Abstract
A powerful approach to address the general factors contributing to ecological speciation is to compare distantly related taxa that inhabit the same selective environments. In this design, similarities among taxa can elucidate general mechanisms of the process whereas differences may uncover specific factors important to the process for individual taxa. Herein, we present evidence of parallel patterns of morphological and behavioral variation among host-associated populations of two species of cynipid gall wasps, Belonocnema treatae and Disholcaspis quercusvirens, that each exhibit a life cycle intimately tied to the same two host plant environments, Quercus geminata and Q. virginiana. Across both gall-former species we find consistent differences in body size and gall morphology associated with host plant use, as well as strong differences in host plant preference, a measure of habitat isolation among populations. These consistent differences among taxa highlight the important role of host plant use in promoting reproductive isolation and morphological variation among herbivorous insect populations–a prerequisite for ecological speciation.
Introduction
Throughout the modern synthesis, biologists described a central role for ecological adaptation in the speciation process [1]–[3]. However, it was not until the recent renaissance of empirical studies that specific ecological barriers have been shown experimentally to contribute to reproductive isolation through a process termed ecological speciation [4]. Specifically, ecological speciation describes a process in which reproductive isolation evolves as a by-product of divergent natural selection among environments [5]. Examples of ecological speciation are present across a diverse set of taxa (e.g., insects [6]–[10], snails [11], fishes [12], [13], and plants [14]). These studies have documented that a diversity of prezygotic and postzygotic reproductive barriers can arise as a result of divergent ecological adaptation [4], [15]. Barriers examined include temporal isolation [16], sexual isolation [17], cryptic isolation [18], and extrinsic (ecological) postzygotic isolation [19], [20].
To determine the generality of environment-specific factors that may promote (or impede) ecological speciation, it is necessary to examine ecological divergence across multiple taxa experiencing similar environments [21]. Thus far, the majority of studies addressing parallel ecological speciation have compared geographically separate populations of individual species in different versus similar environments [7], [9], [13], [22], [23]. These studies have suggested a central role for divergent selection in promoting speciation [4]. A complementary and powerful approach to understand the process of ecological speciation is to examine more distantly related taxa that inhabit the same selective environments. Similarities among taxa in their response to divergent selection may then suggest specific hypotheses that can then be tested to elucidate general mechanisms of the process, whereas differences may uncover specific factors important to the biology of each species. Evidence for parallel ecological speciation produced by comparing more distantly related taxa is rare but includes studies of multiple lizard species inhabiting two soil color habitats [24] and multiple herbivorous insect species inhabiting similar sets of host plants [25]–[28].
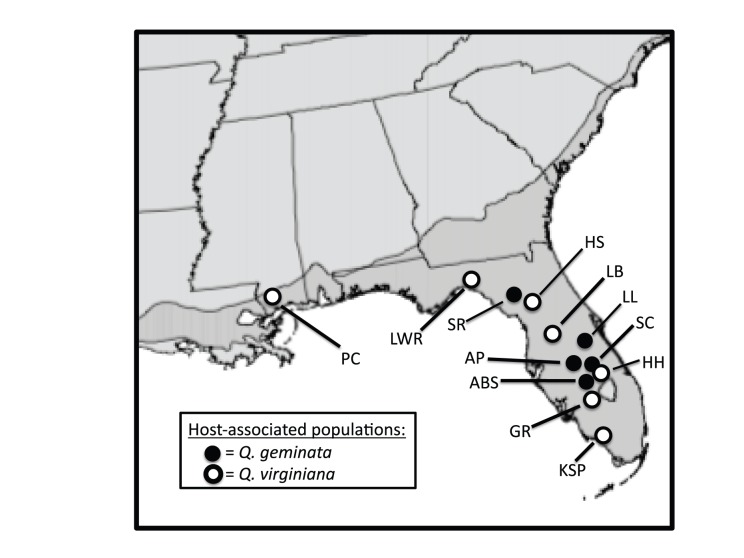
Across the southeastern United States a diverse community of host-specific gall formers (Hymenoptera: Cynipidae) inhabits two sister species of live oak, Quercus virginiana and Q. geminata. These oak species overlap in geographic range (Figure 1) but occupy different microhabitats. Specifically, Q. virginiana occurs in moister, more nutrient rich, and higher pH sites than Q. geminata [29]. The two species also differ in leaf morphology, flowering time, and growth and photosynthetic rates [29]. Thus within each species of cynipid, populations of gallers resident on the alternative oak species may experience different environmental challenges and thus may undergo divergent natural selection related to the host plant and (or) the habitat occupied by the alternative host plants. With a minimum of eight gall wasp species [30] spanning six different genera [31] inhabiting both oak species, this system provides an opportunity to test for the parallel effects of divergent host use across a community of gall formers.
Figure 1. Sampling localities across southeastern USA.
Map of host-associated populations of two species of gall formers on live oak used in the present study. Darker grey shading along coastal USA and Florida illustrates the general distribution of both host plants. See Tables 2 and 3 for population abbreviations.
Herein we examine evidence for parallel host-associated differences in adult morphology and behavior among multiple populations for each of two cynipid species, Belonocnema treatae [32] and Disholcaspis quercusvirens [33], that each inhabit Q. virginiana and Q. geminata. For each cynipid species we examine a main prediction of ecological speciation theory: allopatric populations inhabiting different host plant species will exhibit greater differences in ecologically important traits (e.g., body size [34] and gall morphology [35]) and express greater reproductive isolation (here, habitat isolation due to host preference) than will allopatric populations inhabiting the same host plant. Thus, we specifically sampled both gall-former species from distinct allopatric stands of each host plant species (Figure 1). By comparing multiple allopatric pairs of populations within each gall wasp species that occur in ecologically similar habitats (i.e., host plant species), we can control for host plant independent divergence (i.e., due to genetic drift, sexual conflict, and certain forms of sexual selection) whereas pairs of populations in ecologically different habitats may diverge due to any of these processes as well as from host plant associated divergent selection [7], [22].
