Abstract
Many clinicians hoped that the completion of the Human Genome Project would result in “individualized drug therapy,” i.e., determining the right medication at the right dose 100% of the time based upon the individual's genetics. The pharmacogenomic prediction of drug efficacy and safety has not become a reality due to continuing realization of the complexity dictating the human–drug interaction. New methods of metabolomics, proteomics, and transcriptomics that account for this complexity hold promise for translational researchers hoping to increase drug efficacy and decrease drug toxicity.
Keywords: CYP2D6, Metabolomics, Pharmacogenomics, Proteomics, Transcriptomics
Introduction
Pharmacogenomics is the study of the interaction between the human genome and clinical drug response. Upon completion of the Human Genome Project, many expected rapid advancement toward “individualized drug therapy.” However, despite continued discovery of the genetic polymorphisms affecting drug pharmacokinetics and pharmacodynamics, individualized drug therapy has not been widely achieved in clinical practice [1, 2]. Drug–drug interactions, environmental interactions, and polymorphism in drug targets limit the ability to predict drug response in the individual patient [3]. Due to the complexity of xenobiotic interaction with the human body, drug response cannot be predicted by genetic polymorphism alone; rather, response must be viewed as a complete biologic system. The objective of this manuscript is to: (a) describe the translational issues that have limited the application of genotype analysis from predicting individual patient phenotype and (b) introduce an approach that integrates targeted metabolomic panels with specific genotyping assays to improve predictive efficiency of drug response.
Part A: Predicting Medication Response Based on Genotype
The difficulty in predicting phenotype based upon genotype is a product of the complexity of the human–drug interaction. Each stage of drug absorption, distribution, metabolism, and elimination (ADME) is subject to genetic polymorphism and subsequent phenotype variation. There is further polymorphism in drug targets and cellular signaling mechanisms that ultimately mediate the response to a medication (Fig. 1).
Fig. 1.
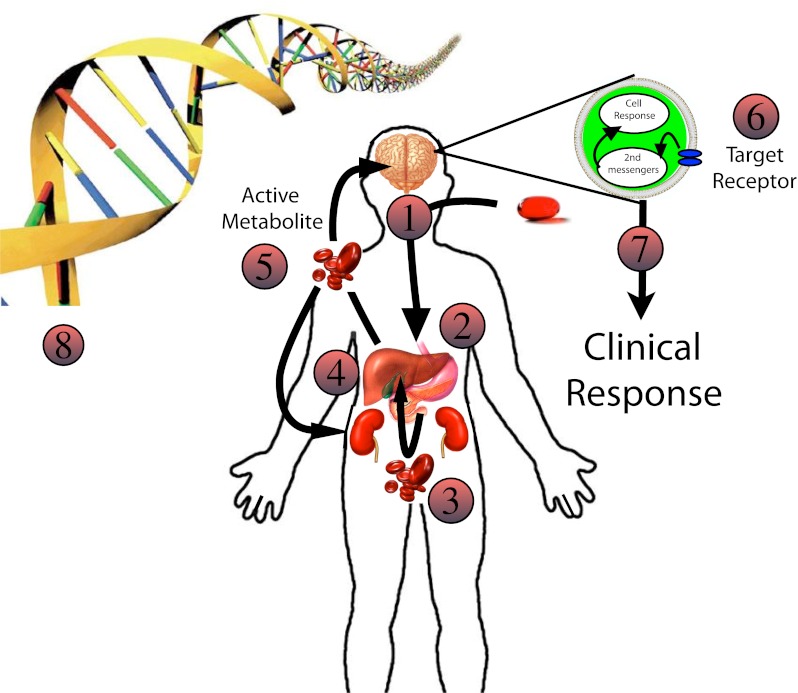
The complexity of drug response in the individual patient: 1 Drug ingested—Variations in drug dose may affect efficacy. 2 Drug absorption—Variations in the local gastrointestinal environment (acid concentration, absorption surface, bacteria, gut motility, etc) may affect the amount of drug available for absorption. 3 Translocation into blood—Drug crosses the intestinal epithelium, some is metabolized at the intestinal brush border; then the drug and its metabolites enter the portal vein and travel to the liver. Polymorphisms in drug transporters as well as changes in blood flow may affect drug delivery to the liver. 4 Hepatic DMEs—If there is low enzyme capacity, the drug may be shunted to other DMEs resulting in alternative metabolites with variable activity. Drug inhibitors or inducers may change the percentages of drug metabolites entering systemic circulation. 5 Systemic circulation—After leaving the liver, drug metabolites are sent to target organs and cleared by the kidneys. Blood flow and transporters may affect the amount of active metabolite reaching the drug target and the subsequent drug clearance. 6 Target receptor—The expression of drug target receptors may affect the clinical response. Variations in overall number, affinity for the drug, and the presence of inhibitors may result in variable clinical response. 7 Clinical response—May be affected by upstream variations as well as changes in downstream second messenger systems leading to variable phenotypic response. 8 Genetic polymorphism—Each step may be effected by gene polymorphism. In addition, local DNA/RNA environmental factors may affect transcription and translation. Given that each step is dependent on multiple genes, the range of phenotypes expressed is enormous for complex diseases. Ultimately, it is unlikely for identification of single mutations to determine individual patient phenotypes
The Importance of ADME
Clinical efficacy and toxicity of pharmaceutical therapy are affected by all stages of this pharmacokinetic model (Fig. 1). When a drug is ingested, absorption may be affected by the dose, enteric bacteria colonization, local pH variability, co-morbid intestinal pathology, among other factors. Transporters are often required to facilitate drug absorption into the portal circulation. These transporters are affected by the same genetic variability as other proteins in this model resulting in a range of drug delivery to the liver [4–6]. Measurement of baseline metabolic products (metabolomics) may reflect an individual's metabolic capacity and risk of toxicity when drugs are introduced [7–10]. Drug distribution is dependent upon multiple factors including drug transporters and body habitus resulting in variability in the amount of drug reaching the target tissue. Elimination is similarly affected by transporter polymorphism, local biochemical conditions, such as pH, and alterations in glomerular filtration rate. Decreased elimination increases the exposure to pharmaceutical agents and increases the risk of toxic effects. All of these factors contribute to the ultimate drug response phenotype experienced by the individual patient. Hepatic metabolism is a point of major intersection in drug therapy and therefore much research has focused on variability in these enzymes.
