Abstract
Ischemic stroke is a leading cause of morbidity and mortality worldwide. Elevated plasma aldosterone levels are an independent cardiovascular risk factor and are thought to contribute to hypertension, a major risk factor for stroke. Evidence from both experimental and human studies supports a role for aldosterone and/or the mineralocorticoid receptor (MR) in contributing to detrimental effects in the cerebral vasculature and to the incidence and outcome of ischemic stroke. This article reviews the evidence, including the protective effects of MR antagonism. Specifically, the effects of aldosterone and/or MR activation on cerebral vascular structure and on immune cells will be reviewed. The existing evidence suggests that aldosterone and the MR contribute to cerebral vascular pathology and to the incidence and outcome of stroke. We suggest that further research into the signaling mechanisms underlying the effects of aldosterone and MR activation in the brain and its vasculature, especially with regard to cell-specific actions, will provide important insight into causes and potential treatments for cerebrovascular disease and stroke.
Keywords: Aldosterone, Mineralocorticoid receptor, Stroke, Vascular remodelling
Background
Elevated plasma aldosterone level is an independent cardiovascular risk factor [1,2]. The mineralocorticoid receptor (MR) is known to be expressed in brain [3], blood vessels [4-6] and heart [7,8] as well as its classical site of expression in epithelial tissues such as the distal nephron. The MR is a member of the nuclear receptor superfamily and comprises an N-terminal domain, a central DNA-binding domain and a hinge region linked to a C-terminal ligand-binding domain. The MR has two physiological ligands, aldosterone and cortisol (corticosterone in rodents). It is established that in epithelial tissues aldosterone requires the enzyme 11β-hydroxysteroid dehydrogenase (11-βHSD2) to activate the MR, since 11-βHSD2 metabolises cortisol to cortisone [9]. Cortisol and corticosterone circulate at 100-1000 times the concentration of aldosterone, thus in the absence of 11-βHSD2 and under conditions of normal cortisol levels, the MR would be occupied by cortisol [10]. Co-localisation of 11-βHSD2 and the MR has been demonstrated in the vasculature (i.e. in endothelial and smooth muscle cells) [11-13], suggesting that aldosterone interacts with the MR in the vasculature.
Patients with primary aldosteronism (characterized by an overproduction of aldosterone) suffer stroke and cardiovascular events more frequently [14] than essential hypertensive patients despite having lower blood pressure, suggesting that elevated plasma aldosterone increases the risk of these events in a blood pressure-independent manner. Ischemic stroke is caused by interruption of blood flow to the brain, and deleterious stimuli which alter cerebral vascular structure and function will ultimately adversely influence cerebral blood flow [15]. Therefore, in humans with underlying cardiovascular risk factors, detrimental vascular actions of aldosterone, perhaps acting via the MR, may contribute to the pathophysiology of hypertension and stroke. The purpose of this article is to review evidence for a contributing role of aldosterone and the MR in stroke in human and experimental studies. Deleterious cerebral vascular actions of aldosterone and MR activation, including arterial remodeling, and recent evidence regarding effects on immune cells following ischemic stroke will be discussed.
Aldosterone and the MR
Aldosterone, synthesized from cholesterol in the adrenal cortex, targets the distal nephron of the kidney to promote sodium and water retention, and potassium excretion, thus modulating electrolyte and fluid homeostasis and blood pressure [2]. Given its well known actions on the MR expressed in epithelial cells, aldosterone was traditionally thought to have an exclusive role in the kidney. However mounting evidence suggests that MR is also expressed in non-epithelial tissues, including the brain, vasculature, cardiomyocytes and immune cells such as macrophages [16]. Indeed, both aldosterone production and MR expression have been detected in the brain [3], blood vessels [4-6] and heart [7,8].
