Abstract
Outcomes following unintentional, supratherapeutic ingestions of a patient’s own beta-blocker (BB) or calcium channel blocker (CCB) have not been well studied. A retrospective review of all poison control center (PCC) charts from January 2007 through December 2009 yielded 4,099 cases involving a BB or CCB. Of these, 436 (10.6%) met inclusion criteria. Data abstracted included patient age/gender, medication(s) involved, dose(s), time interval between ingestions, symptoms, and outcome. Exclusion criteria included intentional ingestions, ingesting someone else’s medication, and ingestion intervals >12 h. Outcomes were defined as the development of symptoms, management site, hospital admission, and death. Mean age was 65.1 years (range 2–91; SD 17.9); 284 (65.1%) were women. Eighty-two (18.8%) cases resulted in ED evaluation; 44 (53.7%) of these were referred in by the PCC. Symptoms developed in 44 (10.1%) cases and 32 (7.3%) were admitted due to the ingestion. Of those admitted, five (15.6%) received treatment (three intravenous fluids, one glucagon, one calcium). Of the 343 (78.7%) cases initially observed on site, three (0.9%) were later referred to an ED; none required treatment. There was one death under extenuating circumstances. The validity of data abstraction was determined for six variable using 43 charts [0.97; 95% CI (0.91–0.99)]. Based on a retrospective analysis of PCC cases, home observation of asymptomatic patients following unintentional supratherapeutic ingestions of their own BB or CCB was safe in most cases. Further, prospective study is required to identify risks factors for becoming symptomatic or requiring treatment.
Keywords: beta-Blocker toxicity, Calcium channel blocker toxicity, Poison center utilization, Management of unintentional ingestions
Introduction
beta-Blocker (BB) and calcium channel blocker (CCB) toxicities are common poisonings associated with significant morbidity, prompting the development of consensus guidelines by the American Association of Poison Control Centers (AAPCC) [1, 2]. In 2009, US poison control centers (PCCs) handled approximately 4.2 million total calls, including over 95,000 human exposures to cardiovascular drugs; the substance category with the third fastest rate of increased exposures [3]. Interestingly, 70.2% of all human exposure calls involved unintentional ingestions, with over 87,000 calls involving a patient who “inadvertently took [or was given] a medication twice.”
In 2009, patients 50 years of age or older accounted for 255,292 (10.3%) of all human exposures reported to PCCs and 37.8% of reported fatalities with a known patient age [3]. Between 1990 and 2000, US residents in the 50-to-54 age group experienced the largest percentage in population growth at 55% [4]. Overall, the number of US residents greater than 49 years of age increased from 63.9 million to 76.5 million, accounting for an average increase of 25.1% growth compared to the overall population increase of 13.2%. With this shift toward more elderly Americans, we can anticipate an increase in the use of prescription medications as well as adverse drug reactions [5–8]. The number of PCC calls involving the elderly has increased over the past several years [9].
We reviewed our PCC records to determine the clinical effects, required treatments, and final outcomes following unintentional ingestions of BB and/or CCB medications in patients utilizing a regional PCC. We analyzed these data to review our current management guidelines, including the ability to monitor some of these patients at home. It was our hypothesis that home monitoring via PCC follow-up calls would be safe in all asymptomatic patients who unintentionally ingest a supratherapeutic amount of their own BB and/or CCB. We also hoped to further emphasize the role PCCs may play in saving community and health care resources by encouraging the public’s use of PCCs, minimizing unnecessary emergency department (ED) visits, and justify the use of home monitoring in asymptomatic patients with unintentional ingestions.
Methods
We conducted a retrospective chart review of our PCC’s charts by running a Crystal Reports® (2010, Williamsville, NY, USA) search on all calls received between January 1, 2007 and December 31, 2009. This 3 year study period yielded a total of 391,691 calls. These total calls were then filtered for humans exposed to either a BB or CCB by using the AAPCC generic codes for these substances (112,000 and 262,000 respectively) resulting in a total of 4,099 (1%) charts. Of these charts, 436 (10.6%) met inclusion criteria (see below) and were manually reviewed by investigators who underwent standard training for systematic electronic chart review.