Genomics of Drug Metabolizing Enzymes
The liver plays the central role in drug metabolism in the human body. The vast majority of pharmaceuticals are metabolized by the phase 1 cytochrome P450 system (CYP); therefore, much pharmacogenomic work has focused on genetic polymorphisms of these enzymes. It is estimated that more than 90% of known drugs are metabolized by CYPs 1A2, 2C9, 2C19, 2D6, 2E1, and 3A4 [11]. Polymorphisms in CYP enzymes once seemed likely to be responsible for the majority of variability in drug response between patients. Indeed, numerous genetic variants have been identified, and these may be associated with variation in drug metabolism. These polymorphisms may lead to adverse drug effects and increased or decreased clinical drug response.
New CYP polymorphisms are discovered every year. Many major genetic variations affecting these enzymes have been identified although the phenotype is often discordant from the expected CYP genotype [12–15]. Reasons for discordance have not been fully characterized though several of the contributing factors will be discussed in the sections below. In addition to the expense and time required for CYP genotyping, not all allele variants have been identified, and undiscovered variants may contribute significantly to enzyme expression in certain populations. This has obvious implications for the predictive efficacy of currently available genotyping assays. For instance, the Roche AmpliChip, the only FDA-approved microarray assay, detects 33 distinct CYP2D6 alleles [16]. Unfortunately, this represents only a quarter of the known alleles to date [17]. Therefore, it is probable, even likely, that clinically significant alleles have yet to be identified, and current screens have predictive shortcomings.
In addition to unrecognized polymorphisms, very few pharmaceuticals are metabolized by a single CYP isoenzyme. The CYP enzyme system has inherent redundancy that alters clinical response to drugs when enzyme activity is altered by genetic polymorphism or drug interactions. When one isoform is not available (due to inhibition, downregulation, or non-expression) drugs may be shunted through alternative CYP enzymes resulting in alternative metabolites with varying affinity for the target receptor. Samer et al. elegantly demonstrated this phenomenon by inhibiting CYP 3A4 and showing that oxycodone is then metabolized by CYP2D6 to a more potent metabolite [18]. Thus, characterization of the full spectrum of CYP metabolic capacity and understanding of the drug affinities for specific enzymes are necessary to predict the metabolite profile in an individual patient.
The liver is the site of many phase 2 drug metabolizing enzymes (DMEs) that facilitate drug conjugation to enhance elimination. Polymorphism in these enzymes generally does not significantly affect drug clearance because the rate-limiting metabolism is typically dictated by phase 1 DMEs. However, alterations in the conjugation rate of reactive intermediates may lead to increased toxicity of metabolites [19]. This was first demonstrated with the association of peripheral neuropathy in isoniazid “slow acetylators” [20, 21]. Isoniazid is primarily metabolized by N-acetylation. “Slow acetylators” shunt isoniazid through an alternative CYP isoenzyme to form isonicotinic acid, a neurotoxin that is typically produced in only small amounts by “rapid acetylators” [19].
Finally, patients with the same genotype may not have similar DME activity due to altered gene expression from drug and environmental factors. For instance, smoking affects the clinical efficacy of multiple drugs through several mechanisms [22–24]. Induction of CYP1A2 by active [25, 26] and passive [27, 28] smoke inhalation can decrease serum theophylline concentrations. Epigenetic modifications such as DNA methylation, histone modification, and expression of non-coding RNAs that contribute to DNA gene expression have also been increasingly recognized as important in gene expression and likely affect drug efficacy [29]. While effort is underway to characterize these factors in a “human epigenome project,” these DNA-environmental factors are not readily predictable at this time. Further characterization of these factors is necessary before we can predict translation of genes into the DMEs responsible for drug metabolism.