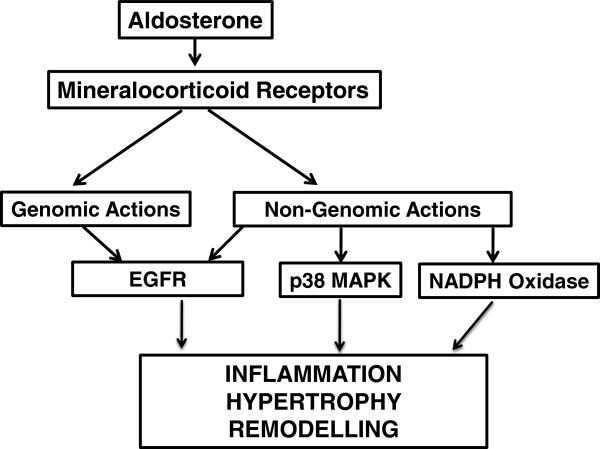
The signalling actions of aldosterone may be either genomic or non-genomic (Figure 1). Genomic actions reflect the classic model of aldosterone action and involve it binding to the MR in the cytoplasm, resulting in MR release from chaperone proteins, dimerization of the receptor and translocation to the nucleus where it binds to hormone response elements on promoters leading to activation of gene transcription [17]. By contrast, rapid, non-genomic actions of aldosterone occur when it binds to MR or other receptors on the cell surface [17] (e.g. G protein coupled receptor 30 [GPR30], and possibly the angiotensin II type 1 receptor [AT1R]) [18,19] to activate second messenger systems.
Figure 1.
Schematic diagram illustrating examples of genomic and non-genomic pathways contributing to vascular remodelling following mineralocorticoid receptor activation by aldosterone. Figure based on text and ref [39].
Effects of MR antagonism on stroke outcome
MR antagonists appear to have beneficial effects in experimental models of stroke. Spironolactone and the more MR-selective compound, eplerenone [20] can both markedly prevent stroke from occurring in stroke-prone spontaneously hypertensive rats (SHRSP) maintained on a 1% NaCl/stroke-prone diet [21,22]. In both studies, control SHRSP showed signs of stroke and died by 16 [21] or 18 [22] weeks of age, whereas all SHRSP treated with spironolactone, and >75% treated with eplerenone, appeared normal and survived until 19 weeks of age before they were culled. Severe cerebrovascular and parenchymal lesions in brains of SHRSP were also reduced in both spironolactone- and eplerenone-treated animals [21,22], suggesting that MR inhibition protects against stroke incidence through actions on both vascular and parenchymal tissues. Additionally, MR antagonism appears to improve outcome after experimental ischemic stroke. Specifically, pretreatment with either spironolactone [23] or eplerenone [24] reportedly reduces infarct size following middle cerebral artery occlusion in SHRSP [23] and in mice [24].
The aforementioned studies provide strong evidence for a role of MR in increasing the incidence and adverse outcomes in rodent models of stroke, although the cellular location(s) of the MR involved was unknown. However, a recent and important study by Frieler and colleagues [25] has provided evidence that MR expressed on myeloid cells (i.e. non-lymphocytic leukocytes) may play an important role in post-stroke outcome. Following cerebral ischemia, a smaller infarct volume was produced in myeloid cell MR-deficient mice compared with control mice, consistent with MR-expressing circulating immune cells mediating post-ischemic neuronal injury [25]. Interestingly, the same group also provided evidence that myeloid cell MR expression regulates macrophage polarization [26], in that deletion of myeloid MR results in an M2 (or ‘alternate’, as opposed to M1 or ‘classical/pro-inflammatory’) macrophage polarization [26].
Macrophage response profile appears to occur according to the polarization state and thus depends on two main groups of T lymphocyte-derived cytokines. For example, Th1-type cytokines (e.g. IFNγ) induce M1 macrophages to display classical pro-inflammatory responses needed for killing parasites, whereas Th2-type cytokines (e.g. interleukins 4, 5, 13), which induce the M2 macrophage phenotype, are also important in the immune response but tend to instead exert an anti-inflammatory action [27]. In addition to myeloid MR activation promoting a proinflammatory M1 state, aldosterone also stimulates several M1 mediators to be generated by peritoneal thioglycolate–elicited macrophages (e.g. TNFα, RANTES, IL-12) with the observation that TNFα expression may be inhibited by eplerenone indicating that the pro-inflammatory action of aldosterone was MR-mediated [26]. In addition, a number of M1 markers (IL-1β, TNFα, IL-6, MCP1, MIP1α) were elevated in the ischemic brain hemisphere of control mice, but these changes were suppressed in myeloid MR-deficient mice. Similarly, there was a marked increase in immunoreactive Iba1+ cells in the ischemic core of control mice (indicative of activated microglia and macrophages), which was significantly weaker in myeloid MR-deficient mice [25]. Together, these findings suggest that MR activity may play an important role in regulating immune cell function during the inflammatory response following cerebral ischemia, thus worsening stroke outcome.