Methods
(a) Ethics Statement
A permit to perform fieldwork and make collections at Archibold Biological Station (ABS), Florida, was approved prior to our work. No other permits were required as all other field sites were along public roadways in Florida and Mississippi, USA. Moreover, collections did not involve any endangered or protected species.
(b) Study System and Sampling
Belonocnema treatae (Mayr) and Disholcaspsis quercusvirens (Ashmead) (Hymenoptera: Cynipidae) are both ecologically specialized gall formers that attack certain species of ‘live oaks’ in the genus Quercus [9], [10], [30], [31], [36], [37]. The ‘live oaks’ (series Virentes) are a lineage of five to seven oak species forming a nearly continuous distribution from the tropics (Costa Rica) to the temperate regions (southeastern United States) [29]. The two sister species, Q. virginiana and Q geminata, overlap geographically in the southeastern United States, where Q. geminata is restricted to this region, but Q. virginiana stretches further up the Atlantic coast to Virginia and further west and south along the Gulf coast into Texas and Mexico [29]. We have previously demonstrated host specificity among populations of B. treatae on Q. virginiana and Q. geminata, as measured by host preference [9], [10].
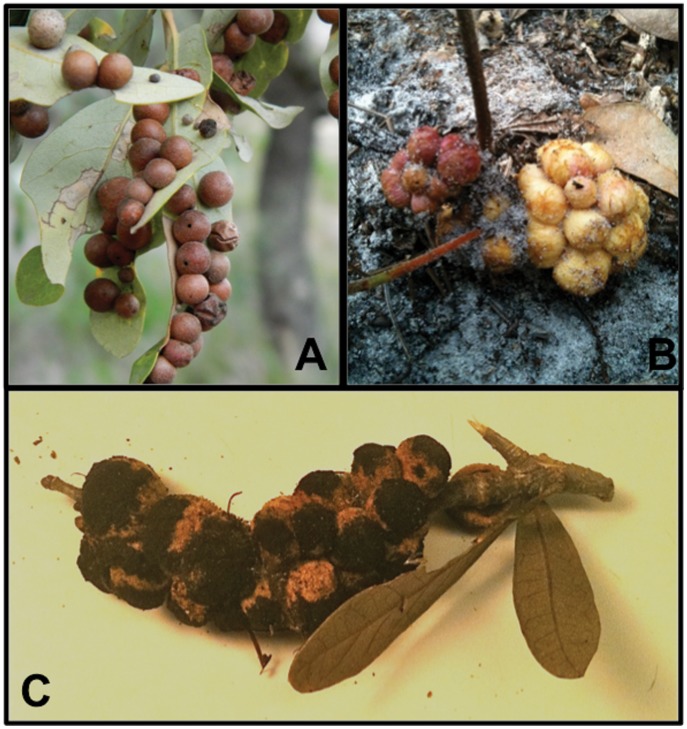
Each gall wasp species exhibits cyclic parthenogenesis (heterogony) whereby spatially and temporally segregated asexual and sexual generations alternate to complete a yearly life cycle [37], [38]. Specifically, the asexual generation of B. treatae develops within single-chambered spherical leaf galls (Figure 2A) on the underside of newly flushed leaves during the summer and fall and emerges in the winter. The sexual generation then develops within multi-chambered galls on oak roots (Figure 2B). Males and females emerge in the spring corresponding with spring bud break [37] and (or) leaf flushes following defoliation by insect herbivores [39]. Similarly, the asexual generation of D. quercusvirens develops within woody compound stem galls (Figure 2C) during early shoot expansion in the summer and fall and emerges in the winter [30], [38]. The woody compound stem galls of the asexual generation comprise clusters of many single-chambered globular galls. While detailed information is lacking on the sexual generation of D. quercusvirens, Platt [38] reports that the sexual generation develops within bud galls initiated beneath bud scales and males and females emerge in the spring. Comparisons presented in this study involve both the asexual and sexual generations of B. treatae and the asexual generation of D. quercusvirens.
Figure 2. Galls induced on live oaks by B. treatae and D. quercusvirens.
(A) Spherical, unilocular leaf galls housing the asexual generation of B. treatae on the host plant Q. virginiana; (B) multi-chambered root gall housing the sexual generation of B. treatae on the host plant Q. geminata; and (C) compound stem gall housing the asexual generation of D. quercusvirens on the host plant Q. virginiana.
We collected B. treatae root galls in the spring of 2010 and 2012 and B. treatae leaf galls and D. quercusvirens stem galls in the fall of 2010. Adult gall formers were reared from galls housed individually by cynipid species, host plant, and locality and husbanded under common conditions (12∶12 light:dark, 23°C) at the University of Notre Dame. Host preference assays were performed within 48 hours of emergence in the same conditions as the rearing environments.
More generally, given the diverse community of cynipid gall formers reported in the historical literature on one or both of these two sister oak species, the patterns revealed for the two species studied here can generate a framework for further testing across all species (26, 40).
Thus, the full suite of cynipid species on each host plant at each locality was collected and is documented in Table 1.
Table 1. Cynipid gall wasp species that inhabit the live oaks, Q. virginiana and Q. geminata, across the southeastern USA.
