Abstract
Ladybirds are a hot-spot for the invasion of male-killing bacteria. These maternally inherited endosymbionts cause the death of male host embryos, to the benefit of female sibling hosts and the bacteria that they contain. Previous studies have shown that high temperatures can eradicate male-killers from ladybirds, leaving the host free from infection. Here we report the discovery of two maternally inherited sex ratio distorters in populations of a coccinellid, Coccinella undecimpunctata, from a hot lowland region of the Middle East. DNA sequence analysis indicates that the male killing is the result of infection by Wolbachia, that the trait is tetracycline sensitive, and that two distinct strains of Wolbachia co-occur within one beetle population. We discuss the implications of these findings for theories of male-killing and suggest avenues for future field-work on this system.
Introduction
Male-killing bacteria are ultra-selfish maternally transmitted endosymbionts whose spread within host populations depends on the damage they do to these hosts [1]. Although some male-killers act late in development, here we focus on male-killers that act early in development and whose dynamics do not entail significant levels of horizontal transmission [2]. All known agents of early male-killing are bacterial, with these agents being taxonomically diverse (including Rickettsia [3], Flavobacteria [4], Spiroplasma, [5], [6], Wolbachia [7] and γ-Proteobacteria [8]. The diversity of the agents associated with male-killing sets the male-killing strategy apart from the other ultra-selfish manipulations of host reproduction, most of which are caused by Wolbachia [9].
Early male-killers have been found in a wide range of insect species, including Coleoptera, Lepidoptera, Diptera, Hemiptera and Hymenoptera [10]. Some groups, such as aphidophagous coccinellids, milkweed bugs and nymphalid butterflies, particularly of the genus Acraea, are especially prone to invasion [11].
Three characteristics of aphidophagous ladybirds make them prone to invasion by male-killers [12]. First, they lay eggs in tight clutches, predisposing them to strong interactions among sibling larvae [13]. Second, ladybirds are highly cannibalistic [14], with neonate larvae habitually consuming any unhatched eggs in their clutch, whether these are viable or not [13]. The potential for sibling egg cannibalism has imposed selection for embryos to develop and hatch rapidly [13]. As a result, neonate larvae are poorly resourced and show high mortality from starvation when they fail to find and subdue their first aphid prey [9]. In egg clutches laid by females infected with male-killing bacteria, male eggs fail to hatch and so are available to be eaten by infected female siblings, which thereby gain significant extra resources before they disperse to find aphid prey. They are, therefore, able to search for longer and subdue larger prey than are larvae from uninfected clutches [15], [16], [17]. Finally, the aphid prey of ladybirds is highly ephemeral due to rapid population increases and crashes [18]. Thus, ladybird larvae are often confronted with local resource scarcity, thus magnifying the benefit of sibling cannibalism.
Of the ladybirds possessing these traits – laying eggs in clutches, exhibiting sibling cannibalism, and feeding on aphids – about half of those surveyed (13 of 30) have been found to be infected with male-killers. Conversely, none of 12 surveyed species lacking one or more of these traits has been found to be infected with male-killing endosymbionts [19]. However, it should be noted that Weinert et al., 2007 [20], assaying 21 species of European coccinellids, did find inherited symbionts of three clades (Spiroplasma, Rickettsia and Wolbachia) from Chilocorus bipustulatus and Halyzia sedecimguttata, both of which lack one or more of the three characteristics. However, in neither species was the phenotype caused by the bacteria ascertained [19].
In addition to the behavioural and ecological prerequisites for invasion and persistence of male-killers, the environmental conditions must also be conducive. Specifically, high temperature may, by suppressing growth or killing the bacteria, prevent their spread within a host species [19]. For instance, Drosophila bifasciata can be cured of male-killing Wolbachia by culturing the flies at 26°C instead of 21°C [21]. Similarly, Drosophila equinoxialis could be cured of male-killing Spiroplasma by exposing eggs to relatively high temperatures (34–40°C) [22]. Using, artificially transferred Spiroplasma into Drosophila melanogaster, Sakaguchi and Poulson [23] showed that high temperature exposure produced a full cure from infection.
High temperature impacts on male-killers have been demonstrated in three species of coccinellids: Adalia bipunctata infected with Rickettsia [24], Adalia decempunctata infected with Rickettsia [25], and Coleomegilla maculata infected with Flavobacterium [26]. In these cases, egg hatch rates increased, either completely or partially, and sex ratios became less female biased, tending towards 1∶1, following high temperature treatment (25–30°C). Furthermore, molecular investigations confirmed the absence of the bacteria in temperature cured lines. In some cases, curing was partial, as in A. decempunctata, where Rickettsia was found in some male progeny, leading to the deduction that male death depends on the bacterial density in the host [25]. As a result of these experiments showing that high temperature can cure hosts of male-killer infections, it has been hypothesized that male-killers may be rare in hot climates [27].
In all aphidophagous coccinellid species previously tested, male-killing bacteria have been shown to be sensitive to high temperature [24], [25], [26]. Furthermore, all coccinellid populations found to date to be infected with male-killing bacteria have been from temperate or Mediterranean climates. This may result from research bias, or may be a consequence of the temperature sensitivity of male-killing bacteria, thus restricting the distribution of coccinellid-infecting male-killers to geographical regions lacking very high temperatures [27].
The mode by which coccinellids become infected with male-killing bacteria is not known. However, there is phylogenetic evidence to suggest that horizontal transmission of male-killing bacteria does occur, although probably very rarely [1]. This means that it is possible for one species of coccinellid to be invaded by two or more different types of male-killing bacteria. However, as the intracellular environment in which bacteria in a particular host species live and are vertically transmitted seems to be essentially the same, and as the bacteria employ the same strategy of ultra-selfish manipulation, the competitive exclusion principle would suggest that only a single male-killer should survive in a particular host population. Indeed, models of the invasion dynamics of early male-killers show that two male-killers cannot occupy the same population at equilibrium, unless there is some degree of male-killer suppression [28]. Randerson et al. [28] demonstrated that the ‘strongest’ male-killer: i.e. the most efficient in terms of high vertical transmission, low direct cost on infected females and high male-killing efficiency, would out-compete and exclude ‘weaker’ male-killers, unless the host can evolve resistance against the strong male-killer, which then allows the two to coexist in the same host population. Despite these theoretical findings, two or more male-killers have been recorded from some host populations without any indication that suppressor genes have evolved in the host. In the butterfly Acraea encedon, two strains of male-killing Wolbachia have been reported from Tanzanian populations [29]. In the coccinellid Adalia bipunctata, Majerus et al. [30] reported that four male-killers (a Rickettsia, a Spiroplasma and two distinct strains of Wolbachia) were found in a single sample collected from a single location in Moscow.