Role of aldosterone in stroke outcome
Animal studies
Whilst it is likely that much of the evidence reviewed above implicating roles for MR activity in stroke indirectly reflects actions of its main endogenous agonist, aldosterone, there is nevertheless additional evidence that more directly implicates a role for this mineralocorticoid in stroke outcome. Treatment with deoxycorticosterone acetate (DOCA), often in combination with uninephrectomy and salt treatment, is commonly used to model pathology caused by aldosterone excess. However, even administration of DOCA alone to normal rats (i.e. in the absence of uninephrectomy and salt treatment) can result in an increased cerebral infarct size following permanent middle cerebral artery (MCA) occlusion, an effect that was associated with increases in both vessel wall thickness and wall: lumen ratio, and decreased lumen and outer diameters of the MCA [28]. Thus, increased levels of mineralocorticoids may contribute to stroke outcome, and such an effect may at least partially result from effects on cerebral blood vessel structure.
Further evidence for a role of aldosterone in outcome after stroke comes from studies of standard anti-hypertensive therapies. Angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II (Ang II) receptor blockers (ARBs) are frontline therapy for hypertension treatment [29], however their beneficial effects in stroke may include the suppression of aldosterone levels and be partly blood pressure-independent [30]. For example, in SHRSP maintained on a high salt diet, the ACEI captopril [31-33] and the AT1R anatagonist losartan [33] increase post-stroke survival [31,32] and restore cerebral blood flow autoregulation [33], yet neither drug exerted an anti-hypertensive effect. In contrast, treatment with the diuretic, hydralazine, significantly lowered blood pressure but was inferior to captopril in improving post-stroke survival [31,32], consistent with the protection by ACEIs and ARBs being blood pressure-independent. In these studies, SHRSP treated with either captopril or losartan had lower plasma aldosterone levels than control SHRSP. Furthermore, when plasma aldosterone levels were restored to normal in captopril-treated SHRSP by infusion from implanted minipumps, the beneficial post-stroke effects of captopril were lost [31,32], as would be expected if the protection by captopril involved suppression of plasma aldosterone levels. Interestingly, although yet to be directly assessed in cerebral vessels, mesenteric arteries from SHRSP are reported to contain significantly higher aldosterone levels at an early age when compared to control rats [34].
Despite experimental evidence that short-term benefits of combination ARB and ACEI therapy may be due to suppression of elevated aldosterone levels, findings from clinical trials using ARBs and ACEIs indicate that, in some patients on combined ARB/ACEI therapy, plasma aldosterone levels can increase over the long term [35]. Thus it is likely that this phenomenon, termed ‘aldosterone breakthrough’, will have important clinical consequences given the non-epithelial actions of aldosterone in organs such as the brain, vasculature, heart and immune system, and may therefore need to be addressed perhaps by additional MR antagonism.
Human studies
Clinical evidence appears to be consistent with data from experimental models indicating an important role for elevated aldosterone levels in stroke outcome [36], perhaps due to elevated aldosterone levels even in the absence of hypertension. For example, the incidence of cerebrovascular events (e.g. stroke, aneurysm, subarachnoid hemorrhage) in patients with glucocorticoid remediable aldosteronism is associated with elevated aldosterone levels and suppressed renin activity [37]. Similarly, a large retrospective study found an association between high aldosterone levels and the risk of stroke and transient ischemic attack that was independent of blood pressure and other risk factors [38]. Furthermore, patients with primary aldosteronism suffer more strokes than patients with essential hypertension, despite having lower blood pressure [39], and they have much higher rates of stroke than age-, sex-, and blood pressure-matched essential hypertensives [14]. Indeed, in recently published clinical studies, relative aldosterone excess (i.e. an increased aldosterone-to-renin ratio) has been identified as a predictor of stroke/transient ischemic attack, during both normal and high sodium intake [40].