| Cynipid species | Q. virginiana | Q. geminata | Reference |
| Andricus quercusfoliatus (Ashmead 1881) | HS, LWR, HH, LB | LL, ABS, AP, SR | [33] |
| Andricus quercuslanigera (Ashmead 1881) | HS, LWR, HH, PC, | AP, ABS, SR | [33] |
| Bassettia pallida (Ashmead 1896) | HH | ABS | [67] |
| Belonocnema treatae (Mayr 1881) | HS, LB, PC, HH, KSP, GR | AP, ABS, SR, SC, LL | [32], [37] |
| Callirhytis quercusbatatoides (Ashmead 1881) | HH, HS | ABS, SR | [33] |
| Disholcaspis quercusvirens (Ashmead 1881) | HH, HS, LB, LWR, | LL, ABS, SR, SC | [33] |
| Neuroterus christi (Melika & Abrahamson 1997) | HH | ABS, SC | [31] |
| Neuroterus quercusminutissimus (Ashmead 1885) | HS, LB | LL, AP, ABS, SR | [68] |
(c) Cynipid Body Size and Gall Morphology
For both species, the body size of adult wasps developing on each host plant species was indexed by measuring the length of the right hind tibia. For B. treatae, we compared the body size of asexual generation adults among populations distributed within and between host plant species. This analysis complements previous comparisons of the sexual generation [9]. For D. quercusvirens, we compared the body size of asexual generation adults among populations distributed within and between host plant species. We then compared measures of gall morphology among host-affiliated populations of B. treatae for both the sexual and asexual generations. Following emergence of the sexual generation, we dissected and counted the number of chambers within each multi-chambered root gall (Figure 2B). To compare gall size among populations of the sexual generation of B. treatae we combined new data from collections made in 2010 and 2012 with previous data collected in 2010 and thus expanded the number of populations and sample sizes reported previously by Egan et al. [9]. Following emergence of the asexual generation, the diameter of each spherical leaf gall (Figure 2A) was measured with digital calipers. Gall morphology for D. quercusvirens was compared among populations for the asexual generation. For each compound stem gall both the number and the average diameter of each individual stem gall forming the compound gall was measured (Figure 2C).
(d) Host Preference Assays
For both gall-former species, host preference trials were conducted within 25×8 cm clear-plastic cups stocked with a fresh cutting of each host plant (Q. virginiana and Q. geminata) collected within the native range of the cynipids. A single individual female of either B. treatae or D. quercusvirens was aspirated into each cup and then observed at 5-minute intervals for 1 hour for a total of 12 observations. At each time point, we recorded the location (on Q. virginiana, on Q. geminata, or on the cup) of each individual. Host preference was calculated for each individual as the relative time spent on one host plant species divided by the total time spent on both host plants during the trials (e.g., for Q. virginiana = [# of observations on Q. virginiana]/[# of observations on Q. virginiana+# of observations on Q. geminata]). We performed, in total, 116 preference assays on sexual generation B. treatae females distributed across six populations (Table 2) and 107 preference assays for asexual D. quercusvirens distributed across five populations (Table 3). The host plant preference of sexual generation female B. treatae has been previously documented by Egan et al. [10].
Table 2. Populations of B. treatae associated with Q. geminata (Qg) and Q. virginiana (Qv) used in the present study, along with study site location and the population means ± SE and sample sizes (in parentheses) for each trait measured (HTL = hind tibia length [asexual generation], CPG = chambers per root gall, LGD = leaf gall diameter, and HP = host preference of sexual generation females for Q. virginiana [ = 1 − host preference for Q. geminata]).
| Population | Host plant | Latitude | Longitude | HTL (mm) | CPG | LGD | HP |
| Avon Park (AP) | Qg | 27° 36′ 00′′ N | 81° 30′ 42′′ W | 1.22±0.01(15) | 8.53±1.55(16) | 6.45±0.09(352) | 0.19±0.03(16) |
| Lake Lizzie (LL) | Qg | 28° 13′ 39.6′′ N | 81° 10′ 47.9′′ W | 1.20±0.02(12) | n/a | 5.88±0.04(999) | n/a |
| Archibold Biol. Station (ABS) | Qg | 27° 10′ 57′′ N | 81° 21′ 08′′ W | 1.38±0.03(20) | 9.92±1.31(37) | 6.22±0.04(820) | 0.21±0.03(22) |
| Scrub field (SC) | Qg | 27° 30′ 48′′ N | 81° 20′ 16′′ W | n/a | 7.19±1.88(10) | n/a | 0.21±0.03(20) |
| Hickory Hammock (HH) | Qv | 27° 24′ 09′′ N | 81° 06′ 42′′ W | 1.19±0.02(20) | 1.47±0.68(73) | 5.50±0.04(760) | 0.85±0.03(22) |
| High Springs (HS) | Qv | 29° 50′ 37.9′′ N | 82° 37′ 54.6′′ W | 1.05±0.02(12) | n/a | 4.93±0.04(289) | n/a |
| Leesburg (LB) | Qv | 28° 40′ 2.5′′ N | 81° 51′ 11.7″ W | 1.11±0.02(9) | n/a | 4.92±0.06(204) | n/a |
| Picayune (PC) | Qv | 30° 31′ 37.8′′ N | 89° 40′ 52.4′′ W | 1.08±0.04(15) | 3.26±2.12(6) | 5.06±0.05(326) | n/a |
| Koreshan State Park (KSP) | Qv | 26° 26′ 04′′ N | 81° 48′ 56′′ W | n/a | 1.83±0.76(17) | n/a | 0.72±0.03(18) |
| Gatorama (GR) | Qv | 26° 55′ 30′′ N | 81° 18′ 44′′ W | n/a | 1.01±0.49(19) | n/a | 0.67±0.03(18) |
Table 3. Populations of D. quercusvirens associated with Q. geminata (Qg) and Q. virginiana (Qv) used in the present study, along with study site location and the population means ± SE and sample sizes (in parentheses) for each trait measured (HTL = hind tibia length [asexual generation], GPC = galls per compound stem gall, MGD = mean gall diameter per individual gall that forms compound gall, HP = host preference of the asexual generation females for Q. virginiana [ = 1 − host preference for Q. geminata]).
