Abstract
Background
There are presently no available therapeutic options for peanut-allergic patients.
Objective
To investigate the safety, efficacy, and immunologic effects of peanut sublingual immunotherapy (SLIT).
Methods
After a baseline oral food challenge (OFC) of up to 2g of peanut powder (~50% protein) (median successfully consumed dose [SCD] 46mg), 40 subjects, aged 12–37 (median 15) years, were randomized 1:1 across 5 sites to daily peanut or placebo SLIT. A 5g OFC was performed after 44 weeks followed by unblinding; placebo subjects then crossed over to higher dose peanut SLIT, followed by a subsequent crossover Week 44 5g OFC. Week 44 OFCs from both groups were compared to baseline OFCs; subjects successfully consuming 5g or at least 10-fold more peanut powder than the baseline OFC threshold were considered responders.
Results
After 44 weeks of SLIT, 14/20 (70%) subjects receiving peanut SLIT were responders compared to 3/20 (15%) subjects receiving placebo (p<0.001). In peanut-SLIT responders, median SCD increased from 3.5mg to 496mg. After 68 weeks of SLIT, median SCD significantly increased to 996mg (compared to week 44, p=0.05). The median SCD at the Week 44 crossover OFC was significantly higher than baseline (603mg vs 71mg; p=0.02). 7/16 (44%) crossover subjects were responders; median SCD increased from 21mg to 496mg among responders. Of 10,855 peanut doses through Week 44 OFCs, 63.1% were symptom-free; excluding oral/pharyngeal symptoms, 95.2% were symptom-free.
Conclusions
Peanut SLIT safely induced a modest level of desensitization in a majority of subjects compared to placebo. Longer duration of therapy showed statistically significant increases in the SCD.
Keywords: peanut allergy, sublingual immunotherapy, desensitization, food allergy
INTRODUCTION
Peanut allergy prevalence is increasing, with significant effects on health-related quality of life.(1) Peanuts and tree nuts are the most common triggers of severe and fatal food-induced anaphylactic reactions,(2;3) and peanut allergy is less commonly outgrown than allergy to other major food allergens; thus significant, lifelong changes in dietary habits are required. Given the ever-present fear of severe allergic reactions from accidental ingestions and food product contamination,(4–6) the potential for severe or fatal reactions,(3;7) the need for strict elimination diets and difficulty interpreting food labels,(8;9) a diagnosis of food allergy has significant medical, nutritional and psychosocial implications for affected individuals and families.(10–14) Additionally, there is a substantial economic impact, as investigators have also reported increased health care expenditures associated with food allergy.(15–17) Standard clinical care for peanut allergy currently includes strict dietary elimination and ready access to injectable epinephrine in case of accidental ingestions; there are presently no broadly available therapeutic options for food-allergic patients. Traditional subcutaneous immunotherapy has proven unsafe for peanut allergy,(18) but novel immunomodulatory approaches such as oral immunotherapy (OIT) are under investigation and have shown promise as therapeutic options.(19;20) However, further study is warranted before these approaches become part of mainstream clinical care.(21)
Sublingual immunotherapy (SLIT) has demonstrated clinical efficacy in treatment of asthma and allergic rhinitis associated with a favorable safety profile.(22–24) SLIT has also been utilized for treatment of allergy to several foods including kiwi, hazelnut, peach, milk and most recently peanut.(25–31)
In this study we examined the clinical effects and safety profile of peanut SLIT in what is to date the first multicenter, randomized, placebo-controlled trial. We present data on the primary end point of the study, the percentage of desensitized subjects, as well as several secondary end points including tolerability of up-dosing, differences in response between treatment dosing arms, safety profile, and immunologic outcomes.
METHODS
Study design
The first phase of the study was a randomized, double-blind, placebo-controlled peanut SLIT trial through 44 weeks. The second phase was an unblinded additional 120 weeks of lower dose peanut SLIT treatment for the initial active subjects, and 164 weeks of higher dose peanut SLIT for the placebo subjects following crossover to active therapy. For an illustration of the study protocol, see Figure E1 in the Online Repository at www.jacionline.org. The data presented in this manuscript include information through Week 68 for Peanut SLIT subjects and through Week 44 after initiation of crossover higher dose peanut SLIT therapy for Placebo Crossover subjects (which corresponds to 88 weeks after study entry and 44 weeks after the Week 44 Crossover OFC).
The primary end point was the percentage of desensitized subjects measured by the 5g peanut powder (~2.5g peanut protein) oral food challenge (OFC) performed 44 weeks after initiation of therapy (Week 44 Unblinding OFC). Responders were defined as those who could consume, without dose-limiting symptoms, either a cumulative dose of 5g or a 10-fold increase in the amount of peanut powder compared to their baseline OFC. Key secondary end points included (1) the percentage of subjects tolerating the 16–36 week build-up stage, (2) immunologic end points, including immunologic changes in IgE, IgG4, skin prick test (SPT) and basophil activation, and (3) incidence of serious adverse events.
Subject recruitment
Forty subjects were recruited from 5 US sites (New York, NY, Baltimore, MD, Little Rock, AR, Denver, CO, Durham, NC; the North Carolina subjects moved with the investigative team from Duke to the University of North Carolina-Chapel Hill in March 2012). A cohort of subjects aged 18–40 years was enrolled initially; after 20 weeks of therapy and Data Safety Monitoring Board (DSMB) review, subjects aged 12 to 40 years were enrolled. The study was conducted with investigational new drug approval from the US Food and Drug Administration. A NIAID DSMB and local Institutional Review Boards approved study procedures, and written informed consents were obtained.
Subject selection and randomization
Inclusion criteria required a clinical history or physician diagnosis of peanut allergy, positive peanut SPT (wheal diameter >3mm after subtracting saline control) or detectable peanut-specific IgE (PN-IgE) (≥0.35 kilounits of antibody per liter [kUA/L]), and a positive baseline double-blind, placebo-controlled food challenge (DBPCFC) to peanut (defined as objective allergic symptoms at a cumulative dose of ≤2g of peanut powder). Exclusion criteria included a history of severe anaphylaxis to peanut, defined as involving hypoxia, hypotension or neurologic compromise; asthma with FEV1<80% predicted, or clinical features of moderate or severe persistent asthma and >500 µg/d fluticasone or fluticasone equivalent; history of intubation; or other significant nonallergic medical conditions.
Qualifying subjects were randomized 1:1 to receive either peanut SLIT or placebo. Assignments were centrally prepared, stratified by site and sequentially provided to unblinded site pharmacists. Primary investigators, clinical and laboratory staff, subjects, and families remained blinded through Week 44 of the study’s first phase.
