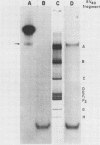
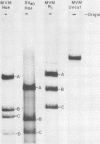
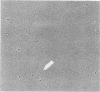
Abstract
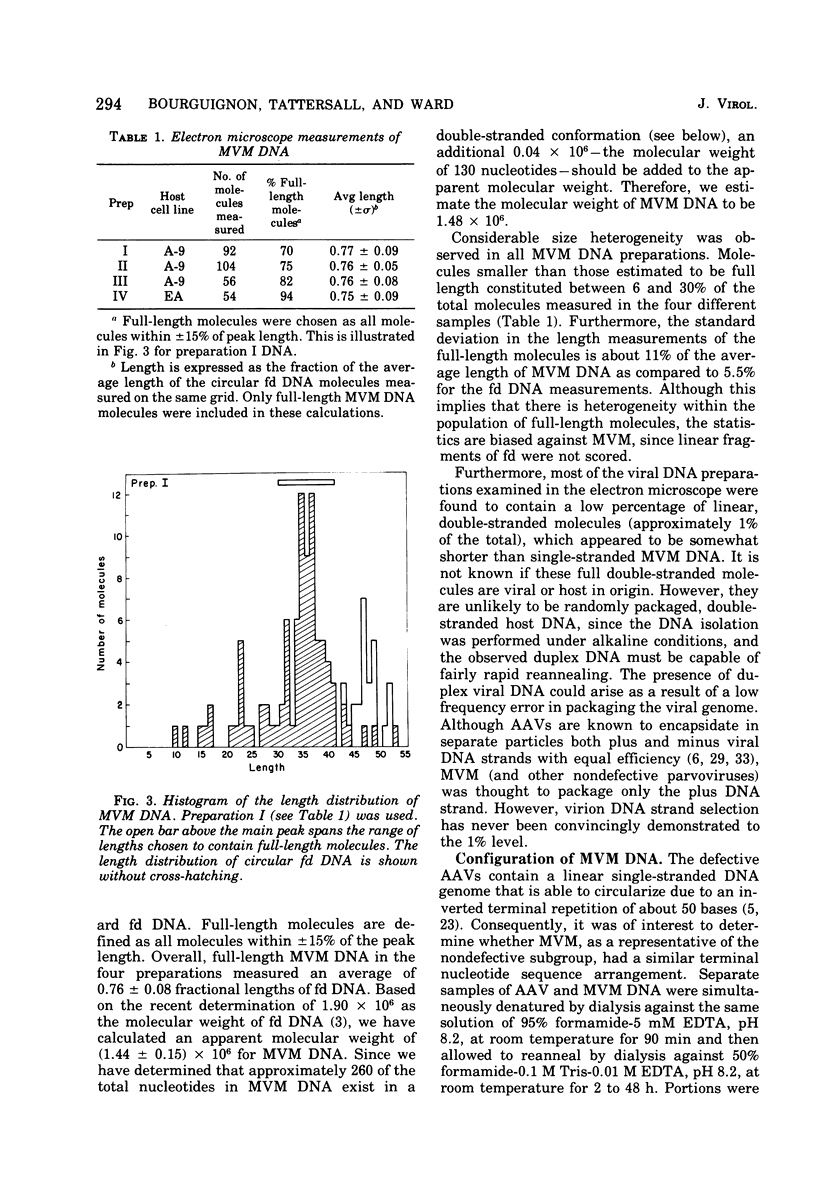
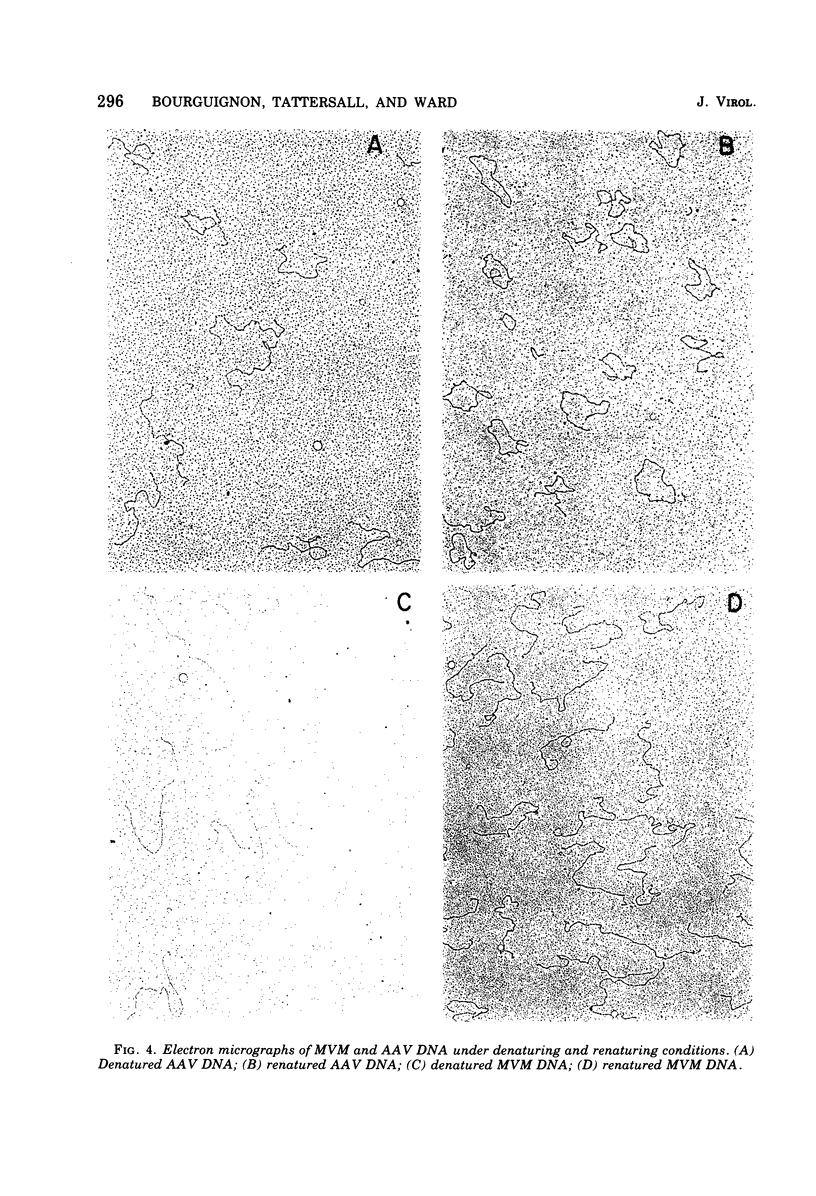
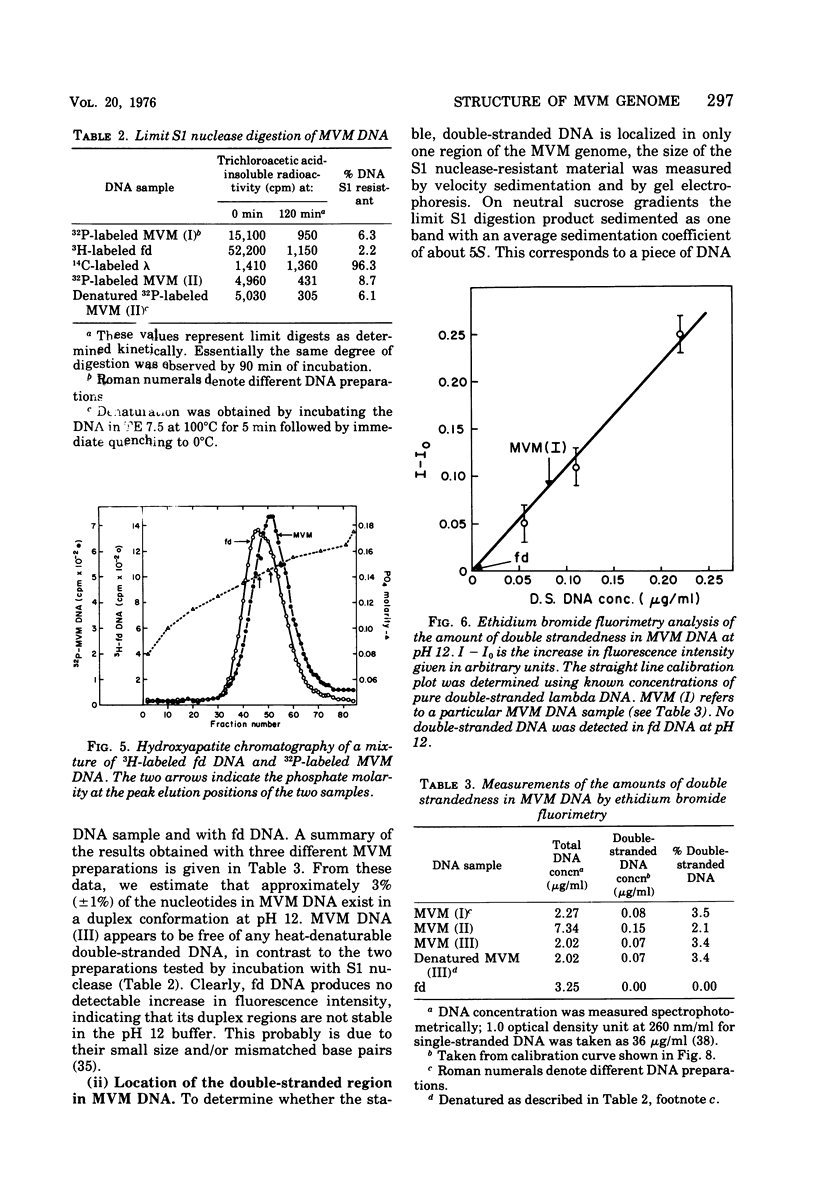
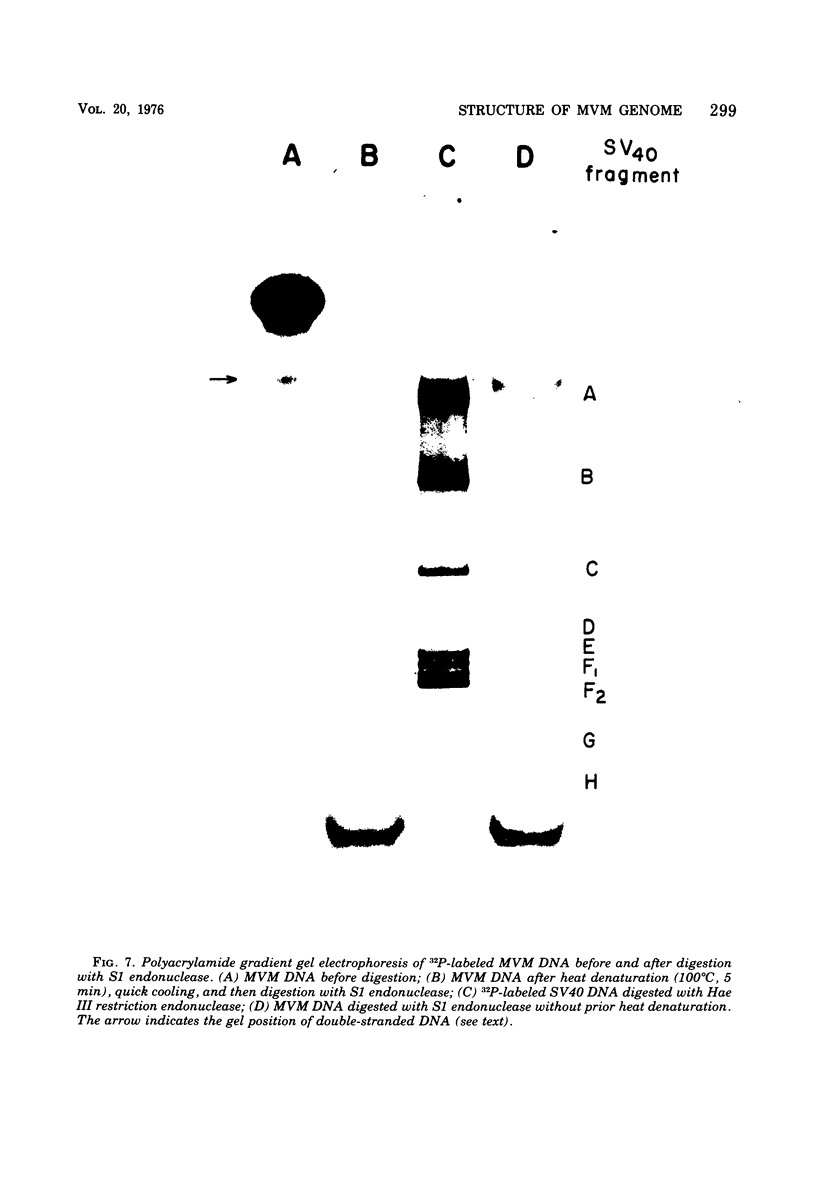
The genome of the nondefective parvovirus minute virus of mice (MVM) is a linear DNA molecular weight 1.48 x 10(6), which is single stranded for approximately 94% of its length. In contrast to the genomes from defective parvoviruses MVM DNA does not contain a detectable inverted terminal redundancy. A combination of enzymatic and physical techniques has shown that the molecule contains a stable hairpin duplex of approximately 130 base pairs located at the 5' terminus of the genome. MVM DNA is efficiently utilized as a template-primer by a number of DNA polymerases, including reverse