| Population | Host plant | Latitude | Longitude | HTL(mm) | GPC | MGD | HP |
| Lake Lizzie (LL) | Qg | 28° 13′ 39.6′′ N | 81° 10′ 47.9′′ W | 1.48±0.01 (38) | 9.50±2.35(12) | 10.32±0.19(192) | 0.32±0.03(17) |
| Archibold Biol. Station (ABS) | Qg | 27° 10′ 57′′ N | 81° 21′ 08′′ W | 1.53±0.01(28) | 6.48±1.79(21) | 8.03±0.18(124) | 0.41±0.04(27) |
| Sewanee River at Branford (SR) | Qg | 29° 57′ 18.4′′ N | 82° 55′ 43.7 ′′W | 1.56±0.03(34) | 7.39±0.81(28) | 8.41±0.16(207) | n/a |
| Scrub field (SC) | Qg | 27° 30′ 48′′ N | 81° 20′ 16′′ W | 1.61±0.02(12) | 8.31±2.27(13) | 9.91±0.24(79) | 0.37±0.04(25) |
| Hickory Hammock (HH) | Qv | 27° 24′ 09′′ N | 81° 06′ 42′′ W | 1.41±0.02(9) | 6.06±0.96(16) | 6.49±0.16(104) | 0.65±0.05(19) |
| High Springs (HH) | Qv | 29° 50′ 37.9′′ N | 82° 37′ 54.6′′ W | 1.37±0.02(8) | 7.96±1.45(27) | 5.55±0.08(234) | n/a |
| Leesburg (LB) | Qv | 28° 40′ 2.5′′ N | 81° 51′ 11.7″ W | 1.33±0.03(12) | n/a | n/a | n/a |
| Luther Wilson Road (LWR) | Qv | 30° 19′ 4.8′′ W | 83° 45′ 38.3′′ W | 1.39±0.01(30) | 9.61±1.55(26) | 6.91±0.10(252) | 0.67±0.05(19) |
(e) Statistical Analyses
For each gall-former species, populations on the same host plant species offer comparisons between ecologically similar environments (hereafter, “same-host pairs”) while pairs of populations on different host plants offer comparisons between ecologically dissimilar environments (hereafter, “different-host pairs”). To test for differences in body size and gall morphology among host-associated populations within each species, we generated datasets in the form of matrices of pairwise comparisons among same-host and different-host populations. To compare elements between matrices, Mantel tests [41] were run in the “ecodist” package in R version 2.11.1 with 10,000 randomizations and one-tailed hypothesis testing.
To test for differences in host plant preferences of individual females among populations, we conducted an ANOVA on individual relative preference for Q. virginiana ( = 1 − preference for Q. geminata), with population treated as a random effect, followed by a Tukey’s HSD test to compare means among populations. Since ANOVA determines only whether different populations vary from each other, we then compared each population’s relative preference for its native host to a value of 0.5 by means of a one-sample t-test. The value 0.5 indicates no preference, that is, equal time spent on each of the two host plants, whereas a significant difference implies a preference for a specific host plant. All ANOVA and t-tests were run in the program JMP version 5.0.1a (SAS Institute).
Results
(a) Parallel Patterns of Phenotypic Divergence
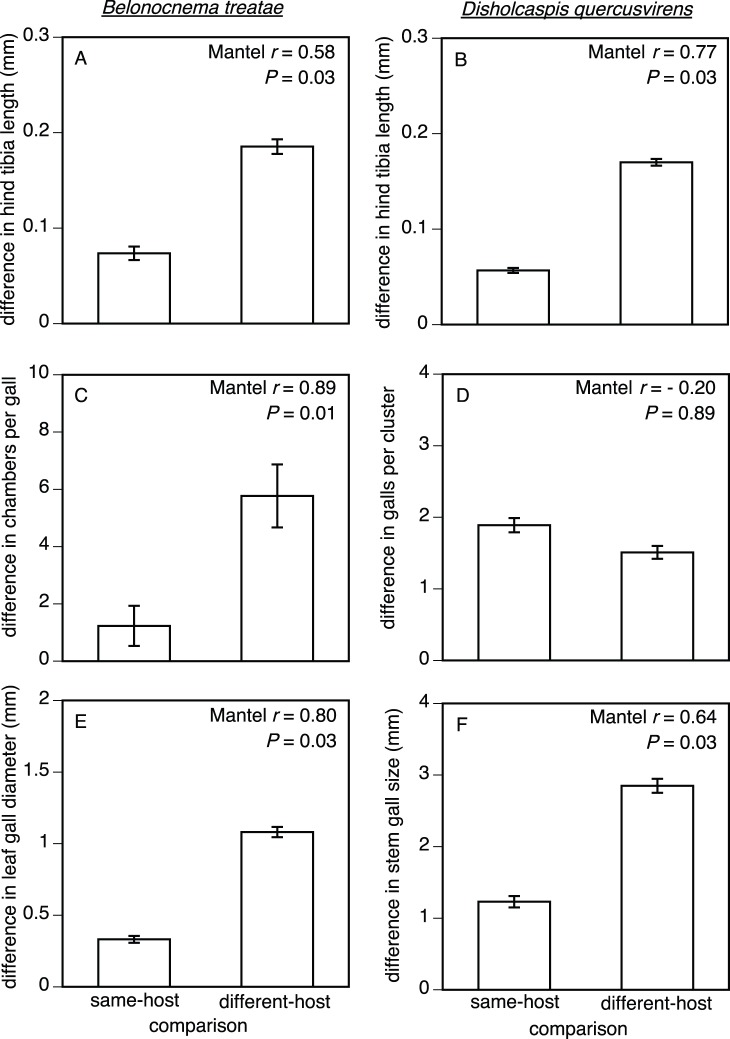
Adult B. treatae from the asexual generation exhibited greater differences in body size among populations in different-host comparisons than in same-host comparisons (Mantel r = 0.58, P = 0.031; Figure 3A). Differences among populations in chamber number per root gall of the sexual generation (Mantel r = 0.89, P = 0.012; Figure 3C) and diameter of the leaf galls produced by the asexual generation (Mantel r = 0.80, P = 0.028; Figure 3E) were also associated with different host plant use. Thus, asexual generation populations of B. treatae on Q. geminata produced larger females and larger leaf galls whereas sexual generation populations of B. treatae produced root galls with more chambers on Q. geminata than did sexual populations of B. treatae on Q. virginiana (Table 2).
Figure 3. Parallel phenotypic patterns among populations of B. treatae (left) and among populations of D. quercusvirens (right) across the same two host plants, Q. virginiana and Q. geminata.
All values compare mean (± SE) of pairwise differences among populations for each species: (A & B) illustrate body size differences, (C & D) illustrate gall structure differences, and (E & F) illustrate gall size differences. See Tables 2 and 3 for sample sizes per population.