Study protocol
Subjects were instructed to remain on a peanut-free diet throughout the entire study and required to carry an epinephrine autoinjector. Solicited dosing symptoms were recorded on a daily basis. Other unsolicited adverse events were separately recorded. All escalation dosing was observed in monitored research units equipped with emergency medications. Study drug was administered sublingually, held for 2 minutes, and then swallowed.
Escalation dosing
Dosing started at 0.000165 µg of peanut protein (1 pump of a 1:20,000,000 w/v dilution) or placebo (see the Methods section for study medication details and Table E1 in the Online Repository for full escalation dosing). Escalation through 660 µg occurred every 2 weeks, with 3 doses attempted at a minimal interval of 30 minutes. If subjects failed 3-dose escalations after 3 consecutive biweekly attempts, one or two dose biweekly escalations were allowed subsequently. Subjects were monitored for 30 minutes after dosing (if no symptoms or only oral/pharyngeal symptoms occurred) or longer depending on symptoms. After each observed dose, subjects continued the same daily dose at home for 2 weeks. After the 660 µg was achieved, single dose increases occurred followed by 2 weeks of maintenance therapy.
Maintenance dosing
During the first phase, subjects took a minimum dose of 165 µg and a maximum maintenance dose of 1386 µg of peanut protein or placebo (420 µL) at home on a daily basis for the maintenance period until the Week 44 Unblinding 5g DBPCFC. After unblinding, subjects on active peanut SLIT continued on maintenance dosing with a 10g OFC after approximately 1 year of maintenance therapy. Placebo subjects crossed over to active peanut SLIT and were escalated to a maximum maintenance dose of 3696 µg (1120 µL). A 5g Crossover OFC was performed following 44 weeks of SLIT therapy. For more details about the OFCs, see the Methods section in the Online Repository.
Endpoint titration skin prick testing
Endpoint titration SPTs were performed with serial ten-fold dilutions of peanut extract at the start of the study and then following approximately 1 year of maintenance peanut SLIT therapy. For details, see the Methods section in the Online Repository.
Immunologic studies
Basophil activation
Basophil activation was evaluated at baseline and at Weeks 29 and 44 by CD63 up-regulation by flow cytometry.(32)
Immunoglobulins
Total IgE was measured by immunoassay, and PN-IgE and peanut-specific IgG4 (PN-IgG4) were measured using the ImmunoCAP 100 (Thermo Fisher Scientific, Waltham, MA) at baseline and at Weeks 29, 44, and 68.
Statistical analysis
A sample size of 17 per arm was required to provide 90% power to detect, with a two-sided 5% level of significance test, a difference between a 5% desensitization rate for placebo-treated versus a 50% rate for SLIT-treated subjects. The required sample size and analysis used unconditional exact binomial methods (StatXact6). All randomized cases, irrespective of achieved dose, were used in the intent-to-treat analysis of the primary end point with those failing to demonstrate desensitization to peanut consumption at the unblinding time point considered failures in the binary assessment. We increased the sample size to 20 individuals per arm to accommodate potential dropouts or noncompliant cases. With the sample size of 20 per arm, the power is 82% to detect a difference if the true placebo SLIT desensitization rate is 10%, and the peanut SLIT rate is 50%.
RESULTS
Study Participants
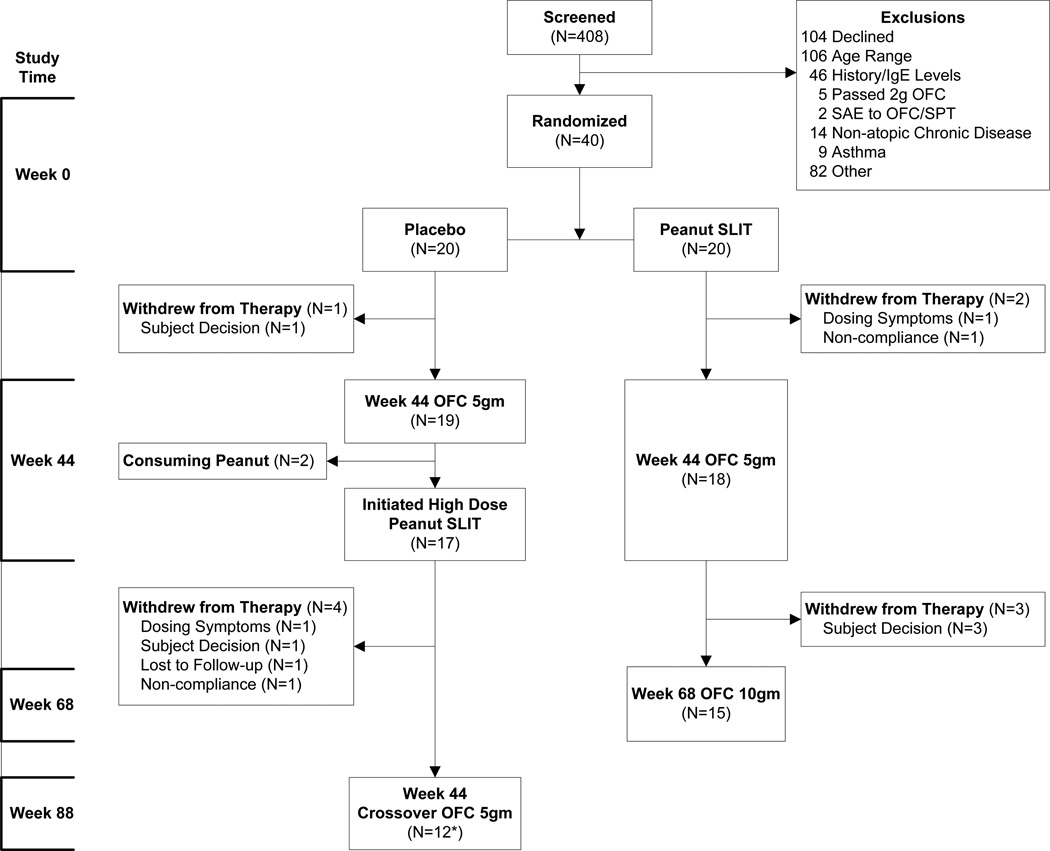
Forty subjects (8 per institution) were enrolled (20 Peanut SLIT, 20 Placebo), and 68% were male with a median age of 15.0 years (range 12.2–36.8 years) (Figure 1). Most subjects had a history of other food allergies (78%), asthma (58%), or allergic rhinitis (73%), and less than half had atopic dermatitis (47.5%). There were no statistical differences in baseline characteristics between treatment groups (Table 1).
