Abstract
Oligodendroglial cells undergo rapid transcriptional and dynamic morphological transformation in order to effectively myelinate neuronal axons. Olig1, a basic helix-loop-helix transcription factor, functions to promote the transcription of myelin-specific genes and promotes differentiation and (re)myelination. While the role for nuclear Olig1 is well established, the function for cytoplasmic Olig1 remains uncertain. We observe that translocation of Olig1 into the cytosol highly correlates with differentiation of oligodendrocytes both in vivo and in vitro. By enforcing expression of a nuclear-specific form of Olig1 into OPCs in a null-Olig1 background, we demonstrate that nuclear Olig1 is sufficient to facilitate MBP expression, but with greatly diminished membrane volume and area. We demonstrate that serine 138 in the helix-loop-helix domain of Olig1 is phosphorylated and that this form resides in the cytosol. Mutating serine 138 to alanine restricts Olig1 to the nucleus, facilitating MBP expression but limiting membrane expansion. However, a serine to aspartic acid mutation results in the cytoplasmic localization of Olig1 enhancing membrane expansion. Our results suggest a novel role for a phosphorylated cytosolic Olig1 in membrane expansion and maturation of oligodendrocytes.
Keywords: Olig1, phosphorylation, oligodendrocyte, differentiation
INTRODUCTION
Oligodendrocytes derive from progenitor cells and undergo a series of developmental changes prior to attaining the mature myelinating phenotype. These changes include myelin-specific gene expression and the essential morphological transformation for myelin sheet formation (Liu et al., 2003; Richter-Landsberg, 2000). In recent years, valuable insight has been provided concerning the transcriptional program necessary for the generation of oligodendroglial lineage cells and the differentiation of the oligodendrocyte precursor cell (OPC) into a mature myelin-forming oligodendrocyte (Emery, 2010). In particular, two basic helix-loop-helix transcription factors, Olig1 and Olig2, were identified to play essential roles in determining the oligodendroglial lineage and in the generation and maturation of oligodendrocytes (Ligon et al., 2006a; Lu et al., 2000; Takebayashi et al., 2000; Zhou et al., 2000). Olig1/2 are responsive to Sonic Hedgehog, a ventrally expressed morphogen, which is both sufficient and necessary to induce Olig gene expression for the generation of OPCs (Lu et al., 2000). Olig1/2 knockout mice fail to develop cells of the oligodendroglial lineage (Lu et al., 2002; Zhou and Anderson, 2002), which is thought to be attributed specifically to Olig2 function, as the Olig2-null mouse displays a similar impairment in the generation of oligodendroglia (Lu et al., 2002; Takebayashi et al., 2002).
It is widely held that Olig1 functions to promote differentiation of oligodendrocytes (Lu et al., 2001); however, it is unclear whether this role occurs during development or is specific to the repair/remyelination process. The null mouse in one particular study displays defects in myelin gene expression and myelination while maintaining a seemingly normal pool of OPCs until postnatal day 14, as the mice die prematurely (Xin et al., 2005). However, another study reports normal development of oligodendrocytes and myelination in the null mouse, but a significant requirement for Olig1 in remyelination after demyelinating insult (Arnett et al., 2004). It is thought that Olig1 participates in differentiation by promoting the expression of several myelin genes, including MBP, PLP, and MAG as well as suppressing GFAP, an astrocytic gene (Li et al., 2007; Xin et al., 2005). The localization of Olig1/2 is observed in the nucleus of OPCs during development; however, although Olig2 remains in the nucleus of OPCs in the adult mouse. The localization of Olig1/2 is observed in the nucleus of OPCs during development; however, although Olig2 remains in the nucleus of OPCs in the adult mouse, Olig1 is localized to the cytoplasm (Arnett et al., 2004). In a murine demyelination model and within tissue from multiple sclerosis patients, Olig1 is localized in the nucleus of OPCs as in development (Arnett et al., 2004; Balabanov and Popko, 2005). Recently, it was reported that cytosolic Olig1 is expressed in human oligodendrocytes upon process outgrowth and maintained at a high expression level during membrane maintenance prior to myelination (Othman et al., 2011). Could Olig1 in the cytosol contribute to oligodendrocyte maturation during development and in the remyelination process, and what mechanisms underlie the translocation of Olig1 from the nucleus to the cytosol?
In this study, we confirm that Olig1 translocation into the cytosol highly correlates with the differentiation of oligodendrocytes, the termination of the cell cycle and the phosphorylation of Olig1 both in vivo and in vitro. This suggests three major possibilities for cytosolic Olig1 function: (1) promoting oligodendrocyte differentiation, (2) inhibiting proliferation of OPCs, and (3) functioning in an alternative manner still yet unidentified. To investigate these possibilities, we initiated studies by enforcing expression of a nuclear-specific form of Olig1 into OPCs with a null-Olig1 background, and demonstrate that nuclear Olig1 is sufficient to facilitate MBP expression, similar to wildtype Olig1, but with greatly diminished membrane expansion. To further investigate the possible role of cytoplasmic Olig1 in the growth and expansion of the oligodendroglial membrane, we identify the phosphorylation of serine 138 in the helix-loop-helix domain of Olig1 and that this form resides in the cytosol. Mutating serine 138 to alanine restricts Olig1 to the nucleus, promoting MBP expression but limiting membrane expansion similar to the nuclear-specific form of Olig1. However, a serine to aspartic acid mutation, mimicking the charge associated with phosphorylation, results in the cytoplasmic localization of Olig1 enhancing membrane expansion. These results suggest a dual role for nuclear and cytoplasmic Olig1 in the myelin gene expression and morphological transformation of the oligodendrocyte.