These 436 cases involving unintentional, supratherapeutic ingestions of a patient’s own medication(s) were recorded on a data abstraction sheet. Recorded data included PCC case number, date of call, patient age and gender, medication(s) involved, time interval between the intended and unintentional ingestions, dose, any other ingested medications, the presence of symptoms (hypotension, bradycardia, subjective dizziness, or lightheadedness), abnormal serum glucose, confounders or other potential reasons for the patient’s symptoms (e.g., other identified active medical issues), referral to an ED, disposition if referred to an ED, coded effects, and survival. Outcomes were defined as the development of symptoms, abnormal glucose, management site, hospital admission, and death.
All charts involving a patient called to our PCC after an unintentional ingestion of their own BB and/or CCB, within the study period, were subject to enrollment. Inclusion criteria required that the patient must already be taking the BB or CCB chronically (i.e., not a new medication) and that the pills involved were the patient’s (i.e., not the same medications but a different dose or formulation prescribed to another person). Exclusion criteria included intentional ingestions, ingestion of someone else’s medication, and a time interval of more than 12 h. We prospectively decided that repeated ingestions occurring more than 12 h apart, regardless of formulation, introduced minimal risk for clinical effects.
A master list was generated with unique subject numbers assigned to each chart. The collected data were secured in password-protected electronic computer files by a PCC director with access to all records. Descriptive statistics were used with continuous variables and reported as means with standard deviations. Categorical variables and outcomes are reported as percentages. Forty-three (9.8%) cases were reviewed by a second investigator to ensure reliability of data abstraction from PCC charts. The result of this re-analysis is reported as percent agreement. This study received institutional review board approval.
Results
A total of 436 charts met inclusion criteria, of which 424 (97.2%) had complete data. All 12 cases with missing information contained sufficient data concerning outcomes and were therefore included in the final analysis. The subjects’ mean age was 65.1 years (range 2–91; SD 17.9). Women represented a majority of the cases with a total of 284 (65.1%). Six variables were re-assessed in 43 (9.8%) of the charts by a second reviewer. These included amount of drug ingested, time interval between ingestions, heart rate, blood pressure, coded outcome, and clinical effects (i.e., symptoms). The percent agreement for data abstraction for these six variables was 97%.
A total of 15 different medications were involved in the 436 included charts (Table 1). Metoprolol was the most common agent involved and accounted for 133 (30.5%) of all cases. A minority of cases (n = 99; 22.7%) involved a non-immediate release formulation. A total of 289 cases (66.3%) included the unintentional ingestion of a BB and 258 (59.2%) involved a BB only. One hundred seventy-eight cases (40.8%) involved a CCB and 147 (33.7%) involved only a CCB. Thirty-one (7.1%) of all included cases involved the ingestion of both a BB and CCB.
Table 1.
Medications involved in all included cases (N = 436)
| beta-Blockers (n = 289) | Calcium channel blockers (n = 178) |
|---|---|
| BB only=258 | CCB only = 147 |
| Metoprolol 133 (IR = 102; NIR = 31) | Diltiazem 61 (NIR = 44; IR = 17) |
| Atenolol 78 | Amlodipine 54 (2 combined with benazepril) |
| Carvedilol 30 | Verapamil 31 (NIR = 19; IR = 12) |
| Propranolol 22 (IR = 17; NIR = 5) | Nifedipine 23 (IR = 12; NIR = 11) |
| Labetalol 13 | Felodipine 7 |
| Sotalol 7 | Isradipine 1 |
| Nadolol 4 | Nisoldipine 1 |
| Nebivolol 4 |
Two patients took two different BBs; therefore, there are a total of 289 subjects and 291 total BBs
BB beta-blocker, CCB calcium channel blocker, IR immediate release formulation, NIR non-immediate release formulation
A total of 11 different medications were involved in the 44 cases which resulted in symptoms (Table 2). Metoprolol was the most common agent involved, accounting for 13 (29.5%) of these cases. Thirty-two cases (72.7%) involved immediate release formulations. Thirty-one cases (70.5%) included the unintentional ingestion of a BB; 28 (63.6%) involved a BB only. Sixteen cases (36.6%) involved a CCB; 13 (29.5%) involved only a CCB. Three (6.8%) of the symptomatic cases involved the ingestion of a BB and CCB.
Table 2.