Figure 1. Enrollment and Disposition of Subjects.
The 40 subjects who passed screening were randomized onto placebo or peanut SLIT. After the Week 44 oral food challenge, the study was unblinded. Subjects in the original Peanut SLIT group continued on maintenance peanut SLIT therapy and received a Week 68 oral food challenge. Original placebo recipients were offered a higher dose peanut SLIT from week 44 to week 88, and then an oral food challenge.
Table 1.
Baseline Characteristics
| Treatment | ||
|---|---|---|
| Placebo (N=20) |
Peanut SLIT (N=20) |
|
| % | % | |
| Male Gender | 70.0 | 65.0 |
| Additional Food Allergy | 70.0 | 85.0 |
| Physician Diagnosis Asthma | 60.0 | 55.0 |
| Allergic Rhinitis | 75.0 | 70.0 |
|
Median [Q1, Q3] |
Median [Q1, Q3] |
|
| Age (years) | 16.0 [13.5,18.5] |
14.0 [13.0,18.0] |
| Baseline Atopic Dermatitis Total Score | 0.0 [0.0,1.5] |
0.0 [0.0,3.0] |
| Baseline Peanut Endpoint Titer | 2000 [200,20000] |
2000 [1100,11000] |
| Baseline Skin Prick Test Peanut Score (mm) | 12.0 [9.0,16.5] |
13.3 [9.5,17.5] |
| Baseline Peanut IgE (kUA/L) | 22.5 [3.3,77.7] |
31.3 [3.2,42.4] |
| Baseline OFC Dose at First Symptom (mg) | 6.0 [3.5,71.0] |
6.0 [1.0,46.0] |
| Baseline OFC Successfully Consumed Dose (mg) | 71.0 [3.5,196.0] |
21.0 [1.0,146.0] |
Assessment of clinical desensitization
Week 44 Unblinding 5g DBPCFC
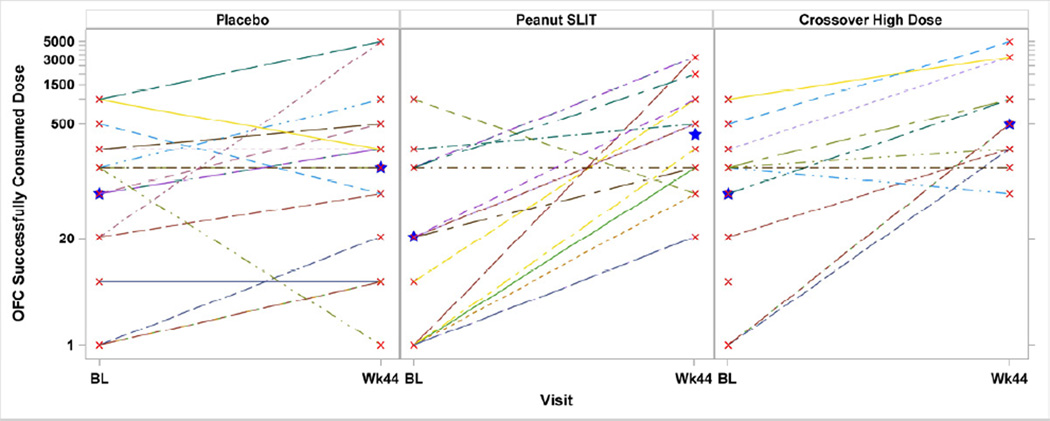
Three subjects (2 Peanut SLIT, 1 Placebo) withdrew prior to the Week 44 Unblinding OFC; per protocol, these subjects were treated as non-responders. A significantly higher response rate was noted in the active treatment group: 14 of 20 (70%) subjects on Peanut SLIT were responders per study criteria versus only 3 of 20 (15%) receiving placebo (p<0.001, 95% CI [22.2%, 77.6%]). Among the 14 responders in the Peanut SLIT group, the successfully consumed dose (SCD) was <500 mg for 8 subjects, 996 mg for 2 subjects, 1996 mg for 1 subject, and 3256 mg for 3 subjects (median 496 mg) (Figure 2). Among the 3 responders in the Placebo group, 1 successfully consumed 21 mg, and the other 2 successfully consumed 5000 mg and passed the challenge (Figure 2). For more details regarding the responders in the Placebo group, please see the Results section in the Online Repository.
Figure 2. Oral Food Challenge Successfully Consumed Dose by Treatment Group.
Oral food challenge doses successfully consumed were compared to initial 2g baseline successfully consumed doses after 44 weeks of therapy from study entry for randomized, initially treated group; and after 44 weeks of therapy from crossover initiation for Crossover High Dose group. The median oral food challenge successfully consumed dose at Week 44 was significantly higher than at baseline oral food challenge for Peanut SLIT subjects (21mg vs 371mg; p=0.01) but was not for Placebo subjects (71mg vs 146mg; p=0.14). Star identifies the median.
The median SCD at Week 44 was significantly higher than the baseline OFC for Peanut SLIT subjects (371 mg vs 21 mg, respectively; p=0.01) but not for Placebo subjects (146 mg vs 71 mg, respectively; p=0.14). However, the median SCD after 44 weeks of therapy was not significantly different between treatment groups (p=0.16).
Baseline characteristics (age, atopic dermatitis total score, peanut endpoint titer, peanut SPT score, PN-IgE, baseline OFC dose at first symptom, baseline OFC SCD) were examined in Peanut SLIT subjects to identify predictors of response. Only the SCD at the baseline OFC was significantly different between responders compared to non-responders, 3.5 mg versus 246 mg, respectively (p=0.008).