Gender effects on aldosterone levels and MR antagonism in stroke
Human studies
Postmenopausal hypertension is largely dependent on mineralocorticoid receptor activation and is selectively sensitive to mineralocorticoid receptor antagonists [41]. Furthermore, in premenopausal women, plasma aldosterone levels are lower than in men [42], but after menopause there is no gender difference in plasma aldosterone levels [43,44].
Experimental studies
Spironolactone and eplerenone were without effect on infarct size in female SHRSP despite a higher relative expression of MR in cerebral arteries in females than males [45], highlighting a potential sex difference in the utility of MR antagonists for stroke therapy. Another study using Wistar rats found that females were less sensitive to central MR antagonism (using the MR antagonist RU28318) than males [46]. Thus, in contrast to findings in human studies where MR appears to be important in post-menopausal women, in experimental studies females appear to be less sensitive to MR antagonism.
Complex roles of aldosterone and the MR in the brain
Despite the evidence that aldosterone contributes to the incidence and outcome of stroke, and that MR inhibition may be protective under these pathological conditions, the MR is nevertheless likely to be important in the normal physiology of the brain. The receptor is most highly expressed in the hippocampus where it is plays a role in behavioral, cognitive and neuroendocrine regulation [47], and pharmacological evidence suggests it is neuroprotective in that it can play a role in neuronal cell survival after cerebral ischemia [48]. Indeed, as cerebral ischemia is known to increase MR expression in the hippocampus of humans [49], rats [48] and gerbils [50], this aspect of MR signaling could be beneficial for promoting neuronal survival. Furthermore, there is evidence that in mice with forebrain overexpression of the MR, neuronal death following transient global cerebral ischemia is reduced compared to controls [47].
One mechanism whereby the MR could reduce neuronal cell death is through reversing the pro-apoptotic effect of the glucocorticoid receptor (GR). Activation of the GR increases neuronal cell death in a hippocampal culture system, and this effect was significantly reduced in the presence of aldosterone. Although it was not examined whether that effect was MR-dependent, the addition of either spironolactone or RU28318 was found to increase neuronal cell death [51]. Thus, the effects of MR (and GR) on neuronal survival could occur via influencing the expression of regulatory proteins associated with apoptosis, and their overall effect (i.e. protective or detrimental) may be the opposite in hippocampus and cortex – the two locations in the brain where MR are normally expressed. For example, in rat hippocampus, GR activation increases the ratio of the pro-apoptotic protein, Bax, relative to the anti-apoptotic proteins, Bcl-2 and Bcl-xL, resulting in increased neuronal cell death, whereas in that study the opposite effect was found after MR activation [52]. However, MR expression occurred after cerebral ischemia in the striatum, and spironolactone treatment increased expression of neuroprotective factors and the number of migrating neuroblasts in that region [53].
Aldosterone, MR, and cerebral artery remodelling
Blood vessels, including cerebral arteries, can undergo hypertrophy (i.e. increased cross-sectional area) and/or exhibit remodeling (inward or outward changes in diameter) in response to hypertension and other chronic stimuli [54]. Several types of cellular processes and signaling mechanisms may be associated with vascular remodeling, including fibrosis, rearrangement of vascular smooth muscle cells, or increased responsiveness to epidermal growth factor (EGF). EGF binds to the EGF receptor (EGFR) to activate cell growth and proliferation [23]. Inward remodeling in the cerebral vasculature can lead to a reduced lumen diameter and a less flexible vessel wall, which consequently impairs the ability of the cerebral vessel to dilate in response to ischemia [23].
Aldosterone, which is also reported to cause vascular remodelling by increasing media thickness [55], increases extracellular matrix proteins such as fibronectin in carotid arteries, an effect that is inhibited by eplerenone [56], suggesting cerebral vascular remodeling in response to elevated aldosterone is MR-dependent. MR located on vascular smooth muscle cells (VSMC) mediates contraction of the mesenteric artery in response to Ang II, and increased contraction in response to KCl and U46619 in aged mice was also dependent on MR in VSMC. Although MR expression in VSMC had no effect on lumen diameter or wall-to-lumen ratio, mesenteric arteries from mice lacking VSMC MR developed less spontaneous myogenic tone compared to controls [57]. These findings indicate an important role for VSMC MR in vascular reactivity. Increased expression of EGFR mRNA has been reported in rat cerebral vessels from SHRSP compared to control rats [23]. Spironolactone treatment resulted in increased lumen and outer diameters of the MCAs in SHRSP, as well as a reduced wall/lumen ratio without lowering blood pressure [58,59], suggesting that spironolactone alters cerebral vascular structure without a change in blood pressure.