transcriptases. Polymerases lacking 5' to 3' exonuclease activity yield a duplex DNA product with a molecular weight 1.96 times that of the viral genome, in which the newly synthesized complementary strand is covalently attached to the template. This duplex contains an internal "nick" that can be sealed by DNA ligase to produce a self-complementary single-strand circle. The MVM DNA duplex is cleaved twice by EcoR-RI restriction endonuclease to yield three distinct fragments in molar amounts. These results suggest that the initiation of DNA synthesis in vitro occurs at a point within 100 bases of the 3' end of the genome, using the 3' terminus of viral DNA as a primer, and that the sequence of nucleotides in the genome is not permuted.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Berkowitz S. A., Day L. A. Molecular weight of single-stranded fd bacteriophage DNA. High speed equilibrium sedimentation and light scattering measurements. Biochemistry. 1974 Nov 5;13(23):4825–4831. doi: 10.1021/bi00720a022. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Kelly T. J., Jr Letter: Visualization of the inverted terminal repetition in adeno-associated virus DNA. J Mol Biol. 1974 Jan 15;82(2):267–271. doi: 10.1016/0022-2836(74)90344-1. [DOI] [PubMed] [Google Scholar]
- Berns K. I. Molecular biology of the adeno-associated viruses. Curr Top Microbiol Immunol. 1974;65:1–20. doi: 10.1007/978-3-642-65875-4_1. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford R., Weinstein I. B. Transformation of a rat epithelial-like cell line by murine sarcoma virus. J Natl Cancer Inst. 1972 Aug;49(2):379–385. [PubMed] [Google Scholar]