Week 68 10g DBPCFC
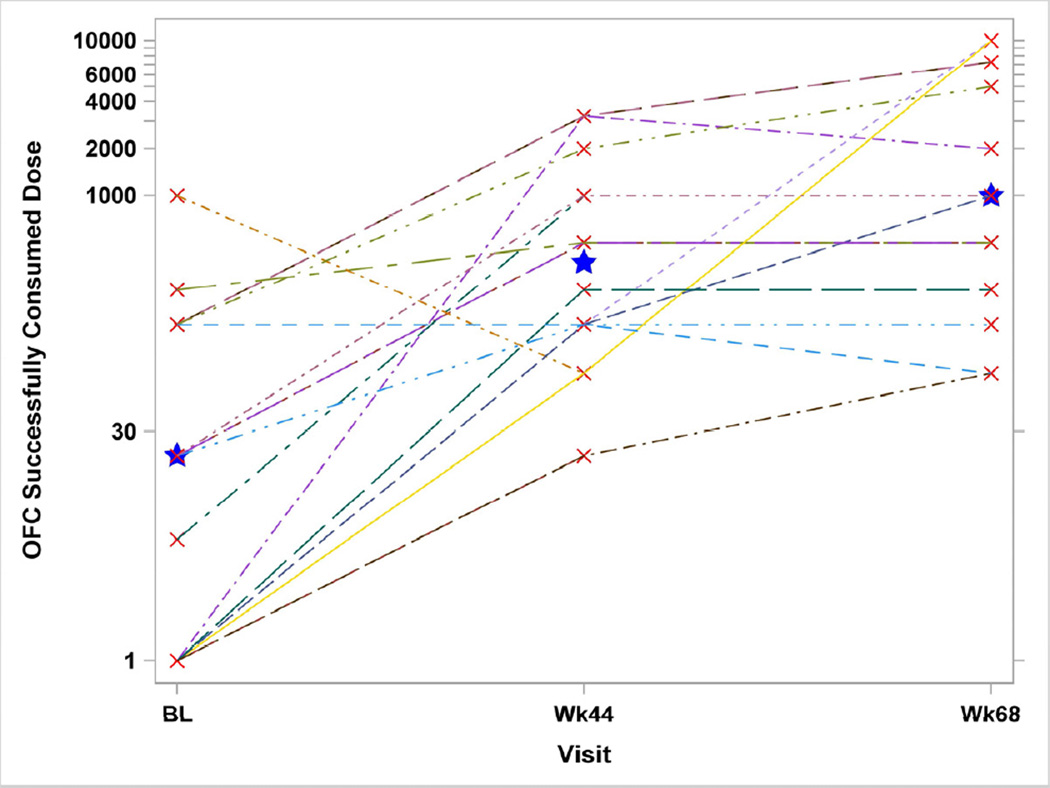
All Week 44 responders still being followed were Week 68 responders: no Week 44 non-responders converted to responders at Week 68. For the 15 Peanut SLIT subjects who underwent the Week 68 OFC, the SCD compared to the Week 44 Unblinding OFC declined for 2 subjects, increased for 7 subjects, and remained the same for 6 subjects. Two subjects successfully consumed 10g of peanut powder, and 3 others consumed 5g. The median SCD increased to 996 mg, and this was significantly higher than at Week 44 (p=0.05) and at Baseline (p=0.009) (Figure 3).
Figure 3. Oral Food Challenge Successfully Consumed Dose for Peanut SLIT Subjects Who Have Week 68 Oral Food Challenge.
The oral food challenge successfully consumed doses of subjects are shown by the oral food challenge doses successfully consumed at Baseline, Week 44 and Week 68 for Peanut SLIT subjects. At Week 68, the median dose successfully consumed increased to 996 mg, and this was significantly higher than at Week 44 (p=0.05) and at Baseline (p=0.009). Star identifies the median.
Week 44 Crossover 5g DBPCFC in initial Placebo group
Seventeen Placebo subjects crossed over to active SLIT at Week 44, escalating to a higher peanut SLIT dose than the initial active group. Among these 17 subjects (Crossover High Dose subjects), 12 completed the Week 44 Crossover OFC. Four discontinued dosing prior to this OFC and were counted as non-responders per protocol; 1 missed this OFC (not evaluable) but continued dosing and was challenged at Week 68. The median SCD at the Week 44 Crossover OFC was significantly higher than at the Baseline OFC (603 mg vs 71 mg; p=0.02) (Figure 2).
The number of responders among the Crossover High Dose subjects with an evaluable result was 7/16 (44%). Among the 7 responders in this group, the SCD was <500 mg for 4 subjects, 996 mg for 1 subject, 3246 mg for 1 subject, and 4996 mg for 1 subject (median 496 mg). None of the baseline characteristics, as described above, was significantly different between Crossover High Dose responders and non-responders.
Among the 17 Crossover High Dose subjects who initiated peanut SLIT active dosing, 15 (88%) were able to attain the maximum maintenance dose of 3696 mg. One subject reached a dose of 6.6 mg and discontinued dosing due to adverse symptoms, while the other reached 165 mg and was lost to follow-up.
Immunologic changes
Immunoglobulins
Baseline PN-IgE levels were not statistically different between Peanut SLIT [median 31.3 kUA/L (range 0.4–154.1 kUA/L)] and Placebo subjects [median 22.5 kUA/L (range, 0.6–207.5 kUA/L)] (see Table E2 in the Online Repository). Median PN-IgE levels among Peanut SLIT subjects increased significantly between baseline and Week 44 (p=0.035), but not between Weeks 44 and 68. (p=0.21). The change from baseline to Week 44 in PN-IgE levels was not statistically significant (p=0.43) among Placebo subjects or Crossover High Dose subjects (p=0.07) (see Figure E2 in the Online Repository). At Week 44, no statistically significant differences in median PN-IgE levels were detected between Peanut SLIT and Placebo subjects, Peanut SLIT responders and non-responders, or Crossover High Dose and Peanut SLIT subjects.