Medications involved in symptomatic cases (N = 44)
| beta-Blockers (n = 31) | Calcium channel blockers (n = 16) |
|---|---|
| BB only = 28 | CCB only = 13 |
| Metoprolol 13 (IR = 10; NIR = 3) | Diltiazem 8 (NIR = 7; IR = 1) |
| Atenolol 10 | Verapamil (NIR) 4 |
| Propranolol 5 | Amlodipine 2 |
| Carvedilol 1 | Isradipine 1 |
| Labetalol 1 | Nifedipine 1 |
| Sotalol 1 |
BB beta-blocker, CCB calcium channel blocker, IR immediate release formulation, NIR non-immediate release formulation
Of the 436 included cases, 82 (18.8%) were evaluated in an ED. Forty-four (53.7%) of these cases were referred for ED evaluation by PCC staff, and the remaining 38 (46.3%) presented to an ED without PCC involvement. Symptoms developed in 44 (10.1%) of the 436 cases and 32 (7.3%) were admitted as a direct result of the ingestion. Three other subjects were admitted to the hospital but were excluded from analysis due to being admitted for unrelated issues (one for an international normalized ratio of 11 and two for hypertensive urgency).
A total of 32 patients were admitted to a hospital. These included nine (23.7%) of the 38 subjects who self-triaged to an ED, 22 (40%) of the 55 subjects referred to an ED by the PCC, and one (0.3%) of the 343 subjects initially managed on site. This last patient was admitted despite remaining asymptomatic (see below).
Only three (0.9%) out of 343 subjects initially followed on site were later referred to an ED. Two subjects developed symptoms at home that were identified during follow-up calls. The first subject experienced lightheadedness, identified 1 h later during the first follow-up call, and was sent to the ED by EMS. The other subject developed asymptomatic hypotension (87/49) and decreased heart rate (80 to 67 bpm), identified on initial PCC follow-up call 2.5 h after the ingestion, and was sent to the ED. A third subject, following initial PCC observation, contacted their primary care provider (PCP) and, despite remaining asymptomatic, was referred to an ED for evaluation. A fourth patient self-triaged to a PCP for evaluation but was still managed on site without developing symptoms. None of these four subjects required treatment. One was admitted after PCP evaluation, despite remaining asymptomatic, and was discharged within 24 h.
Of the 32 admitted subjects, five (15.6%) received a treatment intervention; one received glucagon, one received calcium gluconate, and three received intravenous fluids. Therefore, overall, a treatment was recorded in 1.1% (five out of 436) of all cases. All recorded treatments were coded as appropriate by PCC staff.
The glucagon infusion was used to treat a 52-year-old man with a prior history of atenolol “intolerance” who unintentionally ingested 75 mg of his atenolol at one time, instead of his normal 25 mg dose. He self-triaged to an ED where he developed hypotension (systolic blood pressure 72 mmHg), bradycardia (50 bpm), and dizziness. He was given glucagon 5 mg IV, with normalization of vital signs, and then admitted. He remained hemodynamically stable, required no further treatment, and was discharged within 24 h of admission. Another asymptomatic patient received one ampule of IV calcium gluconate for transient hypotension.
Of the 424 subjects with a recorded age, 351 (82.8%) were ≥50 years of age. Of these 351 patients, 32 (9.1%) developed symptoms and 31 (8.8%) were admitted to the hospital. This compares to 10.1% (n = 44) of all patients to develop symptoms and 7.3% (n = 32) to be admitted. All patients receiving a treatment (n = 5) were ≥50 years of age.
There was only one death (0.2%) reported in the 436 reviewed charts. This case involved a 90-year-old woman who ingested four doses of her daily medications: diltiazem (controlled delivery) 240 mg, atenolol 50 mg, glyburide 5 mg, warfarin 2 mg, furosemide 40 mg, lisinopril 5 mg, potassium chloride 20 mg, zaroxolyn 5 mg, and hydralazine 25 mg. She presented with normal blood pressure (134/60), bradycardia (37 bpm), and confusion of unknown duration. Her confusion was thought to have been responsible for the ingestions. After her family initiated a do-not-resuscitate directive, she developed shock and died within 3 h of the reported time of ingestion.
Discussion
Previous studies related to BB and CCB toxicity were used to help develop evidence-based consensus guidelines for the management of these poisonings, but these included minimal cases involving unintentional supratherapeutic ingestions of a patient’s own medication [1, 2]. Within these reviews, deaths following an unintentional ingestion of one’s own medication are few and involve confounders such as pre-existing morbidity (e.g., congestive heart failure) or substance abuse (e.g., cocaine) [1].