Here, we report the discovery of two male-killing strains of Wolbachia in a population of a coccinellid, Coccinella undecimpunctata, from hot regions of lowland Egypt and Jordan. This finding indicates that climate is less of a limiting factor for the distribution of male-killers in ladybirds.
Materials and Methods
Sample Collection and Culturing
200 individuals of adult Coccinella undecimpunctata L. were collected from Abo-Rawash, Giza, Egypt, in September 2004 and July 2005. An additional 50 individuals were collected in Amman, Jordan, by Professor T. F. Allawi, in October 2004. They were grouped in 10 individuals and housed in 9 cm stock Petri-dishes, and allowed to mate freely. Individual pairs were removed to clean dishes in which to lay eggs for the establishment of individual matrilines. Reproductive adults and larvae were allowed to feed on pea aphids (Acrythosiphon pisum), following methods described by Majerus and Kearns [31]. Eggs were harvested daily by removing adults to clean dishes, leaving the eggs in situ. Immature stages were reared in a controlled temperature room at 21°C and 16L: 8D. An artificial diet [32] was used to maintain non-reproductive adults. No specific permits were required for the described field studies. The samples location is not privately-owned or protected in any way. The field studies did not involve endangered or protected species.
Phenotypic Indicators of Male-killing
Two phenotypic indicators of male-killing were assessed for each matriline: the egg hatch rate and the sex ratio of progeny. Once neonate larvae had dispersed, the eggs within each clutch were categorized as hatched, unhatched and yellow (which is indicative of there having been no embryonic development), or unhatched and grey (indicative of some embryonic development) [24]. Progeny sex ratios (given as proportion male) were obtained for each family by determining the sex of all progeny, using sex-specific ventral abdominal sternite traits, visualized microscopically under CO2 anaesthesia. Families were split into four categories: 1. Sex ratio (SR) = low egg hatch rate (<0.5) and all progeny female. 2. Incomplete sex ratio (iSR) = low egg hatch rate (<0.5) and a statistically significant female bias amongst progeny. 3. Normal (N) = high egg hatch rate (>0.5) and progeny sex ratio not significantly different from 1∶1. 4. Not ascertained (?N) = high egg hatch rate (>0.5) and progeny sex ratio significantly different from a 1∶1 sex ratio.
In some instances, matrilines initially designed as SR or iSR subsequently showed increased hatch rates and sex ratio (proportion males), and these were described as revertant families [4]. In general, these lines were removed from the study stocks, unless otherwise stated. No obvious case of progressive sex ratio [33] was observed in the study stocks.
Inheritance of the SR Trait
Female offspring of SR and iSR lines were crossed with males from N families, to test the inheritance of the sex ratio trait. Pairs and their offspring were treated as before.
Susceptibility of the SR Trait to Antibiotics
The effect of tetracycline, a broad-spectrum antibiotic, on females showing the SR trait was investigated. If the causative agent of the sex distortion was a bacterium, tetracycline treatment should produce a partial [34] or full [24], [35] cure of the trait. Eggs were collected from three females from an SR line for up to three weeks. The egg hatch rates of these females were recorded. Once it had been confirmed from egg hatch rates that these females remained less than 50%, females were fed for two hours per day on a diet of golden syrup containing 10% w/v tetracycline, being otherwise fed on aphids [24]. The time of exposure to tetracycline was varied from 1–4 weeks for different females. Five females from normal (N), presumably uninfected lines were treated in the same way as controls. The egg hatch rates after treatment and the progeny sex ratios from the eggs laid before and after treatments were recorded. Subsequently, molecular assays on progeny from treated females were used to assess the presence or absence of bacteria. For two of the treated SR families, one female resulting from an egg laid after tetracycline treatment was mated to a male from an N line, and her progeny reared, with the sex ratio of progeny being recorded.
Identification of the Male-killing Agent
Genomic DNA was extracted from adult ladybirds, following a fast protocol modified by Majerus et al. [36] from Walsh et al. [37]. Successes of extractions were tested using general insect primers (COI gene) C1-J-1751f and C1-N-2191r [38], to check for the presence of ladybird DNA.
Modified versions of the standard PCR protocols for male-killers [1], [3], [36], [39] were used to assay two females from matriline B (SR) for bacteria. 16S rDNA general eubacterial primers 27f and 1495r [40], were used. Product bands were sliced from the agarose gel and purified using Qiaquick PCR purification kit (Qiagen), and directly sequenced using dye-labelled terminators in a cycle-sequencing reaction (Applied Biosystems). The products were then visualised on an ABI 377 automated sequencing machine. Initial identification of the sequence was established through a BLAST search [41].
Relation between the SR Trait and the Putative Causative Agent
Following identification of Wolbachia as a candidate male-killer, samples from all matrilines, irrespective of their sex ratio status, were assayed for Wolbachia using primers wsp81F and wsp691R [42] (Table 1). From the first Egyptian sample (2004) 39 SR or iSR females from three SR lines, 16 N or ?N females and five N males from three N lines were assayed. In addition, ten females produced by an SR line female before administration of tetracycline and eight females and two males produced by females from this SR line after tetracycline treatment were assayed. From the second Egyptian sample (2005) and the Jordanian sample, 28 SR or iSR line females, ten N females and five N line males were assayed. In addition, five females produced by SR line females before administration of tetracycline and six females and two males produced by SR line females after tetracycline treatment were assayed. DNA samples extracted from two previously known Wolbachia-infected Adalia bipunctata [30] were used as positive controls, while negative controls were prepared using the same PCR premix, except that sterile distilled water replaced the genomic DNA. PCR reactions were carried out in a Techne Progene PCR machine with a heated lid. All PCR cycling conditions were as described by Majerus [43]. PCR products were then run on a horizontal 1% agarose gel, beside an appropriate marker containing DNA fragments of known size (1 kb DNA ladder). Following electrophoresis, the gels were visualised and photographed using a Biogene UV trans-illuminator.