Immune cell MR as mediators of stroke damage
In addition to the concept that MR activity regulates infiltrating myeloid cell function during the inflammatory response following cerebral ischemia [25], there is further evidence for an effect of aldosterone on immune cells [60-62]}. Aldosterone stimulated leukocyte adhesion to human coronary artery endothelial cells that was abolished by spironolactone [61]. Immune cell (ie. monocytes/macrophages, as demonstrated by monocyte/macrophage specific antigen MOMA-2 immunostaining) infiltration in aorta in response to aldosterone was also prevented by Treg adoptive transfer [62]. Aldosterone augmented both CD8+ T cell activation by dendritic cells (which express MR), as well as IL-17 (pro-inflammatory cytokine) release by CD4+ T cells – effects that were inhibited by spironolactone and eplerenone [60]. These results were obtained from the use of bone marrow-derived dendritic cells, nevertheless they support the very new concept that aldosterone, perhaps acting via MR, can modulate dendritic cell function and promote T cell activation. Indeed, in association with their smaller cerebral infarct volume (discussed above), myeloid cell MR-deficient mice also have fewer activated microglia, macrophages and pro-inflammatory mediators (IL-1β and TNFα) in the ischemic core compared to control mice [25], implicating immune cell-expressing MR as important mediators of inflammation in ischemic stroke.
Conclusions and future directions
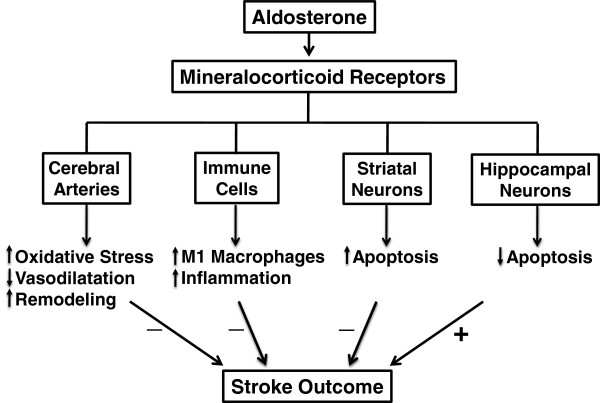
It is now established in experimental stroke studies that activation of the MR contributes to a worse outcome following stroke, as well as altered structure of cerebral blood vessels. Clinical findings also indicate that elevated plasma levels of aldosterone may be a predictor of increased stroke risk (Figure 2). Nevertheless, although these data provide useful insight for the future development of novel stroke therapies, it is noteworthy that both aldosterone, and particularly the MR, may actually exert protective actions in certain brain regions following stroke (Figure 2). Therefore, in order to develop safe and effective therapies targeting these pathways, further research is needed to clarify the cellular and molecular mechanisms underlying the effects of aldosterone and MR activation in the brain and vasculature under physiological conditions as well as during cerebrovascular disease and stroke. The use of cell-specific MR-deficient mice in future studies will thus be crucial to this area of research. There is also a need to clarify if the clinical utility of MR antagonists is likely to be sex-specific.
Figure 2.
Schematic diagram illustrating the potential cellular targets and ultimate effects of mineralocorticoid receptor activation by aldosterone on stroke outcome. ‘↑’ = increased; ‘↓’ = decreased; ‘+’ = improved; ‘−’ = worsened.
Abbreviations
MR: Mineralocorticoid receptor; 11β-HSD: 11β-hydroxysteroid dehydrogenase; GPR30: G-protein coupled receptor 30; AT1R: Angiotensin II type 1 receptor; SHRSP: Stroke prone spontaneously hypertensive rats; DOCA: Deoxycorticosterone acetate; MCA: Middle cerebral artery; ACEIs: Angiotensin converting enzyme inhibitors; Ang II: Angiotensin II; ARBs: Ang II receptor blockers; GR: Glucocorticoid receptor; EGF: Epidermal growth factor; EGFR: EGF receptor.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
QND wrote the first draft and revised the manuscript. TVA, MJY and GRD revised the manuscript. CGS and SC drafted and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Quynh N Dinh, Email: quynh.nhu.dinh@gmail.com.