MATERIALS AND METHODS
Animals
C57BL/6 mice were obtained from the Animal Facility Center of the Third Military Medical University, People’s Republic of China and paired to produce offspring. The timed postnatal mouse pups (P1, P7, P14, and P21) were anesthetized with 1% pentobarbital and transcardially perfused with 4% PFA in PBS. The brains were removed and dehydrated in 30% sucrose at 4°C for 24–48 h. Serial coronal sections (20 µm) were obtained using a cryostat microtome (MS 1900, Leica). The Olig+/− mice (Lu et al., 2002; Xin et al., 2005) were paired to produce offspring for OPC cultures using immunopanning techniques after PCR genotyping at P8–9. All animal studies were performed in accordance with the Health Guide for the Care and Use of Laboratory Animals in Third Military Medical University.
OPC Purification
The rat OPCs were propagated as our previously described (Niu et al., 2010). Briefly, mixed cells were isolated from cortex of P1–P3 Sprague–Dawley (SD) rats and enriched in OPC-growth medium followed by two passages to enrich cell numbers. To examine gene expression during differentiation, 500 µL of single-cell suspensions were plated into 24-well plates with coated glass cover slips (4.5 × 104 cells/well) and 3 mL (3 × 105/mL) of single-cell suspensions were plated into 60 mm dishes. After 12 h incubation in the OPC-growth medium, the OPCs were induced to differentiate using OPC-differentiation medium (DMEM/F12 + 1% N2-supplement + 1% fetal bovine serum + 5 mg/mL insulin). At the indicated time points, the cells were fixed with 4% paraformaldehyde (PFA) for immuofluorescence (IF), and the cells in dishes were harvested for immunoprecipitation (IP) and/or Western blotting.
The mouse OPCs were prepared by immunopanning from P8–9 mouse cortices as previously described (Chan et al., 2004). Briefly, panning dishes were incubated with secondary and primary antibodies sequentially. Mouse brain cerebral hemispheres were diced and dissociated with papain at 37°C for 75 min. After trituration, cells were resuspended in a panning buffer and then incubated at room temperature sequentially on three immunopanning dishes: Ran-2, GalC, and PDGFαR. PDGFαR positive mouse OPCs were released from the final panning dish by 0.25% trypsin-EDTA (Invitrogen). The purified mouse OPCs (4.5 × 104 cells/well) were plated into 24-well plates with coated cover slips or 60 mm dishes and infected with lentivirus for 48 h and then cultured with OPC-differentiation medium for another 4 days. The protein collected for Western blotting analysis, and the cells were fixed for immunocytochemistry.
Plasmid Vector Construction and OPC Transfection/Transduction
Wild-type mouse Olig1 was subcloned into pEGFP and pWPI vectors. The NLS-Olig1 sequence was subcloned into the pWPI vector. pEGFP-Olig1-S138A, pEGFP-Olig1-S138D constructs were generated using Stratagene’s Quik Change site-directed mutagenesis Kit following the manufacturer’s instructions. Four plasmid vectors (pEGFP, pEGFP-Olig1-S138A, pEGFP-Olig1-S138D, and pEGFP-wt-Olig1) were transfected into OPCs using Amaxa Nucleofector Kit (Lonza) for rat oligodendrocytes according to the manufacturer’s instruction. After electroporation, the OPCs were plated into 24-well plates with Poly-D-Lysine coated cover slips and cultured with OPC differentiation-medium at indicated time points. Three lentivirus (EGFP-vector, NLS-Olig1, and wt-Olig1) were produced using 293FT cells. After transfecting the 293 FT cells, the cells were transferred into DMEM medium and cultured for 48 h before collecting the supernatant. The supernatant was supplemented with 1% N2-supplement, 10 ng/mL PDGF-AA (Peprotech) and 10 ng/mL bFGF (Peprotech) to generate the lentivirus infection medium. The purified mouse OPCs in 24-well plates or 60 mm dishes were infected with 500 µL or 3 mL of the lentivirus infection medium separately for 48 h before cultured with OPC differentiation-medium for indicated experiments.
Immunocytochemistry, Morphometric Analysis, and Quantification
Cells on coverslips or the brain sections were incubated in primary antibodies overnight at 4°C followed by the fluorescence-conjugated secondary antibodies incubated at room temperature (RT) for 1 h. All primary antibodies used and the respective dilutions are provided in Table 1. Cell nuclei were stained with DAPI (0.1 µg/mL in PBS). The immunoreactivity was determined using a 20× objective lens on a fluorescence microscope (Olympus BX-60) and a confocal laser-scanning microscope (Olympus) with an excitation wavelength appropriate for FITC (488 nm), Cy3 (552 nm), or Cy5 (625 nm). Cell counting was conducted on nine randomly chosen fields for each sample (Media Cybernetics, Silver Spring, MD). Morphometric analysis was performed using the Sholl analysis on the Pro Plus image analysis system by using standard concentric circles to evaluate the diameter and area of oligodendroglia (Rajasekharan et al., 2009). Briefly, the program superimposes a grid of five concentric circles with increasing radii on an oligodendrocyte cell body, and then measures the number of intersections and the membrane expansion generated by the oligodendrocytes with each circle. At least nine representative fields were randomly acquired at 400× magnifications from each of two experiments performed in triplicate.