Table 1. The number of individuals molecularly assayed for Wolbachia using primers wsp81F and wsp691R.
| Sample | Linename | Linestatus | ♀ | ♂ | Total |
| Egyptian2004 | A | SR | 13 | 0 | 39 SR♀, 16 N♀, 5 ♂ |
| B | SR | 13 | 0 | ||
| F | iSR | 13 | 0 | ||
| D | N | 6 | 2 | ||
| E | N | 5 | 2 | ||
| G | N | 5 | 1 | ||
| Egyptian2005 | E1 | SR | 5 | 0 | 15 SR♀, 3N♀, 2 ♂ |
| E2 | SR | 5 | 0 | ||
| E3 | SR | 5 | 0 | ||
| E4 | N | 4 | 2 | ||
| Jordanian2005 | J2 | SR | 5 | 0 | 13 SR♀, 6N♀, 3 ♂ |
| J3 | iSR | 4 | 0 | ||
| J4 | SR | 4 | 0 | ||
| J1 | N | 3 | 2 | ||
| J5 | N | 3 | 1 |
Phylogenetic Analysis
DNA from SR lines was amplified with Wolbachia specific primers wsp81F and wsp691R [42] for the wsp gene. Products were sequenced as described above for the 16S rDNA gene and the resultant partial sequence was subjected to a BLAST search [41]. The sequences were then manually aligned with a selected group of wsp sequences (see Fig. 1 for details), including those known to cause male-killing (including the only known two male-killing Wolbachia strains in ladybirds, which are in Adalia bipunctata), at least one strain causing each of the other reproductive manipulations of hosts, and those showing closest homology to the sequence under investigation. Phylogenetic analysis was performed using Mega version 3.1 [44]. Phylogenetic trees were constructed using maximum parsimony and support was obtained from 500 bootstrap replicates; seed 64238 (program Mega 3.1). Gaps or missing data were dealt with by complete deletion.
Figure 1. Egg hatch rates and progeny sex ratios (± standard errors) of matrilines of C. undecimpunctata.
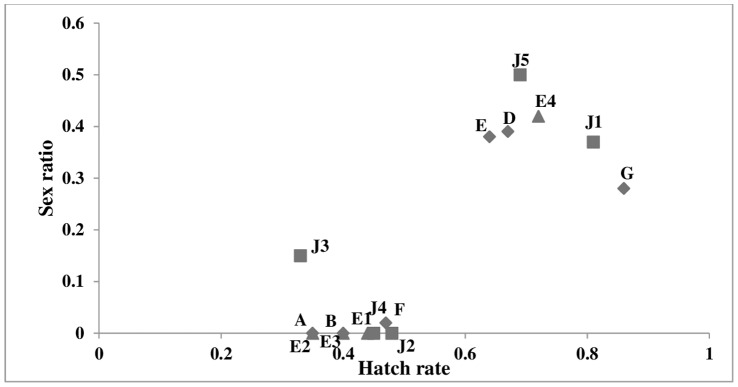
Hatch rate was measured by comparing the number of hatched eggs (H) to the total number of laid eggs (hatched+grey+yellow). The matrilines are labelled as follows: Egypt 2004 - A-G with diamond symbol; Egypt 2005 - E1–E4 with triangle symbol; and Jordan - J1–J5 with square symbol.
Statistical Analyses
Using StatXact 8 software, Fisher’s exact test was used to test the significance of deviation from 1∶1 sex ratios among family progeny. Bonferroni correction was used to correct for multiple comparisons among the different families. The association between the F1 and F2 sex ratios and the F1 sex ratio and hatch rate were evaluated using a simple linear regression model to report slope, associated error of estimate and P-value, as well as the correlation coefficient. The heterogeneity between lines was investigated using a Chi-square test to test the differences in proportions of sex ratio and hatch rates. Sex ratio changes subsequent to antibiotic treatments were also evaluated with a simple linear model which looked at information that we combined over different observations periods.
Results
Phenotypic Results
Sixteen matrilines were obtained from the Egyptian and the Jordanian collections, one of which failed to produce sufficient offspring for analysis. From these lines, nine lines were designated as SR lines, having low egg hatch rates and absence of male progeny (3 from Egyptian 2004 collection, 3 from the Egyptian 2005 collection, and 3 from the Jordanian collection) (Table 2). The rest of were designated as normal lines due to their normal sex ratio (Figure 1).
Table 2. Progenic sex ratios and sex ratio status of F1 females of C. undecimpunctata of 2004 Egyptian sample (A-G), 2005 Egyptian sample (E1–E4) and Jordanian sample (J1–J5) lines.