Polymorphisms in Drug Targets
Independent of drug–CYP interactions, clinical response to drugs is affected by polymorphism in the drug targets [30]. An attempt to characterize the variability in warfarin doses based upon CYP2C19 and the drug target vitamin K epoxide reductase complex (VKORC1) polymorphisms demonstrated that VKORC1 had a greater effect on the warfarin dose requirement then CYP polymorphisms [31]. Polymorphisms in the beta-2 adrenergic receptor have been associated with variation in the phenotypic response to asthma pharmaceuticals [32]. Opioid receptor variants have been associated with decreased signaling in the cellular pathway determining pain perception [33]. Downstream drug target polymorphisms must be characterized to understand the drug and metabolite interactions that lead to clinical response.
Monogenetic Versus Polygenetic Diseases
In order for a genotype to completely predict a phenotype, drug response must be independent of upstream and downstream genetic and environmental variations. A simple mathematical calculation reveals that increasing the number of genes involved increases the variability in resulting phenotype. For instance, under ideal Mendelian conditions, if only one gene represented by two alleles determines the phenotype, the Hardy–Wienberg equilibrium dictates a 1:2:1 proportion of the four possible genotypes expressed. However, as more genes contribute to the trait, the number of potential genotypes increases so that 2 genes with 2 alleles result in 16 genotypes, 3 genes with 2 alleles result in 64 genotypes, and so on.
Phenotype can be predicted by genotype only in the simplest drug response or toxicity interactions. These relationships are present in monogenic diseases or in extreme phenotypes that have little or no biologic redundancy in the system [30, 34]. An example of a simple genetically identifiable phenotype is Gaucher's disease. This is a monogenic disease in which identification of a mutant allele results in an enzyme deficiency and thus, disease. This glucocerebrosidase enzyme can be replaced restoring the phenotype to near normal. No significant protein redundancy exists in this system; therefore, inability to make a functional protein directly leads to disease.
More complex diseases such as coronary artery disease or obesity are dependent upon many genes (polygenic) and environmental factors leading to a wide range of phenotypes that are virtually impossible to predict with genomics alone. Drug response is not as simple as Gaucher's disease but also not as complex as obesity due to the occurrence of major funnel points in the human ADME model. Funnel points represent areas of the human–drug interaction where numerous upstream variables convene and subsequent downstream phenotype is determined by only a few factors. Phenotyping of funnel points accounts for the complexity of the upstream contributory factors. Hepatic DME activity represents an important funnel point in the human–drug interaction.
The expression of human CYP450s can be characterized as high-penetrance predominantly monogenic (hPpM) traits [30]. hPpM traits are primarily determined by a single gene with redundancy within the biologic output leading to preservation of the phenotype when a deficiency of one enzyme occurs. Clinical examples of this phenomenon are toxicity associated with HLA polymorphisms in patients given carbamazepine [35, 36], CYP2D6 deficiency leading to ineffective analgesia with codeine [37, 38], or increased CYP2D6 activity leading to opioid toxicity [39–41]. In these cases, genotypic characterization allowed for prediction of drug efficacy or toxicity at the extremes of the phenotypic distribution. Perfect prediction of phenotype is not possible with these traits, but identification of the extreme phenotypes, represented by complete lack of efficacy or severe drug toxicity, may be achieved due to failure of redundancy at these extremes of gene expression.
Given the above limitations, we must realize that genotype cannot be expected to predict phenotype in complex diseases. However, functional studies at complex funnel points may allow for phenotypic characterization that ultimately predicts the human–drug response at these intersections. Functional studies represent a shortcut around full characterization of each of the contributing factors at complex intersections.
Part B: A Systems-Based Approach
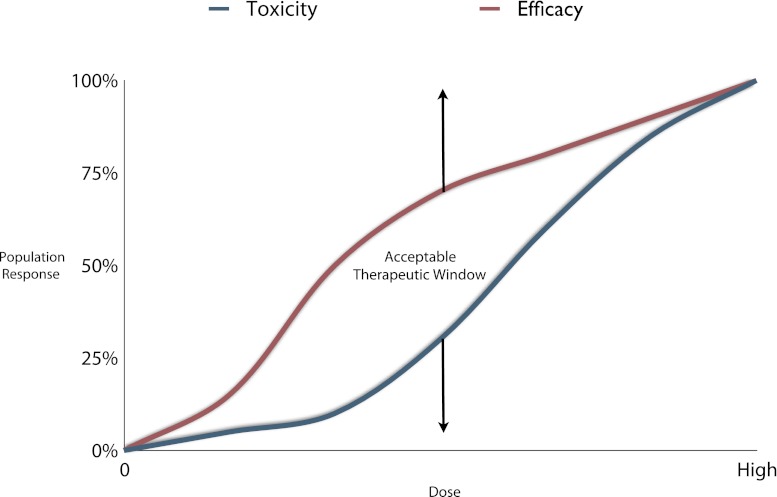
While further research is necessary to identify isolated enzyme or target polymorphisms that predict drug efficacy and toxicity, these polymorphisms are likely to represent only a minority of clinically significant variations. Warfarin dose requirements were only 41% predictable when researchers focused upon demographics, CYP, and drug target genotypes [31]. Integrating pharmacogenomic and functional studies into panels, here forth referred to as biologic systems panels (BSPs), may allow for more efficient prediction of individual drug response and limitation of toxicity. Characterization of phenotype at complex intersections and genotypes at simple funnel points integrated in a pharmacokinetic and pharmacodynamic model can improve drug therapy by excluding patients at risk of toxicity and allowing for increased doses in other drug-resistant patients. This approach may serve to broaden the acceptable therapeutic window (Fig. 2). Below we will suggest a BSP approach for study of predictive efficacy and toxicity of drug therapy.