TABLE 1.
Primary Antibodies
| Primary antibodies | |||
|---|---|---|---|
| Antigen | Source | Dilution | Supplier |
| Olig1 | Rabbit | 1:1000 (ICC) | Abcam |
| Olig1 | Mouse | 1:100 (ICC) | Santa Cruz |
| 1:1000 (WB) | |||
| 10 µL/300 µg(IP) | |||
| PDGFRα | Rabbit | 1:100 (ICC) | Santa Cruz |
| 10 µL/dish (Panning) | |||
| NG2 | Rabbit | 1:200 (ICC) | Millipore |
| O4 | Mouse | 1:100 (ICC) | Sigma |
| CC1 | Mouse | 1:200 (ICC) | Millipore |
| MBP | Goat | 1:500 (ICC) | Santa Cruz |
| 1:1000 (WB) | |||
| Menin | Rabbit | 1:1000 (WB) | Bethyl |
| Tublin | Mouse | 1:2000 (WB) | Millipore |
| β-actin | Mouse | 1:2000 (WB) | Santa Cruz |
| Phospho-serine | Rabbit | 1:500 (WB) | Millipore |
| 10 µL/300 µg (IP) | |||
Immunoprecipitation and Western blotting
The nuclear/cytoplasmic fractionation was performed following the protocol by Mo (Mo et al., 2008). SDS-PAGE Western-blotting was carried out as described previously (Niu et al., 2010). β-actin was used as a loading control for the total protein loaded, menin was used as an internal control for the nuclear protein, and tubulin for the cytoplasmic protein. The primary antibodies are listed in Table 1. Proteins were transferred to polyvinylidene difluoride membranes, and visualized by chemiluminescence (ECLplus, GE Healthcare). Band intensity was quantified using the Image Pro Plus image analysis system.
Statistical Analysis
Statistical analyses were performed using One- or two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Comparisons between two experimental groups were made using Student’s t test. A probability of P < 0.05 was considered statistically significant.
RESULTS
The Translocation of Olig1 from the Nucleus to Cytosol Correlates with Oligodendroglial Differentiation and Myelination
As previously described by Arnett (2004), the cytoplasmic localization of Olig1 during development and after demyelination highly correlates with differentiation of oligodendrocytes. To examine the possible function of this translocation event, we initially examined the localization of Olig1 by immunostaining. During the development of the mouse brain, Olig1 localizes to the nucleus at postnatal day 1 (P1), transfers to the cytosol at p7, and by P14, 84.3% of the OPCs exhibit cytoplasmic Olig1. By P21 only a few remaining cells display nuclear Olig1 (Fig. 1A). Concomitantly, the translocation of Olig1 correlates with the expression of myelin basic protein (MBP) and oligodendrocyte myelination (Fig. 1A). We further examined whether Olig1 translocation occurs during specific developmental stages of the OPC. Olig1 (red) is localized to the nucleus in PDGFαR+ cells (green) at P1 representing early OPCs, and in NG2+ cells (green) at P9 representing OPCs. Partial translocation into cytoplasm can be observed in weakly stained NG2+ cells (green) at P9, and may represent more mature OPCs. Cytoplasmic localization of Olig1 is clearly observed in CC1+ cells (green) at P14 and represent immature and mature oligodendrocytes (Fig. 1B). Quantification of the cytoplasmic localization of Olig1 inversely correlates with proliferating OPCs as Ki67 positive cells display nuclear Olig1. Oligodendroglial cells with expression of cytoplasmic Olig1 display a significant decrease in Ki67 positive cells, correlating with cell cycle exit (Fig. 1C,D).
Fig. 1.
Localization of Olig1 from nucleus to cytoplasm in oligodendroglial cells during development. (A) Olig1 (red) is localized in the nucleus of OPCs at postnatal day 1(P1) and begins to translocate into the cytoplasm at P7. By P14, the majority of the oligodendroglial cells display cytoplasmic Olig1 with the concomitant expression of MBP (green). (B) Olig1 (red) is localized in the nucleus (arrowhead) of PDGFRα+ cells (green) at P1 and NG2+ cells (green) at P9, transfers into the cytoplasm (thin arrow) of weak NG2+ cells at P9, and is specifically localized to the cytoplasm (thin arrow) in CC1+ cells (green) at P14. (C) Nuclear localization of Olig1 correlates with proliferating OPCs as Ki67 positive (arrowhead). Oligodendroglial cells with cytoplasmic Olig1 display a Ki67 negative nucleus (thin arrow). (D) Quantification of cytoplasmic and nuclear Olig1 cells during development, the cytoplasmic localization of Olig1 inversely correlates with proliferating OPCs as Ki67 positive cells display nuclear Olig1. Data represent mean ± SEM (4 animals in each group) **P < 0.01, *P < 0.05.
To further resolve the relationship between the translocation of Olig1 and the differentiation of oligodendroglia, we examined Olig1 localization in vitro. Similar to in vivo observations, Olig1 (red) localizes to the nucleus when OPCs are actively proliferating and expressing PDGFRα (green). Upon stimulation of differentiation for three days (O4+, green) and five days (MBP+, green), Olig1 (red) predominantly localizes into the cytosol and subsequently produces expansive membrane sheets (Fig. 2A). Quantification illustrates that the percentage of cytoplasmic Olig1 increases upon differentiation and after 3 days in differentiation medium, 88.7% of the all Olig1 positive cells contain cytoplasmic Olig1 (Fig. 2C). Additionally, the vast majority of Ki67 positive OPCs display nuclear Olig1, suggesting that cells with cytoplasmic Olig1 have exited the cell cycle (Fig. 2B). By extracting nuclear and cytoplasmic fractions from the oligodendroglia, we find that the nuclear Olig1 levels are significantly decreased whereas the cytoplasmic Olig1 levels are increased following differentiation (Fig. 2D,E). While we observe a high correlation between differentiation and membrane expansion of the oligodendrocyte with the cytoplasmic localization of Olig1, demonstrating a direct link remains unclear.
