Abstract
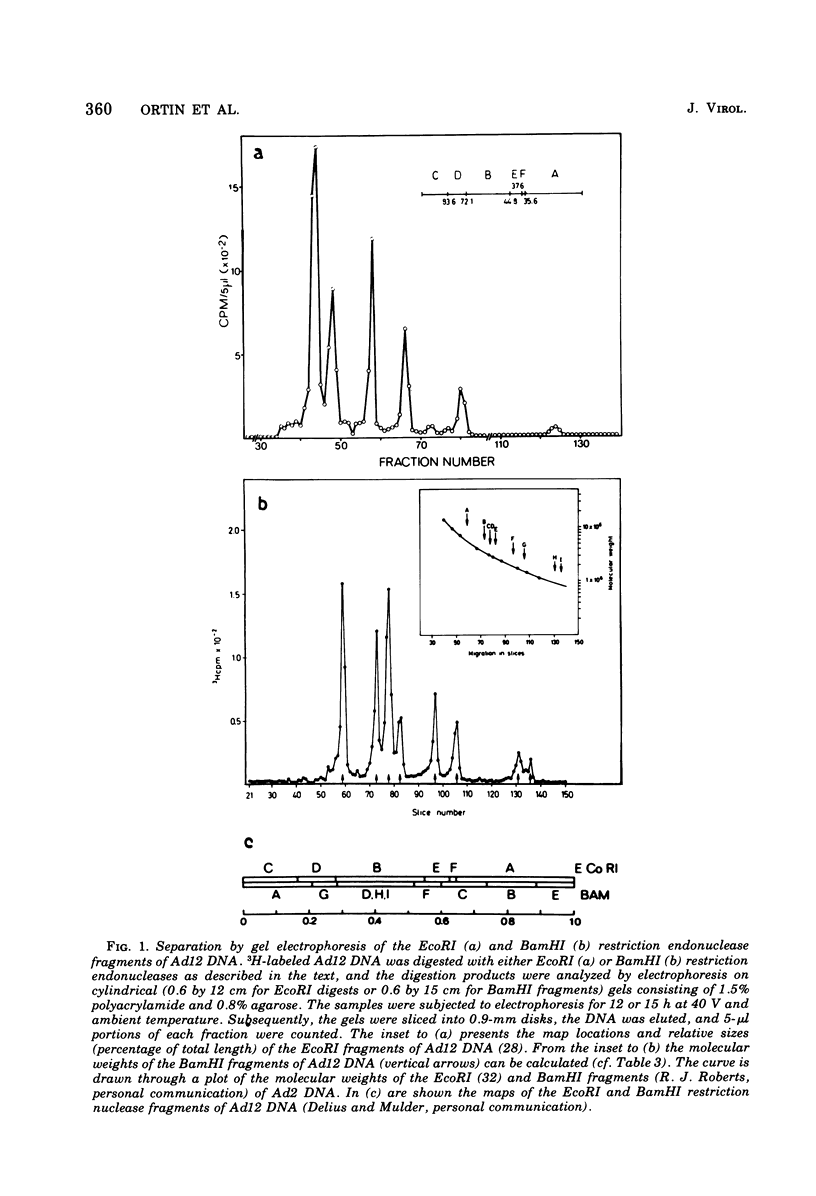
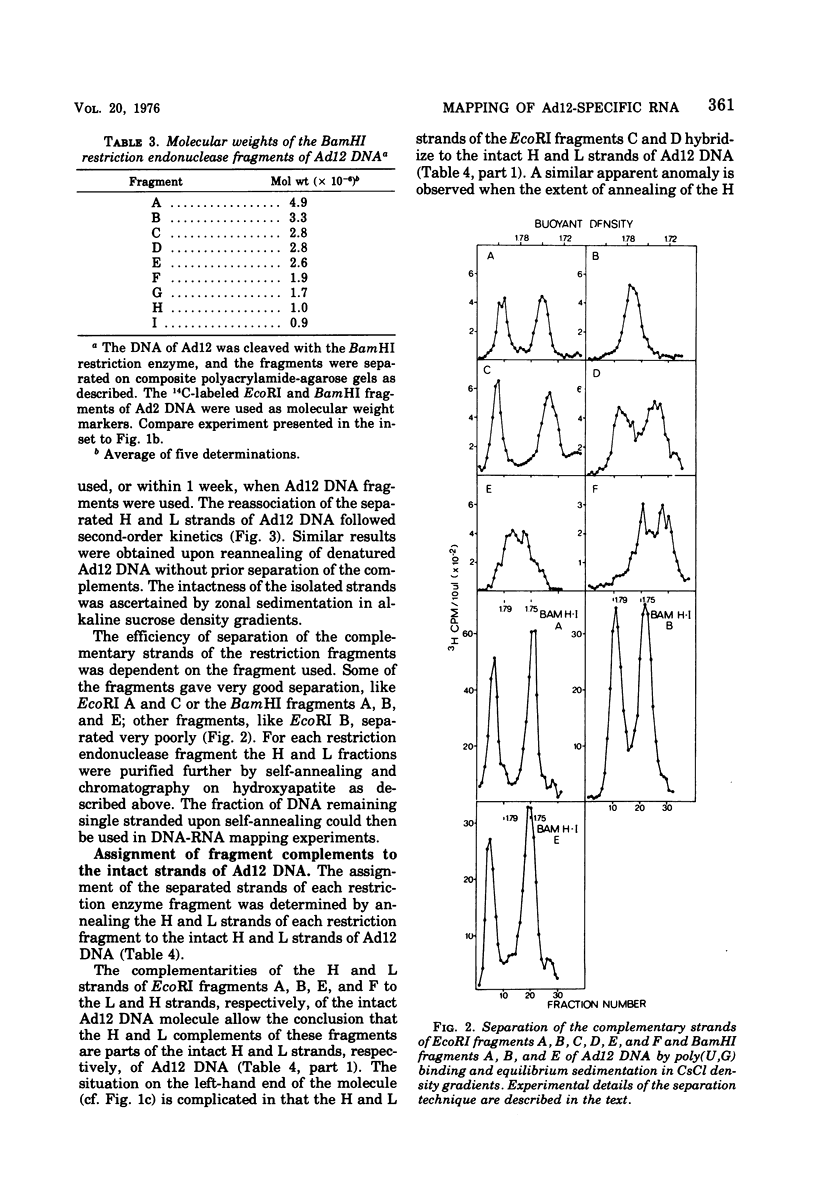
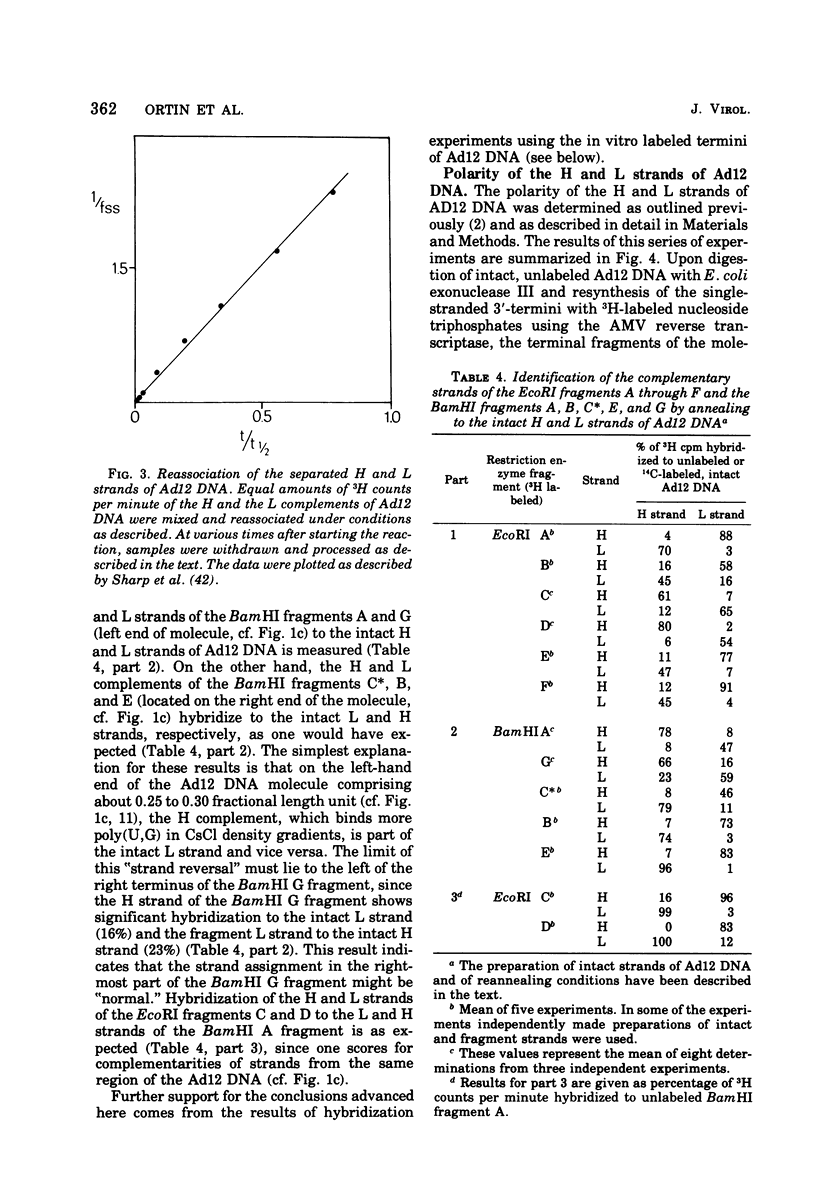
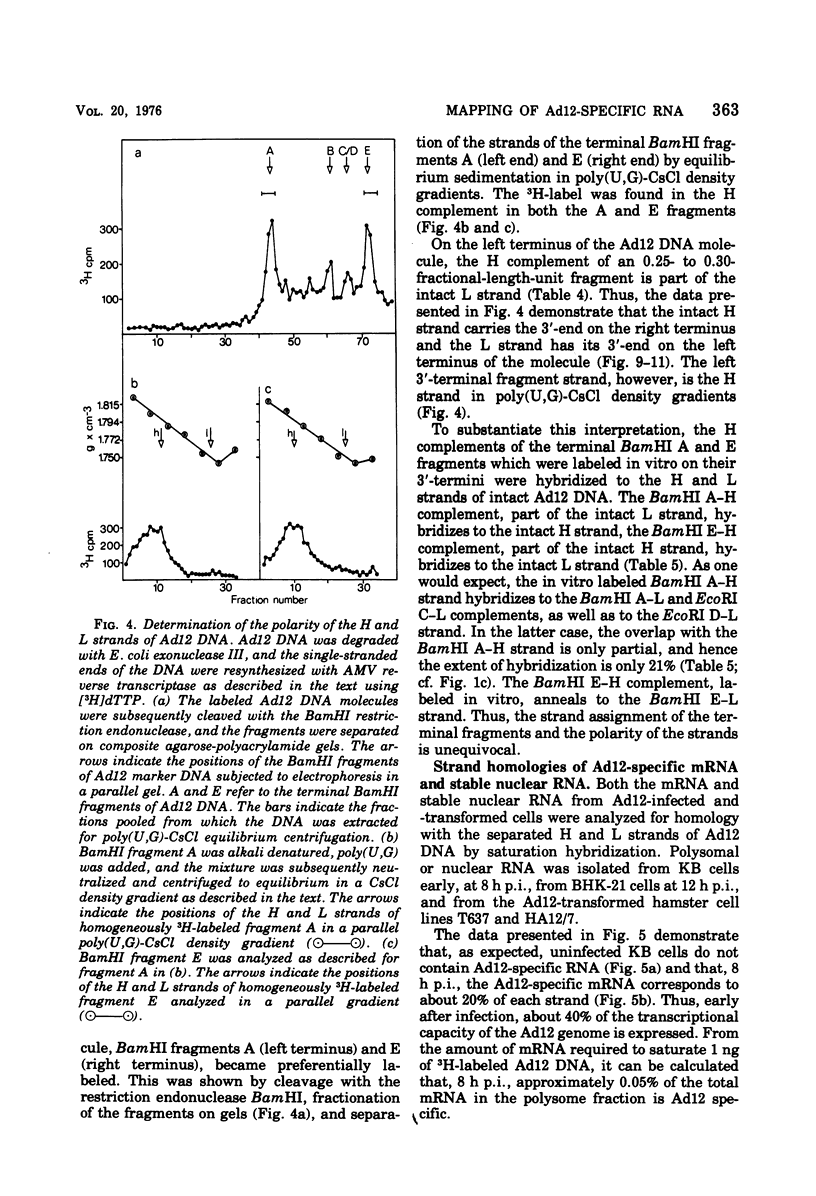
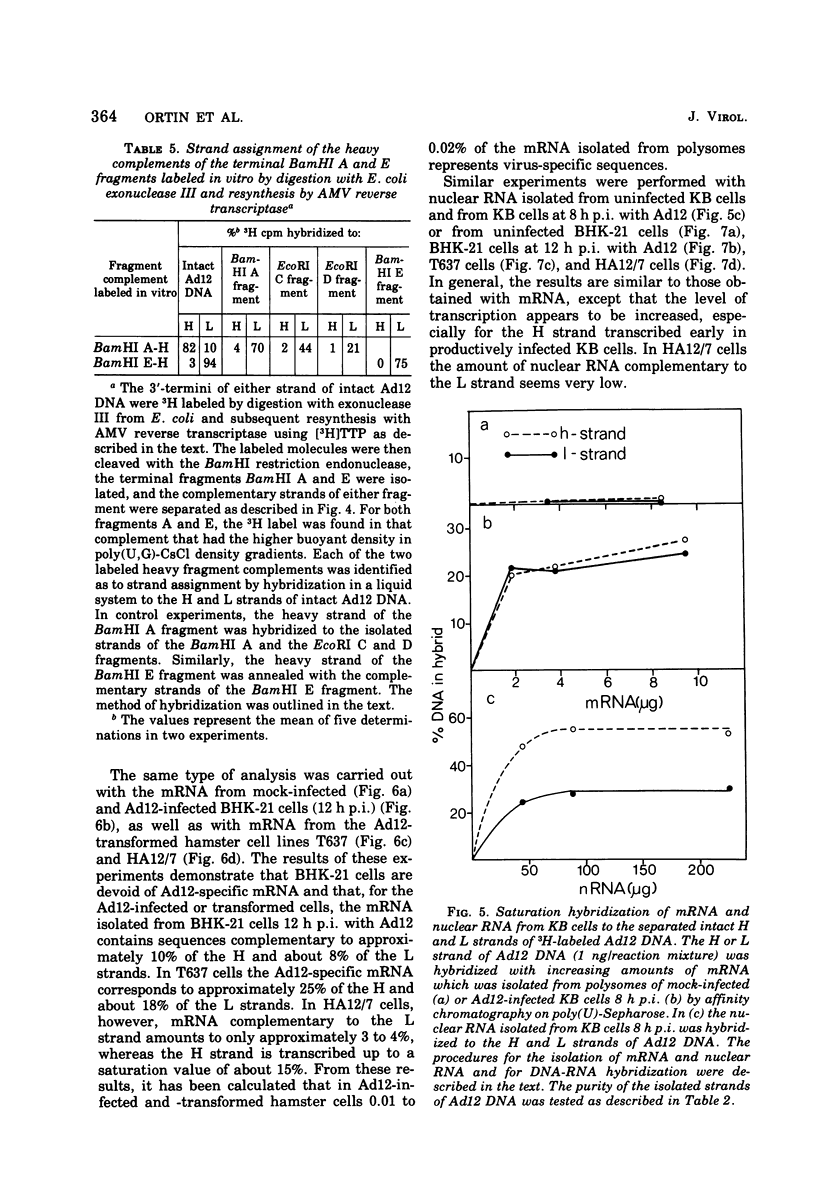
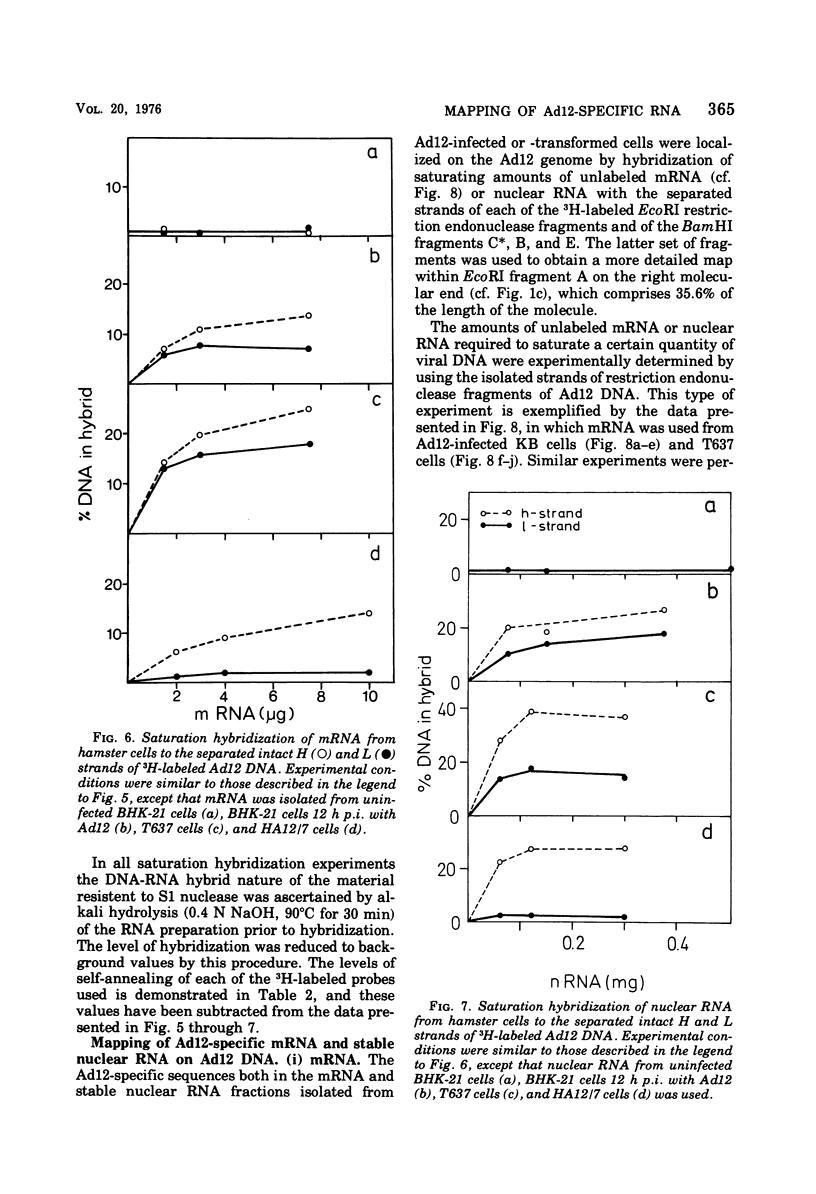
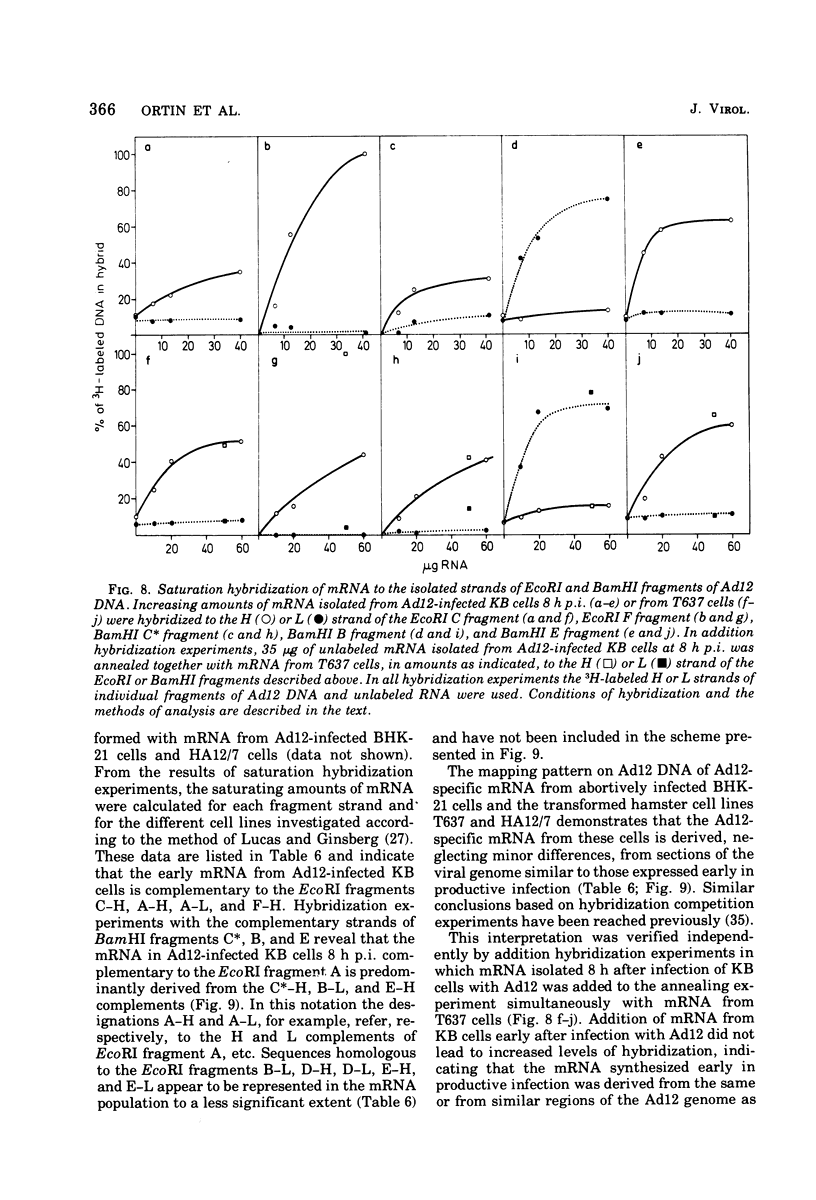
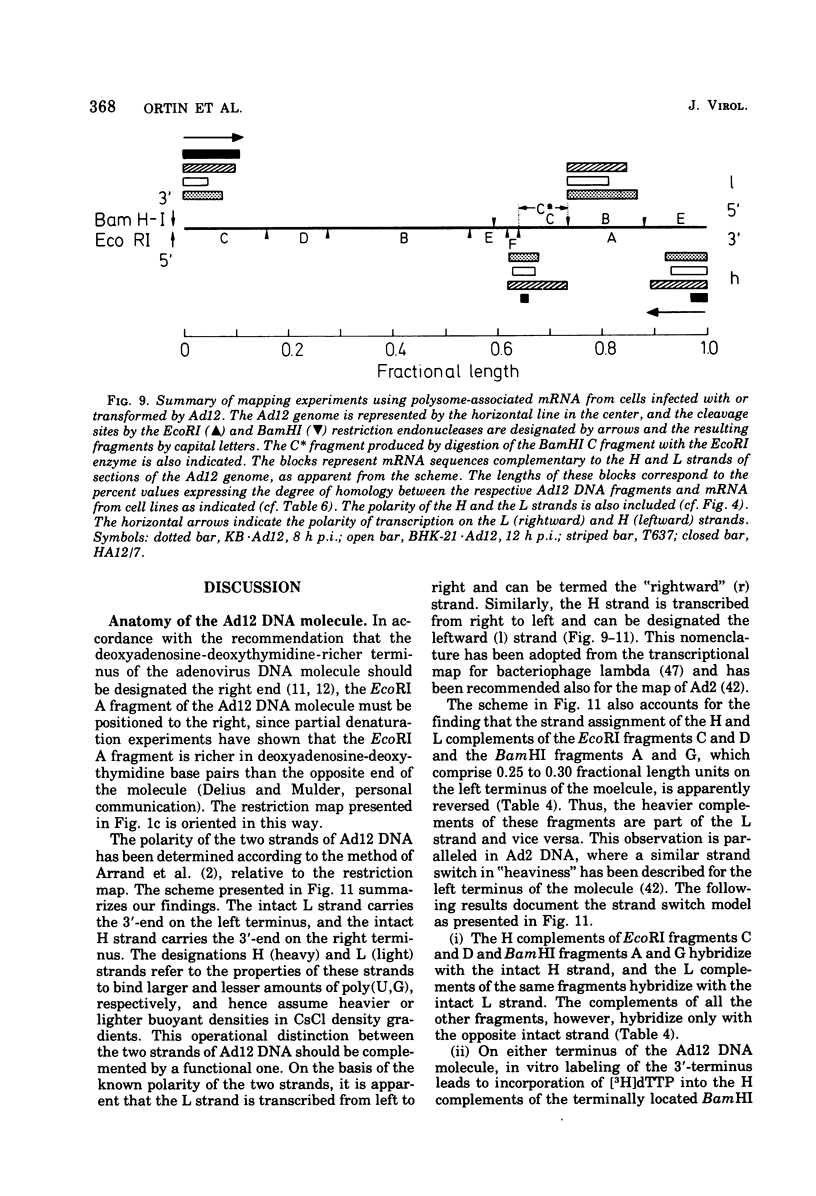
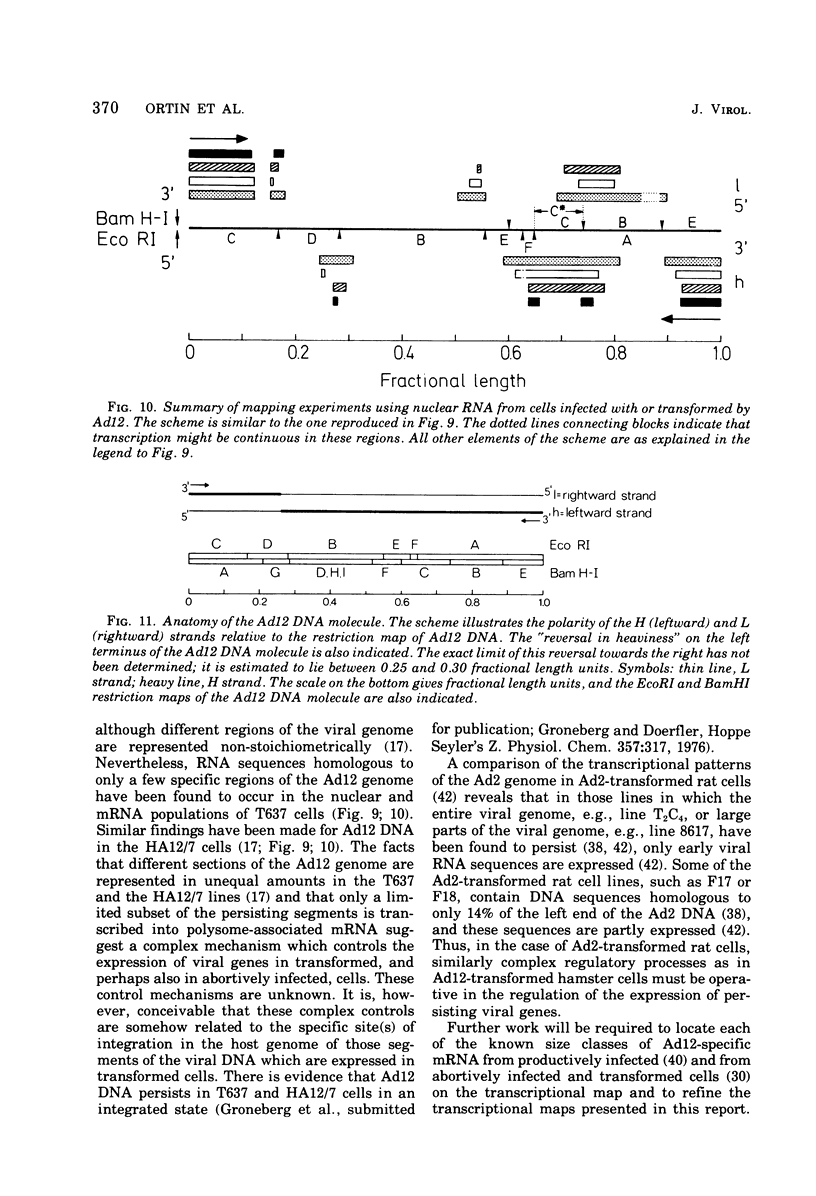
The adenovirus type 12-specific mRNA and the stable nuclear RNA from productively infected KB cells, early postinfection, from abortively infected BHK-21 cells, and from the adenovirus type 12-transformed hamster lines T637 and HA12/7 have been mapped on the genome of adenovirus type 12. The intact separated heavy (H) and light (L) strands of adenovirus type 12 DNA have been used to determine the extent of complementarity of the mRNA or nuclear RNA from different cell lines to each of the strands. More precise map positions have been obtained by the use of the H and L complements of the fragments