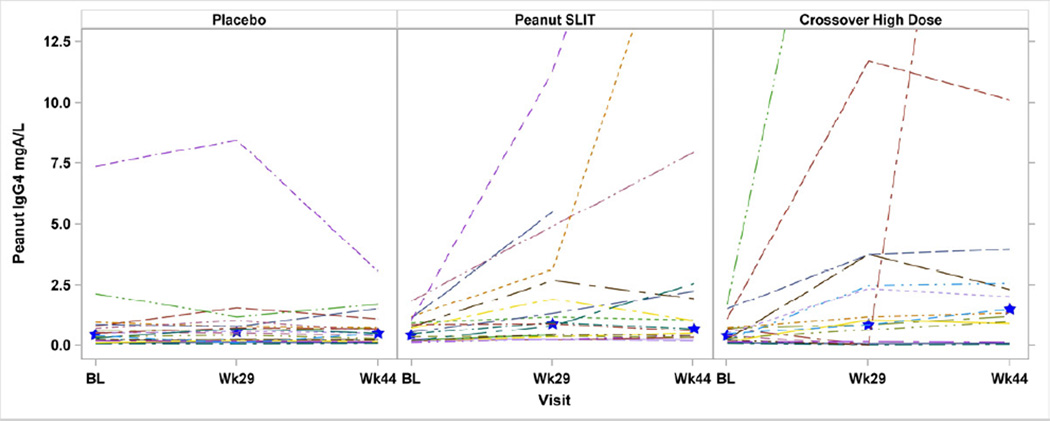
Baseline PN-IgG4 levels were not significantly different between treatment groups (see Table E2 in the Online Repository). PN-IgG4 levels increased significantly among Peanut SLIT subjects between baseline and Week 44 (p=0.001), but not between Weeks 44 and 68 (p=0.42). Placebo subjects had no significant change from baseline to Week 44 in median PN-IgG4 levels (p=0.99). A statistically significant increase in PN-IgG4 was noted in Crossover High Dose subjects from baseline to Week 44 (p<0.001). The median change from baseline to Week 44 in PN-IgG4 was significantly different in Peanut SLIT subjects compared to Placebo subjects: 0.3 milligrams of antibody per liter (mgA/L) vs 0.0 mgA/L (p<0.001) (Figure 4). There were no statistically significant differences in median change from baseline to Week 44 in PN-IgG4 between Peanut SLIT responders and non-responders (p=0.33) or between Crossover High Dose responders and non-responders (p=0.07). There was no statistically significant difference in median PN-IgG4 levels at Week 44 or in median change from baseline to Week 44 in PN-IgG4 between Crossover High Dose subjects and Peanut SLIT subjects.
Figure 4. Change in Peanut-Specific IgG4 During SLIT.
Levels were compared after 44 weeks of therapy from study entry for the randomized, initially treated group; and after 44 weeks of therapy from crossover initiation for the Crossover High Dose group. Median increase in PN-IgG4 from baseline to Week 44 was statistically significantly higher Crossover High Dose subjects (p<0.001) and in Peanut SLIT compared to placebo (0.3 mgA/L vs 0.0 mgA/L; p<0.001). Star identifies the median.
Basophil activation
A repeated measures analysis of percent CD63 positivity (%CD63+) data from Weeks 29 and 44, with study visit and baseline %CD63+ as covariates, found the %CD63+ was significantly lower for Peanut SLIT subjects compared to Placebo subjects for the 10−2 µg/mL crude peanut stimulant (Δ=−19.2, p=0.008) and the 10−3 µg/mL crude peanut stimulant (Δ=−11.9, p=0.049 (see Figure E3 in the Online Repository), indicating a weak effect on basophil activation. Similar analyses performed between responders and non-responders in the Peanut SLIT group, as well as between Crossover High Dose and Peanut SLIT subjects and between responders and non-responders in the Crossover High Dose group revealed no significant differences.
Peanut SPT and titrated SPT
No difference in baseline median peanut SPT score was detected between treatment groups (Peanut SLIT 13.3 mm, Placebo 12.0 mm; p=0.43). Peanut endpoint titration SPT was performed at baseline and at Week 68. There was no statistically significant difference between Peanut SLIT responders and non-responders in baseline peanut SPT score or area under the curve analysis for endpoint titration SPT. However, at Week 68 the median change from baseline for the area under the SPT endpoint titration curve was improved for Week 44 responders (−17.0) vs. non-responders (1.0) (p=0.03) (see Figure E4 in the Online Repository).
Dosing symptoms, safety, and adverse events
During the study’s first phase (baseline to Week 44 Unblinding challenge), 99.4% of 6029 placebo doses were symptom-free, whereas only 59.9% of 5825 Peanut SLIT doses were symptom-free (Table 2). However, upon exclusion of oral/pharyngeal symptoms, 94.7% of doses were symptom-free in Peanut SLIT subjects. Only 127 of 11,854 total doses (1.1%) required treatment during the first phase: 125 (1.1%), oral antihistamine only; 1 (0.01%), albuterol only; and 1 (0.01%), epinephrine and oral antihistamine.
Table 2.
Pre-Week 44 Oral Food Challenge Dosing Symptom Summary
| Placebo | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit Type | # Doses | %Symptom Free |
%Symptom Free excluding oral/phary |
% Oral/Phary Symptoms |
% Skin | % Resp. | % GI | % Other | % Symptoms > .5 Hour |
% Treated | % Mild | % Moderate | % Severe |
| Escalation | 469 | 96.38 | 98.93 | 2.77 | 0.43 | 0.85 | 0.00 | 0.43 | 0.21 | 0.00 | 1.07 | 0.00 | 0.00 |
| Clinic | 130 | 100.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Home | 5433 | 99.65 | 99.78 | 0.18 | 0.02 | 0.02 | 0.02 | 0.17 | 0.07 | 0.06 | 0.20 | 0.02 | 0.00 |
| All | 6032 | 99.40 | 99.72 | 0.38 | 0.05 | 0.08 | 0.02 | 0.18 | 0.08 | 0.05 | 0.27 | 0.02 | 0.00 |
| Peanut SLIT | |||||||||||||
| Visit Type | # Doses |
%Symptom Free |
%Symptom Free excluding oral/phary |
% Oral/Phary Symptoms |
% Skin | % Resp. | % GI | % Other |
% Symptoms > .5 Hour |
% Treated | % Mild | % Moderate | % Severe |
| Escalation | 471 | 75.37 | 92.14 | 21.87 | 2.12 | 4.03 | 1.27 | 1.06 | 2.34 | 1.91 | 7.64 | 0.21 | 0.00 |
| Clinic | 143 | 63.64 | 95.10 | 33.57 | 2.80 | 1.40 | 0.00 | 1.40 | 2.10 | 0.00 | 4.90 | 0.00 | 0.00 |
| Home | 5211 | 58.47 | 94.95 | 38.76 | 1.29 | 2.00 | 0.90 | 1.65 | 1.27 | 2.21 | 4.99 | 0.06 | 0.00 |
| All | 5825 | 59.97 | 94.73 | 37.27 | 1.39 | 2.15 | 0.91 | 1.60 | 1.37 | 2.13 | 5.20 | 0.07 | 0.00 |
| Crossover High Dose | |||||||||||||
| Visit Type | # Doses |
%Symptom Free |
%Symptom Free excluding oral/phary |
% Oral/Phary Symptoms |
% Skin | % Resp. | % GI | % Other |
% Symptoms > .5 Hour |
% Treated | % Mild | % Moderate | % Severe |
| Escalation | 474 | 74.47 | 93.88 | 23.84 | 0.63 | 3.80 | 0.63 | 1.27 | 0.42 | 1.69 | 5.91 | 0.21 | 0.00 |
| Clinic | 61 | 75.41 | 96.72 | 24.59 | 0.00 | 0.00 | 1.64 | 1.64 | 1.64 | 0.00 | 3.28 | 0.00 | 0.00 |
| Home | 4495 | 65.72 | 96.00 | 32.28 | 0.71 | 0.27 | 0.67 | 2.51 | 0.53 | 3.09 | 3.98 | 0.02 | 0.00 |
| All | 5030 | 66.66 | 95.81 | 31.39 | 0.70 | 0.60 | 0.68 | 2.39 | 0.54 | 2.92 | 4.16 | 0.04 | 0.00 |
In the Crossover High Dose subjects, 66.7% of 5030 doses from build-up to Week 44 Crossover OFC were symptom-free, increasing to 95.8% when excluding oral-pharyngeal symptoms. One hundred forty-seven doses (2.9%) required treatment: 146 (2.9%), oral antihistamine only; and 1 (0.02%), oral antihistamine and albuterol. From the Week 44 Unblinding OFC to Week 68, Peanut SLIT subjects took an additional 2083 doses, of which 64.2% were symptom-free; 98.9% were symptom-free when oral-pharyngeal symptoms were excluded. Only one dose (0.05%) required treatment with an antihistamine.
