Wright JT, Mendelsohn R, Peterson MGE, Doty S, et al. Maturational changes in dentin mineral properties. Bone. 2007;40:1399–407. doi: 10.1016/j.bone.2006.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Yerramshetty JS, Lind C, Akkus O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone. 2006;39:1236–43. doi: 10.1016/j.bone.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 639.Kuhn LT, Grynpas MD, Rey CC, Wu Y, Ackerman JL, Glimcher MJ. A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif Tissue Int. 2008;83:146–54. doi: 10.1007/s00223-008-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Donnelly E, Boskey AL, Baker SP, van der Meulen MC. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A. 2010;92:1048–56. doi: 10.1002/jbm.a.32442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Lowenstam HA. Minerals formed by organisms. Science. 1981;211:1126–31. doi: 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- 642.Lowenstam HA, Weiner S. Mineralization by organisms and the evolution of biomineralization. In: Biomineralization and biological metal accumulation. Westbroek P, de Jong EW, (Eds.) D Reidel Pub Co Dordrecht, Holland 1983; 191-203. [Google Scholar]
- 643.Plate U, Tkotz T, Wiesmann HP, Stratmann U, Joos U, Höhling HJ. Early mineralization of matrix vesicles in the epiphyseal growth plate. J Microsc. 1996;183:102–7. doi: 10.1046/j.1365-2818.1996.67430.x. [DOI] [PubMed] [Google Scholar]
- 644.Stratmann U, Schaarschmidt K, Wiesmann HP, Plate U, Höhling HJ. Mineralization during matrix-vesicle-mediated mantle dentine formation in molars of albino rats: a microanalytical and ultrastructural study. Cell Tissue Res. 1996;284:223–30. doi: 10.1007/s004410050582. [DOI] [PubMed] [Google Scholar]
- 645.Jahnen-Dechent W, Schinke T, Trindl A, Müller-Esterl W, Sablitzky F, Kaiser S, et al. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- 646.Schinke T, McKee MD, Karsenty G. Extracellular matrix calcification: where is the action? Nat Genet. 1999;21:150–1. doi: 10.1038/5928. [DOI] [PubMed] [Google Scholar]
- 647.Jahnen-Dechent W, Schäfer G, Heiss A, Grötzinger J. Systemic inhibition of spontaneous calcification by the serum protein a2-HS glycoprotein/fetuin. Z Kardiol. 2001;90:4756. doi: 10.1007/s003920170042. [DOI] [PubMed] [Google Scholar]
- 648.Avery JK. Oral development and histology. Thieme Medical Publishers Inc.: New York USA 2001; 3:435. [Google Scholar]
- 649.ten Cate AR. Oral histology: development, structure and function. Mosby-Year Book. Saint Louis USA. 1998;5:497. [Google Scholar]
- 650.Xue J, Zhang L, Zou L, Liao Y, Li J, Xiao L, et al. High-resolution X-ray microdiffraction analysis of natural teeth. J Synchrotron Radiat. 2008;15:235–8. doi: 10.1107/S0909049508003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Gaft M, Shoval S, Panczer G, Nathan Y, Champagnon B, Garapon C. Luminescence of uranium and rare-earth elements in apatite of fossil fish teeth. Palaeogeogr. Palaeocl. 1996;126:187–93. doi: 10.1016/S0031-0182(96)00079-X. [DOI] [Google Scholar]
- 652.Huang CM, Zhang Q, Bai S, Wang CS. [FTIR and XRD analysis of hydroxyapatite from fossil human and animal teeth in Jinsha Relict, Chengdu] Guang Pu Xue Yu Guang Pu Fen Xi. 2007;27:2448–52. [PubMed] [Google Scholar]
- 653.Ho SP, Yu B, Yun W, Marshall GW, Ryder MI, Marshall SJ. Structure, chemical composition and mechanical properties of human and rat cementum and its interface with root dentin. Acta Biomater. 2009;5:707–18. doi: 10.1016/j.actbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Bosshardt DD, Selvig KA. Dental cementum: the dynamic tissue covering of the root. Periodontol 2000. 1997;13:41–75. doi: 10.1111/j.1600-0757.1997.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 655.Ho SP, Senkyrikova P, Marshall GW, Yun W, Wang Y, Karan K, et al. Structure, chemical composition and mechanical properties of coronal cementum in human deciduous molars. Dent Mater. 2009;25:1195–204. doi: 10.1016/j.dental.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Yamamoto T, Li M, Liu Z, Guo Y, Hasegawa T, Masuki H, et al. Histological review of the human cellular cementum with special reference to an alternating lamellar pattern. Odontology. 2010;98:102–9. doi: 10.1007/s10266-010-0134-3. [DOI] [PubMed] [Google Scholar]
- 657.Margolis HC, Beniash E, Fowler CE. Role of macromolecular assembly of enamel matrix proteins in enamel formation. J Dent Res. 2006;85:775–93. doi: 10.1177/154405910608500902. [DOI] [PubMed] [Google Scholar]
- 658.Vieira A, Hancock R, Limeback H, Schwartz M, Grynpas MD. How does fluoride concentration in the tooth affect apatite crystal size? J Dent Res. 2003;82:909–13. doi: 10.1177/154405910308201112. [DOI] [PubMed] [Google Scholar]
- 659.Cui FZ, Ge J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J Tissue Eng Regen Med. 2007;1:185–91. doi: 10.1002/term.21. [DOI] [PubMed] [Google Scholar]
- 660.Jandt KD. Probing the future in functional soft drinks on the nanometre scale—towards tooth friendly soft drinks. Trends Food Sci Technol. 2006;17:263–71. doi: 10.1016/j.tifs.2005.11.016. [DOI] [Google Scholar]
- 661.Chen H, Chen Y, Orr BG, Holl MM, Majoros I, Clarkson BH. Nanoscale probing of the enamel nanorod surface using polyamidoamine dendrimers. Langmuir. 2004;20:4168–71. doi: 10.1021/la0303005. [DOI] [PubMed] [Google Scholar]
- 662.Rönnholm E. The amelogenesis of human teeth as revealed by electron microscopy. II. The development of the enamel crystallites. J Ultrastruct Res. 1962;6:249–303. doi: 10.1016/S0022-5320(62)80036-7. [DOI] [PubMed] [Google Scholar]
- 663.Nylen MU, Eanes ED, Omnell KA. Crystal growth in rat enamel. J Cell Biol. 1963;18:109–23. doi: 10.1083/jcb.18.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Miake Y, Shimoda S, Fukae M, Aoba T. Epitaxial overgrowth of apatite crystals on the thin-ribbon precursor at early stages of porcine enamel mineralization. Calcif Tissue Int. 1993;53:249–56. doi: 10.1007/BF01320910. [DOI] [PubMed] [Google Scholar]
- 665.Daculsi G, Menanteau J, Kerebel LM, Mitre D. Length and shape of enamel crystals. Calcif Tissue Int. 1984;36:550–5. doi: 10.1007/BF02405364. [DOI] [PubMed] [Google Scholar]
- 666.Jodaikin A, Weiner S, Traub W. Enamel rod relations in the developing rat incisor. J Ultrastruct Res. 1984;89:324–32. doi: 10.1016/S0022-5320(84)80048-9. [DOI] [PubMed] [Google Scholar]
- 667.Brès EF, Hutchison JL. Surface structure study of biological calcium phosphate apatite crystals from human tooth enamel. J Biomed Mater Res. 2002;63:433–40. doi: 10.1002/jbm.10254. [DOI] [PubMed] [Google Scholar]
- 668.Schroeder L, Frank RM. High-resolution transmission electron microscopy of adult human peritubular dentine. Cell Tissue Res. 1985;242:449–51. doi: 10.1007/BF00214561. [DOI] [PubMed] [Google Scholar]
- 669.Brès EF, Voegel JC, Frank RM. High-resolution electron microscopy of human enamel crystals. J Microsc. 1990;160:183–201. doi: 10.1111/j.1365-2818.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 670.Robinson C, Connell S, Kirkham J, Shorea R, Smith A. Dental enamel—a biological ceramic: regular substructures in enamel hydroxyapatite crystals revealed by atomic force microscopy. J Mater Chem. 2004;14:2242–8. doi: 10.1039/b401154f. [DOI] [Google Scholar]
- 671.Warf RD, Watson RR. Calcium phosphate—nutrition in prevention of early childhood dental caries. In: Wild-type food in health promotion and disease prevention: the Columbus concept. de Meester F, Watson RR, Eds. Humana Press: Totowa NJ, USA 2008; 343-53. [Google Scholar]
- 672.Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6:84–108. doi: 10.1177/10454411950060020701. [DOI] [PubMed] [Google Scholar]
- 673.Diekwisch TG, Berman BJ, Gentner S, Slavkin HC. Initial enamel crystals are not spatially associated with mineralized dentine. Cell Tissue Res. 1995;279:149–67. doi: 10.1007/BF00300701. [DOI] [PubMed] [Google Scholar]
- 674.Aoba T. Recent observations on enamel crystal formation during mammalian amelogenesis. Anat Rec. 1996;245:208–18. doi: 10.1002/(SICI)1097-0185(199606)245:2<208::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 675.Beniash E, Metzler RA, Lam RSK, Gilbert PUPA. Transient amorphous calcium phosphate in forming enamel. J Struct Biol. 2009;166:133–43. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–61. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 677.Sydney-Zax M, Mayer I, Deutsch D. Carbonate content in developing human and bovine enamel. J Dent Res. 1991;70:913–6. doi: 10.1177/00220345910700051001. [DOI] [PubMed] [Google Scholar]
- 678.Rey C, Renugopalakrishnan V, Shimizu M, Collins B, Glimcher MJ. A resolution-enhanced Fourier transform infrared spectroscopic study of the environment of the CO3(2-) ion in the mineral phase of enamel during its formation and maturation. Calcif Tissue Int. 1991;49:259–68. doi: 10.1007/BF02556215. [DOI] [PubMed] [Google Scholar]
- 679.Takagi T, Ogasawara T, Tagami J, Akao M, Kuboki Y, Nagai N, et al. pH and carbonate levels in developing enamel. Connect Tissue Res. 1998;38:181–7, discussion 201-5. doi: 10.3109/03008209809017035. [DOI] [PubMed] [Google Scholar]