Populations of D. quercusvirens exhibited greater differences in asexual female body size in the different-host comparisons than in same-host comparisons (Mantel r = 0.77, P = 0.028; Figure 3B) but did not exhibit differences in galls per compound gall associated with host use (Mantel r = −0.20, P = 0.885; Figure 3D). The average size per individual gall within a compound gall was associated with host use (Mantel r = 0.64, P = 0.031; Figure 3F). Thus, populations of D. quercusvirens on Q. geminata produced larger asexual females and larger individual galls within clusters than did populations of D. quercusvirens on Q. virginiana (Table 3).
(b) Parallel Patterns in Habitat Isolation
Sexual generation female B. treatae exhibit geographic variation in relative host plant preference among populations (ANOVA on female host preference: F 5,110 = 113.4, P<0.001) that is associated with host plant of origin (Tukey’s HSD test: [AP = ABS = S]<{[GR = KSP]<HH}, P<0.05; Table 2, Figure 4A). All populations differed from a no preference expectation of equal time spent on each host plant (t-test of population mean versus 0.50: all t-values >5.40, df ranged from 29–41, all P-values <0.001). Further details can be found in Egan et al. [10].
Figure 4. Parallel behavioral patterns in host preference among (A) B. treatae and (B) D. quercusvirens populations associated with Q. virginiana or Q. geminata.
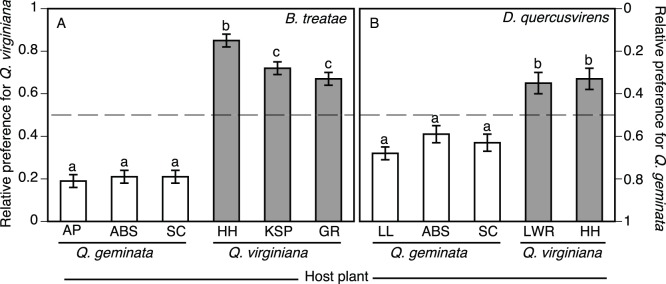
Mean (± SE) illustrates the proportion of time spent on Q. virginiana by each population; however, note reversed y-axis on right (mean time on Q. geminata = 1– mean time on Q. virginiana). The dashed line highlights no preference (defined as 50% of time spent on each host). Each population differs significantly from 50% (P<0.001). See Tables 2 and 3 for locality information and sample sizes per population. Letters above bars denote significant differences among means from Tukey’s HSD test (P<0.05).
In parallel, D. quercusvirens exhibited geographic variation in relative host plant preference among populations as evidenced by a significant population effect (ANOVA: F 4,99 = 13.89, P<0.001). This difference was associated with the population’s host plant of origin (Tukey’s HSD test: [LL = S = ABS]<[HH = LWR], P<0.05; Table 3, Figure 4B). Each population expressed preference for its native host, as all populations differed from a no preference expectation of equal time spent on each host plant (t-test of population mean versus 0.50: LWR tdf = 18 = 3.40, P = 0.002; ABS tdf = 26 = −2.44, P = 0.011; HH tdf = 16 = 2.78, P = 0.007; LL tdf = 16 = −5.19, P<0.001; SC tdf = 22 = −3.76, P = 0.001).
Discussion
A consistent and repeated pattern of variation in trait values among populations inhabiting the same environmental gradient suggests an important role for ecology in generating morphological variation and promoting reproductive isolation among populations–a prerequisite for ecological speciation [7], [8], [21], [24], [26]. However, evidence for similar patterns of variation among populations within two or more species distributed across the same environmental gradient is uncommon, in part because it is rarely tested [24]–[28]. In the present study we provide evidence from two cynipid gall wasp species representing different genera that differences in host plant use within each species are associated with parallel differences in adult and gall morphology between cynipid species. Previously, we demonstrated that partial reproductive isolation, arising as a by-product of significant habitat preference, is exhibited by populations of B. treatae [9], [10]. Our results support the perspective that patterns of host plant use play a central role in promoting both morphological variation and reproductive isolation among populations of herbivorous insect species.
The morphological variation exhibited by cynipid populations on each host plant may be explained by the hypothesis of heritable adaptive divergence among populations. Alternatively, non-genetic effects of the host environment represents a competing hypothesis to explain the observed patterns as all individuals measured in the present study developed on their native hosts. Reciprocal transplant experiments, repeated across populations of each gall-former species, will be needed to distinguish genetic and non-genetic contributions to the observed differences in trait expression and to assess the adaptive nature of trait variation through measurements of the mean fitness of each population on the two host plant species. Our results justify further exploration of the causal basis of variation in morphology (and behavior) within populations of B. treatae and D. quercusvirens and suggest that the hypothesis of host plant driven divergence should be examined for the full suite of cynipid species that comprises the community of gall formers on these two species of live oak. While the present study was not designed to evaluate the relative contributions of adaptive differentiation and plasticity to observed trait variation, we note that populations of both cynipid species exhibited consistent differences across host plant populations even though there is evidence of significant variation in leaf morphology among populations of each oak species and genetic structure is present among populations of each host plant [29]. These consistent differences across variable plant populations exhibited by populations of both cynipid species argues against plasticity as the sole explanation for the patterns of morphological divergence observed within each gall former species [40].
As with patterns of variation observed for morphological traits, our results documenting parallel differences in host plant preference among B. treatae and D. quercusvirens populations may reflect genetic and (or) non-genetic contributions to variation in behavior. However, no evidence to date suggests that host plant preference in cynipids is influenced by the effects of single generation non-genetic rearing conditions [42]. More generally, the role of insect larval conditioning in influencing adult host selection behavior has been thoroughly investigated and gauged as subtle in effect and (or) rare among insects [42], including among other hymenopterans [43], [44] and herbivores [45]. However, even if the behavioral differences we observed among B. treatae and D. quercusvirens populations represent plasticity arising from rearing conditions, habitat isolation would still be predicted to contribute to reproductive isolation as individual females of both species were averse to settling on the alternative host plant and chose to spend more than twice as much time on their own host plant. Thus, regardless of the underlying basis of variation for host preference, our results suggest that choice of host plant promotes reproductive isolation among populations of each species. Lastly, our results are consistent with a growing appreciation for the role of initial environmentally induced differences (i.e. plasticity) promoting divergence and speciation [46], [47], including divergent host use among herbivorous insects [48].