A total of 240 adverse events were reported; 234 (97.5%) were unrelated to study product (e.g., infections [upper respiratory], gastrointestinal disorders [diarrhea], nervous system disorders [headaches]). Thirty-six percent were in the Peanut SLIT subjects, 31% in the Placebo subjects, and 33% were in the Crossover High Dose subjects. Eighty-six percent were judged to be mild in severity. One dose-related serious adverse event occurred during the build-up stage in a Peanut SLIT subject. Five minutes after taking the daily dose at home, the subject developed erythema, pruritus, and oral symptoms. Diphenhydramine was administered at home without improvement, and the reaction progressed to include urticaria and coughing. Epinephrine was administered at home, and the subject transported to the study site. After treatment and monitoring, further dosing was discontinued.
Four additional subjects on Peanut SLIT discontinued dosing for the following reasons: noncompliance with therapy; perceived lack of efficacy; opportunity to participate in another food allergy treatment study; and fear of subsequent OFCs. Of the 5 Placebo/Crossover subjects who discontinued dosing, one each did so for the following reasons: poorly controlled asthma; anxiety with ongoing dosing; pregnancy; lack of desire to continue dosing; and loss to follow-up. (Figure 1).
DISCUSSION
This is the first multicenter, randomized, placebo-controlled trial of peanut SLIT. The study achieved its primary efficacy end point, demonstrating that treatment with peanut SLIT induces a statistically significant degree of desensitization in a majority of subjects, compared to placebo. Desensitization was also observed in subjects originally randomized to placebo who subsequently crossed over and received treatment with higher dose peanut SLIT.
Although these results are encouraging, none of the subjects treated with lower or lower dose peanut SLIT were able to ingest 5g of peanut powder without symptoms during the desensitization challenge, suggesting a modest desensitization effect conferred by 44 weeks of peanut SLIT may not provide clinically relevant protection. Interestingly, a subset of subjects challenged again after 68 weeks of treatment demonstrated incremental, statistically significant increases in the SCD, with three subjects consuming 5g of peanut powder without symptoms and two successfully consuming 10g. These data suggest continued long-term therapy with peanut SLIT might confer reduced reactivity to peanut following further desensitization, allowing for protection from accidental ingestions, which are reactions to less than 100mg of peanut protein in general.
The clinical effect of peanut SLIT was independent of baseline or quantitative changes in peanut-specific antibody levels. Specifically, clinical improvement was not associated with a reduction in PN-IgE levels at 44 or 68 weeks, consistent with some(33–36) but not all(20;37–39) studies of allergen immunotherapy. Active peanut SLIT but not placebo increased the PN-IgG4 levels, but there was no difference between responders and non-responders. Although statistically significant inhibition of basophil reactivity in vitro was detected in Peanut SLIT subjects compared to Placebo subjects, these finding were not significant when comparing Peanut SLIT or Crossover High Dose responders and non-responders. Peanut SLIT responders compared to non-responders, however, experienced significant suppression in the peanut SPT size by week 68. These data suggest that the desensitization observed in our study may have been mediated by reduced mast cell reactivity, but further mechanistic studies are warranted with long-term therapy.
Other studies have demonstrated that blocking IgG antibody,(40) regulatory T cells,(40–42) and salivary IgA(40;43) are associated with therapeutic effects of SLIT with aeroallergens and food allergens, but we did not examine these parameters specifically in this analysis. We were unable to identify subject characteristics that would predict therapeutic response to peanut SLIT; the only factor that was significantly different between responders and non-responders was the SCD at the baseline OFC in subjects during the first phase. However, since a successful response was defined as a 10-fold increase from the baseline SCD, subjects with a lower dose at baseline had to consume a lesser absolute amount of peanut powder at Week 44 to be considered a responder. Therefore, this finding may reflect our definition of a responder rather than being a true predictor of response to therapy. Although limited by the small sample size, this dose effect was not significant in the Crossover High Dose cohort.
In Peanut SLIT subjects, the majority of dose-related symptoms involved only the oropharyngeal mucosa. Subjects in the initial Peanut SLIT and Crossover High Dose arms reported symptoms that required treatment following 1 and 3% of doses, respectively, generally including only an oral antihistamine. These findings suggest an overall favorable safety profile of peanut SLIT. However, one subject experienced Grade 1 anaphylaxis(44) within 5 minutes following a home dose of 66 µg after safely consuming the same build-up dose without symptoms in the clinical research unit. Treatment included self-administration of diphenhydramine and epinephrine with urgent evaluation by study staff. The subject recovered without sequelae, but further dosing was discontinued. Similar sentinel events have been reported in other SLIT protocols.(45;46) Ten subjects were unable to complete the protocol, including 3 during the initial build-up period, primarily for reasons including poor compliance/loss of motivation, anxiety, perceived lack of efficacy, and poorly controlled asthma. Before peanut SLIT could be considered for use in the general population, further study is necessary to better understand the safety profile and develop methods to increase adherence.
