Abstract
A 29-year-old man with vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal defects, and limb defects (VACTERL) presented with headache, photophobia, and worsening nystagmus. He had near-normal visual acuity and visual fields, absent stereopsis, and see-saw nystagmus. Brain MRI revealed a thin remnant of the optic chiasm but normal-sized optic nerves. Functional MRI during monocular visual stimulation demonstrated non-crossing of the visual evoked responses in the occipital cortex, confirming achiasma. These findings have not previously been reported in VACTERL.
The formation of the optic chiasm by the optic nerves has fascinated anatomists for centuries and continues to be vigorously studied today (1). In congenital achiasma, there are few or no crossing fibers, leading to decreased vision, strabismus, and nystagmus (2–7). Although achiasma usually occurs in the absence of other developmental anomalies, we report a case of achiasma in the setting of a congenital syndrome involving vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula with atresia, renal defects, and limb defects (VACTERL), a finding not previously described.
Case Report
A 29-year-old normally pigmented Caucasian man with VACTERL was evaluated for a 2-year history of gradually worsening headache, blurred vision, and nystagmus. As a child, he had mild infantile nystagmus with relatively normal visual function. His axial and appendicular abnormalities, including tracheoesophageal fistula, cleft palate, shortened radius, and finger abnormalities, had been surgically repaired. Attention deficit disorder had been diagnosed when he was a child and bipolar affective disorder in adulthood. Even so, he completed high school and worked full-time.
Best-corrected visual acuities were 20/20 in the right eye and 20/25 in the left eye with a small left relative afferent pupillary defect. Anterior and posterior segments were normal. Goldmann visual fields were slightly restricted bilaterally, left greater than right. Color perception was normal by Hardy-Rand-Rittler pseudoisochromatic plates. Stereopsis was absent by the Titmus stereo test. He had a dissociated vertical deviation.
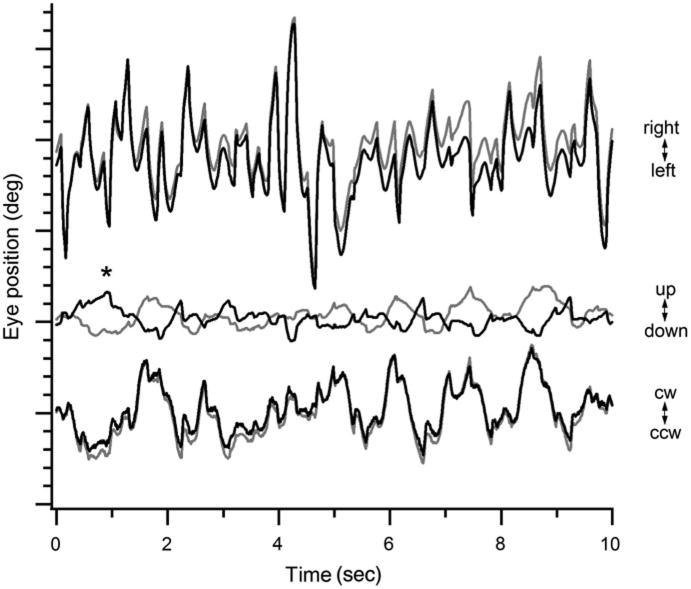
Extraocular movements were full with prominent pendular nystagmus that manifested cyclical depression and extorsion of 1 eye and concomitant elevation and intorsion of the contralateral eye, consistent with a see-saw pattern (Fig. 1). Because the subject's eye positions could not be accurately calibrated on infrared oculography due to the nystagmus, the recordings reflected eye positions over time with approximation of the amplitude values. A trial of gabapentin did not reduce the amplitude of the nystagmus (data not shown).
Fig. 1.
See-saw nystagmus in achiasma as demonstrated by 60-Hz infrared oculography. The horizontal (top), vertical (middle), and torsional (bottom) dimensions of eye movement over 10 seconds are displayed, with each major tick on the y-axis representing 10 and each minor tick representing 2. At the * there was intorsion and elevation of the right eye (black traces) and extorsion and depression of the left eye (gray traces), findings consistent with see-saw nystagmus. This was immediately followed by extorsion and depression of the right eye and intorsion and elevation of the left eye. CW, clockwise; CCW, counterclockwise.