Fig. 2.
Population response versus toxicity: The area between the curves represents the therapeutic range in which efficacy is outweighed by the toxicity. At higher doses, the efficacy and toxicity lines begin to converge making the risk of toxicity not worth the increased efficacy. BSPs may allow exclusion of patients at risk of toxicity and allow for increased doses in drug-resistant patients. This can increase the area between these lines thereby increasing efficacy and decreasing toxicity for a specific drug
Phenotyping to Integrate Complex Pathway Function
Phenotyping of an individual's DME activity has advantages over genotyping in complex pharmaceutical systems. As discussed above, genotyping cannot readily predict phenotype in biologic systems dependent upon multiple genes and environmental factors. Phenotyping takes genome interactions into account and functionally characterizes the metabolic enzyme in question. Phenotyping of the CYP system may be accomplished using a drug “cocktail” that contains several probe drugs allowing characterization of multiple CYP enzymes at the same time [42, 43]. Taken together, this can account for CYP redundancy. New methods allow for quantitative serum measurements of a probe drug and their metabolites [32, 43–45] after administration of “microdoses” of probe drugs. This phenotyping approach can be considered targeted “metabolomics.” Advantages of this serum-based approach include development of a metabolic curve within several hours compared to the more time-consuming urinary clearance methods [46]. Microdosing allows for administration of small probe drug doses minimizing the possibility of clinical effects from probe drugs as well as decreasing the risk of interaction between the probe drugs themselves [47]. Serum measurements are more precise and are not prone to the same pH-dependent inaccuracies associated with urinary metabolic ratio studies [46]. Serum testing is ideal for emergency department (ED) phenotyping since many subjects do not spend adequate time in the ED for urinary studies, and many subjects in this venue have co-morbid diseases that may affect urinary studies.
Metabolomic phenotyping accounts for variability in absorption, CYP gene expression, transporters, as well as the redundancy in the system allowing for better characterization of the true individual patient metabolic potential. Metabolomics represents a sensitive quantitative approach that accounts for upstream and downstream variables limiting the predictive efficacy of DME genotyping. This approach may be a more efficient method to predict dose and toxicity associated with an individual's metabolic capacity.
Genomic, Transcriptomic, and Proteomic Characterization of Simple Determinants of Drug Response
The next major intersection of drug therapy occurs at the drug target site. Major drug classes target specific physiologic receptors such as the alpha- and beta-adrenergic receptors, u-opioid receptors, serotonin, or dopamine transporters. Identification of polymorphism in drug targets may help to improve drug efficacy. Characterization of these polymorphisms can be achieved with genomics using PCR or microarray methods, transcriptomics utilizing microarray assays to determine changes in gene expression [48], and proteomics using a variety of methods to determine the protein variability in human disease [49, 50]. Polymorphisms can affect binding, and their identification can be rapidly performed with these screening assays. Using transcriptomics and proteomics requires measurement of gene products, RNA, and proteins, respectively, within the target tissue limiting their application if the target is only present in an in situ organ. Drug-receptor pharmacodynamic interactions are simpler than determining receptor expression for several reasons. There is little redundancy in many of these interactions since pharmaceutical agents target specific receptors or proteins at a cellular level. Simple ions or chemicals rather than proteins mediate downstream cellular signaling following receptor binding thus eliminating much of the variability associated with downstream transcription and translation. However, subtle changes in the local cellular environment may still have implications for phenotypic expression. Identification of polymorphisms associated with extreme phenotypes is likely to represent the highest clinical utility and is more likely to have genotype–phenotype concordance. For instance, opiate receptor polymorphisms contribute significantly to pain perception and likely contribute to efficacy, independent of metabolic capacity [37]. Identification of this genetic variant may improve analgesic effect from prescribed opioids since u-receptor binding is dose dependent. Opioid potency is likely to be more predictable via genotyping in opioid-naive patients prior to changes in expression of cellular receptors and downstream signaling machinery associated with chronic use. Since chronic users have proven themselves tolerant to the clinical effects at lower doses, decreased efficacy can only be explained by changes in receptor number or downstream cellular signaling. A transcriptomic or proteomic approach would be favorable in chronic users, though brain tissue samples are not readily available. Utilizing the extreme phenotype approach, however, genotype analysis can identify polymorphisms that are associated with extreme non-responder or phenotypes predisposed to toxicity.