Parallel Phenotypic Differences Associated with Host Use
Phenotypic differences among populations within each species provide evidence of a response to the different host plant environments. However, the two herbivore species need not show the same pattern (i.e., one species might be bigger while the other species smaller on a specific plant species). The phenotypic differences we observed among populations within each cynipid species are in the same direction, suggesting a similar response to selection or a similar plasticity response in each gall-former species to the alternative host plant environment. To assess the general importance of each type of response in structuring phenotypic variation within and among the cynipid gall former community centered on these two oak species will require further testing of the species indicated in Table 1.
Interestingly, each of the two species tested here attack a different set of plant tissues within the host plant, but both species exhibit parallel body size differences: populations of each species are bigger on Q. geminata [9]. Second, and likely correlated with body size, gall size differs among populations for both species: individuals on Q. geminata generate larger galls than individuals on Q. virginiana. Differences in these traits suggest the hypothesis of adaptation to the host plant environment. The two host plants differ in a suite of morphological traits, some of which may be important to the biology of each cynipid. For example, the morphology of the leaf, which is the critical location for oviposition and gall induction for B. treatae, differs among the two host plants; Q. geminata has leaves with thicker midveins and denser trichomes and is overall more “thick and leathery” as measured by mass per area [29]. Similarly, for the stem-galling D. quercusvirens, host plants differ in stem diameter, root to shoot ratio, and above ground biomass [29]. Equally important to both cynipid species, host plants have been shown to differ in flowering phenology, as measured by male flowering date, and another trait associated with phenology, the number of leaf flushes when there is periodic initiation of new growth [29].
Parallel Patterns of Habitat Isolation among Populations
Most importantly, both cynipid species exhibited habitat isolation via host plant preference. Habitat isolation arising from habitat preferences can reduce dispersal between populations inhabiting contrasting habitats and promote adaptive divergence [6], [7], [49]–[52]. Habitat preference is a critical aspect of reproductive isolation among herbivorous insect specialists, who tend to oviposit, feed, rest, develop, and mate on their host plants [53]. Habitat isolation for host-specific phytophagous insect species explicitly describes the process by which the differing habitat preferences of insect populations associated with alternative host plants reduces the frequency of encounters and thus the likelihood of mating between individuals from the differing host-associated populations. In the present study, we demonstrated that multiple populations of both gall-former species, inhabiting either of two closely related oak species, express strong preferences for their natal host plant species. Our results are consistent with partial habitat isolation evolving in these gall wasps as a by-product of adaptation to different hosts, as has now been demonstrated to be an important component to reproductive isolation in a number of plant–insect systems (Timema stick insects [50], leaf beetles [7], [54], pea aphids [55], ladybird beetles [56], Rhagoletis fruit flies [6], and Eurosta galling flies [49]).
An underlying assumption of our measurement of host preference is that the time spent on a host plant is correlated with the gall former’s subsequent mating and oviposition decisions, as has been shown in other host-associated and ecologically divergent insect populations [57]–[59]. We note that residence time as defined in our study is a conservative measure of host preference, as in two other specialist insect species that form host-associated populations, Eurosta solidaginis and Rhagoletis pomonella, insects are more likely to sit on the alternate host plant than to mate or oviposit on it [6], [57], [58]. Finally, for the six populations of B. treatae that we examined we have established the association between population level host preference and positive assortative mating [9], [10].
Parallel Divergence and the Opportunity for Multiplicative Effects
In light of the regional overlap of the oak species [29], [60] and their associated gall wasp community (Table 1), our results suggest the hypothesis that habitat isolation may play a role in the evolution of reproductive isolation in this entire group [26]. Moreover, gall-former communities offer great systems to investigate parallel speciation in a tritrophic context [61]–[64], as global surveys find an average of approximately five parasitoid species per gall former (range: 1–120; [65]). Specifically here, the asexual generation of B. treatae is attacked by a community of up to 24 natural enemies [66], while the asexual generation of D. quercusvirens is attacked by a community of 10 natural enemies [38]. Thus, this study system also provides an opportunity to investigate the hypothesis of parallel sequential speciation for an entire community [62], [64].
Conclusions
This study has documented parallel patterns of morphological and behavioral variation among host-associated populations of two gall-forming species. All populations of both gall-former species examined showed significant habitat preference, which can decrease the probability of mating between members of populations that reside on alternative host plants. These patterns support predictions of ecological speciation theory and are consistent with the hypothesis of host plant driven diversification in the species-rich and ecologically diverse Cynipidae.
Acknowledgments
We gratefully acknowledge M. Deyrup and the Archibold Biological Station for providing lab space and help with host plant identification. We thank E. Silverfine for editorial comments, T. Powell and S. Berlocher for collection locality information, and K. Prior and T. Powell for assistance in processing gall samples. We also would like to thank W. J. Etges, S. H. Heard, T. Craig, and an anonymous reviewer for useful comments that greatly improved the manuscript.
Funding Statement
The research was partially funded by a University of Notre Dame - FRSP Initiation Grant. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mayr E (1947) Ecological factors in speciation. Evolution 1: 263–288. [Google Scholar]
- 2.Dobzhansky T (1951) Genetics and the origin of species (3rd edition). New York: Columbia University Press.
- 3.Simpson GG (1953) The major features of evolution. New York: Columbia University Press.