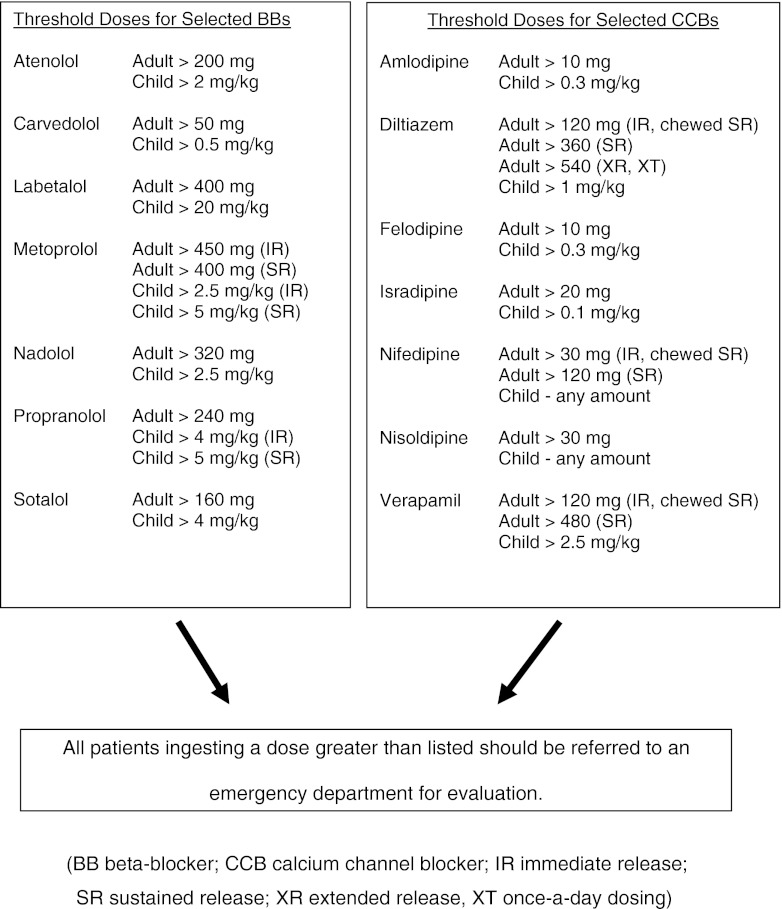
We used the AAPCC treatment algorithms to further analyze our data in terms of ingested doses (Fig. 1) [1, 2]. Although these resources were not specifically designed to triage all potential patients, including those with “significant underlying cardiovascular disease” or those “taking … another cardiodepressant drug,” we used the referral threshold doses to compare our PCC’s initial recommendations based on the reported amount of ingested medications (Table 3) as well as for those subjects who developed symptoms (Table 4).
Fig. 1.
AAPCC dose limitations for included medications (limited version; adapted from references [1, 2]). BB beta-blocker, CCB calcium channel blocker, IR immediate release, SR sustained release, XR extended release, XT once-a-day dosing
Table 3.
All included cases that would have failed the BB and/or CCB outpatient treatment algorithm(s) base on dose alone (n = 436)
| Would have been sent in for ED evaluation based on ingested dose only | BBs involved in cases of failed lgorithm (n = 26) | CCBs involved in cases of failed algorithm (n = 51) |
|---|---|---|
| Yes = 68 | Atenolol = 5 | Amlodipine = 28 |
| Carvedilol = 2 | Diltiazem = 11 | |
| No = 348 | Labetalol = 7 | Felodipine = 1 |
| Unknown = 16 | Metoprolol = 8 | Nicardipine = 1 |
| Not Included in original algorithm = 4 (Nebivolol) | Sotalol = 4 | Nifedipine = 6 |
| Verapamil = 4 |
BB beta-blocker, CCB calcium channel blocker, Unknown unknown total dose ingested
Table 4.