| Line | H | G | Y | Hatch rate | Progeny | Fisher’s exact test | Sex ratio status | ||
| ♀ | ♂ | Sex ratio | |||||||
| A | 42 | 6 | 72 | 0.35 | 20 | 0 | 0 | F = 14.4, d.f. = 1, p<0.001 | SR |
| B | 21 | 0 | 31 | 0.40 | 4 | 0 | 0 | F = 2.22, d.f. = 1, p = 0.214 | SR |
| D | 82 | 2 | 38 | 0.67 | 25 | 16 | 0.39 | F = 0.98, d.f. = 1, p = 0.374 | N |
| E | 90 | 4 | 47 | 0.64 | 34 | 21 | 0.38 | F = 1.533, d.f. = 1, p = 0.250 | N |
| F | 67 | 4 | 71 | 0.47 | 42 | 1 | 0.02 | F = 27.78, d.f. = 1, p<0.001 | iSR |
| G | 113 | 2 | 16 | 0.86 | 55 | 22 | 0.28 | F = 7.341, d.f. = 1, p<0.01 | N |
| E1 | 42 | 0 | 53 | 0.44 | 15 | 0 | 0 | F = 10.12, d.f. = 1, p<0.01 | SR |
| E2 | 63 | 2 | 115 | 0.35 | 22 | 0 | 0 | F = 16.03, d.f. = 1, p<0.001 | SR |
| E3 | 66 | 4 | 95 | 0.4 | 30 | 0 | 0 | F = 22.62, d.f. = 1, p<0.001 | SR |
| E4 | 94 | 7 | 30 | 0.72 | 36 | 27 | 0.42 | F = 0.786, d.f. = 1, p = 0.478 | N |
| J1 | 74 | 2 | 15 | 0.81 | 27 | 16 | 0.37 | F = 1.403, d.f. = 1, p = 0.278 | N |
| J2 | 21 | 0 | 23 | 0.48 | 3 | 0 | 0 | F = 1.645, d.f. = 1, p = 0.4 | SR |
| J3 | 52 | 2 | 104 | 0.33 | 16 | 3 | 0.15 | F = 4.8, d.f. = 1, p = 0.025 | iSR |
| J4 | 29 | 1 | 34 | 0.45 | 2 | 0 | 0 | F = 1.222, d.f. = 1, p = 0.5 | SR |
| J5 | 33 | 0 | 15 | 0.69 | 2 | 2 | 0.5 | F = 0.185, d.f. = 1, p = 0.514 | N |
Hatch rate was measured by comparing the number of hatched eggs (H) to the total number of laid eggs (hatched (H)+grey (G)+yellow (Y)). Sex ratio is given as the proportion of male offspring. Significance p-value using Bonferroni correction was 0.01.
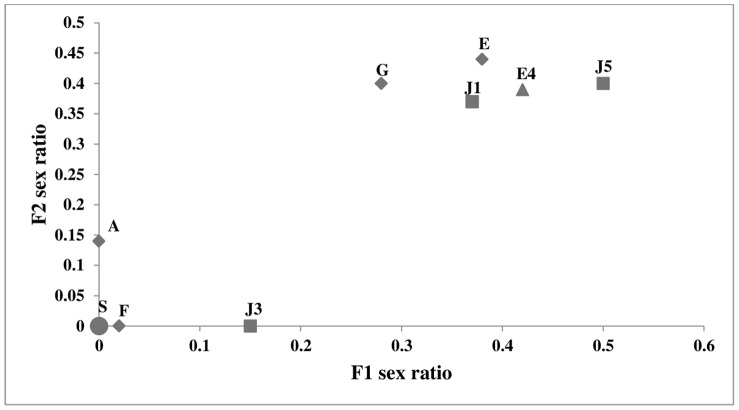
Lines that had produced all or almost all female progeny in the F1 also did so in the F2, and lines with normal F1 sex ratios remained normal in the F2(Figure 2). The strong correlation between the F1 and F2 sex ratios (r2 = 0.86, slope = 0.93±0.11; P<0.0001) demonstrates essentially perfect maternal inheritance of the male-killing effect (Figure 2).
Figure 2. C. undecimpunctata matrilines females & F1 females sex ratio (proportion of male offspring) with standard error.
Matrilines are labeled as in Figure 1. The letter “S” and the circle symbol represent all lines that have zero sex ratio in both F1 and F2 from the three collections (B, E1, E2, E3, J2 & J4).
The F1 progeny sex ratios of all matrilines showed significant heterogeneity (χ2 11 = 65, P<0.0001 )Furthermore, the progeny sex ratio was significantly correlated with egg hatch rate (r 2 = 0.68, slope = 0.91±0.11, P<0.001), such that clutches with hatch rates less than 50% were made up almost entirely of females (Fig. 1).
Effect of Tetracycline Treatment
The sex ratios of progeny produced before and after administration of tetracycline to females from an SR line are given in Table 3a. Families reared from F1 females produced by SR mothers after tetracycline treatment all produced high egg hatch rates and normal progeny sex ratios. The treatment resulted in an initial decrease in egg hatch rates, with hatch rates close to zero for two to five days (characteristic of ladybirds when treated with tetracycline) [45], followed by an increase in egg hatch rates to greater than 0.5. The sex ratio before antibiotic treatment was significantly different from that after antibiotic treatment in females 2 (p<0.05), 3 (p<0.01) and 4 (p<0.05), but not in females 1 (p = 0.27) or 5 (p = 0.91).
Table 3. Effect of tetracycline treatment on Egyptian lines (sex ratio is proportion of males to all offspring).
| Female | Period of treatment | Pre-treatmentegg hatch rate | Pre-treatmentprogeny | Sex ratio | Post-treatment egg hatch rate | Post-treatment progeny | Sex ratio | Sexes in F3 | Sex ratio | |
| a | 1 | 1 week | 0.36 | 8♀, 0♂ | 0 | 0.59 | 2♀, 1♂ | 0.33 | ||
| 2 | 3 weeks | 0.34 | 18♀, 0♂ | 0 | 0.64 | 29♀, 20♂ | 0.41 | 24♀, 26♂ | 0.5 | |
| 3 | 4 weeks | 0.32 | 10♀, 0♂ | 0 | 0.72 | 9♀, 10♂ | 0.52 | 6♀, 6♂ | 0.5 | |
| b | 4 | 3 weeks | 0.40 | 9♀, 0♂ | 0 | 0.62 | 21♀, 14♂ | 0.4 | 9♀, 8♂ | 0.47 |
| 5 | 4 weeks | 0.35 | 8♀, 0♂ | 0 | 0.70 | 2♀, 2♂ | 0.5 | 2♀, 2♂ | 0.5 | |
| c | N1 | 4 weeks | 0.61 | 8♀, 5♂ | 0.8 | 0.60 | 2♀, 2♂ | 0.5 | ||
| N2 | 4 weeks | 0.77 | 5♀, 3♂ | 0.37 | 0.67 | 4♀, 3♂ | 0.43 | |||
| N3 | 4 weeks | 0.67 | 8♀, 4♂ | 0.33 | 0.70 | 5♀, 4♂ | 0.44 | |||
| N4 | 4 weeks | 0.80 | 7♀, 4♂ | 0.36 | 0.74 | 5♀, 3♂ | 0.37 | |||
| N5 | 4 weeks | 0.72 | 4♀, 3♂ | 0.42 | 0.69 | 6♀, 4♂ | 0.4 |
a) Results from the 2004 Egyptian F1 females from SR lines. b) Results from two 2005 F1 females from line 3 (bearing a second male-killing strain). c) Results from 5 F1 females from the 2004 Egyptian N lines. Sexes in F3 refer to the progeny of one F2, post-treatment female mated to an unrelated male.