- 680.Lowenstam HA, Weiner S. Transformation of amorphous calcium phosphate to crystalline dahillite in the radular teeth of chitons. Science. 1985;227:51–3. doi: 10.1126/science.227.4682.51. [DOI] [PubMed] [Google Scholar]
- 681.Selvig KA. Periodic lattice images of hydroxyapatite crystals in human bone and dental hard tissues. Calcif Tissue Res. 1970;6:227–38. doi: 10.1007/BF02196203. [DOI] [PubMed] [Google Scholar]
- 682.Selvig KA. Electron microscopy of dental enamel: analysis of crystal lattice images. Z Zellforsch Mikrosk Anat. 1973;137:271–80. doi: 10.1007/BF00307434. [DOI] [PubMed] [Google Scholar]
- 683.Cuisinier FJG, Steuer P, Senger B, Voegel JC, Frank RM. Human amelogenesis: high resolution electron microscopy of nanometer-sized particles. Cell Tissue Res. 1993;273:175–82. doi: 10.1007/BF00304624. [DOI] [PubMed] [Google Scholar]
- 684.Cuisinier FJG, Steuer P, Brisson A, Voegel JC. High resolution electron microscopy study of crystal growth mechanisms in chicken bone composites. J Cryst Growth. 1995;156:443–53. doi: 10.1016/0022-0248(95)00237-5. [DOI] [Google Scholar]
- 685.Houllé P, Voegel JC, Schultz P, Steuer P, Cuisinier FJG. High resolution electron microscopy: structure and growth mechanisms of human dentin crystals. J Dent Res. 1997;76:895–904. doi: 10.1177/00220345970760041101. [DOI] [PubMed] [Google Scholar]
- 686.Mann S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature. 1993;365:499–505. doi: 10.1038/365499a0. [DOI] [Google Scholar]
- 687.Kirkham J, Zhang J, Brookes SJ, Shore RC, Wood SR, Smith DA, et al. Evidence for charge domains on developing enamel crystal surfaces. J Dent Res. 2000;79:1943–7. doi: 10.1177/00220345000790120401. [DOI] [PubMed] [Google Scholar]
- 688.Smith TM, Tafforeau P. New visions of dental tissue research: tooth development, chemistry and structure. Evol Anthropol. 2008;17:213–26. doi: 10.1002/evan.20176. [DOI] [Google Scholar]
- 689.Warshawsky H, Nanci A. Stereo electron microscopy of enamel crystallites. J Dent Res. 1982;61(Spec No):1504–14. [PubMed] [Google Scholar]
- 690.Warshawsky H. Organization of crystals in enamel. Anat Rec. 1989;224:242–62. doi: 10.1002/ar.1092240214. [DOI] [PubMed] [Google Scholar]
- 691.Xu C, Yao X, Walker MP, Wang Y. Chemical/molecular structure of the dentin-enamel junction is dependent on the intratooth location. Calcif Tissue Int. 2009;84:221–8. doi: 10.1007/s00223-008-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Arsenault AL, Robinson BW. The dentino-enamel junction: a structural and microanalytical study of early mineralization. Calcif Tissue Int. 1989;45:111–21. doi: 10.1007/BF02561410. [DOI] [PubMed] [Google Scholar]
- 693.Hayashi Y. High resolution electron microscopy in the dentino-enamel junction. J Electron Microsc (Tokyo) 1992;41:387–91. [PubMed] [Google Scholar]
- 694.Hayashi Y. High resolution electron microscopic study on the human dentine crystal. J Electron Microsc (Tokyo) 1993;42:141–6. [PubMed] [Google Scholar]
- 695.Bodier-Houllé P, Steuer P, Meyer JM, Bigeard L, Cuisinier FJG. High-resolution electron-microscopic study of the relationship between human enamel and dentin crystals at the dentinoenamel junction. Cell Tissue Res. 2000;301:389–95. doi: 10.1007/s004410000241. [DOI] [PubMed] [Google Scholar]
- 696.Takano Y, Hanaizumi Y, Ohshima H. Occurrence of amorphous and crystalline mineral deposits at the epithelial-mesenchymal interface of incisors in the calcium-loaded rat: implication of novel calcium binding domains. Anat Rec. 1996;245:174–85. doi: 10.1002/(SICI)1097-0185(199606)245:2<174::AID-AR6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 697.Dong W, Warshawsky H. Lattice fringe continuity in the absence of crystal continuity in enamel. Adv Dent Res. 1996;10:232–7. doi: 10.1177/08959374960100021901. [DOI] [PubMed] [Google Scholar]
- 698.Wang R, Hu Y, Ng C. Microstructure and interfacial fracture at the cementum-enamel junctions in equine and bovine teeth. J Mater Res. 2006;21:2146–55. doi: 10.1557/jmr.2006.0265. [DOI] [Google Scholar]
- 699.Ho SP, Goodis H, Balooch M, Nonomura G, Marshall SJ, Marshall GW. The effect of sample preparation technique on determination of structure and nanomechanical properties of human cementum hard tissue. Biomaterials. 2004;25:4847–57. doi: 10.1016/j.biomaterials.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 700.Paine ML, White SN, Luo W, Fong H, Sarikaya M, Snead ML. Regulated gene expression dictates enamel structure and tooth function. Matrix Biol. 2001;20:273–92. doi: 10.1016/S0945-053X(01)00153-6. [DOI] [PubMed] [Google Scholar]
- 701.Finke M, Parker DM, Jandt KD. Influence of soft drinks on the thickness and morphology of in situ acquired pellicle layer on enamel. J Colloid Interface Sci. 2002;251:263–70. doi: 10.1006/jcis.2002.8428. [DOI] [PubMed] [Google Scholar]
- 702.Barbour ME, Parker DM, Jandt KD. Enamel dissolution as a function of solution degree of saturation with respect to hydroxyapatite: a nanoindentation study. J Colloid Interface Sci. 2003;265:9–14. doi: 10.1016/S0021-9797(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 703.Lippert F, Parker DM, Jandt KD. Susceptibility of deciduous and permanent enamel to dietary acid-induced erosion studied with atomic force microscopy nanoindentation. Eur J Oral Sci. 2004;112:61–6. doi: 10.1111/j.0909-8836.2004.00095.x. [DOI] [PubMed] [Google Scholar]
- 704.Barbour ME, Parker DM, Allen GC, Jandt KD. Human enamel erosion in constant composition citric acid solutions as a function of degree of saturation with respect to hydroxyapatite. J Oral Rehabil. 2005;32:16–21. doi: 10.1111/j.1365-2842.2004.01365.x. [DOI] [PubMed] [Google Scholar]
- 705.LeGeros RZ. Calcium phosphates in demineralization and remineralization processes. J Clin Dent. 1999;10:65–73. [Google Scholar]
- 706.Walker G, Cai F, Shen P, Reynolds C, Ward B, Fone C, et al. Increased remineralization of tooth enamel by milk containing added casein phosphopeptide-amorphous calcium phosphate. J Dairy Res. 2006;73:74–8. doi: 10.1017/S0022029905001482. [DOI] [PubMed] [Google Scholar]
- 707.Lippert F, Parker DM, Jandt KD. In situ remineralisation of surface softened human enamel studied with AFM nanoindentation. Surf Sci. 2004;553:105–14. doi: 10.1016/j.susc.2004.01.040. [DOI] [Google Scholar]
- 708.Lippert F, Parker DM, Jandt KD. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J Colloid Interface Sci. 2004;280:442–8. doi: 10.1016/j.jcis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 709.Lippert F, Parker DM, Jandt KD. Toothbrush abrasion of surface softened enamel studied with tapping mode AFM and AFM nanoindentation. Caries Res. 2004;38:464–72. doi: 10.1159/000079628. [DOI] [PubMed] [Google Scholar]
- 710.Hannig C, Hannig M. Natural enamel wear--a physiological source of hydroxylapatite nanoparticles for biofilm management and tooth repair? Med Hypotheses. 2010;74:670–2. doi: 10.1016/j.mehy.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 711.Busch S. Regeneration of human tooth enamel. Angew Chew Int Ed 2004; 43:1428-31. [DOI] [PubMed] [Google Scholar]
- 712.Onuma K, Yamagishi K, Oyane A. Nucleation and growth of hydroxyapatite nanocrystals for nondestructive repair of early caries lesions. J Cryst Growth. 2005;282:199–207. doi: 10.1016/j.jcrysgro.2005.04.085. [DOI] [Google Scholar]
- 713.He L, Feng Z. Preparation and characterization of dicalcium phosphate dihydrate coating on enamel. Mater Lett. 2007;61:3923–6. doi: 10.1016/j.matlet.2006.12.059. [DOI] [Google Scholar]
- 714.Li L, Pan H, Tao J, Xu X, Mao C, Gu X, et al. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–84. doi: 10.1039/b806090h. [DOI] [Google Scholar]
- 715.Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42:88–97. doi: 10.1159/000113161. [DOI] [PubMed] [Google Scholar]
- 716.Wang X, Xia C, Zhang Z, Deng X, Wei S, Zheng G, et al. Direct growth of human enamel-like calcium phosphate microstructures on human tooth. J Nanosci Nanotechnol. 2009;9:1361–4. doi: 10.1166/jnn.2009.C157. [DOI] [PubMed] [Google Scholar]
- 717.Roveri N, Battistella E, Bianchi CL, Foltran I, Foresti E, Iafisco M, et al. Surface enamel remineralization: biomimetic apatite nanocrystals and fluoride ions different effects. J Nanomater. 2009;•••:746383. [Google Scholar]
- 718.Roveri N, Foresti E, Lelli M, Lesci IG. Recent advancements in preventing teeth health hazard: the daily use of hydroxyapatite instead of fluoride. Recent Patents on Biomed. Eng. 2009;2:197–215. [Google Scholar]
- 719.Yin Y, Yun S, Fang J, Chen H. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem Commun (Camb) 2009;21:5892–4. doi: 10.1039/b911407f. [DOI] [PubMed] [Google Scholar]
- 720.Peters MC, Bresciani E, Barata TJE, Fagundes TC, Navarro RL, Navarro MFL, et al. In vivo dentin remineralization by calcium-phosphate cement. J Dent Res. 2010;89:286–91. doi: 10.1177/0022034509360155. [DOI] [PubMed] [Google Scholar]
