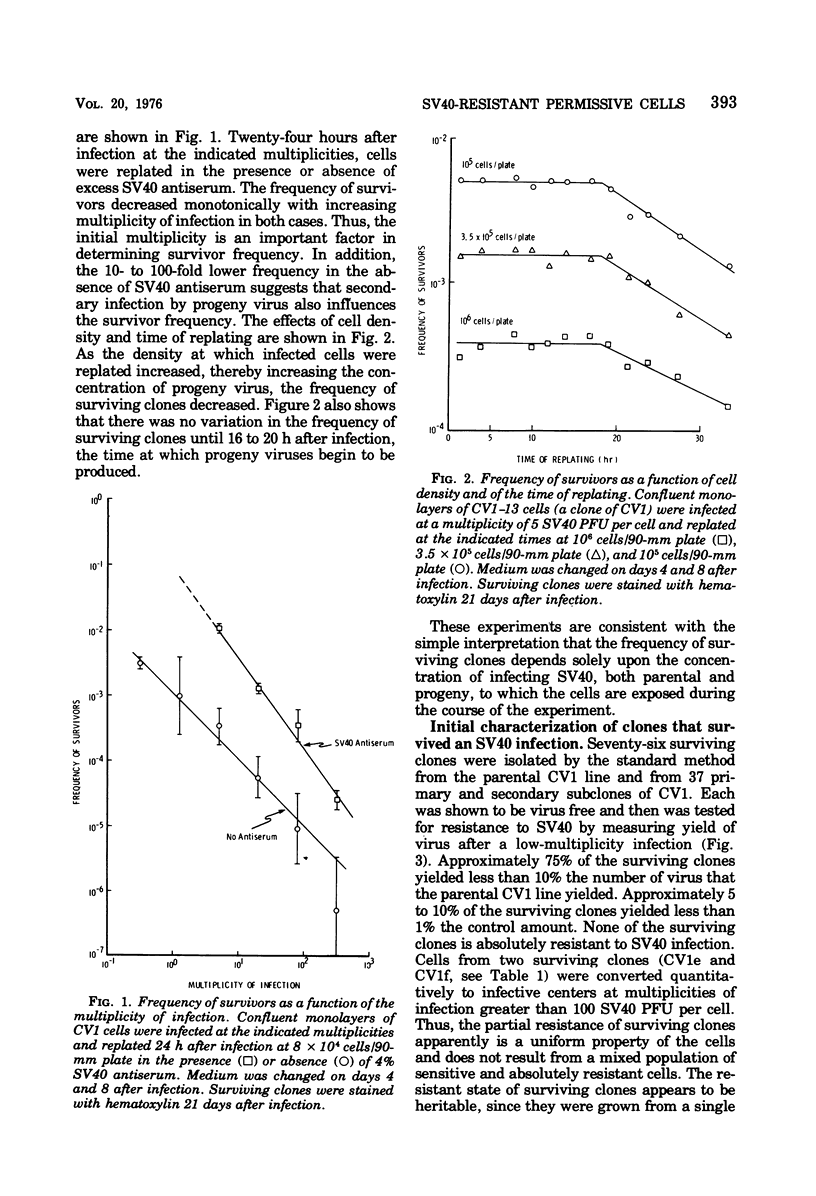
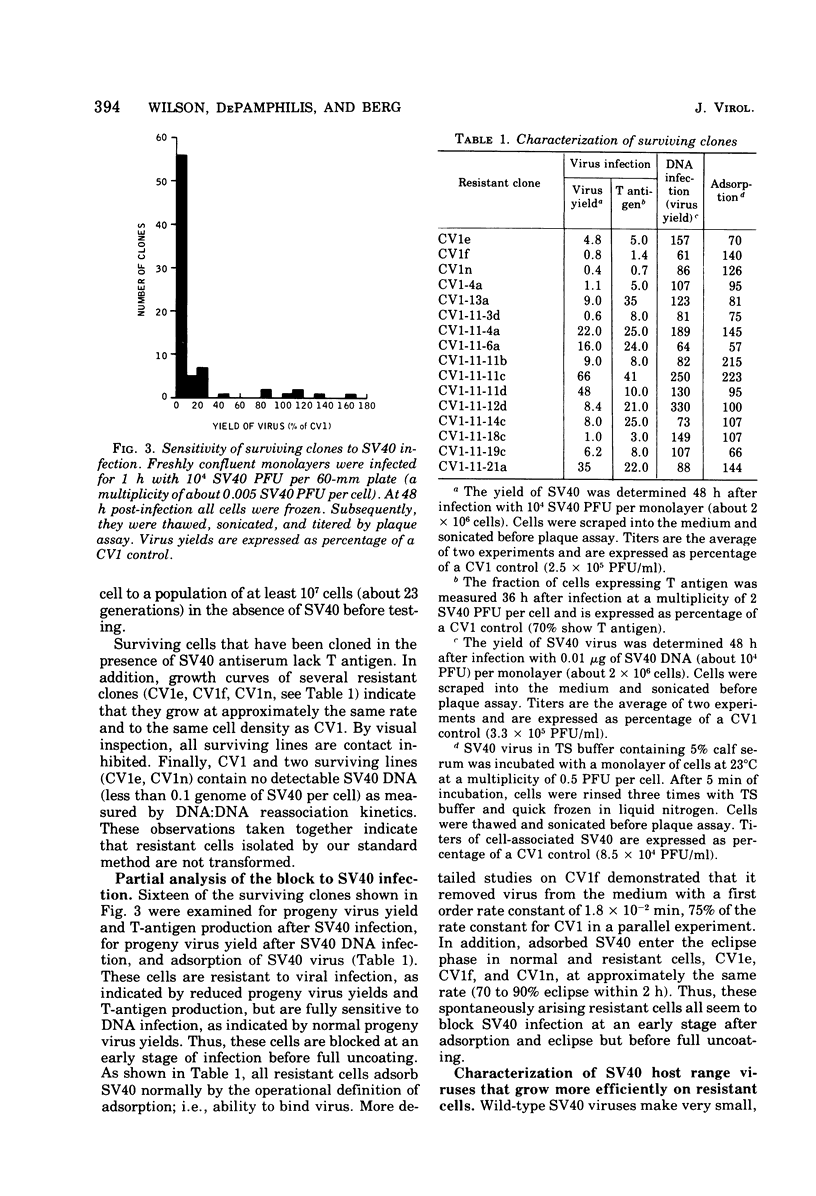
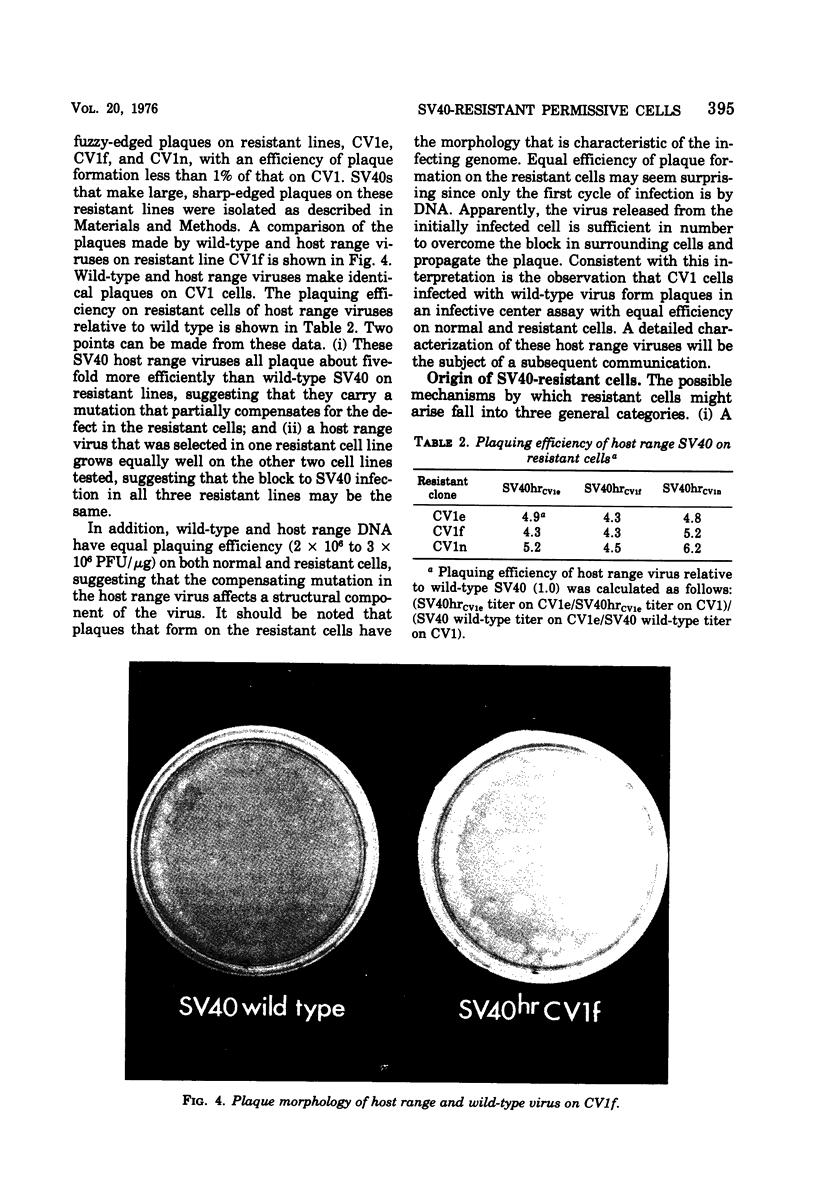
Abstract
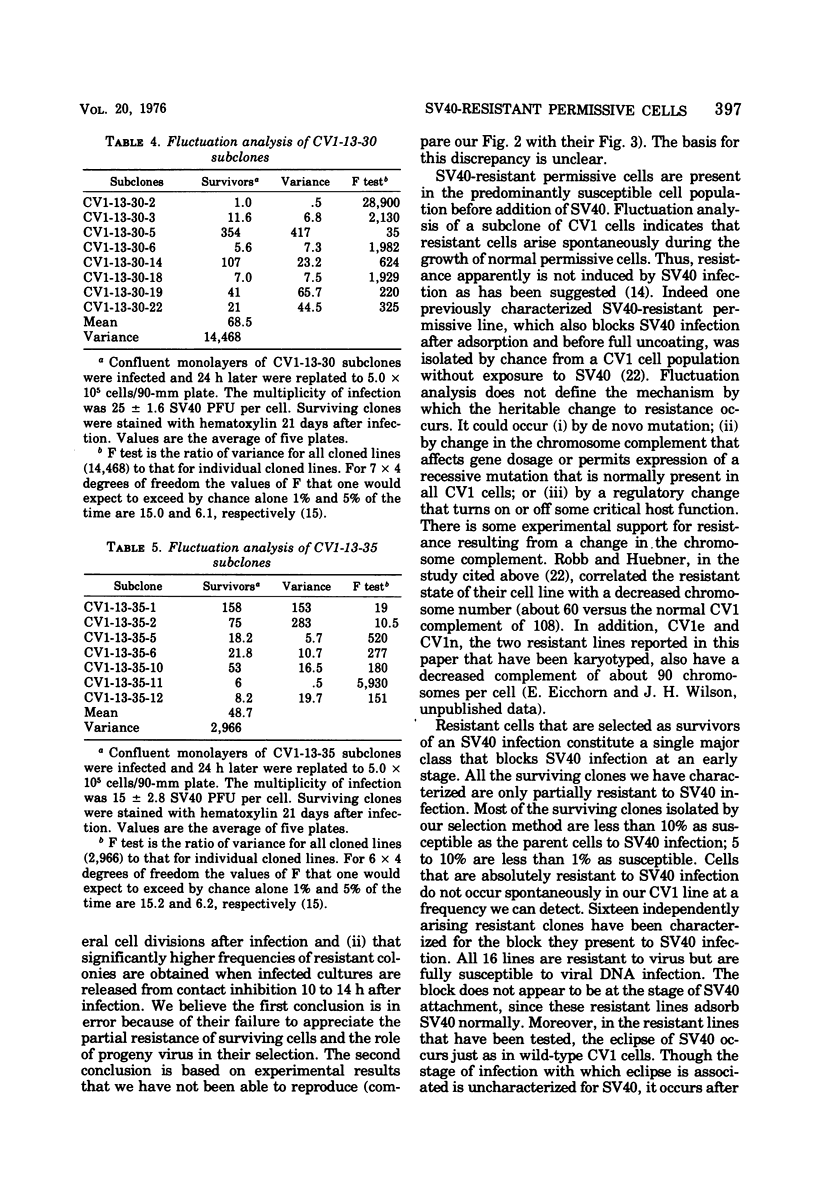
A fraction of permissive cells survive simian virus 40 (SV40) infection. The frequency of such surviving cells depends only upon the concentration of infecting virus, both parental and progeny, to which the cells are exposed during the course of selection. Surviving clones, which can be freed of virus by cloning in the presence of SV40 antiserum, are indistinguishable from parental cells in their growth of characteristics and display no SV40 antigen; thus they are not transformed. Most surviving clones are less than 10% as susceptible as parental cells to SV40 infection; 5 to 10% are less than 1% as susceptible. None of these SV40-resistant clones is absolutely resistant to SV40 infection. Analysis of 16 independently arising resistant