Although several studies of OIT for food allergy have been published,(19;20)few rigorous trials have investigated SLIT.(29;30) In an ongoing, single-center placebo controlled clinical trial, Kim et al evaluated peanut SLIT in pediatric subjects.(25) Similar to the current study, they utilized a 1:1 randomization scheme, assigned a 2mg per day maintenance dose, and performed a per-protocol interim analysis of desensitization as the primary efficacy end point, measured by a 2.5g peanut protein (5g peanut powder) DBPCFC after 52 weeks of therapy. Interestingly, both studies (1) met their primary statistical end point; (2) showed significant variation in the clinical desensitization effect size; (3) demonstrated evidence of skin test (i.e., mast cell) suppression; and (4) observed increases in allergen-specific IgG4 levels among actively treated subjects. Although formal statistical comparisons are not possible, Kim et al reported a several-fold higher median tolerated dose and increased suppression of peanut-induced basophil activation. It is possible that by enrolling pediatric subjects aged 1–11 years and treating with 2mg per day, Kim et al may have capitalized on factors, including young age and less established immune deviation, that enabled a larger therapeutic effect. Alternatively, or in combination, the differences may be due to the overestimation of effect size that may occur in single-center interventional trials, as compared to multicenter trials.(47;48) Although promising, given the relatively modest clinical and immunologic effect observed in peanut-allergic subjects treated with SLIT, more study is needed to determine if this approach is clinically useful as a potential treatment for peanut allergy.
There are limitations to our study. First, our definition of success may not accurately predict therapeutic response to peanut SLIT, as there were subjects who had large increases in SCD from baseline (i.e. 500mg to >2g). However, these subjects were labeled as non-responders by definition since they failed to consume a 10-fold increase in SCD from baseline because they were starting from a relatively higher baseline SCD. Although these subjects were non-responders by study definition, they may have experienced some clinical benefit from peanut SLIT in protection from accidental exposures given the large increases in SCD from baseline. Second, subjects receiving active SLIT reported symptoms, primarily oropharyngeal pruritus, approximately 40% of the time whereas almost all placebo doses were symptom-free, a finding that could have potentially affected the blinding of the study. This concern is mitigated by measuring the primary outcome variable with a DBPCFC, the results of which are less influenced by knowledge of treatment assignment. Third, although done for safety reasons, we did not enroll peanut-allergic patients who had a history of life-threatening reactions. It is possible these patients may have a different therapeutic response, which is important to consider because these subjects may be more likely to seek treatment if an effective therapy becomes available. Therefore, it will be important in future clinical trials with peanut SLIT to include all severities of peanut-allergic subjects to potentially eliminate the bias seen in this study by not including those with severe anaphylaxis. Fourth, there was a high dropout rate in this trial, primarily due to logistical or personal reasons. Fifth, as in other SLIT studies(25;28;30) due to volume and potency constraints of available materials, dosing options for SLIT are limited. As a result, the doses given in SLIT are minuscule (mg) when compared to the much larger gram doses given in OIT. Finally, our results reflect only desensitization, and offer no insight into long-term tolerance or incorporation of the food into the normal diet.
Interestingly, two subjects in the placebo group developed spontaneous tolerance to peanut during the course of the trial. Data from observational natural history studies suggest spontaneous tolerance occurs in up to 20% of children with peanut allergy.(49–51) These and other studies have shown that tolerance development generally can be expected only in young children and those with low IgE levels. However, it remains possible that such observational studies may underestimate the rate of spontaneous tolerance development, especially in adolescents and adults.(52) This must be kept in mind when interpreting the results of clinical trials lacking an adequate control group.
In summary, we report the initial results of the first multicenter, randomized clinical trial of peanut SLIT. This potential therapy was relatively safe, and 44 weeks of treatment was sufficient to produce clinical desensitization in some subjects. The immunomodulatory effects of peanut SLIT studied here were modest during the first year of the study. Additional clinical benefits are noted when higher doses and longer treatment courses were used. Further investigation of SLIT as a treatment for food allergy is warranted.
Supplementary Material
Clinical implications.
Peanut sublingual immunotherapy safely induces some level of clinical desensitization in a majority of treated subjects when compared to placebo. Further study to determine whether it is a therapeutic option is warranted.
Acknowledgements
Additional Site Investigators: FM Atkins, DYM Leung, TT Perry.
Coordinators and support: D Brown, J Gau, K Mudd, S Driggers, P Steele, J Kamilaris, S Carlisle, A Hiegel, J Straw, P Mayfield, L Christie, M Groetch, J Slinkard, and S Leung.
We thank J Poyser, for managing the project for CoFAR (NIAID). We thank the families who kindly participated. We thank the staff of the clinical research units at each institution and the Statistical and Clinical Coordinating Center, without whose participation the study could not have been done. We thank Greer (Lenoir, NC) and Thermo Fisher Scientific (Waltham, MA) for supplying reagents.
Sources of support: NIH-NIAID U19AI066738 and U01AI066560. The project was also supported by Grant Numbers UL1 RR025780 from the National Center for Research Resources (NCRR)/National Institutes of Health (NIH) and UL1 TR000154 from the NIH/National Center for Advancing Translational Sciences (National Jewish); Grant Numbers UL1 TR000067 (Mount Sinai), UL1 TR000039 (Arkansas), UL 1 RR024128 (North Carolina) and UL1 RR 025005 (Johns Hopkins) from the NCRR. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Abbreviations
- OIT
oral immunotherapy
- SLIT
sublingual immunotherapy
- OFC
oral food challenge
- SPT
skin prick test
- DSMB
Data Safety Monitoring Board
- PN-IgE
peanut-specific IgE
- kUA/L
kilounits of antibody per liter
- DBPCFC
double-blind, placebo-controlled food challenge
- PN-IgG4
peanut-specific IgG4
- SCD
successfully consumed dose
- mgA/L
milligrams of antibody per liter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food: 2001–2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 4.Ford LS, Taylor SL, Pacenza R, Niemann LM, Lambrecht DM, Sicherer SH. Food allergen advisory labeling and product contamination with egg, milk, and peanut. J Allergy Clin Immunol. 2010;126:384–385. doi: 10.1016/j.jaci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Burks AW, Sampson HA. Clinical Features of Acute Allergic Reactions to Peanut and Tree Nuts in Children. Pediatrics. 1998;102:e6. doi: 10.1542/peds.102.1.e6. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM et al. Allergic Reactions to Foods in Preschool-Aged Children in a Prospective Observational Food Allergy Study. Pediatrics. 2012;130:e25–e32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark S, Bock SA, Gaeta TJ, Brenner BE, Cydulka RK, Camargo CA. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113:347–352. doi: 10.1016/j.jaci.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 8.Altschul AS, Scherrer DL, Munoz-Furlong A, Sicherer SH. Manufacturing and labeling issues for commercial products: relevance to food allergy. J Allergy Clin Immunol. 2001;108:468. doi: 10.1067/mai.2001.117794. [DOI] [PubMed] [Google Scholar]