- 721.Orsini G, Procaccini M, Manzoli L, Giuliodori F, Lorenzini A, Putignano A. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J Clin Periodontol. 2010;37:510–7. doi: 10.1111/j.1600-051X.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- 722.Uysal T, Amasyali M, Koyuturk AE, Ozcan S, Sagdic D. Amorphous calcium phosphate-containing orthodontic composites. Do they prevent demineralisation around orthodontic brackets? Aust Orthod J. 2010;26:10–5. [PubMed] [Google Scholar]
- 723.Sá Roriz Fonteles C, Zero DT, Moss ME, Fu J. Fluoride concentrations in enamel and dentin of primary teeth after pre- and postnatal fluoride exposure. Caries Res. 2005;39:505–8. doi: 10.1159/000088187. [DOI] [PubMed] [Google Scholar]
- 724.Wiegand A, Krieger C, Attin R, Hellwig E, Attin T. Fluoride uptake and resistance to further demineralisation of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin Oral Investig. 2005;9:52–7. doi: 10.1007/s00784-005-0306-7. [DOI] [PubMed] [Google Scholar]
- 725.Waszkiel D, Opalko K, Lagocka R, Chlubek D. Fluoride and magnesium content in superficial enamel layers of teeth with erosions. Fluoride. 2004;37:271–7. [Google Scholar]
- 726.Vieira APGF, Hancock R, Dumitriu M, Schwartz M, Limeback H, Grynpas MD. How does fluoride affect dentin microhardness and mineralization? J Dent Res. 2005;84:951–7. doi: 10.1177/154405910508401015. [DOI] [PubMed] [Google Scholar]
- 727.Driessens FCM. Relation between apatite solubility and anti-cariogenic effect of fluoride. Nature. 1973;243:420–1. doi: 10.1038/243420a0. [DOI] [PubMed] [Google Scholar]
- 728.Moreno EC, Kresak M, Zahradnik RT. Fluoridated hydroxyapatite solubility and caries formation. Nature. 1974;247:64–5. doi: 10.1038/247064a0. [DOI] [PubMed] [Google Scholar]
- 729.McClendon JF. Fluorapatite and teeth. Science. 1966;151:151. doi: 10.1126/science.151.3707.151-b. [DOI] [PubMed] [Google Scholar]
- 730.Wang X, Klocke A, Mihailova B, Tosheva L, Bismayer U. New insights into structural alteration of enamel apatite induced by citric acid and sodium fluoride solutions. J Phys Chem B. 2008;112:8840–8. doi: 10.1021/jp802492d. [DOI] [PubMed] [Google Scholar]
- 731.Kierdorf U, Kierdorf H. Deer antlers - a model of mammalian appendage regeneration: an extensive review. Gerontology. 2011;57:53–65. doi: 10.1159/000300565. [DOI] [PubMed] [Google Scholar]
- 732.Price J, Faucheux C, Allen S. Deer antlers as a model of Mammalian regeneration. Curr Top Dev Biol. 2005;67:1–48. doi: 10.1016/S0070-2153(05)67001-9. [DOI] [PubMed] [Google Scholar]
- 733.Yue Z, Deng X, Feng H. The mechanisms of deer antlers development and regeneration. J Econ Animal. 2005;9:46–9. [Google Scholar]
- 734.Zhao L, Yue Z, Zhang X, Deng X. The deer antlers endochondral ossification and its regulation mechanisms. J Econ Animal. 2006;10:238–41. [Google Scholar]
- 735.Landete-Castillejos T, Garcia A, Gallego L. Body weight, early growth and antler size influence antler bone mineral composition of Iberian red deer (Cervus elaphus hispanicus) Bone. 2007;40:230–5. doi: 10.1016/j.bone.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 736.Huxley J. The relative size of antlers of deer. Proc Zool Soc Lond. 1931;72:819–64. [Google Scholar]
- 737.Kierdorf U, Li C, Price JS. Improbable appendages: Deer antler renewal as a unique case of mammalian regeneration. Semin Cell Dev Biol. 2009;20:535–42. doi: 10.1016/j.semcdb.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 738.Chen PY, Stokes AG, McKittrick J. Comparison of the structure and mechanical properties of bovine femur bone and antler of the North American elk (Cervus elaphus canadensis) Acta Biomater. 2009;5:693–706. doi: 10.1016/j.actbio.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 739.Zioupos P, Wang XT, Currey JD. Experimental and theoretical quantification of the development of damage in fatigue tests of bone and antler. J Biomech. 1996;29:989–1002. doi: 10.1016/0021-9290(96)00001-2. [DOI] [PubMed] [Google Scholar]
- 740.Landete-Castillejos T, Currey JD, Estevez JA, Gaspar-López E, Garcia A, Gallego L. Influence of physiological effort of growth and chemical composition on antler bone mechanical properties. Bone. 2007;41:794–803. doi: 10.1016/j.bone.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 741.Evans LA, McCutcheon AL, Dennis GR, Mulley RC, Wilson MA. Pore size analysis of fallow deer (Dama dama) antler bone. J Mater Sci. 2005;40:5733–9. doi: 10.1007/s10853-005-1118-5. [DOI] [Google Scholar]
- 742.Akhtar R, Daymond MR, Almer JD, Mummery PM. Elastic strains in antler trabecular bone determined by synchrotron X-ray diffraction. Acta Biomater. 2008;4:1677–87. doi: 10.1016/j.actbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 743.Currey JD, Landete-Castillejos T, Estevez J, Ceacero F, Olguin A, Garcia A, et al. The mechanical properties of red deer antler bone when used in fighting. J Exp Biol. 2009;212:3985–93. doi: 10.1242/jeb.032292. [DOI] [PubMed] [Google Scholar]
- 744.Goss RJ. Deer antlers regeneration, function and evolution. Academic Press: New York USA 1983; 316. [Google Scholar]
- 745.Bubenik GA, Bubenik AB, eds. Horns, pronghorns and antlers: evolution, morphology, physiology and social significance. Springer: New York USA 1990; 562. [DOI] [PubMed] [Google Scholar]
- 746.Kierdorf U, Kierdorf H. Antlers as biomonitors of environmental pollution by lead and fluoride: a review. Eur J Wildl Res. 2005;51:137–50. doi: 10.1007/s10344-005-0093-0. [DOI] [Google Scholar]
- 747.Barling PM, Gupta DK, Lim CEL. Involvement of phosphodiesterase I in mineralization: histochemical studies using antler from red deer (Cervus elaphus) as a model. Calcif Tissue Int. 1999;65:384–9. doi: 10.1007/s002239900718. [DOI] [PubMed] [Google Scholar]
- 748.Barling PM, Chong KW. The involvement of phosphohydrolases in mineralization: studies on enzymatic activities extracted from red deer antler. Calcif Tissue Int. 1999;65:232–6. doi: 10.1007/s002239900689. [DOI] [PubMed] [Google Scholar]
- 749.Kierdorf U, Kierdorf H. The fluoride content of antlers as an indicator of fluoride exposure in red deer (Cervus elaphus): a historical biomonitoring study. Fluoride. 2000;33:92–4. doi: 10.1007/s002449910015. [DOI] [PubMed] [Google Scholar]
- 750.Pathak NN, Pattanaik AK, Patra RC, Arora BM. Mineral composition of antlers of three deer species reared in captivity. Small Rumin Res. 2001;42:61–5. doi: 10.1016/S0921-4488(01)00218-8. [DOI] [Google Scholar]
- 751.Yuxia Y, Rui D, Wang Y, Wang S. Calcium and phosphors contents of three-branched and two-branched antler and ossificational antler from sika deer. J Econ Animal. 2002;6:6–8. [Google Scholar]
- 752.Kim HY, Rhyu MR. Sectional composition of minerals in domestic deer antler. Korean J. Food Sci. Technol. 2000;32:31–6. [Google Scholar]
- 753.Li C, Suttie JM, Clark DE. Histological examination of antler regeneration in red deer (Cervus elaphus) Anat Rec A Discov Mol Cell Evol Biol. 2005;282:163–74. doi: 10.1002/ar.a.20148. [DOI] [PubMed] [Google Scholar]
- 754.Meister W. Changes in biological structure of the long bones of white-tailed deer during the growth of antlers. Anat Rec. 1956;124:709–21. doi: 10.1002/ar.1091240407. [DOI] [PubMed] [Google Scholar]
- 755.Muir PD, Sykes AR, Barrell GK. Calcium metabolism in red deer (Cervus elaphus) offered herbages during antlerogenesis: kinetic and stable balance studies. J Agric Sci Camb. 1987;109:357–64. doi: 10.1017/S0021859600080783. [DOI] [Google Scholar]
- 756.Baxter BJ, Andrews RN, Barrell GK. Bone turnover associated with antler growth in red deer (Cervus elaphus) Anat Rec. 1999;256:14–9. doi: 10.1002/(SICI)1097-0185(19990901)256:1<14::AID-AR3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 757.Bubenik GA, Bubenik PG. Palmated antlers of moose may serve as a parabolic reflector of sounds. Eur J Wildl Res. 2008;54:533–5. doi: 10.1007/s10344-007-0165-4. [DOI] [Google Scholar]
- 758.Landete-Castillejos T, Estevez JA, Martínez A, Ceacero F, Garcia A, Gallego L. Does chemical composition of antler bone reflect the physiological effort made to grow it? Bone. 2007;40:1095–102. doi: 10.1016/j.bone.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 759.Baciut M, Baciut G, Simon V, Albon C, Coman V, Prodan P, et al. Investigation of deer antler as a potential bone regenerating biomaterial. J Optoelectron Adv Mater. 2007;9:2547–50. [Google Scholar]
- 760.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 761.Kazama JJ, Amizuka N, Fukagawa M. Ectopic calcification as abnormal biomineralization. Ther Apher Dial. 2006;10:34–8. doi: 10.1111/j.1744-9987.2006.00438.x. [DOI] [Google Scholar]
- 762.Mackarell WW, Moore B, Thomas WT. On the presence of insoluble salts of calcium (oxalate and phosphate) in renal calculi in large amount in a preponderating number of cases, and the bearing of this finding upon calcium metabolism in gout and allied conditions. Biochem J. 1911;5:161–80. doi: 10.1042/bj0050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Frondel C, Prien EL. Carbonate-apatite and hydroxylapatite in urinary calculi. Science. 1942;95:431. doi: 10.1126/science.95.2469.431. [DOI] [PubMed] [Google Scholar]