of adenovirus type 12 DNA which were produced with the EcoRI and BamHI restriction endonucleases. The results of the mapping experiments demonstrate that the mRNA's isolated early from productively and abortively infected and from two lines of transformed cells are derived from the same or similar regions of the adenovirus type 12 genome. The map positions on the adenovirus type 12 genome for the mRNA from the cell lines as indicated correspond to regions located approximately between 0 and 0.1 and 0.74 and 0.88 fractional length units on the L strand and to regions between 0.63 and 0.74 and 0.89 and 1.0 fractional length units on the H strand. The HA12/7 line lacks mRNA complementary to the region between 0.74 and 0.88 fractional length units on the L strand. Similar data are found for the nuclear RNA, except that the regions transcribed are more extensive than those observed in mRNA. The polarity of the H strand has its 3'-end on the right terminus in the EcoRI A fragment, and the L strand has its 3'-end on the left terminus in the EcoRI C fragment. Thus, the H strand is transcribed from right to left (1 = leftward strand); and the L strand is transcribed from left to right (r = rightward strand). The designations H and L refer to the relative heavy and light densities of the two strands in polyuridylic-polyguanylic acid-CsCl density gradients. The EcoRI C-H and D-H complements have been shown to be part of the intact L strand; thus, there is a "reversal in heaviness" on the left terminus of the viral DNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrand J. R., Keller W., Roberts R. J. Extent of terminal repetition in adenovirus 2 DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):401–407. doi: 10.1101/sqb.1974.039.01.052. [DOI] [PubMed] [Google Scholar]
- Bellett A. J. Covalent integration of viral DNA into cell DNA in hamster cells transformed by an avian adenovirus. Virology. 1975 Jun;65(2):427–435. doi: 10.1016/0042-6822(75)90048-3. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Burger H., Ortin J., Fanning E., Brown D. T., Mestphal M., Winterhoff U., Weiser B., Schick J. Integration of adenovirus DNA into the cellular genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):505–521. doi: 10.1101/sqb.1974.039.01.063. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Hellmann W., Kleinschmidt A. K. The DNA of adenovirus type 12 and its denaturation pattern. Virology. 1972 Feb;47(2):507–512. doi: 10.1016/0042-6822(72)90290-5. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U. Absence of replication of the DNA of adenovirus type 12 in BHK21 cells. Virology. 1970 Mar;40(3):754–757. doi: 10.1016/0042-6822(70)90222-9. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- Eron L., Wesphal H., Callahan R. In vitro synthesis of adenovirus core proteins. J Virol. 1974 Aug;14(2):375–383. doi: 10.1128/jvi.14.2.375-383.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Doerfler W. Intracellular forms of adenovirus DNA. V. Viral DNA sequences in hamster cells abortively infected and transformed with human adenovirus type 12. J Virol. 1976 Nov;20(2):373–383. doi: 10.1128/jvi.20.2.373-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Chinnadurai G., Mackey J. K., Green M. A unique pattern of integrated viral genes in hamster cells transformed by highly oncogenic human adenovirus 12. Cell. 1976 Mar;7(3):419–428. doi: 10.1016/0092-8674(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Green M. Effect of oncogenic DNA viruses on regulatory mechanisms of cells. Fed Proc. 1970 May-Jun;29(3):1265–1275. [PubMed] [Google Scholar]
- Green M. Molecular basis for the attack on cancer. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1036–1041. doi: 10.1073/pnas.69.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Khoury G., Howley P., Brown M., Martin M. The detection and quantitation of SV40 nucleic acid sequences using single-stranded SV40 DNA probes. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):147–152. doi: 10.1101/sqb.1974.039.01.020. [DOI] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Sharp P. A., Delius H., Pettersson U. Specific fragmentation of DNA of adenovirus serotypes 3, 5, 7, and 12, and adeno-simian virus 40 hybrid virus Ad2+ND1 by restriction endonuclease R.EcoRI. J Virol. 1974 Jul;14(1):68–77. doi: 10.1128/jvi.14.1.68-77.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Doerfler W. Transcription of the genome of adenovirus type 12. I. Viral mRNA in abortively infected and transformed cells. J Virol. 1975 Jan;15(1):27–35. doi: 10.1128/jvi.15.1.27-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Synthesis of complementary RNA sequences during productive adenovirus infection. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4887–4891. doi: 10.1073/pnas.71.12.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Raska K., Jr, Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. VI. Synthesis of virus-specific RNA. Virology. 1972 Mar;47(3):734–742. doi: 10.1016/0042-6822(72)90563-6. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sugden B., Keller W., Sharp P. A. Transcription of simian virus 40. 3. Mapping of "early" and "late" species of RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3711–3715. doi: 10.1073/pnas.70.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Ortin J., Doerfler W. Transcription of the genome of adenovirus type 12. Viral mRNA in productively infected KB cells. Eur J Biochem. 1975 Oct 15;58(2):283–290. doi: 10.1111/j.1432-1033.1975.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Schick J., Baczko K., Fanning E., Groneberg J., Burger H., Doerfler W. Intracellular forms of adenovirus DNA: integrated form of adenovirus DNA appears early in productive infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1043–1047. doi: 10.1073/pnas.73.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Strohl W. A., Rouse H., Teets K., Schlesinger R. W. The response of BHK21 cells to infection with type 12 adenovirus. 3. Transformation and restricted replication of superinfecting type 2 adenovirus. Arch Gesamte Virusforsch. 1970;31(1):93–112. doi: 10.1007/BF01241669. [DOI] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. 1. Cell killing and T antigen synthesis as correlated viral genome functions. Virology. 1969 Dec;39(4):642–652. doi: 10.1016/0042-6822(69)90003-8. [DOI] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Zimmer S., Raskas H. J. Localization of adenovirus 2 messenger RNA's to segments of the viral genome defined by endonuclease R-R1. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4057–4061. doi: 10.1073/pnas.71.10.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Interactions of adenovirus type 12 with host cell chromosomes. Prog Exp Tumor Res. 1973;18:240–259. doi: 10.1159/000393169. [DOI] [PubMed] [Google Scholar]