The sex ratio of progeny from SR females produced after tetracycline treatment increased rapidly, and converged upon 1∶1. Females from N lines fed on tetracycline showed no significant alteration of egg hatch rate or progeny sex ratio (Table 3b).
Relation between Wolbachia Presence and the SR Trait
All SR females that had not been treated with tetracycline were found to be positive for the presence of Wolbachia. None of the females from N lines, or progeny of females from SR lines after tetracycline treatment, males from either these lines or N lines, or either of the negative controls were found to have Wolbachia present.
Moreover, while females produced by SR females before being treated with antibiotic tested positive for Wolbachia, none of these females and two males produced by these same SR females after tetracycline treatment did. Both negative control samples also tested negative.
Phylogenetic Analysis
The analysis of the 16S rDNA gene from females from the Egyptian 2004 SR matrilines showed that the male-killing causative agent may be a B-type Wolbachia (Accession number DQ993358). Phylogenetic analysis of this wsp sequence (Accession number EF502046) (Fig. 3) shows closest affinity to the two male-killing Wolbachia sequences from A. bipunctata reported by Hurst et al. [7]. This conclusion should be viewed with some caution because the wsp can undergo recombination between different Wolbachia strains, thus affecting phylogenetic patterns.
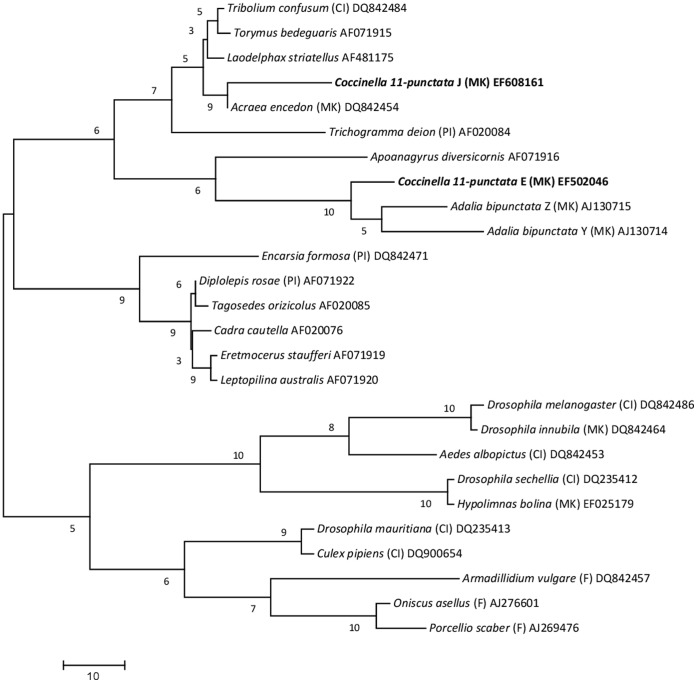
Figure 3. Phylogenetic tree of wsp DNA sequence data of Wolbachia hosted by different species.
Maximum parsimony-based bootstrap analysis of different hosts of Wolbachia including the two Wolbachia strains of the C. undecimpunctata. Bootstrap values are indicated above the branches. Wolbachia strains are represented by the names of their host species, their phenotype where known (CI = cytoplasmic incompatibility; PI = parthenogenesis inducing; F = feminizing; MK = male-killing) and Genebank accession numbers. Suffix letters for A. bipunctata refer to the two different Wolbachia sequences, lodged by Hurst et al. (1999b). The wsp sequences found in this study are the Coccinella 11-punctata E (MK) EF502046 and Coccinella 11-punctata J (MK) EF608161 (in bold). Wolbachia wsp sequences used in constructing this tree were obtained from the Entrez Nucleotide database.
Analysis of the wsp gene revealed that our samples included two distinct strains of Wolbachia strains in our samples (Table 1). One strain (wsp Accession number EF502046) was found in three lines from Egypt in 2004 (A, B and F) and one line from Egypt in 2005 (line 1). A second Wolbachia strain (wsp Accession number EF608161) was found in two lines from Egypt in 2005 (lines 2 & 3) and three lines from Jordan (Lines 2, 3 &4). The sequence of this second strain differed from that of the first strain by 20.97% (of 515 bases) (Figure 3). The wsp sequence from the second strain is most similar to that of the U strain of Acraea encedon, with divergence of 15.13% (of 522 bp), including a central identical region of 387 bp.
On the basis of the limited data in this study, the prevalence of the first Wolbachia strain, which was only recorded from the Egyptian samples (sample size = 50), is 0.4 (3 of 6 in 2004 and 1 of 4 in 2005). The mean fidelity (±se) of vertical transmission (calculated as: 1 - (number of males/number of females) [15] is estimated as 0.987±0.012. The second Wolbachia strain, which was recorded from both Egypt and Jordan, had an overall prevalence of 0.33 (combining 2 of 10 in Egypt and 3 of 5 in Jordan). For this second strain, the vertical transmission is estimated as >0.99 from Egypt and 0.85 from Jordan, giving a mean vertical transmission for this strain of 0.94±0.109. Overall, 60% (9 of 15) of the lines were infected with one of these male-killing Wolbachia strains.