Thiruma V Arumugam, Email: t.arumugam@uq.edu.au.
Morag J Young, Email: morag.young@princehenrys.org.
Grant R Drummond, Email: Grant.Drummond@monash.edu.
Christopher G Sobey, Email: Chris.Sobey@monash.edu.
Sophocles Chrissobolis, Email: Sophocles.Chrissobolis@monash.edu.
Acknowledgements
The authors are supported by project grants from the NHMRC of Australia and the Heart Foundation of Australia. SC is supported by a postdoctoral fellowship from the High Blood Pressure Research Council of Australia. CGS and GRD are Senior Research Fellows of the National Health and Research Council of Australia. TVA is a Future Fellow of the Australian Research Council. Prince Henry’s Institute is supported by the Victorian Government’s Operational Infrastructure Program.
References
- Stier CT Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev. 2002;10:97–107. doi: 10.1097/00045415-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Herrada AA, Campino C, Amador CA, Michea LF, Fardella CE, Kalergis AM. Aldosterone as a modulator of immunity: implications in the organ damage. J Hypertens. 2011;29:1684–1692. doi: 10.1097/HJH.0b013e32834a4c75. [DOI] [PubMed] [Google Scholar]
- Han F, Ozawa H, Matsuda KI, Lu H, De Kloet ER, Kawata M. Changes in the expression of corticotrophin-releasing hormone, mineralocorticoid receptor and glucocorticoid receptor mRNAs in the hypothalamic paraventricular nucleus induced by fornix transection and adrenalectomy. J Neuroendocrinol. 2007;19:229–238. doi: 10.1111/j.1365-2826.2006.01519.x. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Blair IA, Hsieh FY, Takeda R. Production of aldosterone in isolated rat blood vessels. Hypertension. 1995;25:170–173. doi: 10.1161/01.HYP.25.2.170. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation. 2002;105:2212–2216. doi: 10.1161/01.CIR.0000015854.60710.10. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998;273:4883–4891. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- Milik E, Szczepanska-Sadowska E, Maslinski W, Cudnoch-Jedrzejewska A. Enhanced expression of mineralocorticoid receptors in the heart after the myocardial infarct in rats. J Physiol Pharmacol. 2007;58:745–755. [PubMed] [Google Scholar]
- Fuller PJ, Yao Y, Yang J, Young MJ. Mechanisms of ligand specificity of the mineralocorticoid receptor. J Endocrinol. 2012;213:15–24. doi: 10.1530/JOE-11-0372. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP. Mineralocorticoid receptors in the brain and cardiovascular regulation: minority rule? Trends Endocrinol Metab. 2011;22:179–187. doi: 10.1016/j.tem.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol. 2012;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornel L. Colocalization of 11 beta-hydroxysteroid dehydrogenase and mineralocorticoid receptors in cultured vascular smooth muscle cells. Am J Hypertens. 1994;7:100–103. doi: 10.1093/ajh/7.1.100. [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension. 2005;46:1227–1235. doi: 10.1161/01.HYP.0000193502.77417.17. [DOI] [PubMed] [Google Scholar]
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Muller N, Herold MJ, van den Brandt J, Reichardt HM. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007;122:47–53. doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyreff B, Wehling M. Aldosterone: refreshing a slow hormone by swift action. News Physiol Sci. 2004;19:97–100. doi: 10.1152/nips.01596.2003. [DOI] [PubMed] [Google Scholar]
- Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 Expression Is Required for the Mineralocorticoid Receptor-Independent Rapid Vascular Effects of Aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarie CA, Paradis P, Schiffrin EL. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J Mol Med. 2008;86:673–678. doi: 10.1007/s00109-008-0323-5. [DOI] [PubMed] [Google Scholar]