Fig. 2.
Translocation of Olig1 from nucleus to cytoplasm correlates with the differentiation of OPCs to oligodendrocytes in vitro. (A) Olig1 (red) is localized to the nucleus of OPCs when cultured in proliferation medium (PDGFRα+, green); upon induction of differentiation for three days (O4+, green) or five days (MBP+, green), Olig1 translocates from the nucleus to the cytoplasm. (B) OPCs with nuclear Olig1 are Ki67 positive (arrowhead). Oligodendroglial cells with cytoplasmic Olig1 are Ki67 negative (thin arrow). (C) Quantification of cytoplasmic Olig1+ cells upon differentiation of OPCs into oligodendrocytes and the Ki67 positive cells after 8 hours in differentiation medium. Data represent mean ± SEM (three experiments performed in triplicate). **P < 0.01. (D, E) Nuclear and cytoplasmic fractionations were performed and reveal that nuclear Olig1 detection decreases following the differentiation of OPCs into oligodendrocytes with the concomitant increase in the detection of cytoplasmic Olig1.
Nuclear Olig1 is Sufficient to Facilitate MBP Expression and Differentiation but not for Membrane Expansion and Growth
The translocation of Olig1 greatly correlates with the differentiation and membrane expansion of oligodendroglia both in vivo and in vitro; however, demonstrating causation remains uncertain. To determine the role of Olig1 translocation, we enforced expression of a nuclear-specific form of Olig1 and the corresponding controls into OPCs with a null-Olig1 background using a lentiviral vector. Olig1 (purple) containing the nuclear location signal (NLS), specifically localizes to the nucleus while wt-Olig1 localizes in cytosol normally as previously described (Fig. 3A). Comparing the maturation of OPCs with the EGFP-vector, wt-Olig1 and NLS-Olig1, the percentage of MBP+/GFP+ oligodendroglia is significantly enhanced in the presence of the wt-Olig1 and NLS-Olig1; however, no significant difference could be detected between NLS-Olig1 and wt-Olig1 groups (Fig. 3B). It is important to note that wt-Olig1 greatly enhances membrane expansion and sheet formation (MBP+, red) in Olig1−/− mouse OPCs; whereas the NLS-Olig1 and EGFP expressing OPCs display reduced membrane sheet formation (Fig. 3A). Morphometric and quantitative analysis, performed on the expansion of MBP+ membrane area in transduced cells clearly demonstrate that the MBP+ membrane areas are significantly increased in wt-Olig1 expressing cells (Fig. 3C), and to a lesser extent in the NLS-Olig1 expressing cells. Western blotting analyses clearly demonstrates that NLS-Olig1 is sufficient to mimic wt-Olig1 and facilitate MBP expression both at day 2 and 4 (Fig. 3D,E). Is it therefore possible that Olig1 has two specific roles: (1) Nuclear Olig1 facilitates the myelin gene expression and promotes the maturation of OPCs to oligodendrocytes and (2) Cytoplasmic Olig1 contributes to the membrane expansion and growth of oligodendrocytes?
Fig. 3.
Restricting Olig1 localization to the nucleus inhibits the outgrowth and membrane expansion of oligodendroglial cells but does not alter MBP expression. (A) Enforced expression of Olig1 (purple) in OPCs with a null-Olig1 background was accomplished by lentiviral transduction. Oligodendroglial cells expressing wt-Olig1, NLS-Olig1, or Olig1 null cells were induced to differentiate for 2 or 4 days. Olig1 containing a nuclear location signal (NLS) specifically localized to the nucleus (arrow-head), while wt-Olig1 appeared locate in cytoplasm (thin arrow) at Day4. The third panel depicts the morphometric analysis of oligodendrocyte membrane area of MBP+/GFP+ cells. (B, C) Quantification of the number and membrane sheet area of MBP+/GFP+ cells, no significant difference in the percentage of MBP+ cells was observed between the NLS-Olig1 and wt-Olig1 expressing cells; however, a significant increase was observed between both groups when compared with the lenti-vector control (Olig1 null cells). Data represent the mean ± SEM (three experiments performed in triplicate). *P < 0.05. **P < 0.01. (D) Western-blotting, expression of Olig1 and MBP in wt-Olig1, NLS-Olig1, or Olig1 null cells. (E) Quantification of MBP and Olig1 expression, the NLS-Olig1 is sufficient to mimic wt-Olig1 and promote MBP expression both at Day 2 and Day 4 in vitro. **P < 0.01 (3 experiments performed in duplication).