- 4. Rundle H, Nosil P (2005) Ecological speciation. Ecol Lett 8: 336–352. [Google Scholar]
- 5. Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16: 372–80. [DOI] [PubMed] [Google Scholar]
- 6. Feder JL, Opp S, Wazlo B, Reynolds K, Go W, et al. (1994) Host fidelity as an effective premating barrier between sympatric races of the apple maggot fly. P Natl Acad Sci USA 91: 7990–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Funk DJ (1998) Isolating a role for natural selection in speciation: host adaptation and sexual isolation in Neochlamisus bebbianae leaf beetles. Evolution 52: 1744–1759. [DOI] [PubMed] [Google Scholar]
- 8. Nosil P (2007) Divergent host-plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking-sticks. Am Nat 169: 151–162. [DOI] [PubMed] [Google Scholar]
- 9. Egan SP, Hood GR, Feder JL, Ott JR (2012) Divergent host plant use promotes reproductive isolation among cynipid gall wasp populations. Biology Lett 8: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egan SP, Hood GR, Ott JR (2012) Testing the role of habitat isolation among ecologically divergent gall wasp populations. Int J Ecol 2012: 809897. [Google Scholar]
- 11. Rolán-Alvarez E, Johannesson K, Erlandsson J (1997) The maintenance of a cline in the marine snail Littorina saxatilis – the role of home site advantage and hybrid fitness. Evolution 51: 1838–1847. [DOI] [PubMed] [Google Scholar]
- 12. Rundle HD, Nagel L, Boughman JW, Schluter D (2000) Natural selection and parallel speciation in sympatric sticklebacks. Science 287: 306–308. [DOI] [PubMed] [Google Scholar]
- 13. Langerhans RB, Gifford ME, Joseph EO (2007) Ecological speciation in Gambusia fishes. Evolution 61: 2056–2074. [DOI] [PubMed] [Google Scholar]
- 14. Lowry DB, Rockwood RC, Willis JH (2008) Ecological reproductive isolation of coast and inland races of Mimulus guttatus . Evolution 62: 2196–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyne JA, Orr HA (2004) Speciation. Sunderland Massachusetts: Sinauer Press.
- 16. Wood TK, Keese MC (1990) Host plant induced assortative mating in Enchenopa treehoppers. Evolution 44: 619–628. [DOI] [PubMed] [Google Scholar]
- 17. Nagel L, Schluter D (1998) Body size, natural selection, and speciation in sticklebacks. Evolution 52: 209–218. [DOI] [PubMed] [Google Scholar]
- 18. Nosil P, Crespi BJ (2006) Ecological divergence promotes the evolution of cryptic reproductive isolation. P R Soc B 273: 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egan SP (2009) Funk DJ (2009) Ecologically dependent postmating isolation between sympatric ‘host forms’ of Neochlamisus bebbianae leaf beetles. P Natl Acad Sci USA 106: 19426–19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McBride CS, Singer MC (2010) Field studies reveal strong postmating isolation between ecologically divergent butterfly populations. PLoS Biol 8: e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schluter D, Nagel LM (1995) Parallel speciation by natural selection. Am Nat 146: 292–301. [Google Scholar]
- 22. Nosil P, Crespi BJ, Sandoval CP (2002) Host plant adaptation drives the parallel evolution of reproductive isolation. Nature 417: 440–443. [DOI] [PubMed] [Google Scholar]
- 23. Vines TH, Schluter D (2005) Strong assortative mating between allopatric sticklebacks as a by-product of adaptation to different environments. P R Soc B 273: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenblum EB, Harmon LJ (2011) “Same same but different”: replicated ecological speciation at White Sands. Evolution 65: 946–960. [DOI] [PubMed] [Google Scholar]
- 25. Sandoval CP, Nosil P (2005) Counteracting selective regimes and host preference evolution in ecotypes of two species of walking sticks. Evolution 59: 2405–2413. [PubMed] [Google Scholar]
- 26. Stireman III JO, Nason JD, Heard SB (2005) Host-associated genetic differentiation in phytophagous insects: general phenomenon or isolated exceptions? Evidence from a goldenrod–insect community. Evolution 59: 2573–2587. [PubMed] [Google Scholar]
- 27. Nosil P, Sandoval CP (2008) Ecological niche dimensionality and the evolutionary diversification of stick insects. PLoS ONE 3: e1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dickey AM, Medina RF (2010) Testing host-associated differentiation in a quasi-endophage and a parthenogen on native trees. J Evol Biol 23: 945–956. [DOI] [PubMed] [Google Scholar]
- 29. Cavender-Bares J, Pahlich A (2009) Molecular, morphological, and ecological differentiation of sympatric sister oak species, Quercus virginiana and Q. geminata (Fagaceae). Am J Bot 96: 1690–1702. [DOI] [PubMed] [Google Scholar]
- 30. Price PW, Abrahamson WG, Hunter MD (2004) Using gall wasps on oaks to test broad ecological concepts. Conserv Biol 18: 1405–1416. [Google Scholar]
- 31.Melika G, Abrahamson WG (2002) Review of the world genera of oak cynipid wasps (Hymenoptera: Cynipidae: Cynipini). In Melinka G, Thuroczy C editors. Parasitic wasps: evolution, systematics, biodiversity and biological control. Budapest, Hungary: Agroinform. 150–190.
- 32. Mayr G (1881) Die Genera der gallenbewohnenden Cynipiden. Jahresberichte der Communal-Oberrealschule im I. Bezirke. Wien 20: 1–38. [Google Scholar]
- 33. Ashmead WH (1881) On the cynipidous galls of Florida. T Am Entomol Soc 9: 9–15. [Google Scholar]
- 34. Honek A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66: 483–492. [Google Scholar]
- 35. Stone GN, Schönrogge K (2003) The adaptive significance of insect gall morphology. Trends Ecol Evol 18: 512–522. [Google Scholar]
- 36.Krombein KV, Hurd PD, Smith DR, Burks BD (1979) Catalog of Hymenoptera in America north of Mexico, Vol. 1: Symphyta and Aprocrita (Parasitics). Washington D.C.: Smithsonian Institution Press.
- 37. Lund JN, Ott JR, Lyons R (1998) Heterogony in Belonocnema treatae Mayr (Hymenoptera: Cynipidae). P Entomol Soc Wash 100: 755–763. [Google Scholar]
- 38.Platt JE (2009) Life history and management of a bullet gall wasp, Disholcaspis quercusvirens (Hymenoptera: Cynipidae) on Cathedral Live Oak (Quercus virginiana) in Northern Florida. Masters thesis, University of Florida, USA.