Discussion
The results show that C. undecimpunctata is a host to at least 2 different strains of male-killing B-type Wolbachia. The association between the Wolbachia infection and the SR trait was perfect. The Wolbachia was detected in females from all strains with significantly female-biased sex ratios and low hatch rates (SR and iSR), but from none of the strains with normal sex rations (N and ?N). One line (G) from the 2004 Egyptian sample had a significantly female biased sex ration among the F1, but had a high egg hatch rate (0.86). However, the F2 exhibited both a high hatch rate and normal sex ratio, and this line was found to be negative for Wolbachia infection. Thus, the F1 sex ratio bias may have been due to chance or to a factor other than a male killing endosymbiont (e.g., meiotic drive).
Both male-killing strains of Wolbachia identified here are antibiotic sensitive, as antibiotic treatment increased hatch rates of eggs laid by treated females, and restored a 1∶1 progeny sex ratio, and resulted in the production of offspring uninfected with Wolbachia. Moreover, the offspring of tetracycline-treated females produced normal progeny sex ratios.
Although the two Wolbachia strains found in C. undecimpunctata had identical 16S sequences, their wsp sequences were 20% divergent. Using the rapidly evolving wsp gene to infer relationships to other closely related strains, we find that one of the two strains in C. undecimpunctata is closely related to the two different but closely related male-killing Wolbachia strains that occur in the ladybird A. bipunctata [7]. Such a pattern could result from each beetle species having been invaded by closely related strains of Wolbachia that subsequently evolved male-killing within their new ladybird hosts. Alternatively, which is more likely, a male-killing strain may have jumped from one ladybird species to another via lateral transfer.
The other Wolbachia strain found in C. undecimpunctata is most similar to the male-killing Wolbachia in A. encedon [29]. This result supports previous hypotheses that horizontal transmission of male-killers may occur occasionally between hosts from different genera or even orders [7], [46], [47], [48]. Dyson et al. [48] suggested that some Wolbachia strains might specialise on particular host sex determination systems. The apparently close relationship between the second C. undecimpunctata strain and the U strain of A. encedon is thus striking, since most ladybirds, including Coccinella, have an XY sex determination system [13], while butterflies have a ZW sex determination system. If the same Wolbachia strain can cause male-killing in XY male beetles and ZZ male butterflies, this would suggest that some male-killing Wolbachia are generalists when it comes to sex determination systems. This possibility could be tested by transferring Wolbachia between C. undecimpunctata and A. encedon.
This is the first report of a male-killing endosymbiont in any coccinellid species from a hot climate. The daily high temperatures in Amman, Jordan and Giza, Egypt average ∼32°C and ∼34°C, respectively, during the hottest summer months, well above the temperatures shown to kill or debilitate male-killing endosymbionts from more temperate regions [24], [26], [49]. Thus, high temperature is not an insurmountable barrier to infection of coccinellids by male-killing bacteria. Further work on aphidophagous coccinellids from hot climates is likely to extend the number of cases of male-killing infection in this family of beetles. In some coccinellids, such as Harmonia axyridis infected with Spiroplasma, the temperature required to kill male-killing infections is close to the critical temperature at which coccinellids can survive, making cure of the SR trait by temperature difficult [43]. It would be interesting to determine whether the Wolbachia that infects Egyptian C. undecimpunctata can be cured by heat treatment without adversely affecting the beetles. Whether or not this is the case, the Wolbachia strains found in C. undecimpunctata from Egypt and Jordan provide an opportunity to explore the evolution of high-temperature tolerance in Wolbachia. The present findings also indicate that the ecology and evolution of ladybird beetles from hot climates may be as likely to be affected by male-killing endosymbionts as those from more temperate regions.
In previous assays of C. undecimpunctata samples from Britain (n = 47), male-killers have not been found [19]. This may be because of low prevalence and incomplete ascertainment, or it may be because male-killers are absent from British populations of this species. If the latter is the case, this might be due to lack of invasion opportunity, or the result of inherent resistance, making British C. undecimpunctata an unsuitable host for male-killers. Microinjection of one of the male-killing Wolbachia that occurs in C. undecimpunctata in the Middle East, into British individuals could be used to test this latter possibility.
This is the first confirmed case of a heritable male-killing bacterium having been reported from the genus Coccinella. This is noteworthy because the majority of species in this genus are aphidophagous and have the ecological characteristics that should make them liable to infection by such bacteria. Despite this, in previous assays of 19 collections from seven species of Coccinella from temperate or Mediterranean climates (including two previous samples of C. undecimpunctata from England) (M. Majerus, unpubl. data), no male-killers have been detected. The results herein suggest that either further testing of members of this genus would be worthwhile or might reveal that Wolbachia in this genus may not only tolerate, but actually require high temperature for expression and transmission.
The results to date do not allow resolution of whether C. undecimpunctata was invaded by a single strain of Wolbachia that has subsequently diverged, or was invaded by two already different strains of Wolbachia. This question might be resolved by analysis of mtDNA variability associated with hosts of each Wolbachia strain. This is the third finding of more than one male-killing bacterial strain occurring sympatrically in the same host. This instance is similar to that in A. encedon in Tanzania, where two different strains of Wolbachia coexist [29]. The similarity to A. bipunctata, is less clear, for here, in addition to two strains of Wolbachia coexisting, two other phylogenetically disparate male-killers occur [30].
Randerson et al. [28] have tried in their model to explain the observed co-existence of multiple male-killing strains in the same host populations. They proposed the evolution and spread of a costly resistance gene in the host that should weaken the resident (the stronger) male-killer, although this may mean that both the resistance gene and the male-killer may be lost form the population. Then the weaker male-killer may spread at the expenses of the stronger male-killer, since it is tolerant to the effect of the evolved host resistance gene. As a result, the frequency of the stronger male-killer will decrease and in turn the frequency for the resistance gene will decrease as well. Thereafter, the frequency of the stronger male-killer may increase again, in a cyclical manner in response to the resistance gene. Such frequency dependent selection should allow stable coexistence of multiple male-killers in the same host population. However, the cause of male-killers coexistence in a population seems unconfirmed, especially no resistance genes have been reported in C. undecimpunctata.