Biologic Systems Panel
These approaches may be combined in order to improve the predictive efficiency of opioid drug therapy. We are unlikely to fully individualize drug therapy due to the complexity of these biologic systems. A more realistic goal should be to eliminate ineffective therapies and limit major toxicities characterized by extension of therapeutic effects. This may be accomplished by employing functional metabolomic studies with selective genomic, transcriptomic, or proteomic characterization of extreme discordant phenotypes at simpler intersections. If a patient's CYP phenotype is combined with genotyping of the drug target and creatinine clearance to account for variability in elimination, then predictions can be made regarding metabolic capacity, receptor affinity, and area under the curve of the active metabolites. This may allow for more efficient dosing of a drug and improved clinical efficacy in the phenotypic extremes. This approach bypasses much of the upstream and downstream variability associated with CYP polymorphisms. In addition, it examines major intersections of drug therapy identifying potential bottlenecks in the ADME model. This approach cannot be expected to identify all specific toxicities or determine the specific dose needed for the individual patient since it does not account for receptor expression or downstream cellular signaling polymorphisms. However, it may allow for safer and more effective titration in an individual patient.
Clinical Setting
Limited study has been undertaken in clinically relevant patient populations, and population-based genomics studies have found poor correlation with clinical phenotypes. Most studies of CYP genotyping have been undertaken in healthy volunteers. No studies have yet sought to characterize CYP phenotype in ambulatory patient populations, the group most likely to benefit from identification of metabolic variation. The ED is an ideal place to study real-world patient populations with the drug interaction and drug efficacy issues raised above. The majority of ED patients have intravenous access obtained and undergo observation and treatment for several hours. This affords the opportunity to perform both genotyping and phenotyping studies accounting for interactions that are typically ignored in healthy volunteer studies.
CYP2D6 and the u-Opioid Receptor—Rational Targets for BSP Integration
The hepatic CYP2D6 enzyme metabolizes 25% of prescribed drugs. Classes of drugs metabolized by 2D6 are diverse and include antidepressants, antipsychotics, anti-arrhythmics, β-blockers, and analgesics. This enzyme is highly polymorphic affecting at least 50% of the drugs it metabolizes. Polymorphisms in 2D6 have been linked to both ineffective drug therapy [51, 52] and drug toxicity [41, 53, 54]. Population genotypes are divided into four metabolic activities: poor metabolizer (PM), intermediate metabolizer, extensive metabolizer, and ultra-rapid metabolizer (UM). Up to 19% of some ethnicities possess PM enzyme capacity and up to 16% possess UM capacity determined by genotype analysis. Using microarray assays, genotype correlates well with phenotype in healthy volunteer studies. However, significant discordance between genotype and predicted phenotype has been observed in various populations and in subjects receiving other medications [13, 14, 47, 55, 56]. This enzyme is an example of a hPpM trait that is responsible for drug metabolism affecting complex disease states [57]. CYP2D6 is ideal for characterization utilizing a systems approach because phenotype is partially predictable based upon characterization of allelic variation; it is a low-capacity enzyme thus subject to saturation due to drug interaction. CYP redundancy exists, and phenotyping of enzyme activity is readily available.
The most commonly prescribed pharmaceutical in the ED is hydrocodone/acetaminophen [58]. The u-opioid receptor affinity of hydrocodone is two orders of magnitude lower than its CYP2D6 metabolite hydromorphone [59], and thus, potency is largely dependent upon an individual's CYP2D6 enzyme function. Preliminary studies by AF Manini have demonstrated correlation of a u-opioid receptor single nucleotide polymorphism associated with respiratory arrest. Targeted genotyping to identify u-opioid receptor polymorphisms can be integrated with CYP phenotype and glomerular filtration rate (GFR) to predict drug exposure and subsequent response at the cellular level.
Putting It All Together
A BSP that includes characterization of DME phenotypes, opiate receptor polymorphism, and GFR can potentially identify patients that will not have drug efficacy at any dose and those at significant risk of toxicity. As outlined above, phenotyping can be performed rapidly, in the ED or office setting, with pharmacokinetic evaluation of probe drugs. The individual's phenotype will remain largely unchanged unless xenobiotic ingestions significantly change. Likewise, drug target genotyping can be rapidly performed and will not change in a patient's lifetime. Adding a measurement of drug clearance can account for variability of area under the curve and hence drug target exposure, due to decreased elimination. Ultimately, we must gain more knowledge about how upstream factors affect the concentrations at the drug target level. Theoretically, if one factor changes, the entire panel would not have to be repeated due to independence of the individual components of the panel. The BSP concept can serve as a prediction tool for expected active drug concentrations and subsequent pharmacodynamic effects at drug targets. One can see the potential power of this knowledge in an ED setting. An initial investment in individual patient characterization can lead to more efficient prescribing practices and fewer toxic effects.
Future Research Needs and Conclusions
The concept of individualized drug therapy has not become a reality due, in part, to the above limitations. Future studies on predicting drug efficacy must take into account the pharmacogenomic, pharmacokinetic, and pharmacodynamic factors as a system. Continued basic science research is necessary to identify significant polymorphisms at all stages of drug therapy. Researchers hoping to improve drug efficacy and minimize toxicity must utilize a translational approach integrating these factors. The role of targeted metabolomic studies to characterize DME activity is more likely to account for the complexity dictating drug effect.
Genotyping alone is unlikely to predict an individual's response to drug therapy. Characterization of phenotypes at complex intersections in conjunction with selective genotyping at simple intersections may improve medication efficacy and safety.