- 764.Frondel C, Prien EL. Deposition of calcium phosphates accompanying senile degeneration and disease. Science. 1946;103:326. doi: 10.1126/science.103.2672.326. [DOI] [PubMed] [Google Scholar]
- 765.Brancaccio D, Cozzolino M. The mechanism of calcium deposition in soft tissues. Contrib Nephrol. 2005;149:279–86. doi: 10.1159/000085689. [DOI] [PubMed] [Google Scholar]
- 766.Goff AK, Reichard R. A soft-tissue calcification: differential diagnosis and pathogenesis. J Forensic Sci. 2006;51:493–7. doi: 10.1111/j.1556-4029.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 767.Bittmann S, Günther MW, Ulus H. Tumoral calcinosis of the gluteal region in a child: case report with overview of different soft-tissue calcifications. J Pediatr Surg. 2003;38:E4–7. doi: 10.1016/S0022-3468(03)00289-6. [DOI] [PubMed] [Google Scholar]
- 768.Molloy ES, McCarthy GM. Basic calcium phosphate crystals: pathways to joint degeneration. Curr Opin Rheumatol. 2006;18:187–92. doi: 10.1097/01.bor.0000209433.43978.a8. [DOI] [PubMed] [Google Scholar]
- 769.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–64. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 770.Fukagawa M, Kazama JJ, Fukagawa M. The making of a bone in blood vessels: from the soft shell to the hard bone. Kidney Int. 2007;72:533–4. doi: 10.1038/sj.ki.5002440. [DOI] [PubMed] [Google Scholar]
- 771.Achilles W. Crystallization in gel matrices: a new experimental model of calcium stone formation. Contrib Nephrol. 1987;58:59–64. doi: 10.1159/000414488. [DOI] [PubMed] [Google Scholar]
- 772.Achilles W, Jöckel U, Schaper A, Burk M, Riedmiller H. In vitro formation of “urinary stones”: generation of spherulites of calcium phosphate in gel and overgrowth with calcium oxalate using a new flow model of crystallization. Scanning Microsc. 1995;9:577–85, discussion 585-6. [PubMed] [Google Scholar]
- 773.Ross AE, Handa S, Lingeman JE, Matlaga BR. Kidney stones during pregnancy: an investigation into stone composition. Urol Res. 2008;36:99–102. doi: 10.1007/s00240-008-0138-4. [DOI] [PubMed] [Google Scholar]
- 774.Ringdén I, Tiselius HG. Composition and clinically determined hardness of urinary tract stones. Scand J Urol Nephrol. 2007;41:316–23. doi: 10.1080/00365590601154551. [DOI] [PubMed] [Google Scholar]
- 775.Jensen AT, Rowles SL. Magnesium whitlockite, a major constituent of dental calculus. Acta Odontol Scand. 1957;16:121–39. doi: 10.3109/00016355709041096. [DOI] [Google Scholar]
- 776.Le May O, Kaqueler JC, Kodaka T. Electron probe micro-analysis of human dental pulp stones. Scanning Microsc. 1993;7:267–71, discussion 271-2. [PubMed] [Google Scholar]
- 777.Kodaka T, Hirayama A, Mori R, Sano T. Spherulitic brushite stone in the dental pulp of a cow. J Electron Microsc (Tokyo) 1998;47:57–65. doi: 10.1093/oxfordjournals.jmicro.a023559. [DOI] [PubMed] [Google Scholar]
- 778.Hayashizaki J, Ban S, Nakagaki H, Okumura A, Yoshii S, Robinson C. Site specific mineral composition and microstructure of human supra-gingival dental calculus. Arch Oral Biol. 2008;53:168–74. doi: 10.1016/j.archoralbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 779.Zelentsov EL, Moroz TN, Kolmogorov YP, Tolmachev VE, Dragun GN, Palchik NA, et al. The elemental SRXRF analysis and mineral composition of human salivary stones. NIM A. 2001;470:417–21. doi: 10.1016/S0168-9002(01)01088-9. [DOI] [Google Scholar]
- 780.Ortlepp JR, Schmitz F, Mevissen V, Weiss S, Huster J, Dronskowski R, et al. The amount of calcium-deficient hexagonal hydroxyapatite in aortic valves is influenced by gender and associated with genetic polymorphisms in patients with severe calcific aortic stenosis. Eur Heart J. 2004;25:514–22. doi: 10.1016/j.ehj.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 781.Kim KM. Cellular mechanism of calcification and its prevention in glutaraldehyde treated vascular tissue. Z Kardiol. 2001;90(Suppl 3):99–105. doi: 10.1007/s003920170050. [DOI] [PubMed] [Google Scholar]
- 782.Tomazic BB. Physiochemical principles of cardiovascular calcification. Z Kardiol. 2001;90(Suppl 3):68–80. doi: 10.1007/s003920170046. [DOI] [PubMed] [Google Scholar]
- 783.Marra SP, Daghlian CP, Fillinger MF, Kennedy FE. Elemental composition, morphology and mechanical properties of calcified deposits obtained from abdominal aortic aneurysms. Acta Biomater. 2006;2:515–20. doi: 10.1016/j.actbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 784.Fitzpatrick LA, Turner RT, Ritman ER. Endochondral bone formation in the heart: a possible mechanism of coronary calcification. Endocrinology. 2003;144:2214–9. doi: 10.1210/en.2002-0170. [DOI] [PubMed] [Google Scholar]
- 785.Suvorova EI, Buffat PA. Pathological mineralization of cardiac valves: causes and mechanism. J Long Term Eff Med Implants. 2005;15:355–68. doi: 10.1615/JLongTermEffMedImplants.v15.i4.30. [DOI] [PubMed] [Google Scholar]
- 786.Giachelli CM. Ectopic calcification: new concepts in cellular regulation. Z Kardiol. 2001;90(Suppl 3):31–7. doi: 10.1007/s003920170039. [DOI] [PubMed] [Google Scholar]
- 787.Glasmacher B, Nellen E, Reul H, Rau G. In vitro hemocompatibility testing of new materials for mechanical heart valves. Materialwiss Werkstofftech. 1999;30:806–8. doi: 10.1002/(SICI)1521-4052(199912)30:12<806::AID-MAWE806>3.0.CO;2-F. [DOI] [Google Scholar]
- 788.Deiwick M, Glasmacher B, Pettenazzo E, Hammel D, Castellón W, Thiene G, et al. Primary tissue failure of bioprostheses: new evidence from in vitro tests. Thorac Cardiovasc Surg. 2001;49:78–83. doi: 10.1055/s-2001-11711. [DOI] [PubMed] [Google Scholar]
- 789.Pettenazzo E, Deiwick M, Thiene G, Molin G, Glasmacher B, Martignago F, et al. Dynamic in vitro calcification of bioprosthetic porcine valves: evidence of apatite crystallization. J Thorac Cardiovasc Surg. 2001;121:500–9. doi: 10.1067/mtc.2001.112464. [DOI] [PubMed] [Google Scholar]
- 790.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 791.Sensui K, Saitoh S, Kametani K, Makino K, Ohira M, Kimura T, et al. Property analysis of ectopic calcification in the carpal tunnel identification of apatite crystals: a case report. Arch Orthop Trauma Surg. 2003;123:442–5. doi: 10.1007/s00402-003-0574-0. [DOI] [PubMed] [Google Scholar]
- 792.Kim CJ, Choi SK. Analysis of aqueous humor calcium and phosphate from cataract eyes with and without diabetes mellitus. Korean J Ophthalmol. 2007;21:90–4. doi: 10.3341/kjo.2007.21.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Mccarty DJ, Jr., Hogan JM, Gatter RA, Grossman M. Studies on pathological calcifications in human cartilage. I. Prevalence and types of crystal deposits in the menisci of two hundred fifteen cadavera. J Bone Joint Surg Am. 1966;48:309–25. [PubMed] [Google Scholar]
- 794.Laird DF, Mucalo MR, Yokogawa Y. Growth of calcium hydroxyapatite (Ca-HAp) on cholesterol and cholestanol crystals from a simulated body fluid: A possible insight into the pathological calcifications associated with atherosclerosis. J Colloid Interface Sci. 2006;295:348–63. doi: 10.1016/j.jcis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 795.Stock SR, Ignatiev K, Lee PL, Abbott K, Pachman LM. Pathological calcification in juvenile dermatomyositis (JDM): microCT and synchrotron x-ray diffraction reveal hydroxyapatite with varied microstructures. Connect Tissue Res. 2004;45:248–56. doi: 10.1080/03008200490903066. [DOI] [PubMed] [Google Scholar]
- 796.Pachman LM, Boskey AL. Clinical manifestations and pathogenesis of hydroxyapatite crystal deposition in juvenile dermatomyositis. Curr Rheumatol Rep. 2006;8:236–43. doi: 10.1007/s11926-996-0031-5. [DOI] [PubMed] [Google Scholar]
- 797.Hale EK. Metastatic calcification. Dermatology Online J 2003; 9:2 (http://dermatology.cdlib.org/94/NYU/Nov2001/3.html)—accessed in October 2010. [PubMed]
- 798.Alkan O, Tokmak N, Demir S, Yildirim T. Metastatic pulmonary calcification in a patient with chronic renal failure. J Radiology Case Reports. 2009;3:14–7. doi: 10.3941/jrcr.v3i4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Grech P, Ell PJ, Martin TJ, Barrington NA, Martin TJ. Diagnosis in metabolic bone disease. Hodder Arnold H&S: USA 1998; 300. [Google Scholar]
- 800.Mohr W. Apatite diseases. The pathological substrate in dependence of tissue processing and in consideration of pathogenesis. Aktuelle Rheumatol. 2003;28:53–8. doi: 10.1055/s-2003-37165. [DOI] [Google Scholar]
- 801.Gorospe M, Fadare O. Gastric mucosal calcinosis: clinicopathologic considerations. Adv Anat Pathol. 2007;14:224–8. doi: 10.1097/PAP.0b013e31805048ea. [DOI] [PubMed] [Google Scholar]
- 802.Dubois LA, Gray DK, Tweedie EJ. Surgical images: soft tissue. Calcinosis cutis. Can J Surg. 2007;50:217–8. [PMC free article] [PubMed] [Google Scholar]