- Carter B. J., Khoury G., Rose J. A. Adenovirus-associated virus multiplication. IX. Extent of transcription of the viral genome in vivo. J Virol. 1972 Dec;10(6):1118–1125. doi: 10.1128/jvi.10.6.1118-1125.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G. Specific cleavage of adenovirus-associated virus DNA by restriction endonuclease R-EcoRI--characterization of cleavage products. Virology. 1975 Feb;63(2):523–538. doi: 10.1016/0042-6822(75)90325-6. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Adenovirus-associated virus multiplication. 8. Analysis of in vivo transcription induced by complete or partial helper viruses. J Virol. 1972 Jul;10(1):9–16. doi: 10.1128/jvi.10.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Transcription in vivo of a defective parvovirus: sedimentation and electrophoretic analysis of RNA synthesized by adenovirus-associated virus and its helper adenovirus. Virology. 1974 Sep;61(1):182–199. doi: 10.1016/0042-6822(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Coulter M., Flintoff W., Paetkau V., Pulleyblank D., Morgan A. R. In vitro synthesis and detection of deoxyribonucleic acids with covalently linked complementary sequences. Biochemistry. 1974 Apr 9;13(8):1603–1609. doi: 10.1021/bi00705a008. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Follett E. A., Burdon M. G., McGeoch D. J. The DNA of a minute virus of mice. J Gen Virol. 1969 Jan;4(1):37–46. doi: 10.1099/0022-1317-4-1-37. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G. A method for separating DNA fragments by electrophoresis in polyacrylamide concentration gradient slab gels. Anal Biochem. 1974 Mar;58(1):195–207. doi: 10.1016/0003-2697(74)90458-8. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Lebowitz P., Siegel W., Sklar J. Hemophilus aegyptius restriction edonuclease cleavage map of the simian virus 40 genome and its colinear relation with the hemophilus influenzae cleavage map of SV40. J Mol Biol. 1974 Sep 5;88(1):105–123. doi: 10.1016/0022-2836(74)90297-6. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y., Richardson C. C. Strand displacement during deoxyribonucleic acid synthesis at single strand breaks. J Biol Chem. 1971 Apr 25;246(8):2692–2701. [PubMed] [Google Scholar]
- Mayor H. D., Torikai K., Melnick J. L., Mandel M. Plus and minus single-stranded DNA separately encapsidated in adeno-associated satellite virions. Science. 1969 Dec 5;166(3910):1280–1282. doi: 10.1126/science.166.3910.1280. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Crawford L. V., Follett E. A. The DNAs of three parvoviruses. J Gen Virol. 1970 Jan;6(1):33–40. doi: 10.1099/0022-1317-6-1-33. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., Kakefuda T. Linear, single-stranded deoxyribonucleic acid isolated from Kilham rat virus. J Virol. 1971 Jun;7(6):830–835. doi: 10.1128/jvi.7.6.830-835.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Uhlmann A., Geider K. A DNA fragment from the origin of single-strand to double-strand DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1976 Jan;73(1):49–53. doi: 10.1073/pnas.73.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Voss H., Gucker S. Structure of the DNA of bacteriophage fd. II. Isolation and characterization of a DNA fraction with double strand-like properties. J Mol Biol. 1969 Sep 28;44(3):445–458. doi: 10.1016/0022-2836(69)90372-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Pan J., Zain S., Weissman S. M. The mapping and ordering of fragments of SV40 DNA produced by restriction endonucleases. Nucleic Acids Res. 1974 Jun;1(6):727–752. doi: 10.1093/nar/1.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