Acknowledgments
Funding Mechanism Proposal
This work will be used for the Society of Academic Emergency Medicine (SAEM) Research Training Grant (http://www.saem.org/research-training-grant-rtg) and an NIH K23 entitled, Translational Scholar Career Award in Pharmacogenomics and Personalized Medicine (http://grants1.nih.gov/grants/guide/pa-files/PA-11-009.html). The SAEM grant will serve to provide pilot data for the NIH application.
Conflict of Interest
None.
The 'Omics
- Genomics
The study of the human genome encompassed by all human genes and the attendant variability of the contained DNA
- Transcriptomics
The study of how the human genome is expressed. Changes in epigenomic, local cellular environment, and expression of other genes can alter the human “transcriptome”
- Proteomics
The study of proteins and their variability in human disease states. The proteome may vary between different individuals and even different organs and cells within the same individual
- Metabolomics
The quantitative measurement of endogenous and exogenous metabolic products to measure metabolic response or predict disease. This sensitive approach may be altered by minute-to-minute changes and accounts for upstream variations in an individual's genome, transcriptome, and proteome
Definitions
- Allele
One of two DNA segments, or genes, that exist at a single locus. One allele is from the father, and one is from the mother. Alleles follow the Hardy–Weinberg distribution in the population (p2 + pq + q2 = population frequency) where p is the major allele and q is the genetic variant.
- Complex disease
Disease states that are a result of multiple genetic polymorphisms across the entire genome leading to a bell-shaped curve of phenotypic expression. Examples include obesity, pain, and heart disease which are affected by numerous genes with allelic heterogeneity resulting in a wide-range disease.
- Epigenetics
Environmental factors that regulate gene expression but are not inherently part of the genome. These factors include DNA methylation, variations in histone wrapping, RNA silencing, among other factors.
- Genotype
DNA sequence of an individual organism.
- High-penetrance polymorphic disease (hPpM)
Diseases that are determined primarily by a single gene with redundancy within the biologic output leading to preserved phenotype. An example of these relationships is CYP drug metabolism in which other CYP enzymes will contribute to drug metabolism when the target enzyme is deficient. This leads to preservation of phenotype except when a patient has a deficiency at both enzyme loci.
- Monogenic disease
Diseases that have a high penetrance due to single allelic polymorphisms. Examples include cystic fibrosis, Gaucher's disease, and phenylketonuria.
- Penetrance
Percentage of individuals that express a phenotype associated with a genotypic variation.
- Phenotype
Biologic, physiologic, morphologic, or behavioral trait expressed by an organism.
- Polymorphism
Expression of multiple phenotypes in a population.
References
- 1.Nebert DW, Jorge-Nebert L, Vesell ES. Pharmacogenomics and “individualized drug therapy”: high expectations and disappointing achievements. Am J Pharmacogenomics. 2003;3(6):361–370. doi: 10.2165/00129785-200303060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkelmann BR, Herrington D. Pharmacogenomics–10 years of progress: a cardiovascular perspective. Pharmacogenomics. 2010;11(5):613–616. doi: 10.2217/pgs.10.68. [DOI] [PubMed] [Google Scholar]
- 4.Staud F, Ceckova M, Micuda S, et al. Expression and function of p-glycoprotein in normal tissues: effect on pharmacokinetics. Methods Mol Biol. 2010;596:199–222. doi: 10.1007/978-1-60761-416-6_10. [DOI] [PubMed] [Google Scholar]
- 5.Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5(7):703–729. doi: 10.1517/17425250902976854. [DOI] [PubMed] [Google Scholar]
- 6.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuhara K, Ohno A, Ando Y, et al. A 1H NMR-based metabolomics approach for mechanistic insight into acetaminophen induced hepatotoxicity. Drug Metab Pharmacokinet. 2011;26:399–406. doi: 10.2133/dmpk.DMPK-11-RG-005. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Krausz KW, Shah YM, et al. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22(4):699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Schnackenberg LK, Hansen DK, et al. Study of valproic acid-induced endogenous and exogenous metabolite alterations using LC-MS-based metabolomics. Bioanalysis. 2010;2(2):207–216. doi: 10.4155/bio.09.173. [DOI] [PubMed] [Google Scholar]
- 10.O'Connell TM, Watkins PB. The application of metabonomics to predict drug-induced liver injury. Clin Pharmacol Ther. 2010;88(3):394–399. doi: 10.1038/clpt.2010.151. [DOI] [PubMed] [Google Scholar]
- 11.Wang JF, Zhang CC, Chou KC, et al. Structure of cytochrome p450s and personalized drug. Curr Med Chem. 2009;16(2):232–244. doi: 10.2174/092986709787002727. [DOI] [PubMed] [Google Scholar]