Three scenarios seem possible. First, the cases of multiple male-killer existences in a host population may be the result of independent allopatric invasions by different strains of male-killer, followed by migration such that the different male-killers become sympatric. While this is tenable for the two coccinellid cases, for coccinellids can be highly dispersive [13], it seems to be less tenable for A. encedon, which is a highly colonial species showing low female dispersal. Moreover, in A. bipunctata, Tinsley [50] has shown that prevalence of two of the male-killers in this species (Rickettsia and Spiroplasma) show correlations with environmental factors in Scandinavia, suggesting long-term persistence.
Second, the Randerson et al. [28] model may be essentially correct, but the approach to male-killer prevalence equilibrium frequencies may be extremely slow, such that in those host populations that harbour two or more male-killers, equilibrium frequencies have yet to be reached, and the competitive advantage of the ‘strongest’ male-killer has yet to be manifest in the decline and elimination of ‘weaker’ male-killers. While this may be the case, if it is, it makes the prediction of competitive exclusion in the case of male-killers of little value in the field.
Finally, it is possible that biotic and abiotic factors affect the level of the three principle parameters that control male-killer invasion and prevalence (vertical transmission efficiency, direct effect of infection on females, fitness compensation) in coccinellids (1) in such a way that there is no consistently ‘strongest’ male-killer. Here, if the relative fitnesses of different male-killers oscillate such that the ‘strongest’ is first one strain and then the other, coexistence may be maintained for very long periods of time, particularly if there was a mechanism by which male-killer fitness was inversely related to prevalence.
Acknowledgments
We are grateful to Ian Wright who provided technical assistance, in particular by maintaining aphid supplies and to S.S.M. Hassan, R.L. Ware and L.J. Michie who helped to care for cultures. Special thanks for Prof. John Jaenike for his great help and advice through this manuscript.
Funding Statement
BP Egypt and Cambridge Overseas Trusts funded Sherif Elnagdy project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hurst GDD, Hurst LD, Majerus MEN (1997) Cytoplasmic sex-ratio distorters. In Influential Passengers (eds. O'Neill S.L., Hoffmann, A.A. and Werren, J.H.). Pp. 125–54, Oxford University Press: Oxford.
- 2. Hurst LD (1991) The incidences and evolution of cytoplasmic male killers. Proc. R. Soc. Lond. Ser. B 244: 91–99. [Google Scholar]
- 3. Werren JH, Hurst GDD, Zhang W, Breeuwer JAJ, Stouthamer R, et al. (1994) Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J. Bacteriol. 176: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hurst GDD, Hammarton TM, Bandi C, Majerus TMO, Bertrand D, et al. (1997) The diversity of inherited parasites of insects: the male-killing agent of the ladybird beetle Coleomegilla maculata is a member of the Flavobacteria. Genet. Res. 70: 1–6. [Google Scholar]
- 5. Hurst GDD, Schulenburg JHGvd, Majerus TMO, Bertrand D, Zakharov IA, et al. (1999) Invasion of one insect species, Adalia bipunctata by two different male-killing bacteria. Insect Mol. Biol. 8: 133–139. [DOI] [PubMed] [Google Scholar]
- 6. Tinsley MC, Majerus MEN (2006) A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology 132: 757–765. [DOI] [PubMed] [Google Scholar]
- 7. Hurst GDD, Jiggins FM, Schulenberg JHGvd, Bertran D, West SA, et al. (1999) Male-killing Wolbachia in two species of insect. Proc. R. Soc. Lond. Ser. B 266: 735–740. [Google Scholar]
- 8.Majerus MEN (1999) Simbiontes hereditarios causantes de efectos deletéreos en los artrópodos/Deleterious endosymbionts of Arthropods. In The Evolution and Ecology of Arthropods (eds. Melic, A., De Haro, J.J., Méndez, M. and Ribera, I.). 777–806. (In Spanish and English.) Sociedad Entomologica Aragonera: Zaragosa, Spain.
- 9. Majerus MEN, Majerus TMO (2012) Male-killing in the Coccinellidae: testing the predictions. Evol. Ecol. 26: 207–225. [Google Scholar]
- 10. Hurst GDD, Majerus MEN (1993) Why do maternally inherited micro-organisms kill males? Heredity 71: 81–95. [Google Scholar]
- 11. Majerus TMO, Majerus MEN (2010) Discovery and identification of a male-killing agent in the Japanese ladybird Propylea japonica (Coleoptera: Coccinellidae). BMC Evol. Biol. 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majerus MEN, Hurst GDD (1997) Ladybirds as a model system for the study of male-killing symbionts. Entomophaga 42: 13–20. [Google Scholar]
- 13.Majerus MEN (1994) Ladybirds. No. 81, New Naturalist Series. HarperCollins: London.
- 14. Majerus MEN, Majerus TMO (1997) Cannibalism among ladybirds. Bull. Amat. Entomol. Soc. 56: 235–248. [Google Scholar]
- 15. Hurst GDD, Majerus MEN, Walker LE (1993) The importance of cytoplasmic male killing elements in natural populations of the two spot ladybird, Adalia bipunctata (Linnaeus) (Coleoptera: Coccinellidae). Biol. J. Linn. Soc. 49: 195–202. [Google Scholar]
- 16. Hurst GDD, Purvis EL, Sloggett JJ, Majerus MEN (1994) The effect of infection with male-killing Rickettsia on the demography of female Adalia bipunctata L. (two spot ladybird). Heredity 73: 309–316. [Google Scholar]
- 17. Elnagdy SE, Majerus MEN, Lowson Handley L-J (2011) The value of an egg: resource reallocation in ladybirds (Coleoptera: Coccinellidae) infected with male-killing bacteria. J. Evolution. Biol. 24 (10): 2164–2172. [DOI] [PubMed] [Google Scholar]
- 18.Dixon AGF (2000) Insect Predator-Prey Dynamics: Ladybird Beetles and Biological Control. Cambridge University Press: Cambridge.