- 803.Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130:652–60. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.White DJ. Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur J Oral Sci. 1997;105:508–22. doi: 10.1111/j.1600-0722.1997.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 805.Ciftçioğlu N, McKay DS. Pathological calcification and replicating calcifying-nanoparticles: general approach and correlation. Pediatr Res. 2010;67:490–9. doi: 10.1203/PDR.0b013e3181d476ce. [DOI] [PubMed] [Google Scholar]
- 806.Tomazic BB. Characterization of mineral phases in cardiovascular calcification. In: Hydroxyapatite and related materials. Brown PW, Constantz B, Eds. CRC Press: Boca Raton FL, USA 1994; 93-116. [Google Scholar]
- 807.Lee RS, Kayser MV, Ali SY. Calcium phosphate microcrystal deposition in the human intervertebral disc. J Anat. 2006;208:13–9. doi: 10.1111/j.1469-7580.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Rosenthal AK. Update in calcium deposition diseases. Curr Opin Rheumatol. 2007;19:158–62. doi: 10.1097/BOR.0b013e3280145289. [DOI] [PubMed] [Google Scholar]
- 809.Lagier R, Baud CA. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol Res Pract. 2003;199:329–35. doi: 10.1078/0344-0338-00425. [DOI] [PubMed] [Google Scholar]
- 810.Wesson JA, Ward MD. Pathological biomineralization of kidney stones. Elements. 2007;3:415–21. doi: 10.2113/GSELEMENTS.3.6.415. [DOI] [Google Scholar]
- 811.Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics: a review publication of the Radiological Society of North America Inc. 1990; 10:1031-48. [DOI] [PubMed]
- 812.Best JA, Shapiro RD, Kalmar J, Westesson PL. Hydroxyapatite deposition disease of the temporomandibular joint in a patient with renal failure. J Oral Maxillofac Surg. 1997;55:1316–22. doi: 10.1016/S0278-2391(97)90192-0. [DOI] [PubMed] [Google Scholar]
- 813.Garcia GM, McCord GC, Kumar R. Hydroxyapatite crystal deposition disease. Semin Musculoskelet Radiol. 2003;7:187–93. doi: 10.1055/s-2003-43229. [DOI] [PubMed] [Google Scholar]
- 814.Melrose J, Burkhardt D, Taylor TKF, Dillon CT, Read R, Cake M, et al. Calcification in the ovine intervertebral disc: a model of hydroxyapatite deposition disease. Eur Spine J. 2009;18:479–89. doi: 10.1007/s00586-008-0871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.LeGeros RZ, Orly I, LeGeros JP, Gomez C, Kazimiroff J, Tarpley T, et al. Scanning electron microscopy and electron probe microanalyses of the crystalline components of human and animal dental calculi. Scanning Microsc. 1988;2:345–56. [PubMed] [Google Scholar]
- 816.Grases F, Llobera A. Experimental model to study sedimentary kidney stones. Micron. 1998;29:105–11. doi: 10.1016/S0968-4328(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 817.Lieske JC, Norris R, Toback FG. Adhesion of hydroxyapatite crystals to anionic sites on the surface of renal epithelial cells. Am J Physiol Renal Physiol. 1997;273:224–33. doi: 10.1152/ajprenal.1997.273.2.F224. [DOI] [PubMed] [Google Scholar]
- 818.Kirsch T. Determinants of pathological mineralization. Curr Opin Rheumatol. 2006;18:174–80. doi: 10.1097/01.bor.0000209431.59226.46. [DOI] [PubMed] [Google Scholar]
- 819.Giachelli CM. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am J Pathol. 1999;154:671–5. doi: 10.1016/S0002-9440(10)65313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Kirsch T. Physiological and pathological mineralization: a complex multifactorial process. Curr Opin Orthop. 2007;18:425–7. doi: 10.1097/BCO.0b013e3282e6f3de. [DOI] [Google Scholar]
- 821.Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. doi: 10.1016/S1054-8807(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 822.Giachelli CM. Inducers and inhibitors of biomineralization: lessons from pathological calcification. Orthod Craniofac Res. 2005;8:229–31. doi: 10.1111/j.1601-6343.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 823.Giachelli CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–22. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 824.Schmitt CP, Odenwald T, Ritz E. Calcium, calcium regulatory hormones, and calcimimetics: impact on cardiovascular mortality. J Am Soc Nephrol. 2006;17(Suppl 2):S78–80. doi: 10.1681/ASN.2005121338. [DOI] [PubMed] [Google Scholar]
- 825.Azari F, Vali H, Guerquin-Kern JL, Wu TD, Croisy A, Sears SK, et al. Intracellular precipitation of hydroxyapatite mineral and implications for pathologic calcification. J Struct Biol. 2008;162:468–79. doi: 10.1016/j.jsb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 826.Stayton PS, Drobny GP, Shaw WJ, Long JR, Gilbert M. Molecular recognition at the protein-hydroxyapatite interface. Crit Rev Oral Biol Med. 2003;14:370–6. doi: 10.1177/154411130301400507. [DOI] [PubMed] [Google Scholar]
- 827.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–72. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 828.Dey A, Bomans PHH, Müller FA, Will J, Frederik PM, de With G, et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater. 2010;9:1010–4. doi: 10.1038/nmat2900. [DOI] [PubMed] [Google Scholar]
- 829.Lamas GA, Ackermann A. Clinical evaluation of chelation therapy: is there any wheat amidst the chaff? Am Heart J. 2000;140:4–5. doi: 10.1067/mhj.2000.107549. [DOI] [PubMed] [Google Scholar]
- 830.Knudtson ML, Wyse DG, Galbraith PD, Brant R, Hildebrand K, Paterson D, et al. Program to Assess Alternative Treatment Strategies to Achieve Cardiac Health (PATCH) Investigators Chelation therapy for ischemic heart disease: a randomized controlled trial. JAMA. 2002;287:481–6. doi: 10.1001/jama.287.4.481. [DOI] [PubMed] [Google Scholar]
- 831.Ehrlich H, Koutsoukos PG, Demadis KD, Pokrovsky OS. Principles of demineralization: modern strategies for the isolation of organic frameworks. Part I. Common definitions and history. Micron. 2008;39:1062–91. doi: 10.1016/j.micron.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 832.Ehrlich H, Koutsoukos PG, Demadis KD, Pokrovsky OS. Principles of demineralization: modern strategies for the isolation of organic frameworks. Part II. Decalcification. Micron. 2009;40:169–93. doi: 10.1016/j.micron.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 833.Green D, Walsh D, Mann S, Oreffo ROC. The potential of biomimesis in bone tissue engineering: lessons from the design and synthesis of invertebrate skeletons. Bone. 2002;30:810–5. doi: 10.1016/S8756-3282(02)00727-5. [DOI] [PubMed] [Google Scholar]
- 834.Burke DE, de Markey CA, le Quesne PW, Cook JM. Biomimetic synthesis of the bis-indole alkaloid macralstonine. J Chem Soc Chem Communic 1972; 1346-7. [Google Scholar]
- 835.Breslow R. Centenary lecture: biomimetic chemistry. Chem Soc Rev. 1972;1:553–80. doi: 10.1039/cs9720100553. [DOI] [Google Scholar]
- 836.Benyus JM. Biomimicry: innovation inspired by nature. William Morrow, New York 1997; 308. [Google Scholar]
- 837.Watt JC. The behavior of calcium phosphate and calcium carbonate (bone salts) precipitated in various media, with applications to bone formation. Biol Bull. 1923;44:280–8. doi: 10.2307/1536715. [DOI] [Google Scholar]
- 838.Dorozhkin SV, Dorozhkina EI, Epple M. A model system to provide a good in vitro simulation of biological mineralization. Cryst Growth Des. 2004;4:389–95. doi: 10.1021/cg034066s. [DOI] [Google Scholar]
- 839.Izquierdo-Barba I, Salinas AJ, Vallet-RegI M. Effect of the continuous solution exchange on the in vitro reactivity of a CaO-SiO(2) sol-gel glass. J Biomed Mater Res. 2000;51:191–9. doi: 10.1002/(SICI)1097-4636(200008)51:2<191::AID-JBM7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 840.Vallet-Regí M, Perez-Pariente J, Izquierdo-Barba I, Salinas AJ. Compositional variations in the calcium phosphate layer growth on gel glasses soaked in a simulated body fluid. Chem Mater. 2000;12:3770–5. doi: 10.1021/cm001068g. [DOI] [Google Scholar]
- 841.Koutsoukos P, Amjad Z, Tomson MB, Nancollas GH. Crystallization of calcium phosphates: a constant composition study. J Am Chem Soc. 1980;102:1553–7. doi: 10.1021/ja00525a015. [DOI] [Google Scholar]
- 842.Tomson MB, Nancollas GH. Mineralization kinetics: a constant composition approach. Science. 1978;200:1059–60. doi: 10.1126/science.200.4345.1059. [DOI] [PubMed] [Google Scholar]
- 843.Manjubala I, Scheler S, Bössert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2:75–84. doi: 10.1016/j.actbio.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 844.Cai HQ, Li QL, Zhou J, Tang J, Chen H. Biomimetic synthesis and cytocompatibility of agar-hydroxyapatite composites. J Clin Rehabil Tiss Eng Res. 2010;14:410–4. [Google Scholar]
- 845.Yokoi T, Kawashita M, Kikuta K, Ohtsuki C. Biomimetic mineralization of calcium phosphate crystals in polyacrylamide hydrogel: effect of concentrations of calcium and phosphate ions on crystalline phases and morphology. Mater Sci Eng C. 2010;30:154–9. doi: 10.1016/j.msec.2009.09.012. [DOI] [Google Scholar]
- 846.Sadjadi MS, Meskinfam M, Sadeghi B, Jazdarreh H, Zare K. In situ biomimetic synthesis, characterization and in vitro investigation of bone-like nanohydroxyapatite in starch matrix. Mater Chem Phys. 2010;124:217–22. doi: 10.1016/j.matchemphys.2010.06.022. [DOI] [Google Scholar]