clones indicates that they all block SV40 infection at an early stage after adsorption and eclipse but before full uncoating. Viral mutants have been isolated that partially overcome the block to infection in these cells; these host range viruses plaque on resistant lines fivefold more efficiently than wild-type SV40 and have a characteristic plaque morphology. Fluctuation analysis indicates that resistant cells arise spontaneously during the growth of normally susceptible permissive cells. Thus, SV40-resistant cells are selected for, not induced by, SV40 infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butel J. S., Richardson L. S., Melnick J. L. Variation in properties of SV40-transformed simian cell lines detected by superinfection with SV40 and human adenoviruses. Virology. 1971 Dec;46(3):844–855. doi: 10.1016/0042-6822(71)90085-7. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J Virol. 1974 Sep;14(3):509–516. doi: 10.1128/jvi.14.3.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M, Fano U. Bacteriophage-Resistant Mutants in Escherichia Coli. Genetics. 1945 Mar;30(2):119–136. doi: 10.1093/genetics/30.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M. V., Moorhead P. S. Transformation of African green monkey kidney cultures infected with simian vacuolating virus (SV40). Tex Rep Biol Med. 1965 Jun;23(Suppl):242–258. [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Girardi A. J. Prevention of SV40 virus oncogenesis in hamsters. I. Tumor resistance induced by human cells transformed by SV40. Proc Natl Acad Sci U S A. 1965 Aug;54(2):445–451. doi: 10.1073/pnas.54.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Sauer G. Initial stage of transformation of permissive cells by simian virus 40: development of resistance to productive infection. J Virol. 1971 Jul;8(1):7–16. doi: 10.1128/jvi.8.1.7-16.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C., Tegtmeyer P., Dohan C., Jr, Enders J. F. Isolation of AGMK cells partially resistant to SV40: identification of the resistant step. Proc Soc Exp Biol Med. 1972 Nov;141(2):740–746. doi: 10.3181/00379727-141-36863a. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Sauer G., Koprowski H., Defendi V. The genetic heterogeneity of simian virus 40. Proc Natl Acad Sci U S A. 1967 Aug;58(2):599–606. doi: 10.1073/pnas.58.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Snover D., Doermann A. H. Bacterial mutation affecting T4 phage DNA synthesis and tail production. Nature. 1974 Dec 6;252(5483):451–455. doi: 10.1038/252451a0. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- Wilson J. H. Function of the bacteriophage T4 transfer RNA's. J Mol Biol. 1973 Mar 15;74(4):753–757. doi: 10.1016/0022-2836(73)90065-x. [DOI] [PubMed] [Google Scholar]