Abstract
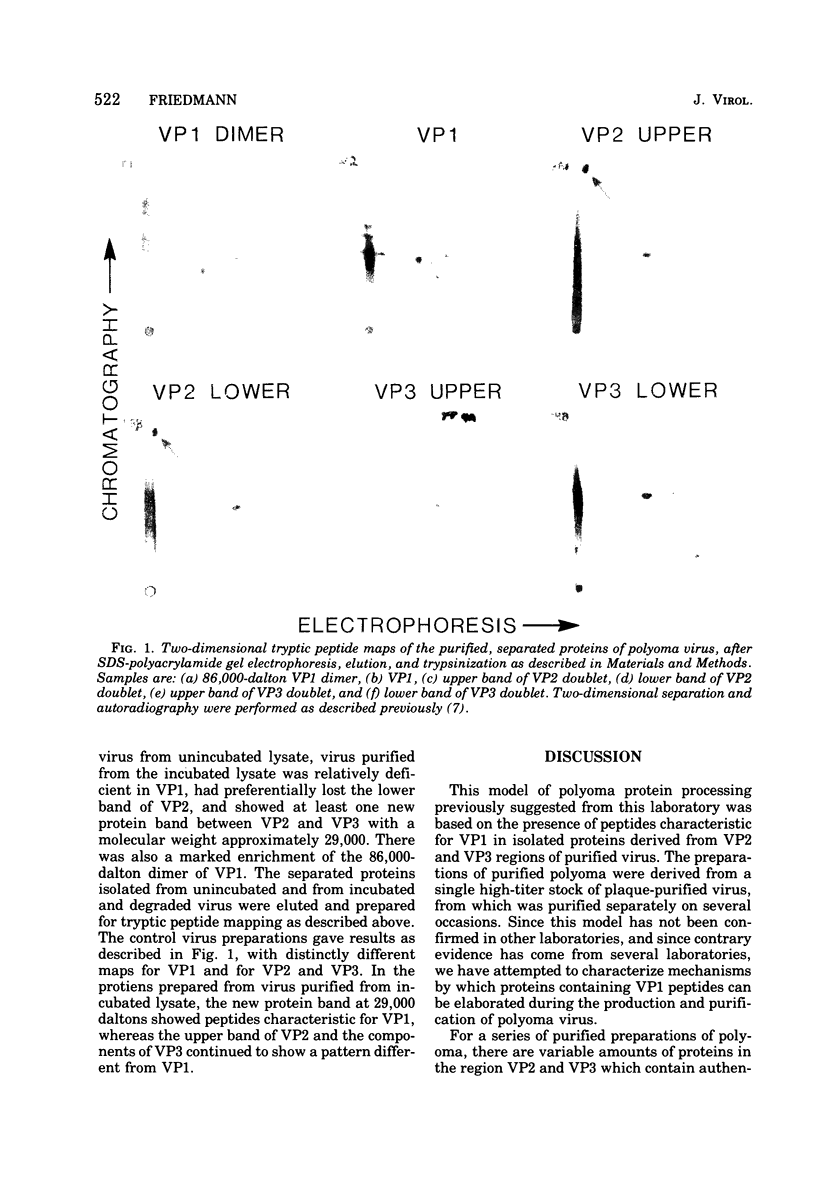
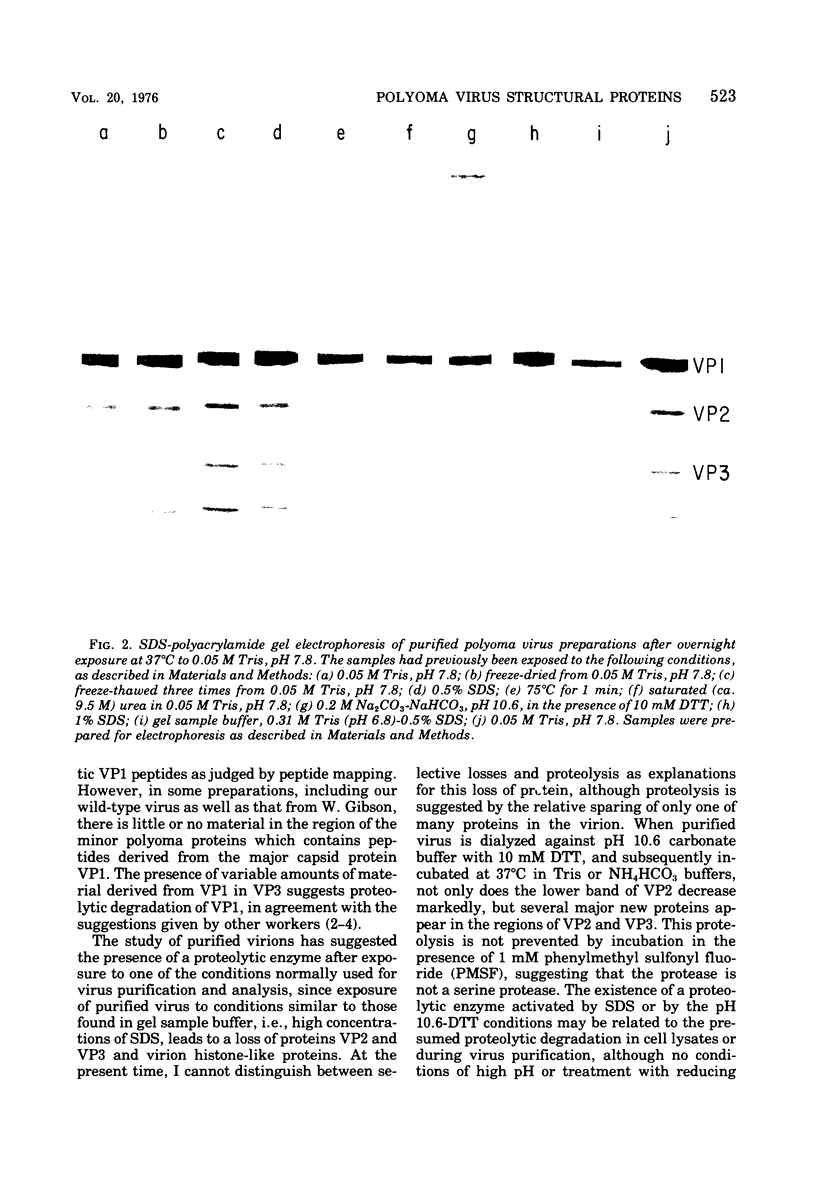
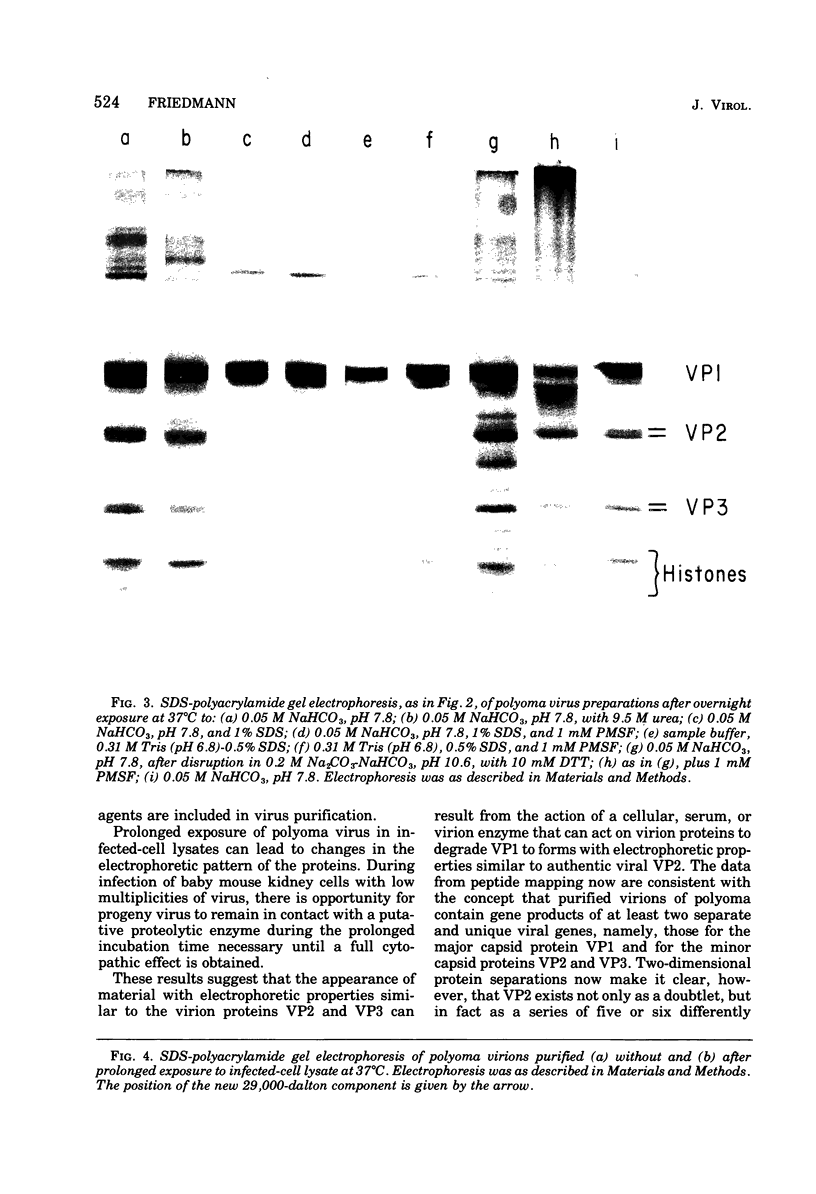
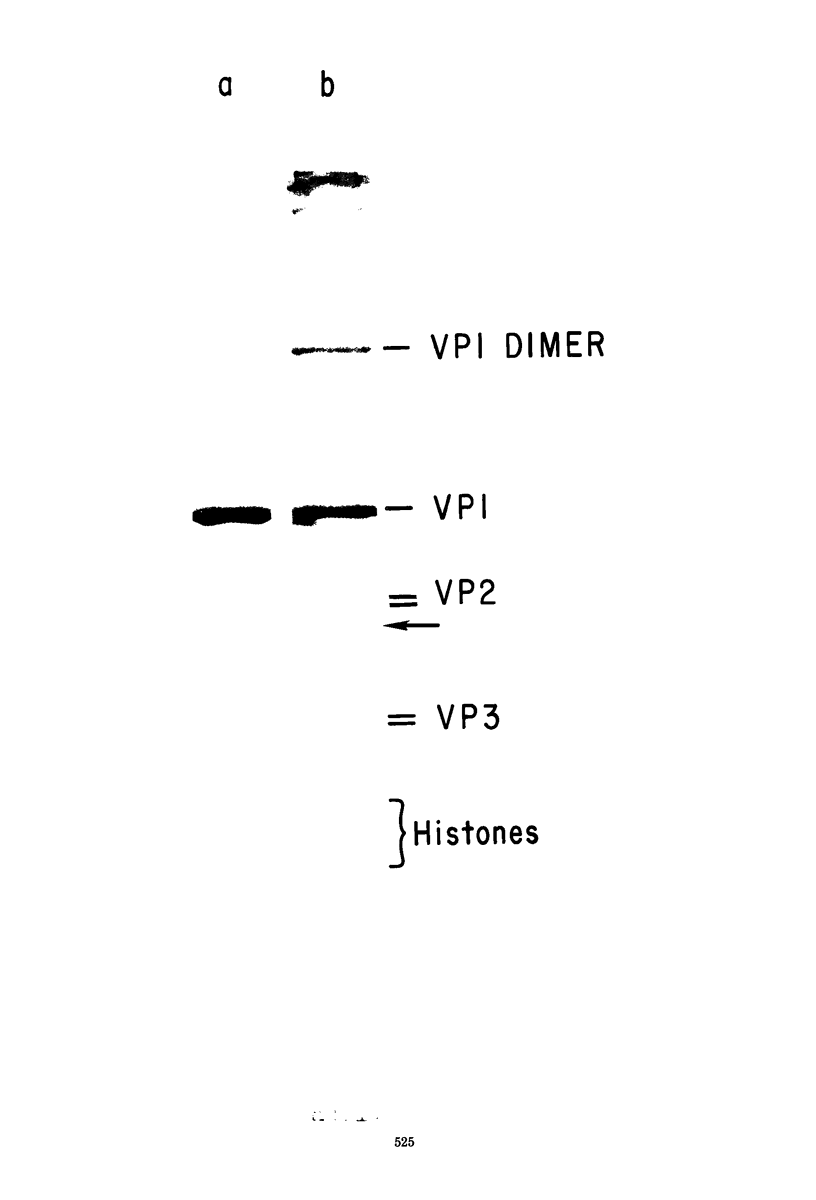
A model has previously been proposed for the genetic relatedness of the structural proteins of polyoma virus, based upon similarities in the peptide maps of the major capsid protein VP1 with the virion proteins VP2 and VP3. Newer evidence suggests that this model is incorrect, and that protein VP1 is a product of one viral gene and that the multiple components of VP2 and VP3 are products of a second viral gene. Two-dimensional peptide maps of several preparations of polyoma purified separately from four separate infected-cell lysates has shown a variable content of VP1 peptides in proteins VP2 and VP3, with some preparations being free of detectable VP1 material in VP2 and VP3. An alternative explanation for the presence of VP1 peptides in the regions of VP2 and