Brain MRI demonstrated absence of the optic chiasm and otherwise normal anatomy, including the size of the optic nerves and optic tracts, pituitary gland, corpus callosum, and septum pellucidum (Fig. 2). On the fast imaging employing steady-state acquisition (FIESTA) sequence, which provided 1-mm axial slices with no skip (0.5-mm overlap between consecutive slices), a thin band could be recognized at the normal location of the optic chiasm (Fig. 2C), which had a signal intensity consistent with connective tissue on the T2 sequence (not shown).
Fig. 2.
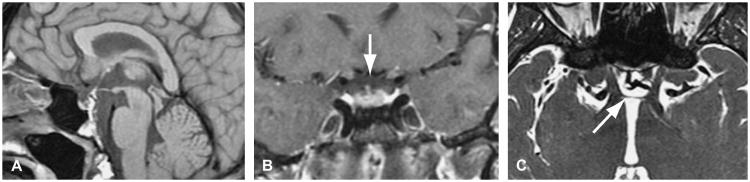
Precontrast T1 sagittal (A) and coronal (B) MRI studies do not show an optic chiasm. Axial fast imaging employing steady-state acquisition sequence (C) shows cerebrospinal fluid as intensely white and outlines a thin dark band at the expected location of the optic chiasm (arrow).
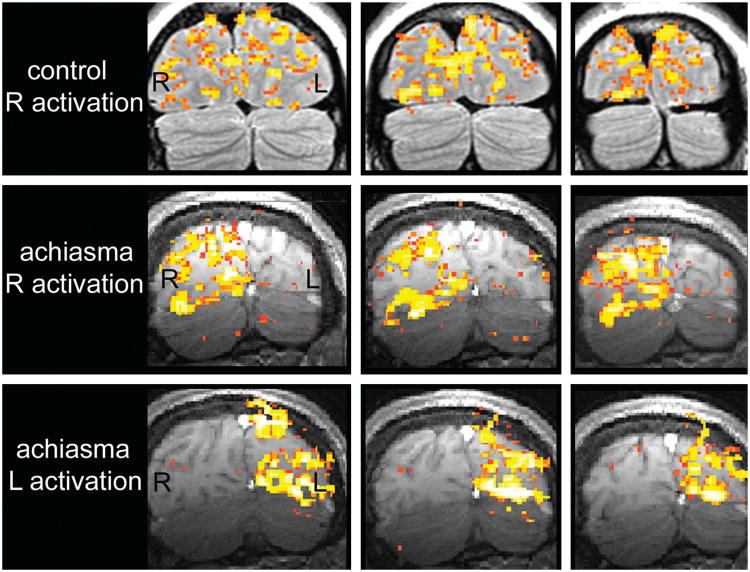
We attempted pattern and flash on-off visual evoked potentials, but responses were poor bilaterally because of significant nystagmus-related artifacts. Blood oxygenation level-dependent (BOLD) functional MRI (fMRI) with monocular presentation of pattern-reversal checkerboard visual stimuli did demonstrate functional non-segregation of the visual pathway, with right eye visual stimulation resulting in neuronal activity restricted to the right visual cortex and left eye visual stimulation resulting in neuronal activity restricted to the left visual cortex (Fig. 3). A subject with a normal optic chiasm should exhibit activity in the bilateral visual cortex in response to monocular visual stimulation due to decussation of the nasal retinogeniculate fibers.
Fig. 3.
Functional MRI during monocular pattern-reversal checkerboard presentation. Coronal images through the visual cortex during right eye stimulation of a control subject (top), during right eye stimulation of our achiasmatic patient (middle), and during left eye stimulation of our achiasmatic patient (bottom). Right eye stimulation of the control subject activates both occipital lobes. Right eye stimulation of the achiasmatic patient activates only the right occipital lobe. Left eye stimulation of the achiasmatic patient activates only the left occipital lobe. These findings are consistent with non-crossing of retinal axonal fibers at the optic chiasm. R, right; L, left.