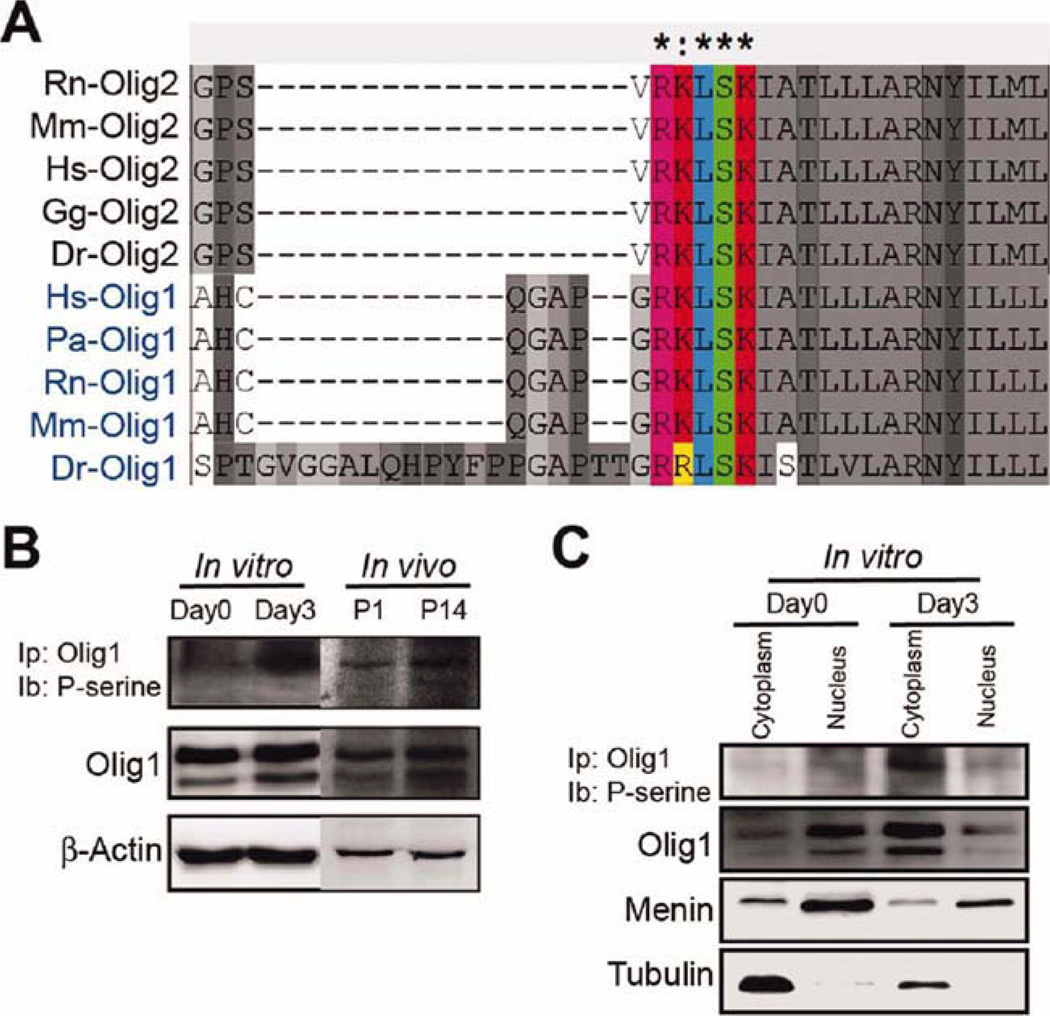
Olig1 Protein can be Phosphorylated and Phosphorylation Correlates with Subcellular Localization and Differentiation
From our data, we hypothesize that Olig1 may have two distinct functions during oligodendroglial maturation. Recently, it has been demonstrated that the function of Olig2 in the neuron-oligodendroglial fate switch depends on the phosphorylation status of Olig2 (Li et al., 2011; Sun et al., 2011). If such a mechanism exists for Olig2, is it possible that the potential role switch of Olig1 is also due to a posttranslational phosphorylation modification? Sequence alignment analysis of Olig1 and Olig2 reveals a possible homologs serine phosphorylation motif (serine-138) in the bHLH domain. This phosphorylation site is conserved across multiple species (Hs, Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus; Gg, Gallus gallus; Dr, Danio rerio; Pa, Papio anubis) and is analogs to serine-147 in Olig2 (Fig. 4A). To verify Olig1 phosphorylation, we performed IP with an anti-Olig1 antibody and probed with an antiphosphoserine antibody. The results clearly reveal that Olig1 is indeed phosphorylated both in vitro and in vivo; and that higher levels of phosphorylated Olig1 correlate with differentiation (Fig. 4B). To further confirm this observation, we performed nuclear and cytoplasmic extractions and examined the phosphorylation status of Olig1 in these fractions. The results clearly demonstrate that during differentiation, phosphorylated Olig1 specifically localizes to the cytosol of oligodendroglia and that during OPC proliferation, phosphorylation is undetectable both in the nucleus and cytosol (Fig. 4C). We conclude that Olig1 phosphorylation correlates with the developmental maturation of oligodendroglia and the specific subcellular localization. How does this posttranslational modification of Olig1 coincide with the translocation from the nucleus to the cytoplasm and what potential function underlies this phosphorylation event?
Fig. 4.