- Menard J. The 45-year story of the development of an anti-aldosterone more specific than spironolactone. Mol Cell Endocrinol. 2004;217:45–52. doi: 10.1016/j.mce.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT Jr. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.HYP.31.1.451. [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier CT Jr. Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12:308–314. doi: 10.1016/S1043-2760(01)00432-5. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Osborn HL, Grekin R, Webb RC. Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R944–950. doi: 10.1152/ajpregu.2001.281.3.R944. [DOI] [PubMed] [Google Scholar]
- Iwanami J, Mogi M, Okamoto S, Gao XY, Li JM, Min LJ, Ide A, Tsukuda K, Iwai M, Horiuchi M. Pretreatment with eplerenone reduces stroke volume in mouse middle cerebral artery occlusion model. Eur J Pharmacol. 2007;566:153–159. doi: 10.1016/j.ejphar.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Frieler RA, Meng H, Duan SZ, Berger S, Schutz G, He Y, Xi G, Wang MM, Mortensen RM. Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke. 2011;42:179–185. doi: 10.1161/STROKEAHA.110.598441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pello OM, Silvestre C, De Pizzol M, Andres V. A glimpse on the phenomenon of macrophage polarization during atherosclerosis. Immunobiology. 2011;216:1172–1176. doi: 10.1016/j.imbio.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47:590–555. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- Meier P, Maillard M, Burnier M. The future of angiotensin II inhibition in cardiovascular medicine. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:15–30. doi: 10.2174/1568006053004994. [DOI] [PubMed] [Google Scholar]
- Hankey GJ. Angiotensin-converting enzyme inhibitors for stroke prevention: is there HOPE for PROGRESS After LIFE? Stroke. 2003;34:354–356. doi: 10.1161/01.STR.0000054261.97525.4B. [DOI] [PubMed] [Google Scholar]
- MacLeod AB, Vasdev S, Smeda JS. The role of blood pressure and aldosterone in the production of hemorrhagic stroke in captopril-treated hypertensive rats. Stroke. 1997;28:1821–1828. doi: 10.1161/01.STR.28.9.1821. discussion 1829. [DOI] [PubMed] [Google Scholar]
- Smeda J, Vasdev S, King SR. Effect of poststroke captopril treatment on mortality associated with hemorrhagic stroke in stroke-prone rats. J Pharmacol Exp Ther. 1999;291:569–575. [PubMed] [Google Scholar]
- Smeda JS, Daneshtalab N. The effects of poststroke captopril and losartan treatment on cerebral blood flow autoregulation in SHRsp with hemorrhagic stroke. J Cereb Blood Flow Metab. 2011;31:476–485. doi: 10.1038/jcbfm.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Inaba S, Furukawa K, Hatakeyama H, Yoneda T, Mabuchi H, Takeda R. Vascular aldosterone in genetically hypertensive rats. Hypertension. 1997;29:45–48. doi: 10.1161/01.HYP.29.1.45. [DOI] [PubMed] [Google Scholar]
- Bomback AS, Toto R. Dual blockade of the renin-angiotensin-aldosterone system: beyond the ACE inhibitor and angiotensin-II receptor blocker combination. Am J Hypertens. 2009;22:1032–1040. doi: 10.1038/ajh.2009.138. [DOI] [PubMed] [Google Scholar]
- Hillaert MA, Lentjes EG, Kemperman H, van der Graaf Y, Nathoe HM, Beygui F, Montalescot G, Doevendans PA, Wassink AM, van Belle E. Aldosterone, atherosclerosis and vascular events in patients with stable coronary artery disease. Int J Cardiol. 2012. The PMID is 22727970. [DOI] [PubMed]
- Litchfield WR, Anderson BF, Weiss RJ, Lifton RP, Dluhy RG. Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension. 1998;31:445–450. doi: 10.1161/01.HYP.31.1.445. [DOI] [PubMed] [Google Scholar]
- Vyssoulis GP, Karpanou EA, Tzamou VE, Kyvelou SM, Michaelidis AP, Gialernios TP, Cokkinos DV, Stefanadis CI. Aldosterone levels and stroke incidence in essential hypertensive patients. Int J Cardiol. 2009;144:171–172. doi: 10.1016/j.ijcard.2008.12.125. [DOI] [PubMed] [Google Scholar]