- 12.Evans WE, Relling MV. Concordance of P450 2D6 (debrisoquine hydroxylase) phenotype and genotype: inability of dextromethorphan metabolic ratio to discriminate reliably heterozygous and homozygous extensive metabolizers. Pharmacogenetics. 1991;1(3):143–148. doi: 10.1097/00008571-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gaedigk A, Bradford LD, Marcucci KA, et al. Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clin Pharmacol Ther. 2002;72(1):76–89. doi: 10.1067/mcp.2002.125783. [DOI] [PubMed] [Google Scholar]
- 14.Ieiri I, Yamada S, Seto K, et al. A CYP2D6 phenotype-genotype mismatch in Japanese psychiatric patients. Pharmacopsychiatry. 2003;36(5):192–196. doi: 10.1055/s-2003-43049. [DOI] [PubMed] [Google Scholar]
- 15.Dumond JB, Vourvahis M, Rezk NL, et al. A phenotype-genotype approach to predicting CYP450 and P-glycoprotein drug interactions with the mixed inhibitor/inducer tipranavir/ritonavir. Clin Pharmacol Ther. 2010;87(6):735–742. doi: 10.1038/clpt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebsamen MC, Desmeules J, Daali Y, et al. The AmpliChip CYP450 test: cytochrome P450 2D6 genotype assessment and phenotype prediction. Pharmacogenomics J. 2009;9(1):34–41. doi: 10.1038/tpj.2008.7. [DOI] [PubMed] [Google Scholar]
- 17.Sim S (2011) CYP2D6 allele nomenclature. Mar 11, 2011. At: http://www.cypalleles.ki.se/cyp2d6.htm. Accessed 7 April 2011
- 18.Samer CF, Daali Y, Wagner M, et al. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol. 2010;160(4):907–918. doi: 10.1111/j.1476-5381.2010.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crettol S, Petrovic N, Murray M. Pharmacogenetics of phase I and phase II drug metabolism. Curr Pharm Des. 2010;16(2):204–219. doi: 10.2174/138161210790112674. [DOI] [PubMed] [Google Scholar]
- 20.Evans DA, Manley KA, Mc KV. Genetic control of isoniazid metabolism in man. Br Med J. 1960;2(5197):485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blum M, Grant DM, McBride W, et al. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. DNA Cell Biol. 1990;9(3):193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- 22.Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917–1921. doi: 10.2146/ajhp060414. [DOI] [PubMed] [Google Scholar]
- 23.Schein JR. Cigarette smoking and clinically significant drug interactions. Ann Pharmacother. 1995;29(11):1139–1148. doi: 10.1177/106002809502901113. [DOI] [PubMed] [Google Scholar]
- 24.Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Lee BL, Benowitz NL, Jacob P., 3rd Cigarette abstinence, nicotine gum, and theophylline disposition. Ann Intern Med. 1987;106(4):553–555. doi: 10.7326/0003-4819-106-4-553. [DOI] [PubMed] [Google Scholar]
- 26.Gardner MJ, Tornatore KM, Jusko WJ, et al. Effects of tobacco smoking and oral contraceptive use on theophylline disposition. Br J Clin Pharmacol. 1983;16(3):271–280. doi: 10.1111/j.1365-2125.1983.tb02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo PR. Effect of passive smoking on theophylline clearance in children. Ther Drug Monit. 2001;23(5):503–505. doi: 10.1097/00007691-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Matsunga SK, Plezia PM, Karol MD, et al. Effects of passive smoking on theophylline clearance. Clin Pharmacol Ther. 1989;46(4):399–407. doi: 10.1038/clpt.1989.158. [DOI] [PubMed] [Google Scholar]
- 29.Brena RM, Huang TH, Plass C. Toward a human epigenome. Nat Genet. 2006;38(12):1359–1360. doi: 10.1038/ng1206-1359. [DOI] [PubMed] [Google Scholar]
- 30.Nebert DW, Zhang G, Vesell ES. From human genetics and genomics to pharmacogenetics and pharmacogenomics: past lessons, future directions. Drug Metab Rev. 2008;40(2):187–224. doi: 10.1080/03602530801952864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namazi S, Azarpira N, Hendijani F, et al. The impact of genetic polymorphisms and patient characteristics on warfarin dose requirements: a cross-sectional study in Iran. Clin Ther. 2010;32(6):1050–1060. doi: 10.1016/j.clinthera.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Qiu YY, Zhang XL, Qin Y, et al. Beta(2)-adrenergic receptor haplotype/polymorphisms and asthma susceptibility and clinical phenotype in a Chinese Han population. Allergy Asthma Proc. 2010;31(5):91–97. doi: 10.2500/aap.2010.31.3371. [DOI] [PubMed] [Google Scholar]
- 33.Oertel BG, Kettner M, Scholich K, et al. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem. 2009;284(10):6530–6535. doi: 10.1074/jbc.M807030200. [DOI] [PubMed] [Google Scholar]
- 34.Nebert DW. Extreme discordant phenotype methodology: an intuitive approach to clinical pharmacogenetics. Eur J Pharmacol. 2000;410(2–3):107–120. doi: 10.1016/S0014-2999(00)00809-8. [DOI] [PubMed] [Google Scholar]
- 35.McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Lin JJ, Lu CS, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126–1133. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 37.Lotsch J, Skarke C, Liefhold J, et al. Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet. 2004;43(14):983–1013. doi: 10.2165/00003088-200443140-00003. [DOI] [PubMed] [Google Scholar]