Symptomatic cases that would have failed the BB and/or CCB outpatient treatment algorithm(s) base on dose alone (n = 44)
| Would have been sent in for ED evaluation based on ingested dose only | BBs involved in cases of failed algorithm (n = 6) | CCBs involved in cases of failed algorithm (n = 8) |
|---|---|---|
| Yes = 14 | Atenolol = 2 | Amlodipine = 2 |
| No = 26 | Labetalol = 1 | Diltiazem = 4 |
| Metoprolol = 2 | Verapamil = 2 | |
| Unknown = 4 | Sotalol = 1 |
BB beta-blocker, CCB calcium channel blocker, Unknown unknown total dose ingested
Our data showed that most (82.8%) of included patients were ≥50 years of age and that this age group represented a majority of the symptomatic (72.7%), admitted (96.9%), and treated (100%) cases. These results follow trends noted in AAPCC and US Census data concerning our aging population.
The major limitation of this study involves the retrospective use of PCC data. Our design introduces the potential for selection bias (reliance on cases voluntarily reported to our PCC) and limited external validity (a single PCC). The reliability of recorded PCC data is compromised by unscripted questioning by PCC staff, which inevitably resulted in a range of documented information. Many independent variables (e.g., dose or symptoms) could not be confirmed. Other variables (e.g., living alone or comorbidities) which may have influenced PCC management decisions were not routinely documented in all charts.
Twenty-five (5.7%) of the included cases involved patients who were lost to follow-up (n = 19; 4.3%) or refused recommended treatments (n = 6; 1.4%), such as ED evaluation. The inability to record the symptoms and outcomes for these cases introduced another limitation. Additionally, it is expected that some subjects meeting inclusion criteria were never reported to our PCC. This may have occurred for several reasons, including remaining on site (e.g., home) without notifying anyone, obtaining advise elsewhere (e.g., PCP), and evaluation in, and possibly admission from, an ED without PCC contact.
We only included cases with a dosing interval of 12 h or less based on the assumption that ingestion intervals > 12 h would be unlikely to result in adverse events. Although this may have introduced selection bias, analysis of our symptomatic cases reveals that the majority (n = 28; 63.6%) of these cases had an ingestion interval of zero. The remaining 16 cases (36.4%) had a mean time interval between ingestions of 7.5 h (range 1–12 h).
Conclusions
Based on a retrospective analysis of PCC cases, home observation of asymptomatic patients following unintentional supratherapeutic ingestions of their own BB or CCB was safe in most cases. Further, prospective study is required to identify risks factors for becoming symptomatic or requiring treatment.
Acknowledgments
Conflict of Interest
The authors report no potential conflicts of interest.
References
- 1.Olson KR, Erdman AR, Woolf AD, et al. Calcium channel blocker ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Tox. 2005;43:797. doi: 10.1080/15563650500357404. [DOI] [PubMed] [Google Scholar]
- 2.Wax PM, Erdman AR, Chyka PA, et al. β Blocker ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Tox. 2005;43:131. [PubMed] [Google Scholar]
- 3.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Griffin SL. 2009 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th annual report. Clin Tox. 2010;48(10):979. doi: 10.3109/15563650.2010.543906. [DOI] [PubMed] [Google Scholar]
- 4.Meyer J (2001) AGE: 2000; Census 2000 brief. US Census Bureau. Issues October 2001. http://www.census.gov/prod/2001pubs/c2kbr01-12.pdf. Accessed 11 Mar 2011
- 5.Mannesse CK, Derkx FHM, de Ridder MAJ, van der Cammen TJM, Man in’t Veld AJ. Adverse drug reactions in elderly patients as contributing factor for hospital admissions: cross sectional study. BMJ. 1997;315(7115):1057. doi: 10.1136/bmj.315.7115.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moen J, Antonov K, Larsson CA, Lindblad U, Nilsson JLG, Rastam L, Ring L. Factors associated with multiple medications use in difference age groups. Ann Pharmacother. 2009;43(12):1978. doi: 10.1345/aph.1M354. [DOI] [PubMed] [Google Scholar]
- 7.Wessell AM, Nietert PJ, Jenkins RG, Nemeth LS, Ornstein SM. Inappropriate medication use in the elderly: results from a quality improvement project in 99 primary care practices. Am J Geriatr Pharmacother. 2008;6(1):21. doi: 10.1016/j.amjopharm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Aspden P, Wolcott JA, Bootman JL, Cronewett LR, editors. Committee on indentifying and preventing medication errors. Preventing medication errors: quality chasm series. Washington, DC: National Academies; 2007. [Google Scholar]
- 9.AAPCC (2011) http://www.aapcc.org/dnn/NPDSPoisonData/NPDSAnnualReports.aspx. Accessed 5 Apr 2011