- Osmond JM, Rigsby CS, Dorrance AM. Is the mineralocorticoid receptor a potential target for stroke prevention? Clin Sci (Lond) 2008;114:37–47. doi: 10.1042/CS20070155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Kikuya M, Ohkubo T, Mori T, Metoki H, Hara A, Utsugi MT, Hashimoto T, Hirose T, Obara T. et al. Aldosterone-to-renin ratio as a predictor of stroke under conditions of high sodium intake: the Ohasama study. Am J Hypertens. 2012;25:777–783. doi: 10.1038/ajh.2012.33. [DOI] [PubMed] [Google Scholar]
- Olivieri O, Pizzolo F, Ciacciarelli A, Corrocher R, Signorelli D, Falcone S, Blengio GS. Menopause not aldosterone-to-renin ratio predicts blood pressure response to a mineralocorticoid receptor antagonist in primary care hypertensive patients. Am J Hypertens. 2008;21:976–982. doi: 10.1038/ajh.2008.234. [DOI] [PubMed] [Google Scholar]
- Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.CIR.95.1.39. [DOI] [PubMed] [Google Scholar]
- Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens. 1998;16:853–862. doi: 10.1097/00004872-199816060-00017. [DOI] [PubMed] [Google Scholar]
- Rigsby CS, Burch AE, Ogbi S, Pollock DM, Dorrance AM. Intact female stroke-prone hypertensive rats lack responsiveness to mineralocorticoid receptor antagonists. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1754–1763. doi: 10.1152/ajpregu.00145.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Barthelmebs M, Grima M, Imbs JL, De Jong W. Cardiovascular and renal effects of central administration of a mineralocorticoid receptor antagonist in conscious female rats. Eur J Pharmacol. 1999;385:199–202. doi: 10.1016/S0014-2999(99)00705-0. [DOI] [PubMed] [Google Scholar]
- Lai M, Horsburgh K, Bae SE, Carter RN, Stenvers DJ, Fowler JH, Yau JL, Gomez-Sanchez CE, Holmes MC, Kenyon CJ. et al. Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci. 2007;25:1832–1842. doi: 10.1111/j.1460-9568.2007.05427.x. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Johansson IM, Soderstrom I, Lai M, Gido G, Wieloch T, Seckl JR, Olsson T. Mineralocorticoid receptor expression and increased survival following neuronal injury. Eur J Neurosci. 2003;17:1549–1555. doi: 10.1046/j.1460-9568.2003.02587.x. [DOI] [PubMed] [Google Scholar]
- Lai M, Bae SE, Bell JE, Seckl JR, Macleod MR. Mineralocorticoid receptor mRNA expression is increased in human hippocampus following brief cerebral ischaemia. Neuropathol Appl Neurobiol. 2009;35:156–164. doi: 10.1111/j.1365-2990.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Nam YS, Choi JH, Lee IS, Kwon YG, Kang TC, Kim YS, Won MH. Mineralocorticoid and glucocorticoid receptor expressions in astrocytes and microglia in the gerbil hippocampal CA1 region after ischemic insult. Neurosci Res. 2006;54:319–327. doi: 10.1016/j.neures.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10:790–798. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- Oyamada N, Sone M, Miyashita K, Park K, Taura D, Inuzuka M, Sonoyama T, Tsujimoto H, Fukunaga Y, Tamura N. et al. The role of mineralocorticoid receptor expression in brain remodeling after cerebral ischemia. Endocrinology. 2008;149:3764–3777. doi: 10.1210/en.2007-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachofeiro V, Miana M, de Las Heras N, Martin-Fernandez B, Ballesteros S, Fernandez-Tresguerres J, Lahera V. Aldosterone and the vascular system. J Steroid Biochem Mol Biol. 2008;109:331–335. doi: 10.1016/j.jsbmb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: effects of eplerenone. Circulation. 2002;106:2848–2853. doi: 10.1161/01.CIR.0000039328.33137.6C. [DOI] [PubMed] [Google Scholar]
- McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012. The PMID is 22922412. [DOI] [PMC free article] [PubMed]
- Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73:198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 2011;24:708–715. doi: 10.1038/ajh.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF. et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184:191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]