- 847.Dorozhkin SV, Dorozhkina EI. The influence of bovine serum albumin on the crystallization of calcium phosphates from a revised simulated body fluid. Colloids Surf A Physicochem Eng Asp. 2003;215:191–9. doi: 10.1016/S0927-7757(02)00438-7. [DOI] [Google Scholar]
- 848.Dorozhkina EI, Dorozhkin SV. In vitro crystallization of carbonateapatite on cholesterol from a modified simulated body fluid. Colloids Surf A Physicochem Eng Asp. 2003;223:231–7. doi: 10.1016/S0927-7757(03)00221-8. [DOI] [Google Scholar]
- 849.Dorozhkin SV, Dorozhkina EI, Epple M. Precipitation of carbonateapatite from a revised simulated body fluid in the presence of glucose. J Appl Biomater Biomech. 2003;1:200–8. [PubMed] [Google Scholar]
- 850.Dorozhkin SV, Dorozhkina EI. In vitro simulation of vascular calcification by the controlled crystallization of amorphous calcium phosphates onto porous cholesterol. J Mater Sci. 2005;40:6417–22. doi: 10.1007/s10853-005-2154-x. [DOI] [PubMed] [Google Scholar]
- 851.Dorozhkin SV. In vitro mineralization of silicon containing calcium phosphate bioceramics. J Am Ceram Soc. 2007;90:244–9. doi: 10.1111/j.1551-2916.2006.01368.x. [DOI] [Google Scholar]
- 852.Becker A, Epple M. A high-throughput crystallisation device to study biomineralisation in vitro. Mater Res Soc Symp Proc 2005; 873:1-10. [Google Scholar]
- 853.Krings M, Kanellopoulou D, Mavrilas D, Glasmacher B. In vitro pH-controlled calcification of biological heart valve prostheses. Materialwiss Werkstofftech. 2006;37:432–5. doi: 10.1002/mawe.200600010. [DOI] [Google Scholar]
- 854.Wang LJ, Guan XY, Yin HY, Moradian-Oldak J, Nancollas GH. Mimicking the self-organized microstructure of tooth enamel. J Phys Chem C Nanomater Interfaces. 2008;112:5892–9. doi: 10.1021/jp077105+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Howland J, Kramer B. Calcium and phosphorus in the serum in relation to rickets. Am J Dis Child. 1921;22:105–19. [Google Scholar]
- 856.Tisdall FF. The effects of ultra violet rays on the calcium and inorganic phosphate content of the blood serum of rachitic infants. Can Med Assoc J. 1922;12:536–8. [PMC free article] [PubMed] [Google Scholar]
- 857.Hanks JH, Wallace RE. Relation of oxygen and temperature in the preservation of tissues by refrigeration. Proc Soc Exp Biol Med. 1949;71:196–200. doi: 10.3181/00379727-71-17131. [DOI] [PubMed] [Google Scholar]
- 858.Shibata Y, Takashima H, Yamamoto H, Miyazaki T. Functionally gradient bonelike hydroxyapatite coating on a titanium metal substrate created by a discharging method in HBSS without organic molecules. Int J Oral Maxillofac Implants. 2004;19:177–83. [PubMed] [Google Scholar]
- 859.Marques PAAP, Serro AP, Saramago BJ, Fernandes AC, Magalhães MCF, Correia RN. Mineralisation of two phosphate ceramics in HBSS: role of albumin. Biomaterials. 2003;24:451–60. doi: 10.1016/S0142-9612(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 860.Gopikrishna V, Baweja PS, Venkateshbabu N, Thomas T, Kandaswamy D. Comparison of coconut water, propolis, HBSS, and milk on PDL cell survival. J Endod. 2008;34:587–9. doi: 10.1016/j.joen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 861.Hanawa T, Asami K, Asaoka K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J Biomed Mater Res. 1998;40:530–8. doi: 10.1002/(SICI)1097-4636(19980615)40:4<530::AID-JBM3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 862.Rocha LA, Anza E, Costa AM, Oliveira FJ, Silva RF. Electrochemical behavior of Ti/Al2O3 interfaces produced by diffusion bonding. Mater Res. 2003;6:439–44. doi: 10.1590/S1516-14392003000400002. [DOI] [Google Scholar]
- 863.Meuleman N, Tondreau T, Delforge A, Dejeneffe M, Massy M, Libertalis M, et al. Human marrow mesenchymal stem cell culture: serum-free medium allows better expansion than classical alpha-MEM medium. Eur J Haematol. 2006;76:309–16. doi: 10.1111/j.1600-0609.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 864.Okazaki Y, Gotoh E. Corrosion fatigue properties of metallic biomaterials in Eagle’s medium. Mater Trans. 2002;43:2949–55. doi: 10.2320/matertrans.43.2949. [DOI] [Google Scholar]
- 865.Coelho MJ, Cabral AT, Fernande MH. Human bone cell cultures in biocompatibility testing. Part I: osteoblastic differentiation of serially passaged human bone marrow cells cultured in alpha-MEM and in DMEM. Biomaterials. 2000;21:1087–94. doi: 10.1016/S0142-9612(99)00284-7. [DOI] [PubMed] [Google Scholar]
- 866.Mandel S, Tas AC. Brushite (CaHPO4·2H2O) to octacalcium phosphate (Ca8(HPO4)2(PO4)4·5H2O) transformation in DMEM solutions at 36.5°C. Mater Sci Eng C. 2010;30:245–54. doi: 10.1016/j.msec.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 867.Chen C, Lee IS, Zhang SM, Yang HC. Biomimetic apatite formation on calcium phosphate-coated titanium in Dulbecco’s phosphate-buffered saline solution containing CaCl(2) with and without fibronectin. Acta Biomater. 2010;6:2274–81. doi: 10.1016/j.actbio.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 868.Gao YB, Weng WJ, Deng XL, Cheng K, Liu XG, Du PY, et al. Surface morphology variations of porous nano-calcium phosphate/poly(L-lactic acid) composites in PBS. Key Eng Mater. 2006;309-11:569–72. doi: 10.4028/www.scientific.net/KEM.309-311.569. [DOI] [Google Scholar]
- 869.Lewis AC, Kilburn MR, Papageorgiou I, Allen GC, Case CP. Effect of synovial fluid, phosphate-buffered saline solution, and water on the dissolution and corrosion properties of CoCrMo alloys as used in orthopedic implants. J Biomed Mater Res A. 2005;73:456–67. doi: 10.1002/jbm.a.30368. [DOI] [PubMed] [Google Scholar]
- 870.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 871.Preetha A, Banerjee R. Comparison of artificial saliva substitutes. Trends Biomater Artif Organs. 2005;18:178–86. [Google Scholar]
- 872.Sato Y, Sato T, Niwa M, Aoki H. Precipitation of octacalcium phosphates on artificial enamel in artificial saliva. J Mater Sci Mater Med. 2006;17:1173–7. doi: 10.1007/s10856-006-0545-4. [DOI] [PubMed] [Google Scholar]
- 873.Grases F, Ramis M, Costa-Bauzá A. Effects of phytate and pyrophosphate on brushite and hydroxyapatite crystallization. Comparison with the action of other polyphosphates. Urol Res. 2000;28:136–40. doi: 10.1007/s002400050152. [DOI] [PubMed] [Google Scholar]
- 874.Jenness R, Koops J. Preparation and properties of a salt solution which simulates milk ultrafiltrate. Neth Milk Dairy J. 1962;16:153–64. [Google Scholar]
- 875.Spanos N, Patis A, Kanellopoulou D, Andritsos N, Koutsoukos PG. Precipitation of calcium phosphate from simulated milk ultrafiltrate solutions. Cryst Growth Des. 2007;7:25–9. doi: 10.1021/cg050361w. [DOI] [Google Scholar]
- 876.Gao R, van Halsema FED, Temminghoff EJM, van Leeuwen HP, van Valenberg HJF, Eisner MD, et al. Modelling ion composition in simulated milk ultrafiltrate (SMUF). I: Influence of calcium phosphate precipitation. Food Chem. 2010;122:700–9. doi: 10.1016/j.foodchem.2010.03.040. [DOI] [Google Scholar]
- 877.Gao R, van Halsema FED, Temminghoff EJM, van Leeuwen HP, van Valenberg HJF, Eisner MD, et al. Modelling ion composition in simulated milk ultrafiltrate (SMUF). II. Influence of pH, ionic strength and polyphosphates. Food Chem. 2010;122:710–5. doi: 10.1016/j.foodchem.2010.03.038. [DOI] [Google Scholar]
- 878.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–34. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 879.Lu X, Leng Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials. 2005;26:1097–108. doi: 10.1016/j.biomaterials.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 880.Tas AC. Synthesis of biomimetic Ca-hydroxyapatite powders at 37 ° C in synthetic body fluids. Biomaterials. 2000;21:1429–38. doi: 10.1016/S0142-9612(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 881.Landi E, Tampieri A, Celotti G, Langenati R, Sandri M, Sprio S. Nucleation of biomimetic apatite in synthetic body fluids: dense and porous scaffold development. Biomaterials. 2005;26:2835–45. doi: 10.1016/j.biomaterials.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 882.Jalota S, Bhaduri SB, Tas AC. Using a synthetic body fluid (SBF) solution of 27 mM HCO3- to make bone substitutes more osteointegrative. Mater Sci Eng C. 2008;28:129–40. doi: 10.1016/j.msec.2007.10.058. [DOI] [Google Scholar]
- 883.Kim HM, Miyazaki T, Kokubo T, Nakamura T. Revised simulated body fluid. In: Bioceramics 13, Giannini S, Moroni A, Eds. Trans Tech Publ.: Switzerland 2001; 192:47-50. [Google Scholar]
- 884.Oyane A, Kim HM, Furuya T, Kokubo T, Miyazaki T, Nakamura T. Preparation and assessment of revised simulated body fluids. J Biomed Mater Res A. 2003;65:188–95. doi: 10.1002/jbm.a.10482. [DOI] [PubMed] [Google Scholar]
- 885.Müller L, Müller FA. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006;2:181–9. doi: 10.1016/j.actbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 886.Hu K, Yang XJ, Cai YL, Cui ZD, Wei Q. Preparation of bone-like composite coating using a modified simulated body fluid with high Ca and P concentrations. Surf Coat Tech. 2006;201:1902–6. doi: 10.1016/j.surfcoat.2006.02.036. [DOI] [Google Scholar]