Olig1 protein is phosphorylated at serine 138 and the phosphorylation state correlates with the differentiation of OPCs into oligodendrocytes. (A) Sequence alignment of Olig1 and Olig2 genes. A predicted PKA phosphorylation motif containing S138 was found in the Olig1 bHLH domain and conserved among different species (Hs, Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus; Gg, Gallus gallus; Dr, Danio rerio). (B) Immunoprecipitation (IP) revealed that Olig1 is phosphorylated when undergoing differentiation for 3 days in vitro and 14 days in vivo (performed in triplicate). (C) Phosphorylated Olig1 correlated with differentiation and appeared to localize specifically within the cytosol (two experiments performed in duplication). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Serine-138 Mutation Alters the Localization of Olig1 and Specifies Function in Oligodendroglia
Is it possible that the phosphorylation status of Olig1 plays an instructive role in the subcellular localization? To investigate the role of phosphorylation, we constructed two mutants of Olig1 by substituting the serine-138 with alanine (Ala, A) to disrupt phosphorylation or with aspartic acid (Asp, D) to mimic the charge resulting from phosphorylation. Each of the mutants, as well as wildtype Olig1 was constructed with EGFP to allow for the examination of the subcellular localization (Fig. 5A). Surprisingly, upon expression of the mutants of Olig1 into OPCs, we observe that the Olig1-S138A is primarily localized in nucleus, while the Olig1-S138D is detected predominantly in the cytoplasmic compartment at steady state (Fig. 5B). Western blot analyses of the nuclear/cytoplasmic fractions reveal similar results (Fig. 5C). While these results suggest that phosphorylation of Olig1 may be instructive for the OL development, what is the potential role of Olig1 phosphorylation in the specification of function?
Fig. 5.
Point mutations of S138 alter the phosphorylation and localization of Olig1. (A) Two mutant constructs were generated expressing a serine to alanine and a serine to aspartic acid mutation at serine 138 of Olig1. The mutant constructs were linked to GFP in order to allow detection of the protein. (B) After transfection of the constructs into Olig1 null OPCs, cells were cultured for an additional 48 h. OPCs expressing the Olig1-S138A mutation displayed nuclear localized Olig1 (arrowhead), whereas the Olig1-S138D mutation displayed cytoplasmic localization of Olig1 (thin arrow), wt-Olig1 was detected with both nuclear and cytoplasmic localization (nuclear: arrowhead; cytoplasmic: thin arrow) (two experiments performed in triplicate). (C) Olig1 null cells were transfected with the constructs and cultured for an additional 48 h. Nuclear and cytoplasmic fractionation were performed and subjected to Western blot analysis. The results reveal that the Olig1-S138A mutation is primarily localized in nucleus and that the Olig1-S138D mutation is predominantly cytoplasmic at the steady state (experiments performed in duplicate). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To determine the role of Olig1 phosphorylation, we enforced expression of the mutant forms of Olig1 and the corresponding controls into OPCs using an EGFP vector. The OPCs expressing the mutant or wt-Olig1 did not display any observable phenotypic differences (Fig. 5B); however, after OPCs were transferred to the differentiation medium for five days, the oligodendrocytes with wt-Olig1 or Olig1-S138D exhibited more elaborate cell processes and more extensive membrane sheet area than that of oligodendrocytes with Olig1-S138A (Fig. 6A). Morphometric analysis performed on membrane area and diameter in O4+ or GFP+ and MBP+ or GFP+ cells demonstrates a significant difference in the membrane area between Olig1-S138A and Olig1-S138D (Fig. 6B,C). The Western blot analyses revealed that Olig1-S138A is sufficient to facilitate MBP expression, recapitulating the function of wt-Olig1 in nuclei. Interestingly, Olig1-S138D also enhanced MBP expression to certain extent (Fig. 6D). This could be due to the fact that a small fraction of Olig1-S138D is still detected in the nucleus, which could promote MBP expression.
Fig. 6.
Olig1 promotes membrane expansion and maturation of oligodendrocytes. OPCs in a null-Olig1 background were transfected with the phosphorylation mutant Olig1 constructs using Amaxa nucleofection and cultured in OPC-differentiation media for 3 or 5 days. (A) Immunostaining using an anti-O4 antibody (white) on OPCs with the expressing of wt-Olig1, Olig1-S138D, Olig1-S138A constructs after 3 days of differentiation. After 5 days of differentiation, membrane expansion is significantly enhanced in the MBP+ OLs (white) with the expressing of wt-Olig1 or Olig1-S138D compared with OLs with the expressing of Olig1-S138A. (B, C) Morphometric analysis and quantification of membrane sheet area and the diameter of OLs with the expressing of Olig1-S138A, Olig1-S138D, or wt-Olig1 at Day 5 after differentiation. The membrane areas were quantified by tracing the O4 or MBP-positive areas (gray) in the GFP-positive cells (green). The diameters were quantified by measuring the number of processes that intersected with the concentric circles (yellow), reflecting an increase in the diameter of the cells. Data represent mean ± SEM (two experiments performed in triplicate), *P < 0.05, **P < 0.01. (D) Western blot analysis of MBP expression of OLs expressing Olig1-S138A, Olig1-S138D, or wt-Olig1 at Day 5 after differentiation. The result illustrates that Olig1-S138A and wt-Olig1 enahnce MBP expression, and while Olig1-S138D also facilitates MBP expression, it is slightly diminished in comparison.
DISCUSSION
OPCs differentiate into myelin-forming oligodendrocytes via a series of distinct developmental steps (Gobert et al., 2009; Stangel and Hartung, 2002). OPCs need to exit the cell cycle, discontinue migration, express myelin-specific genes, and undergo the essential morphological transformation in order to initiate myelination (Baumann and PhamDinh, 2001; Richter-Landsberg, 2000). As an individual oligodendrocyte has the capacity to form multiple myelin internodes, myelination is not solely dependent on myelin gene expression. We hypothesize that oligodendrocytes must balance both extrinsic cues and intrinsic mechanisms to modulate membrane expansion and process outgrowth to generate the precise number of myelin segments. In order for this to be true, the mechanisms regulating the transcriptional program and the expansion and growth of the membrane cannot be the identical. Identification of these mechanisms would allow for the uncoupling of these processes, and providing valuable insight into the differentiation and myelination by oligodendrocytes. Two distinct and sequential mechanisms would ensure the proper temporal expression of myelin genes and the spatial regulation for the deposition of myelin.