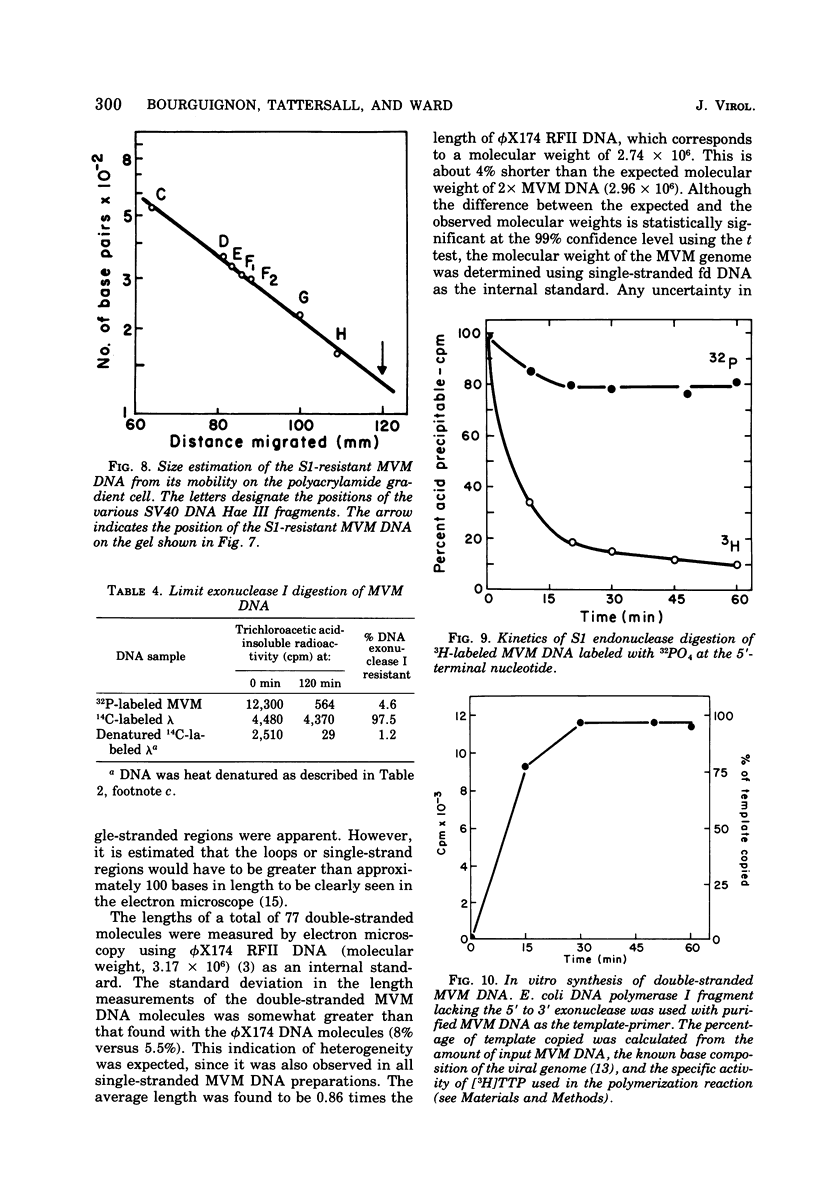
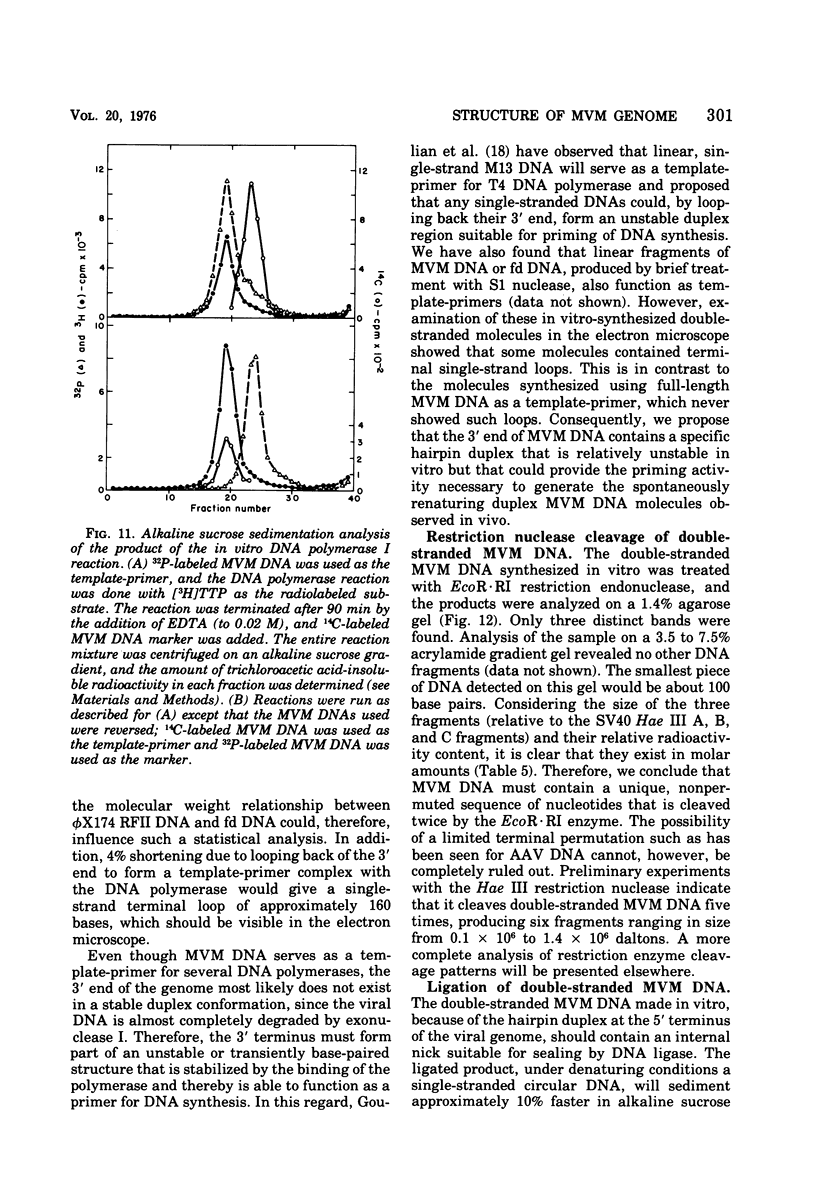
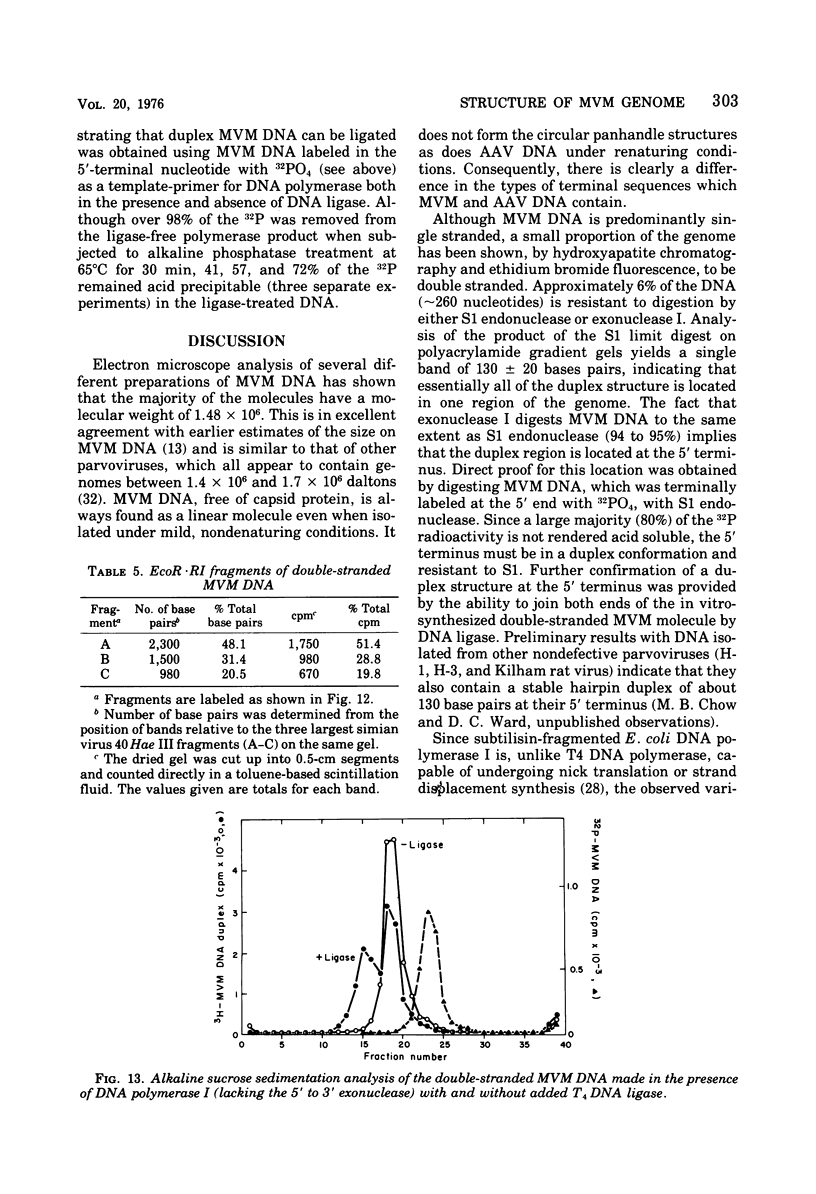
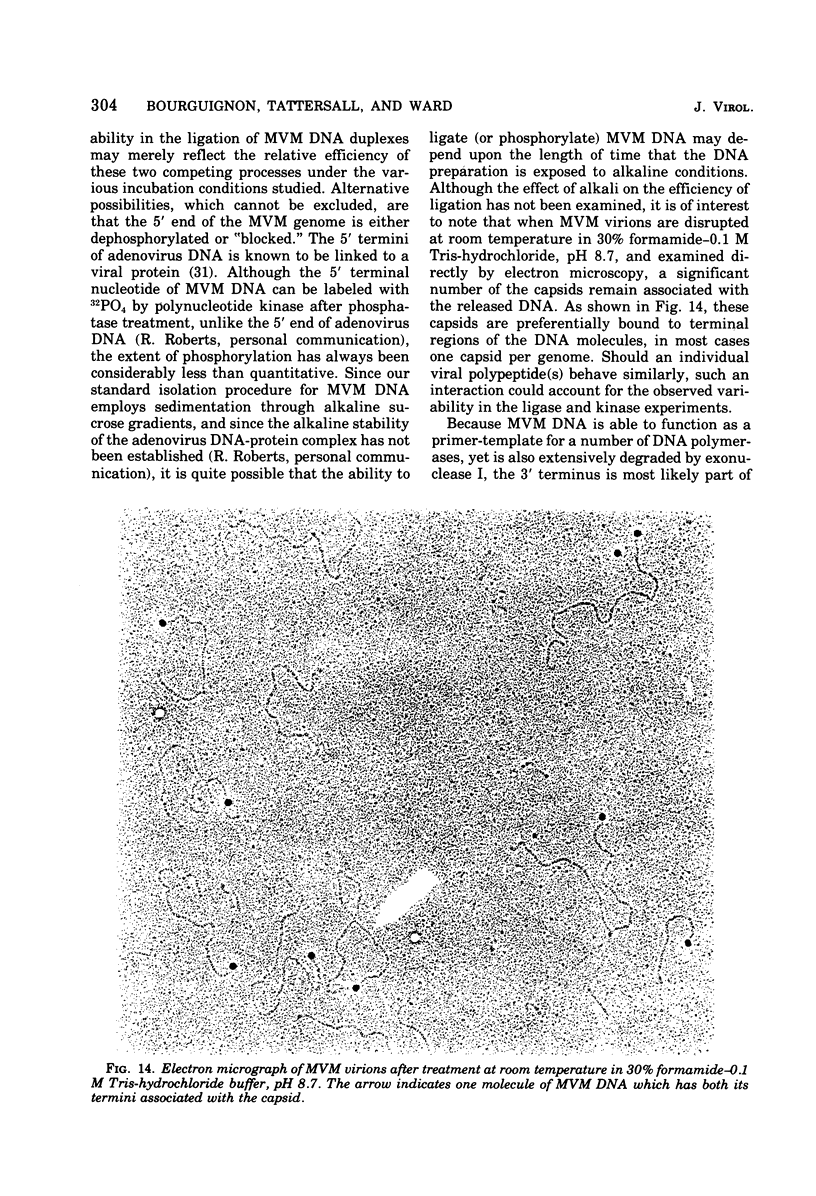
- Tattersall P., Crawford L. V., Shatkin A. J. Replication of the parvovirus MVM. II. Isolation and characterization of intermediates in the replication of the viral deoxyribonucleic acid. J Virol. 1973 Dec;12(6):1446–1456. doi: 10.1128/jvi.12.6.1446-1456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]
- Wiseman R. L., Dunker A. K., Marvin D. A. Filamentous bacterial viruses. 3. Physical and chemical characterization of the If1 virion. Virology. 1972 Apr;48(1):230–244. doi: 10.1016/0042-6822(72)90130-4. [DOI] [PubMed] [Google Scholar]
- Yguerabide J. Nanosecond fluorescence spectroscopy of macromolecules. Methods Enzymol. 1972;26:498–578. doi: 10.1016/s0076-6879(72)26026-8. [DOI] [PubMed] [Google Scholar]