VP3 of some polyoma preparations involves the cleavage of proteins of polyoma virions during exposure to proteolytic enzymes in lysates of infected cells or to endogenous proteolytic activity of virions. Prolonged incubation of infected-cell lysates at 37 degrees C leads to an increase in the amount of 86,000-dalton dimer of VP1, a decrease in the relative amount of VP1, a decrease in or a loss of the lower band of VP2, and the appearance of a new major protein band of approximately 29,000 daltons. Two-dimensional peptide maps of the new 29,000-dalton protein show that it contains some VP1 peptides, indicating that this protein is derived from proteolytic cleavage of VP1. In addition, extensively purified polyoma virus contains a proteolytic activity that can be activated during disruption of the virus with 0.2 M Na2CO3-NaHCO3 (pH 10.6) in the presence of 5 X 10(-3) M dithiothreitol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Eckhart W. Virion proteins of polyoma temperature-sensitive mutants: late mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):243–246. doi: 10.1101/sqb.1974.039.01.031. [DOI] [PubMed] [Google Scholar]
- Friedmann T. Genetic economy of polyoma virus: capsid proteins are cleavage products of same viral gene. Proc Natl Acad Sci U S A. 1974 Feb;71(2):257–259. doi: 10.1073/pnas.71.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Friedmann T. In vitro reassembly of shell-like particles from disrupted polyoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2574–2578. doi: 10.1073/pnas.68.10.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Fried M., Waterfield M. D. Nonhistone virion proteins of polyoma: characterisation of the particle proteins by tryptic peptide analysis by use of ion-exchange columns. Virology. 1975 Aug;66(2):408–419. doi: 10.1016/0042-6822(75)90213-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]