- 9.Joshi P, Mofidi S, Sicherer SH. Interpretation of commercial food ingredient labels by parents of food-allergic children. J Allergy Clin Immunol. 2002;109:1019–1021. doi: 10.1067/mai.2002.123305. [DOI] [PubMed] [Google Scholar]
- 10.Sicherer SH, Noone SA, Munoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001;87:461–464. doi: 10.1016/S1081-1206(10)62258-2. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BL, Noone S, Munoz-Furlong A, Sicherer SH. Development of a questionnaire to measure quality of life in families with a child with food allergy. J Allergy Clin Immunol. 2004;114:1159–1163. doi: 10.1016/j.jaci.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol. 2006;96:415–421. doi: 10.1016/S1081-1206(10)60908-8. [DOI] [PubMed] [Google Scholar]
- 13.King RM, Knibb RC, Hourihane JO'B. Impact of Peanut Allergy on Quality of Life, Stress and Anxiety in the Family. Allergy. 2009;64:461–468. doi: 10.1111/j.1398-9995.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 14.DunnGalvin A, de BlokFlokstra BMJ, Burks AW, Dubois EJ, Hourihane JO'B. Food allergy QoL questionnaire for children aged 0–12 years: content, construct, and cross-cultural validity. Clin Exp Allergy. 2008;38:977–988. doi: 10.1111/j.1365-2222.2008.02978.x. [DOI] [PubMed] [Google Scholar]
- 15.Guest JF, Nagy E. Modelling the resource implications and budget impact of managing cow milk allergy in Australia. Curr Med Res Opin. 2009;25:339–349. doi: 10.1185/03007990802594685. [DOI] [PubMed] [Google Scholar]
- 16.Sladkevicius E, Nagy E, Lack G, Guest JF. Resource implications and budget impact of managing cow milk allergy in the UK. J Med Econ. 2010;13:119–128. doi: 10.3111/13696990903543242. [DOI] [PubMed] [Google Scholar]
- 17.Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011;128:110–115. doi: 10.1016/j.jaci.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Nelson HS, Lahr J, Rule R, Bock SA, Leung DY. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immun. 1997;99:744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 19.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–573. doi: 10.1016/j.jaci.2010.12.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral Immunotherapy for Treatment of Egg Allergy in Children. New Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheikh A, Nurmatov U, Venderbosch I, Bischoff E. Oral immunotherapy for the treatment of peanut allergy: systematic review of six case series studies. Prim Care Resp J. 2012;21:41–49. doi: 10.4104/pcrj.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson DR, Torres Lima M, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 23.Cox LS, Linnemann DseL, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: A comprehensive review. J Allergy Clin Immunol. 2006;117:1021–1035. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Perez N, Ambriz-Moreno MdJ, Canonica GW, Penagos M. Frequency of acute systemic reactions in patients with allergic rhinitis and asthma treated with sublingual immunotherapy. Ann Allergy Asthma Immunol. 2008;101:304–310. doi: 10.1016/s1081-1206(10)60496-6. [DOI] [PubMed] [Google Scholar]
- 25.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: Clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–646. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerzl R, Simonowa A, Ring J, Ollert M, Mempel M. Life-threatening anaphylaxis to kiwi fruit: Protective sublingual allergen immunotherapy effect persists even after discontinuation. J Allergy Clin Immunol. 2007;119:507–508. doi: 10.1016/j.jaci.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 27.Mempel M, Rakoski J, Ring J, Ollert M. Severe anaphylaxis to kiwi fruit: Immunologic changes related to successful sublingual allergen immunotherapy. J Allergy Clin Immunol. 2003;111:1406–1409. doi: 10.1067/mai.2003.1497. [DOI] [PubMed] [Google Scholar]
- 28.Enrique E, Pineda F, Malek T, Bartra J, Basagana M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: A randomized, double-blind, placebo controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073–1079. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Enrique E, Malek T, Pineda F, Palacios R, Bartra J, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a follow-up study. Ann Allergy Asthma Immunol. 2008;100:283–284. doi: 10.1016/S1081-1206(10)60456-5. [DOI] [PubMed] [Google Scholar]
- 30.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–455. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Rivas M, Garrido Fernandez S, Nadal JA, Alonso Diaz de Durana MD, Garcia BE, Gonzalez-Mancebo E, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64:876–883. doi: 10.1111/j.1398-9995.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 32.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009;123:789–794. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morriset M, Moneret-Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. 2007;39:12–19. [PubMed] [Google Scholar]
- 34.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–1269. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 35.Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol. 2008;121:343–347. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE-mediated cow's milk allergy. Allergy. 2004;59:980–987. doi: 10.1111/j.1398-9995.2004.00542.x. [DOI] [PubMed] [Google Scholar]
- 38.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008;122:1154–1160. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LCL, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010 Jul;126:83-91.e.1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 41.O'Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House Dust Mite Sublingual Immunotherapy. Am J Resp Crit Care Med. 2009;180:936–947. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 42.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1159–1162. doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson J, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: Summary report: Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 45.De Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass pollen tablet. Allergy. 2009;64:963–964. doi: 10.1111/j.1398-9995.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 46.Calderon MA, Simons FER, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–311. doi: 10.1111/j.1398-9995.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 47.Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ. 2012;344:e813. doi: 10.1136/bmj.e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-Center Trials Show Larger Treatment Effects Than Multicenter Trials: Evidence From a Meta-epidemiologic Study. Ann Int Med. 2011;155:39–51. doi: 10.7326/0003-4819-155-1-201107050-00006. [DOI] [PubMed] [Google Scholar]
- 49.Neuman-Sunshine DL, Eckman JA, Keet CA, Matsui EC, Peng RD, Lenehan PJ, et al. The natural history of persistent peanut allergy. Ann Allergy Asthma Immunol. 2012;108:326–331. doi: 10.1016/j.anai.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. The natural progression of peanut allergy: Resolution and the possibility of recurrence. J Allergy Clin Immunol. 2003;112:183–189. doi: 10.1067/mai.2003.1517. [DOI] [PubMed] [Google Scholar]
- 51.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–374. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 52.Savage JH, Limb SL, Brereton NH, Wood RA. The natural history of peanut allergy: Extending our knowledge beyond childhood. J Allergy Clin Immunol. 2007;120:717–719. doi: 10.1016/j.jaci.2007.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.