- 38.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48(12):761–804. doi: 10.2165/11318070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Gasche Y, Daali Y, Fathi M, et al. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N Engl J Med. 2004;351(27):2827–2831. doi: 10.1056/NEJMoa041888. [DOI] [PubMed] [Google Scholar]
- 40.Koren G, Cairns J, Chitayat D, et al. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368(9536):704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- 41.Madadi P, Ross CJ, Hayden MR, et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case–control study. Clin Pharmacol Ther. 2009;85(1):31–35. doi: 10.1038/clpt.2008.157. [DOI] [PubMed] [Google Scholar]
- 42.Fuhr U, Jetter A, Kirchheiner J. Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin Pharmacol Ther. 2007;81(2):270–283. doi: 10.1038/sj.clpt.6100050. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka E, Kurata N, Yasuhara H. How useful is the “cocktail approach” for evaluating human hepatic drug metabolizing capacity using cytochrome P450 phenotyping probes in vivo? J Clin Pharm Ther. 2003;28(3):157–165. doi: 10.1046/j.1365-2710.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 44.Alden PG, Plumb RS, Jones MD, et al. A rapid ultra-performance liquid chromatography/tandem mass spectrometric methodology for the in vitro analysis of Pooled and Cocktail cytochrome P450 assays. Rapid Commun Mass Spectrom. 2010;24(1):147–154. doi: 10.1002/rcm.4364. [DOI] [PubMed] [Google Scholar]
- 45.Turpault S, Brian W, Van Horn R, et al. Pharmacokinetic assessment of a five-probe cocktail for CYPs 1A2, 2C9, 2C19, 2D6 and 3A. Br J Clin Pharmacol. 2009;68(6):928–935. doi: 10.1111/j.1365-2125.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank D, Jaehde U, Fuhr U. Evaluation of probe drugs and pharmacokinetic metrics for CYP2D6 phenotyping. Eur J Clin Pharmacol. 2007;63(4):321–333. doi: 10.1007/s00228-006-0250-8. [DOI] [PubMed] [Google Scholar]
- 47.Kummer O, Hammann F, Moser C, et al. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur J Clin Pharmacol. 2011;67(1):63–71. doi: 10.1007/s00228-010-0893-3. [DOI] [PubMed] [Google Scholar]
- 48.Cui Y, Paules RS. Use of transcriptomics in understanding mechanisms of drug-induced toxicity. Pharmacogenomics. 2010;11(4):573–585. doi: 10.2217/pgs.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteaker JR, Lin C, Kennedy J, et al. A targeted proteomics-based pipeline for verification of biomarkers in plasma. Nat Biotechnol. 2011;29:625–634. doi: 10.1038/nbt.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moseley FL, Bicknell KA, Marber MS, et al. The use of proteomics to identify novel therapeutic targets for the treatment of disease. J Pharm Pharmacol. 2007;59(5):609–628. doi: 10.1211/jpp.59.5.0001. [DOI] [PubMed] [Google Scholar]
- 51.Lobello KW, Preskorn SH, Guico-Pabia CJ, et al. Cytochrome P450 2D6 phenotype predicts antidepressant efficacy of venlafaxine: a secondary analysis of 4 studies in major depressive disorder. J Clin Psychiatry. 2010;71:1482–1487. doi: 10.4088/JCP.08m04773blu. [DOI] [PubMed] [Google Scholar]
- 52.Seruga B, Amir E. Cytochrome P450 2D6 and outcomes of adjuvant tamoxifen therapy: results of a meta-analysis. Breast Cancer Res Treat. 2010;122(3):609–617. doi: 10.1007/s10549-010-0902-3. [DOI] [PubMed] [Google Scholar]
- 53.Wuttke H, Rau T, Heide R, et al. Increased frequency of cytochrome P450 2D6 poor metabolizers among patients with metoprolol-associated adverse effects. Clin Pharmacol Ther. 2002;72(4):429–437. doi: 10.1067/mcp.2002.127111. [DOI] [PubMed] [Google Scholar]
- 54.Kirchheiner J, Heesch C, Bauer S, et al. Impact of the ultrarapid metabolizer genotype of cytochrome P450 2D6 on metoprolol pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2004;76(4):302–312. doi: 10.1016/j.clpt.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Shiran MR, Chowdry J, Rostami-Hodjegan A, et al. A discordance between cytochrome P450 2D6 genotype and phenotype in patients undergoing methadone maintenance treatment. Br J Clin Pharmacol. 2003;56(2):220–224. doi: 10.1046/j.1365-2125.2003.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaedigk A, Eklund JD, Pearce RE, et al. Identification and characterization of CYP2D6*56B, an allele associated with the poor metabolizer phenotype. Clin Pharmacol Ther. 2007;81(6):817–820. doi: 10.1038/sj.clpt.6100125. [DOI] [PubMed] [Google Scholar]
- 57.Nebert DW, Vesell ES. Advances in pharmacogenomics and individualized drug therapy: exciting challenges that lie ahead. Eur J Pharmacol. 2004;500(1–3):267–280. doi: 10.1016/j.ejphar.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 58.Raofi S, Schappert SM. Medication therapy in ambulatory medical care: United States, 2003–04. Vital Health Stat. 2006;13(163):1–40. [PubMed] [Google Scholar]
- 59.Volpe DA, Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid Mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]