- 887.Wen Z, Wu C, Dai C, Yang F. Corrosion behaviors of Mg and its alloys with different Al contents in a modified simulated body fluid. J Alloy Comp. 2009;488:392–9. doi: 10.1016/j.jallcom.2009.08.147. [DOI] [Google Scholar]
- 888.Gemelli E, Resende CX, de Almeida Soares GD. Nucleation and growth of octacalcium phosphate on treated titanium by immersion in a simplified simulated body fluid. J Mater Sci Mater Med. 2010;21:2035–47. doi: 10.1007/s10856-010-4074-9. [DOI] [PubMed] [Google Scholar]
- 889.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–15. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 890.Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials. 2009;30:2175–9. doi: 10.1016/j.biomaterials.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 891.Kim HM. Ceramic bioactivity and related biomimetic strategy. Curr Opin Solid State Mater Sci. 2003;7:289–99. doi: 10.1016/j.cossms.2003.09.014. [DOI] [Google Scholar]
- 892.Marques PAAP, Magalhães MCF, Correia RN. Inorganic plasma with physiological CO2/HCO3- buffer. Biomaterials. 2003;24:1541–8. doi: 10.1016/S0142-9612(02)00539-2. [DOI] [PubMed] [Google Scholar]
- 893.Dorozhkina EI, Dorozhkin SV. Surface mineralisation of hydroxyapatite in modified simulated body fluid (mSBF) with higher amounts of hydrogencarbonate ions. Colloids Surf A Physicochem Eng Asp. 2002;210:41–8. doi: 10.1016/S0927-7757(02)00217-0. [DOI] [Google Scholar]
- 894.Marques PAAP, Cachinho SCP, Magalhães MCF, Correia RN, Fernandes MHV. Mineralization of bioceramics in simulated plasma with physiological CO2/HCO3- buffer and albumin. J Mater Chem. 2004;14:1861–6. doi: 10.1039/b403495c. [DOI] [Google Scholar]
- 895.Pasinli A, Yuksel M, Celik E, Sener S, Tas AC. A new approach in biomimetic synthesis of calcium phosphate coatings using lactic acid-Na lactate buffered body fluid solution. Acta Biomater. 2010;6:2282–8. doi: 10.1016/j.actbio.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 896.Sun T, Wang M. Electrochemical deposition of apatite/collagen composite coating on NiTi shape memory alloy and coating properties. Mater Res Soc Symp Proc 2010; 1239:141-6. [Google Scholar]
- 897.Dorozhkin SV, Dorozhkina EI. Crystallization from a milk-based revised simulated body fluid. Biomed Mater. 2007;2:87–92. doi: 10.1088/1748-6041/2/2/005. [DOI] [PubMed] [Google Scholar]
- 898.Miyaji F, Kim HM, Handa S, Kokubo T, Nakamura T. Bonelike apatite coating on organic polymers: novel nucleation process using sodium silicate solution. Biomaterials. 1999;20:913–9. doi: 10.1016/S0142-9612(98)00235-X. [DOI] [PubMed] [Google Scholar]
- 899.Kim HM, Kishimoto K, Miyaji F, Kokubo T, Yao T, Suetsugu Y, et al. Composition and structure of apatite formed on organic polymer in simulated body fluid with a high content of carbonate ion. J Mater Sci Mater Med. 2000;11:421–6. doi: 10.1023/A:1008935924847. [DOI] [PubMed] [Google Scholar]
- 900.Barrere F, van Blitterswijk CA, de Groot K, Layrolle P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBFx5 solution. Biomaterials. 2002;23:1921–30. doi: 10.1016/S0142-9612(01)00318-0. [DOI] [PubMed] [Google Scholar]
- 901.Barrere F, van BC, de GK, Layrolle P. Nucleation of biomimetic Ca-P coatings on ti6A14V from a SBF x 5 solution: influence of magnesium. Biomaterials. 2002;23:2211–20. doi: 10.1016/S0142-9612(01)00354-4. [DOI] [PubMed] [Google Scholar]
- 902.Tas AC, Bhaduri SB. Rapid coating of Ti6Al4V at room temperature with a calcium phosphate solution similar to 10x simulated body fluid. J Mater Res. 2004;19:2742–9. doi: 10.1557/JMR.2004.0349. [DOI] [Google Scholar]
- 903.Dorozhkina EI, Dorozhkin SV. Structure and properties of the precipitates formed from condensed solutions of the revised simulated body fluid. J Biomed Mater Res A. 2003;67:578–81. doi: 10.1002/jbm.a.10127. [DOI] [PubMed] [Google Scholar]
- 904.Sato K. Mechanism of hydroxyapatite mineralization in biological systems. J Ceram Soc Jpn. 2007;115:124–30. doi: 10.2109/jcersj.115.124. [DOI] [Google Scholar]
- 905.Pompe W, Lampenscherf S, Rößler S, Scharnweber D, Weis K, Worch H, et al. Functionally graded bioceramics. Mater Sci Forum. 1999;308-11:325–30. doi: 10.4028/www.scientific.net/MSF.308-311.325. [DOI] [Google Scholar]
- 906.Fan Y, Duan K, Wang R. A composite coating by electrolysis-induced collagen self-assembly and calcium phosphate mineralization. Biomaterials. 2005;26:1623–32. doi: 10.1016/j.biomaterials.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 907.Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001;22:1705–11. doi: 10.1016/S0142-9612(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 908.Yamauchi K, Goda T, Takeuchi N, Einaga H, Tanabe T. Preparation of collagen/calcium phosphate multilayer sheet using enzymatic mineralization. Biomaterials. 2004;25:5481–9. doi: 10.1016/j.biomaterials.2003.12.057. [DOI] [PubMed] [Google Scholar]
- 909.Zhang W, Huang ZL, Liao SS, Cui FZ. Nucleation sites of calcium phosphate crystals during collagen mineralization. J Am Ceram Soc. 2003;86:1052–4. doi: 10.1111/j.1151-2916.2003.tb03422.x. [DOI] [Google Scholar]
- 910.Tampieri A, Celotti G, Landi E, Sandri M, Roveri N, Falini G. Biologically inspired synthesis of bone-like composite: self-assembled collagen fibers/hydroxyapatite nanocrystals. J Biomed Mater Res A. 2003;67:618–25. doi: 10.1002/jbm.a.10039. [DOI] [PubMed] [Google Scholar]
- 911.Wang Y, Yang C, Chen X, Zhao N. Biomimetic formation of hydroxyapatite/collagen matrix composite. Adv Eng Mater. 2006;8:97–100. doi: 10.1002/adem.200500220. [DOI] [Google Scholar]
- 912.Lickorish D, Ramshaw JAM, Werkmeister JA, Glattauer V, Howlett CR. Collagen-hydroxyapatite composite prepared by biomimetic process. J Biomed Mater Res A. 2004;68:19–27. doi: 10.1002/jbm.a.20031. [DOI] [PubMed] [Google Scholar]
- 913.Yunoki S, Ikoma T, Monkawa A, Ohta K, Tanaka J. Preparation and characterization of hydroxyapatite/collagen nanocomposite gel. J Nanosci Nanotechnol. 2007;7:818–21. doi: 10.1166/jnn.2007.504. [DOI] [PubMed] [Google Scholar]
- 914.Nassif N, Gobeaux F, Seto J, Belamie E, Davidson P, Panine P, et al. Self-assembled collagen-apatite matrix with bone-like hierarchy. Chem Mater. 2010;22:3307–9. doi: 10.1021/cm903594n. [DOI] [Google Scholar]
- 915.Zhao F, Yin Y, Lu WW, Leong JC, Zhang W, Zhang J, et al. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23:3227–34. doi: 10.1016/S0142-9612(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 916.Kim HW, Kim HE, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials. 2005;26:5221–30. doi: 10.1016/j.biomaterials.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 917.Kim HW, Knowles JC, Kim HE. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: characterization and antibiotic drug release. J Biomed Mater Res B Appl Biomater. 2005;74:686–98. doi: 10.1002/jbm.b.30236. [DOI] [PubMed] [Google Scholar]
- 918.Chen Z, Li QL, Zen Q, Li G, Jiang H, Liu L, et al. Biomimetic mineralization and bioactivity of phosphorylated chitosan. Key Eng Mater. 2005;288-9:429–32. doi: 10.4028/www.scientific.net/KEM.288-289.429. [DOI] [Google Scholar]
- 919.Li QL, Chen Z, Ou G, Liu L, Jiang H, Zeng Q, et al. Biomimetic synthesis of apatite-polyelectrolyte complex (chitosan-phosphorylated chitosan) hydrogel as an osteoblast carrier. Key Eng Mater. 2005;288-9:75–8. doi: 10.4028/www.scientific.net/KEM.288-289.75. [DOI] [Google Scholar]
- 920.Stupp SI, Ciegler GW. Organoapatites: materials for artificial bone. I. Synthesis and microstructure. J Biomed Mater Res. 1992;26:169–83. doi: 10.1002/jbm.820260204. [DOI] [PubMed] [Google Scholar]
- 921.Stupp SI, Braun PV. Molecular manipulation of microstructures: biomaterials, ceramics, and semiconductors. Science. 1997;277:1242–8. doi: 10.1126/science.277.5330.1242. [DOI] [PubMed] [Google Scholar]
- 922.Stupp SI, Mejicano GC, Hanson JA. Organoapatites: materials for artificial bone. II. Hardening reactions and properties. J Biomed Mater Res. 1993;27:289–99. doi: 10.1002/jbm.820270303. [DOI] [PubMed] [Google Scholar]
- 923.Stupp SI, Hanson JA, Eurell JA, Ciegler GW, Johnson A. Organoapatites: materials for artificial bone. III. Biological testing. J Biomed Mater Res. 1993;27:301–11. doi: 10.1002/jbm.820270304. [DOI] [PubMed] [Google Scholar]
- 924.Liu Y, Layrolle P, de Bruijn J, van Blitterswijk CA, de Groot K. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J Biomed Mater Res. 2001;57:327–35. doi: 10.1002/1097-4636(20011205)57:3<327::AID-JBM1175>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 925.Wang J, Layrolle P, Stigter M, de Groot K. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: physicochemical characteristics and cell attachment. Biomaterials. 2004;25:583–92. doi: 10.1016/S0142-9612(03)00559-3. [DOI] [PubMed] [Google Scholar]