Discussion
Our patient with VACTERL exhibited neuroophthalmologic characteristics typical of achiasma, including decreased visual acuity, lack of stereopsis, intact color vision, and infantile nystagmus with a see-saw pattern (3–8). BOLD fMRI demonstrated functional non-segregation of retinal axon fibers in response to monocular visual stimulation, suggesting the functional absence of an optic chiasm. Brain MRI showed only a thin band of presumed connective tissue in the expected position of the optic chiasm with normal bulk of the optic nerves.
Despite drastic rewiring of the connections and abnormal retinotopic maps in congenital achiasma (2,9,10), individuals with chiasmal malformation typically can adequately perform visually guided activities of daily living without the bitemporal visual field defects often found in patients with acquired lesions of the chiasm. Infantile nystagmus, especially of the see-saw pattern, is a consistent finding (2,3,6,7). This pattern of nystagmus has also been described in acquired lesions of the chiasm (11,12) and mesencephalon (11,13), in cone-rod dystrophy (14), and in Belgian sheepdogs with hereditary achiasma and hemichiasma (9,10). The mechanism of see-saw nystagmus remains unknown but may be related to impaired adaptive vestibular control of eye movement, given that see-saw nystagmus has a waveform similar to that of the ocular tilt reaction, exhibiting dissociated vertical deviation with intorsion of the elevated eye and extorsion of the depressed eye (10).
Functional MRI is a powerful tool to assess non-crossing of retinal axonal fibers at the optic chiasm. It has high spatial resolution, permitting the investigation of anatomic and functional visual pathway organization in humans with chiasmal anomalies. A study of 2 patients has suggested that achiasmatic patients have deranged retinotopic maps in the occipital cortex despite relatively normal visual fields and perception (15). This type of altered anatomy and physiology has also been reported in the lateral geniculate nucleus and visual cortex of achiasmatic Belgian sheepdogs, with dramatic discontinuity of receptive field representations and proximity of neurons that respond to visual stimuli on opposite sides of the visual field (9,10,16)
The Belgian sheepdog model of achiasma is inherited in an autosomal recessive pattern, providing support for a genetic basis for achiasma. Consistent with this idea, the association of relative or complete achiasma with midline malformation syndromes such as VACTERL is not surprising and may be under-reported. Mice with a mutation in sonic hedgehog (Shh) have a phenotype resembling VACTERL (17). The Shh gene is also the major molecule implicated in human holoprosencephaly (18), which is thought to be on the spectrum of midline central nervous system developmental anomalies including septo-optic dysplasia (19).
Acknowledgments
Y.J.L is supported by the National Institute of Neurological Diseases and Stroke (K08 NS044268) and by a Career Award in Biomedical Sciences from the Burroughs Wellcome Foundation.
Footnotes
Helmholtz Institute, Experimental Psychology, Utrecht University, Utrecht, The Netherlands
Contributor Information
Saurabh Prakash, Departments of Ophthalmology, Stanford University School of Medicine, Stanford, California.
Serge Dumoulin, Departments of Psychology, Stanford University School of Medicine, Stanford, California.
Nancy Fischbein, Departments of Radiology, Stanford University School of Medicine, Stanford, California.
Brian A. Wandell, Departments of Psychology, Stanford University School of Medicine, Stanford, California.
Yaping Joyce Liao, Departments of Ophthalmology, Stanford University School of Medicine, Stanford, California.