In recent years, valuable insight has been provided concerning the transcriptional program necessary for the differentiation of the OPC into a mature myelin-forming oligodendrocyte (Emery, 2010). These factors include, MRF, Sox10, Nkx2.2, and YY1 to name a few (Emery et al., 2009; He et al., 2007; Qi et al., 2001; Stolt et al., 2002). In particular, Olig1 and Olig2 were identified to play essential roles in determining both the oligodendroglial lineage and in the generation and maturation of oligodendrocytes (Ligon et al., 2006a,b; Zhou et al., 2000). Olig1, as a transcription factor, is thought to regulate myelin gene expression, but translocation of Olig1 to the cytosol during differentiation suggests that it may have an additional function. Previously, Olig1 was shown to localize in the nucleus of neonatal mice and translocated into the cytoplasm by 2 weeks, when the majority of oligodendrocytes are maturing (Arnett et al., 2004). Recently, it has been reported that O1 positive multipolar OPCs with nuclear expression of Olig1 exhibited fewer processes, while OPCs with cytoplasmic Olig1 possessed more extensive membrane expansion (Othman et al., 2011). Our data further demonstrates that nuclear Olig1 facilitates MBP expression and differentiation but when Olig1 localizes to the cytosol during development, it enhances membrane expansion and process outgrowth (Fig. 3). These findings imply that Olig1 has dual functions in promoting gene expression and membrane expansion via translocation from the nucleus to the cytosol, respectively.
Recently, it was reported that Olig2, a highly homologs transcription factor to Olig1, switches the neuronoligodendroglial fate determination via phosphorylation (Li et al., 2011; Sun et al., 2011; Yates, 2011). Bioinformatic analyses of Olig1 using NetPhosK 1.0 Server (Blom et al., 2004) predicts 20 potential phosphorylation sites, and the serine phosphorylation motif (S138) is the most likely site for PKA, PKC, and PKG phosphorylation. Sequence alignment analysis of Olig1 and Olig2 reveals that this serine (Olig1-S138 and Olig2-S147) resides in the bHLH domain and is conserved across multiple species. Our data identifies that phosphorylation of serine 138 of Olig1 is highly correlative with the translocation of Olig1 and OPC maturation (Fig. 4). Furthermore, the gain or loss of phosphorylation by mutating S138 to aspartic acid or alanine, respectively alters the localization of Olig1 in OPCs and impacts process outgrowth, membrane expansion and MBP expression. While S138D phospho-mimetic Olig1 mutant is predominantly cytoplasmic localization and enhanced membrane expansion, when compared with wt-Olig1, membrane expansion remains diminished (Figs. 5 and 6). Furthermore, Olig1-S138D also increases MBP expression slightly (Fig. 6D), suggesting a small fraction of Olig1-S138D remains partially in nucleus to manipulate MBP expression. These results suggest that the S138D may not be a sufficient mimic of phosphorylation and/or that other potential phosphorylation sites of Olig1 remain to be identified. Upon further analysis of other phosphorylation sites, Olig1-S48 is conserved with Olig2-T43, which regulates neural progenitor proliferation (Sun et al., 2011); Olig1-S55/T202 are also “high-confidence” phosphorylation sites across multiple species; and Olig1-S44/S230 are two likely target sites for glycogen synthase kinase-3 (GSK3), which promotes motor neuron specification by serine phosphorylation (Ma et al., 2008). While these additional sites (and others) may be involved in Olig1 function, they will require further investigation and detailed characterization.
Why would Olig1 have two specific functions during oligodendroglial development? We have demonstrated that the translocation of Olig1 consistently correlates with the differentiation and myelination of oligodendrocytes (Figs. 1 and 2). If two events must occur in an exact time sequence, using one molecule to initiate these two events may be an easy and effective strategy. During the differentiation and myelination by oligodendroglia, myelin gene expression and the membrane expansion are two separate and uncoupled events, which have to occur successively to form a functional myelin sheath along axons. The translocation and switch of function of Olig1 tightly links these two events and prevents overlap. We hypothesize that Olig1 promotes myelin gene expression while in the nucleus (Fig. 3), at this time OPCs receive instruction from an unknown axonal cue(s) stabilizing axonal recognition and contact, after which Olig1 changes the phospho-state (Fig. 4), and translocates into the cytosol to enhance outgrowth of cell processes and/or membrane expansion (Figs. 5 and 6).
What do the membrane expansion and process outgrowth imply in myelination? It is clearly evident that myelination is not an all or none event that is solely controlled by transcriptional programs. Myelination is a graded process, which requires a series of highly orchestrated regulations that balance both the myelin-specific gene expression and the morphological transformation (Emery, 2010; Liu et al., 2003; Richter-Landsberg, 2000). A recent finding showed that individual oligodendrocytes have the capacity to synthesize different numbers of myelin internodes, with lengths that vary from 40 to 400 µm (Chong et al., 2012). This finding suggests that maximizing the myelinogenic capacity of individual oligodendrocyte could facilitate the generation of myelin internodes along axonal tracts, and may offer a more effective strategy for promoting myelination. We propose that enhancing membrane expansion and promoting process outgrowth would help to maximize the myelinogenic capacity, namely to form more and longer myelin internodes, and thereby increase the rate of myelination or the overall extent.