- 926.Zhang Q, Leng Y. Electrochemical activation of titanium for biomimetic coating of calcium phosphate. Biomaterials. 2005;26:3853–9. doi: 10.1016/j.biomaterials.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 927.Bigi A, Boanini E, Bracci B, Facchini A, Panzavolta S, Segatti F, et al. Nanocrystalline hydroxyapatite coatings on titanium: a new fast biomimetic method. Biomaterials. 2005;26:4085–9. doi: 10.1016/j.biomaterials.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 928.Kim HM, Himeno T, Kawashita M, Lee JH, Kokubo T, Nakamura T. Surface potential change in bioactive titanium metal during the process of apatite formation in simulated body fluid. J Biomed Mater Res A. 2003;67:1305–9. doi: 10.1002/jbm.a.20039. [DOI] [PubMed] [Google Scholar]
- 929.Allegrini S, Jr., Rumpel E, Kauschke E, Fanghänel J, König B., Jr. Hydroxyapatite grafting promotes new bone formation and osseointegration of smooth titanium implants. Ann Anat. 2006;188:143–51. doi: 10.1016/j.aanat.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 930.Arnould C, Delhalle J, Mekhalif Z. Multifunctional hybrid coating on titanium towards hydroxyapatite growth: electrodeposition of tantalum and its molecular functionalization with organophosphonic acids films. Electrochim Acta. 2008;53:5632–8. doi: 10.1016/j.electacta.2008.03.003. [DOI] [Google Scholar]
- 931.Iwatsubo T, Kusumocahyo SP, Kanamori T, Shinbo T. Mineralization of hydroxyapatite on a polymer substrate in a solution supersaturated by polyelectrolyte. J Appl Polym Sci. 2006;100:1465–70. doi: 10.1002/app.23496. [DOI] [Google Scholar]
- 932.Bodin A, Gustafsson L, Gatenholm P. Surface-engineered bacterial cellulose as template for crystallization of calcium phosphate. J Biomater Sci Polym Ed. 2006;17:435–47. doi: 10.1163/156856206776374106. [DOI] [PubMed] [Google Scholar]
- 933.Toworfe GK, Composto RJ, Shapiro IM, Ducheyne P. Nucleation and growth of calcium phosphate on amine-, carboxyl- and hydroxyl-silane self-assembled monolayers. Biomaterials. 2006;27:631–42. doi: 10.1016/j.biomaterials.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 934.Storrie H, Stupp SI. Cellular response to zinc-containing organoapatite: an in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials. 2005;26:5492–9. doi: 10.1016/j.biomaterials.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 935.Hench LL. Biomaterials: a forecast for the future. Biomaterials. 1998;19:1419–23. doi: 10.1016/S0142-9612(98)00133-1. [DOI] [PubMed] [Google Scholar]
- 936.Jones JR, Hench LL. Regeneration of trabecular bone using porous ceramics. Curr Opin Solid State Mater Sci. 2003;7:301–7. doi: 10.1016/j.cossms.2003.09.012. [DOI] [Google Scholar]
- 937.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 938.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 939.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 940.Drouet C, Largeot C, Raimbeaux G, Estournès C, Dechambre G, Combes C, et al. Bioceramics: spark plasma sintering (SPS) of calcium phosphates. Adv Sci Technol. 2006;49:45–50. doi: 10.4028/www.scientific.net/AST.49.45. [DOI] [Google Scholar]
- 941.Anderson JM. The future of biomedical materials. J Mater Sci Mater Med. 2006;17:1025–8. doi: 10.1007/s10856-006-0439-5. [DOI] [PubMed] [Google Scholar]
- 942.White AA, Best SM, Kinloch IA. Hydroxyapatite—carbon nanotube composites for biomedical applications: a review. Int J Appl Ceram Technol. 2007;4:1–13. doi: 10.1111/j.1744-7402.2007.02113.x. [DOI] [Google Scholar]
- 943.Kealley C, Elcombe M, van Riessen A, Ben-Nissan B. Development of carbon nanotube-reinforced hydroxyapatite bioceramics. Physica B. 2006;385-6:496–8. doi: 10.1016/j.physb.2006.05.254. [DOI] [Google Scholar]
- 944.Balani K, Anderson R, Laha T, Andara M, Tercero J, Crumpler E, et al. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials. 2007;28:618–24. doi: 10.1016/j.biomaterials.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 945.Dorozhkin SV. Green chemical synthesis of calcium phosphate bioceramics. J Appl Biomater Biomech. 2008;6:104–9. [PubMed] [Google Scholar]
- 946.Salinas AJ, Vallet-Regí M. Evolution of ceramics with medical applications. Z Anorg Allg Chem. 2007;633:1762–73. doi: 10.1002/zaac.200700278. [DOI] [Google Scholar]
- 947.Matsumoto T, Okazaki M, Nakahira A, Sasaki J, Egusa H, Sohmura T. Modification of apatite materials for bone tissue engineering and drug delivery carriers. Curr Med Chem. 2007;14:2726–33. doi: 10.2174/092986707782023208. [DOI] [PubMed] [Google Scholar]
- 948.Mizushima Y, Ikoma T, Tanaka J, Hoshi K, Ishihara T, Ogawa Y, et al. Injectable porous hydroxyapatite microparticles as a new carrier for protein and lipophilic drugs. J Control Release. 2006;110:260–5. doi: 10.1016/j.jconrel.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 949.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113:102–10. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 950.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements: competitive drug carriers for the musculoskeletal system? Biomaterials. 2006;27:2171–7. doi: 10.1016/j.biomaterials.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 951.Fan J, Lei J, Yu C, Tu B, Zhao D. Hard-templating synthesis of a novel rod-like nanoporous calcium phosphate bioceramics and their capacity as antibiotic carriers. Mater Chem Phys. 2007;103:489–93. doi: 10.1016/j.matchemphys.2007.02.069. [DOI] [Google Scholar]
- 952.Barrère F, Mahmood TA, de Groot K, van Blitterswijk CA. Advanced biomaterials for skeletal tissue regeneration: instructive and smart functions. Mater Sci Eng Rep. 2008;59:38–71. doi: 10.1016/j.mser.2007.12.001. [DOI] [Google Scholar]
- 953.Sudo A, Hasegawa M, Fukuda A, Uchida A. Treatment of infected hip arthroplasty with antibiotic-impregnated calcium hydroxyapatite. J Arthroplasty. 2008;23:145–50. doi: 10.1016/j.arth.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 954.Verron E, Khairoun I, Guicheux J, Bouler JM. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov Today. 2010;15:547–52. doi: 10.1016/j.drudis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 955.Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, Siebenrock KA, et al. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 956.Jiang PJ, Wynn-Jones G, Grover LM. A calcium phosphate cryogel for alkaline phosphatase encapsulation. J Mater Sci. 2010;45:5257–63. doi: 10.1007/s10853-010-4568-3. [DOI] [Google Scholar]
- 957.Williams DF. The Williams dictionary of biomaterials. Liverpool University Press: Liverpool UK 1999; 368. [Google Scholar]
- 958.Kohane DS, Langer R. Biocompatibility and drug delivery systems. Chem Sci. 2010;1:441–6. doi: 10.1039/c0sc00203h. [DOI] [Google Scholar]
- 959.Frondel C. Whitlockite: a new calcium phosphate, Ca3(PO4)2. Am Mineral. 1941;26:145–52. [Google Scholar]
- 960.Frondel C. Mineralogy of the calcium phosphates in insular phosphate rock. Am Mineral. 1943;28:215–32. [Google Scholar]
- 961.Li X, Ito A, Sogo Y, Wang X, LeGeros RZ. Solubility of Mg-containing beta-tricalcium phosphate at 25 ° C. Acta Biomater. 2009;5:508–17. doi: 10.1016/j.actbio.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962.Calvo C, Gopal R. The crystal structure of whitlockite from the Palermo Quarry. Am Mineral. 1975;60:120–33. [Google Scholar]
- 963.http://rruff.geo.arizona.edu/doclib/hom/whitlockite.pdf (accessed in October 2010).
- 964.Knowles JC, Callcut S, Georgiou G. Characterisation of the rheological properties and zeta potential of a range of hydroxyapatite powders. Biomaterials. 2000;21:1387–92. doi: 10.1016/S0142-9612(00)00032-6. [DOI] [PubMed] [Google Scholar]
- 965.Kumar R, Prakash KH, Cheang P, Khor KA. Temperature driven morphological changes of chemically precipitated hydroxyapatite nanoparticles. Langmuir. 2004;20:5196–200. doi: 10.1021/la049304f. [DOI] [PubMed] [Google Scholar]
- 966.Sung YM, Lee JC, Yang JW. Crystallization and sintering characteristics of chemically precipitated hydroxyapatite nanopowder. J Cryst Growth. 2004;262:467–72. doi: 10.1016/j.jcrysgro.2003.10.001. [DOI] [Google Scholar]
- 967.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–21. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 968.Reid JW, Hendry JA. Rapid, accurate phase quantification of multiphase calcium phosphate materials using Rietveld refinement. J Appl Cryst. 2006;39:536–43. doi: 10.1107/S0021889806020395. [DOI] [Google Scholar]
- 969.Rogers AF. Dahllite from Tonopah, Nevada; Voelckerite, a new basic calcium phosphate; comments on the chemical composition of apatite and phosphorite. Z Krystallogr Mineral. 1912;52:209–17. [Google Scholar]
- 970.Schaller WT. The probable identity of podolite with dahllite. Am J Sci. 1910;30:309–10. doi: 10.2475/ajs.s4-30.179.309. [DOI] [Google Scholar]
- 971.Schaller WT. On the likely identity of podolite and dahllite. Z Krystallogr Mineral. 1910;48:559–61. [Google Scholar]
- 972.Stanic V, Dimitrijevic S, Antic-Stankovic J, Mitric M, Jokic B, Plecaš IB, et al. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl Surf Sci. 2010;256:6083–9. doi: 10.1016/j.apsusc.2010.03.124. [DOI] [Google Scholar]
- 973.Li Y, Ho J, Ooi CP. Antibacterial efficacy and cytotoxicity studies of copper (II) and titanium (IV) substituted hydroxyapatite nanoparticles. Mater Sci Eng C. 2010;30:1137, 44. doi: 10.1016/j.apsusc.2010.03.124. [DOI] [Google Scholar]