- 39. Hood GR, Ott JR (2010) Developmental plasticity and reduced susceptibility to natural enemies following host plant defoliation in a specialized herbivore. Oecologia 162: 673–683. [DOI] [PubMed] [Google Scholar]
- 40.Heard SH (2012) Use of host-plant trait space by phytophagous insects during host-associated differentiation: the gape-and-pinch model. Int J Ecol 2012, Article ID 192345.
- 41. Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22: 1–19. [Google Scholar]
- 42. Barron AB (2001) The life and death of Hopkins’s host-selection principle. Journal of Insect Behavior 14: 725–737. [Google Scholar]
- 43. Emden HFV, Sponagl B, Wagner E, Baker T, Ganguly S (1996) Hopkins’ ‘host selection principle’, another nail in its coffin. Physiological Entomology 21: 325–328. [Google Scholar]
- 44. Smith MA, Cornell HV (1979) Hopkins host-selection in Nasonia vitripennis and its implications for sympatric speciation. Animal Behavior 27: 365–370. [Google Scholar]
- 45. Janz N, Söderlind L, Nylin S (2008) No effect of larval experience on adult host preferences in Polygonia c-album (Lepidoptera: Nymphalidae): on the persistence of Hopkins’ host selection principle. Physiological Entomology 34: 50–57. [Google Scholar]
- 46. Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, et al. (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25: 459–467. [DOI] [PubMed] [Google Scholar]
- 47. Fitzpatrick B (2012) Underappreciated consequences of phenotypic plasticity for ecological speciation. Int J Ecol 2012: 256017. [Google Scholar]
- 48. Papaj DR, Prokopy RJ (1989) Ecological and evolutionary aspects of learning in phytophagous insects. Ann Rev Entomol 34: 315–350. [Google Scholar]
- 49. Craig TP, Itami JK, Abrahamson WG, Horner JD (1993) Behavioral evidence for host-race formation in Eurosta solidaginis . Evolution 47: 1696–1710. [DOI] [PubMed] [Google Scholar]
- 50. Nosil P, Crespi BJ, Sandoval CP (2006) The evolution of host preference in allopatric versus parapatric populations of Timema cristinae walking-sticks. J Evol Biol 19: 929–942. [DOI] [PubMed] [Google Scholar]
- 51. Edelaar P, Siepielski AM, Clobert J (2008) Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 10: 2462–2472. [DOI] [PubMed] [Google Scholar]
- 52. Bolnick DI, Snowberg L, Patenia C, Lau OL, Stutz WE, et al. (2009) Habitat choice contributes to adaptive divergence between lake and stream populations of three-spine stickleback. Evolution 63: 2004–2016. [DOI] [PubMed] [Google Scholar]
- 53. Funk DJ, Filchak KE, Feder JL (2002) Herbivorous insects: model systems for the comparative study of speciation ecology. Genetica 116: 251–267. [PubMed] [Google Scholar]
- 54. Egan SP, Janson EM, Brown CG, Funk DJ (2011) Postmating isolation and genetically variable host use in ecologically divergent host forms of Neochlamisus bebbianae leaf beetles. J Evol Biol 24: 2217–2229. [DOI] [PubMed] [Google Scholar]
- 55. Via S (1999) Reproductive isolation between sympatric races of pea aphids. I: Gene flow restriction and habitat choice. Evolution 53: 1446–1457. [DOI] [PubMed] [Google Scholar]
- 56. Katakura H, Shioi M, Kira Y (1989) Reproductive isolation by host specificity in a pair of phytophagous ladybird beetles. Evolution 43: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 57.Abrahamson WG, Weis AE (1997) Evolutionary ecology across three trophic levels: goldenrods, gallmakers, and natural enemies. New Jersey: Princeton University Press.
- 58. Craig TP, Horner JD, Itami JK (2001) Genetics, experience and host plant preference in Eurosta solidaginis: implications for host shifts and speciation. Evolution 55: 773–782. [DOI] [PubMed] [Google Scholar]
- 59. Horner JD, Craig TP, Itami JK (2008) The effects of host race, gender, and host-plant dispersion on alighting behavior, mating, and oviposition in Eurosta solidaginis . Entomologia Experimentata et Applicata 128: 274–282. [Google Scholar]
- 60. Cavender-Bares J, Gonzalez-Rodriguez A, Pahlich A, Koehler K, Deacon N (2011) Phylogeography and climatic niche evolution in live oaks (Quercus series Virentes) from the tropics to the temperate zone. J Biogeo 38: 962–981. [Google Scholar]
- 61. Stireman JO, Nason JD, Heard SB, Seehawer JM (2006) Cascading host-associated genetic differentiation in parasitoids of phytophagous insects. Proc R Soc Lond B Biol Sci 273: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Forbes AA, Powell THQ, Stelinski LL, Smith JJ, Feder JL (2009) Sequential sympatric speciation across trophic levels. Science 323: 776–779. [DOI] [PubMed] [Google Scholar]
- 63.Abrahamson WG, Blair CP (2008) Sequential radiation through host race formation: herbivore diversity leads to diversity in natural enemies. In Tilmon KJ editor. Specialization, speciation and radiation: evolutionary biology of herbivorous insects. Berkely: University of California Press. 188–202.
- 64. Feder JL, Forbes AA (2010) Sequential speciation and the diversity of parasitic insects. Ecol Entomol 35: 67–76. [Google Scholar]
- 65. Hawkins BA (1990) Global patterns of parasitoid assemblage size. J Anim Ecol 59: 57–72. [Google Scholar]
- 66.Hall MC (2001) Community structure of parasitoids attacking leaf galls of Belonocnema treatae on Quercus fusiformis. Masters thesis, Department of Biology, Texas State University, San Marcos, USA.
- 67. Ashmead WH (1896) Descriptions of new cynipidous galls and gall wasps in the United States National Museum. Proc US Nat Mus 19: 113–136. [Google Scholar]
- 68. Ashmead WH (1885) On the cynipidous galls of Florida with descriptions of new species. Trans Am Entomol Soc 12: 5–9. [Google Scholar]