Currently, many diseases and abnormalities are considered closely associated with the maldevelopment of oligodendroglial cells and/or the failure of remyelination (Levine et al., 2001; Lin and Bergles, 2004). In this manuscript we demonstrate that nuclear Olig1 is sufficient to promote MBP expression, whereas cytoplasmic Olig1 greatly enhances membrane volume and area. Serine 138 in the helix-loop-helix domain of Olig1 is phosphorylated and this form resides in the cytosol, providing a switch in function during the differentiation and myelination by oligodendrocytes. These findings provide valuable insight for uncoupling myelin gene expression from membrane expansion during the oligodendroglial differentiation and myelination, and may contribute to future therapeutic approaches to promote remyelination after injury and/or disease.
Acknowledgments
Grant sponsor: National Natural Science Foundation of China (NSCF); Grant number: 30870801, 30900465; Grant sponsor: Natural Science Foundation Project of Chongqing; Grant number: CQ CSTC, 2010BB5157.
REFERENCES
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Popko B. Myelin repair: Developmental myelination redux? Nat Neurosci. 2005;8:262–264. doi: 10.1038/nn0305-262. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJ, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert RP, Joubert L, Curchod ML, Salvat C, Foucault I, Jorand-Lebrun C, Lamarine M, Peixoto H, Vignaud C, Fremaux C, Jomotte T, Francon B, Alliod C, Bernasconi L, Abderrahim H, Perrin D, Bombrun A, Zanoguera F, Rommel C, Hooft van Huijsduijnen R. Convergent functional genomics of oligodendrocyte differentiation identifies multiple autoinhibitory signaling circuits. Mol Cell Biol. 2009;29:1538–1553. doi: 10.1128/MCB.01375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Li H, de Faria JP, Andrew P, Nitarska J, Richardson WD. Phosphorylation regulates OLIG2 cofactor choice and the motor neuronoligodendrocyte fate switch. Neuron. 2011;69:918–929. doi: 10.1016/j.neuron.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lu Y, Smith HK, Richardson WD. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J Neurosci. 2007;27:14375–14382. doi: 10.1523/JNEUROSCI.4456-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006a;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006b;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Liu A, Muggironi M, Marin-Husstege M, Casaccia-Bonnefil P. Oligodendrocyte process outgrowth in vitro is modulated by epigenetic regulation of cytoskeletal severing proteins. Glia. 2003;44:264–274. doi: 10.1002/glia.10290. [DOI] [PubMed] [Google Scholar]
- Lu QR, Cai L, Rowitch D, Cepko CL, Stiles CD. Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat Neurosci. 2001;4:973–974. doi: 10.1038/nn718. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog—regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Ma YC, Song MR, Park JP, Henry Ho HY, Hu L, Kurtev MV, Zieg J, Ma Q, Pfaff SL, Greenberg ME. Regulation of motor neuron specification by phosphorylation of neurogenin 2. Neuron. 2008;58:65–77. doi: 10.1016/j.neuron.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo W, Zhang L, Yang G, Zhai J, Hu Z, Chen Y, Chen X, Hui L, Huang R, Hu G. Nuclear beta-arrestin1 functions as a scaffold for the dephosphorylation of STAT1 and moderates the antiviral activity of IFN-gamma. Mol Cell. 2008;31:695–707. doi: 10.1016/j.molcel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Niu J, Mei F, Li N, Wang H, Li X, Kong J, Xiao L. Haloperidol promotes proliferation but inhibits differentiation in rat oligodendrocyte progenitor cell cultures. Biochem Cell Biol. 2010;88:611–620. doi: 10.1139/O09-178. [DOI] [PubMed] [Google Scholar]
- Othman A, Frim DM, Polak P, Vujicic S, Arnason BG, Boullerne AI. Olig1 is expressed in human oligodendrocytes during maturation and regeneration. Glia. 2011;59:914–926. doi: 10.1002/glia.21163. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136:415–426. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C. The oligodendroglia cytoskeleton in health and disease. J Neurosci Res. 2000;59:11–18. doi: 10.1002/(sici)1097-4547(20000101)59:1<11::aid-jnr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stangel M, Hartung HP. Remyelinating strategies for the treatment of multiple sclerosis. Prog Neurobiol. 2002;68:361–376. doi: 10.1016/s0301-0082(02)00105-3. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Meijer DH, Alberta JA, Mehta S, Kane MF, Tien AC, Fu H, Petryniak MA, Potter GB, Liu Z, Powers JF, Runquist IS, Rowitch DH, Stiles CD. Phosphorylation state of Olig2 regulates proliferation of neural progenitors. Neuron. 2011;69:906–917. doi: 10.1016/j.neuron.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Ohtsuki T, Uchida T, Kawamoto S, Okubo K, Ikenaka K, Takeichi M, Chisaka O, Nabeshima Y. Non-overlapping expression of Olig3 and Olig2 in the embryonic neural tube. Mech Dev. 2002;113:169–174. doi: 10.1016/s0925-4773(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99:143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. Development: Directing development through phosphorylation. Nat Rev Neurosci. 2011;12:248–249. doi: 10.1038/nrn3030. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]