- 19.Elnagdy SE (2008) Comparative analysis of bacterial male-killing in coccinellidae from different climatic regions. Unpublished PhD thesis: University of Cambridge, Cambridge.
- 20. Weinert LA, Tinsley MC, Temperley M, Jiggins FM (2007) Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biology Letters 3: 678–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst, GDD Johnson AP, Schulenberg JH and Fuyama Y (2000) Male-killing Wolbachia in Drosophila: a temperature sensitive trait with a threshold bacterial density. Genetics, 165, 699–709. [DOI] [PMC free article] [PubMed]
- 22.Malogolowkin C (1959) Temperature effects on maternally inherited “sex ratio” conditions in Drosophila willistoni and Drosophila paulistorium. Genetics, 43, 274–286. [DOI] [PMC free article] [PubMed]
- 23. Sakaguchi B, Poulson DF (1963) Interspecific transfer of the “sex ratio” condition from Drosophila willistoni to Drosophila melanogaster . Genetics 48: 841–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurst GDD, Majerus MEN, Walker LE (1992) Cytoplasmic male killing elements in Adalia bipunctata (Linnaeus) (Coleoptera: Coccinellidae). Heredity 69: 84–91. [Google Scholar]
- 25. Schulenburg JHGvd, Habig M, Sloggett JJ, Webberley M, Bertrand D, et al. (2001) The incidence of male-killing Rickettsia (α-Proteobacteria) in the 10-spot ladybird, Adalia decempunctata L. (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 67: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurst GDD, Hammarton T, Obrycki JJ, Majerus TMO, Walker LE, et al. (1996) Male-killing in a fifth coccinellid beetle, Coleomegilla maculata. . Heredity 77: 177–185. [DOI] [PubMed] [Google Scholar]
- 27. Majerus MEN (2006) The impact of male-killing bacteria on the evolution of aphidophagous coccinellids. Eur. J. Entomol. 103: 1–7. [Google Scholar]
- 28.Randerson JP, Smith NGC and Hurst LD (2000) The evolutionary dynamics of male-killers and their hosts. Heredity, 84, 152–160. [DOI] [PubMed]
- 29. Jiggins FM, Hurst GDD, Schulenburg JHGvd, Majerus MEN (2001) Two male-killing Wolbachia strains coexist within a population of the butterfly Acraea encedon . Heredity 86: 161–166. [DOI] [PubMed] [Google Scholar]
- 30. Majerus MEN, Schulenburg JHGvd, Zakharov IA (2000) Multiple cause of male-killing in a single sample of the 2 spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity 84: 605–609. [DOI] [PubMed] [Google Scholar]
- 31.Majerus MEN, Kearns PWE (1989) Ladybirds (No. 10 Naturalists’ Handbooks). Richmond Publishing: Slough.
- 32. Majerus MEN, Kearns PWE, Forge H, Ireland H (1989) Ladybirds as teaching aids: 1 Collecting and culturing. J. Biol. Educ. 23: 85–95. [Google Scholar]
- 33. Niijima K, Nakajima K (1981) Abnormal sex ratio in Menochilius sexmaculatus (Fabricius). Bull. Fac. Agric. Tamagawa Univ. 21: 59–67. [Google Scholar]
- 34. Majerus TMO, Majerus MEN, Knowles B, Wheelerm J, Bertrand D, et al. (1998) Extreme variation in the prevalence of inherited male-killing microorganisms between three populations of the ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Heredity 81: 683–691. [Google Scholar]
- 35.Hurst GDD, Bandi C, Sacchi L, Cochrane AG, Betrand D, et al. (1999) Adonia variegata (Coleoptera: Coccinellidae) bears maternally inherited Flavobacteria that kill males only. Parasitology, 118, 125–134. [DOI] [PubMed]
- 36. Majerus TMO, Schulenberg JHGvd, Majerus MEN, Hurst GDD (1999) Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect Mol. Biol. 8: 551–555. [DOI] [PubMed] [Google Scholar]
- 37. Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR based typing of forensic material. Biotechniques 10: 506–513. [PubMed] [Google Scholar]
- 38. Simon C, Frati F, Beckenback A, Crespi B, Liu H, et al. (1994) Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87: 651–701. [Google Scholar]
- 39. Jiggins FM, Hurst GDD, Jiggins CD, Schulenburg JHGvd, Majerus MEN (1998) The butterfly Danus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 120: 439–446. [DOI] [PubMed] [Google Scholar]
- 40. Weisberg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res. 25(17): 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou W, Rousset F, O’Neill S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. Ser. B 2665: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majerus TMO (2001) The evolutionary genetics of male-killing in the Coccinellidae. Unpublished PhD thesis: University of Cambridge, Cambridge.
- 44. Kumar S, Tamura T, Nei M (2004) MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5: 150–163. [DOI] [PubMed] [Google Scholar]
- 45. Majerus MEN, Majerus TMO (2000) Female-biased sex ratio due to male-killing in the Japanese ladybird Coccinula sinensis. Ecol. Entomol. 25: 234–238. [Google Scholar]
- 46.Hurst GDD, Jiggins FM, Majerus MEN (2003) Inherited microorganisms that selectively kill male hosts: the hidden players of insect evolution? In: Insect Symbiosis (Bourtzis, K and Miller, T.A., eds.). Pp. 177–197. CRC Press, Bocan Raton.
- 47. Hurst GDD, Jiggins FM (2000) Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dyson EA, Kamath MK, Hurst GDD (2002) Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): evidence for horizontal transmission of a butterfly male-killer. Heredity, 88, 166–171. [DOI] [PubMed]
- 49.Schulenberg JHGvd (2000) The evolution and dynamics of male-killing in the two-spot ladybird Adalia bipunctata L. (Coleoptera: Coccinellidae). Unpublished PhD thesis: University of Cambridge, Cambridge.
- 50.Tinsley MC (2003) The ecology and evolution of male-killing bacteria in ladybirds. Unpublished PhD thesis: University of Cambridge, Cambridge.