References
- 1.Glaser JS. Romancing the chiasm: vision, vocalization, and virtuosity. J Neuroophthalmol. 2008;28:131–43. doi: 10.1097/WNO.0b013e31817a7b5f. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian P, Bour L, Barth PG. A unique achiasmatic anomaly detected in non-albinos with misrouted retinal-fugal projections. Eur J Neurosci. 1994;6:501–07. doi: 10.1111/j.1460-9568.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian P, Bour LJ, Barth PG, et al. Non-decussating retinal-fugal fibre syndrome: an inborn achiasmatic malformation associated with visuotopic misrouting, visual evoked potential ipsilateral asymmetry and nystagmus. Brain. 1995;118:1195–216. doi: 10.1093/brain/118.5.1195. [DOI] [PubMed] [Google Scholar]
- 4.Brown MC, Southern CL, Anbarasu A, et al. Congenital absence of optic chiasm: demonstration of an uncrossed visual pathway using monocular flash visual evoked potentials. Doc Ophthalmol. 2006;113:1–4. doi: 10.1007/s10633-006-9005-1. [DOI] [PubMed] [Google Scholar]
- 5.Jansonius NM, van der Vliet TM, Cornelissen FW, et al. A girl without a chiasm: electrophysiologic and MRI evidence for the absence of crossing optic nerve fibers in a girl with a congenital nystagmus. J Neuroophthalmol. 2001;21:26–9. doi: 10.1097/00041327-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Korff CM, Apkarian P, Bour LJ, et al. Isolated absence of optic chiasm revealed by congenital nystagmus, MRI and VEPs. Neuropediatrics. 2003;34:219–23. doi: 10.1055/s-2003-42214. [DOI] [PubMed] [Google Scholar]
- 7.Hertle RW, Dell'Osso LF, FitzGibbon EJ, et al. Clinical, radiographic, and electrophysiologic findings in patients with achiasma or hypochiasma. Neuroophthalmology. 2002;26:43–57. [Google Scholar]
- 8.Apkarian P, Bour LJ. See-saw nystagmus and congenital nystagmus identified in the non-decussating retinal-fugal fiber syndrome. Strabismus. 2001;9:143–63. doi: 10.1076/stra.9.3.143.6761. [DOI] [PubMed] [Google Scholar]
- 9.Williams RW, Hogan D, Garraghty PE. Target recognition and visual maps in the thalamus of achiasmatic dogs. Nature. 1994;367:637–39. doi: 10.1038/367637a0. [DOI] [PubMed] [Google Scholar]
- 10.Dell'Osso LF, Williams RW, Jacobs JB, et al. The congenital and seesaw nystagmus in the prototypical achiasma of canines: comparison to the human achiasmatic prototype. Vision Res. 1998;38:1629–41. doi: 10.1016/s0042-6989(97)00337-4. [DOI] [PubMed] [Google Scholar]
- 11.Drachman DA. See-saw nystagmus. J Neurol Neurosurg Psychiatry. 1966;29:356–61. doi: 10.1136/jnnp.29.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis GV, Shock JP. Septo-optic dyspasia associated with see-saw nystagmus. Arch Ophthalmol. 1975;93:137–39. doi: 10.1001/archopht.1975.01010020143011. [DOI] [PubMed] [Google Scholar]
- 13.Halmagyi GM, Aw ST, Dehaene I, et al. Jerk-waveform see-saw nystagmus due to unilateral meso-diencephalic lesion. Brain. 1994;117:789–803. doi: 10.1093/brain/117.4.789. [DOI] [PubMed] [Google Scholar]
- 14.May EF, Truxal AR. Loss of vision alone may result in seesaw nystagmus. J Neuroophthalmol. 1997;17:84–95. [PubMed] [Google Scholar]
- 15.Victor JD, Apkarian P, Hirsch J, et al. Visual function and brain organization in non-decussating retinal-fugal fibre syndrome. Cereb Cortex. 2000;10:2–22. doi: 10.1093/cercor/10.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Hogan D, Garraghty PE, Williams RW. Asymmetric connections, duplicate layers, and a vertically inverted map in the primary visual system. J Neurosci. 1999;19:RC38. doi: 10.1523/JNEUROSCI.19-22-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim PC, Mo R, Hui C. Murine models of VACTERL syndrome: role of sonic hedgehog signaling pathway. J Pediatr Surg. 2001;36:381–84. doi: 10.1053/jpsu.2001.20722. [DOI] [PubMed] [Google Scholar]
- 18.Roessler E, Belloni E, Gaudenz K, et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–60. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 19.Polizzi A, Pavone P, Iannetti P, et al. Septo-optic dysplasia complex: a heterogeneous malformation syndrome. Pediatr Neurol. 2006;34:66–71. doi: 10.1016/j.pediatrneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]