Abstract
Cadmium is one of the elements found to damage antioxidant systems in mammals. To ameliorate cadmium toxicity and to prevent oxidative stress, natural products may be useful. In Indian ethnobotanical practice, a mixture of 17 herbal products is used to fortify the reproductive system of women after parturition and to reverse ovarian oxidative stress. Oral administration of this extract to rats exposed to cadmium was useful in reversing oxidative stress. Two different doses of cadmium (50 ppm and 200 ppm) were given to Wistar rats aged 45 and 65 days. An herbal extract derived from 17 plants was administered orally every day at a dose level of 200 mg/kg of body weight to the rats exposed to cadmium. A battery of enzymes involved in antioxidant activity in the ovary, including superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione-s-transferase (GST) were measured in the control, cadmium-exposed rats without treatment and in the cadmium-exposed rats treated with herbal extract. The reduction in SOD, catalase, GPx and GST activity after cadmium exposure improved significantly in the rats treated with the herbal extract (p < 0.05). The decrease of antioxidant enzymes due to cadmium exposure was reversed significantly with herbal extract administration. The synergistic effect of each bioactive compound in different herbal extracts requires further study.
Keywords: Medicinal plant extract, Cadmium, Superoxide dismutase (SOD), Catalase (CAT), Glutathione peroxidase (GPx), Glutathione-s-transferase (GST), Ovary
Introduction
Cadmium (Cd) is an environmental risk factor with various toxic effects in animals and humans. Cadmium enters the general environment from the natural weathering of materials, forest fires and volcanoes, but much larger amounts are released by human activities [1]. Cadmium chloride used in photography, photocopying, dyeing, calico printing, vacuum tube manufacture pigment production, galvanoplasty, lubricants, ice-nucleation agents and manufacture of special mirrors [2] may easily enter the environment. Long-term ingestion of large amounts of cadmium has been observed in Japan [3]. The exceptionally long half-life of cadmium in the human body, about 30 years [4], emphasizes the need for the effective assessment and treatment of cadmium toxicity. Cadmium is known to affect reproductive organs [5,6]. In the blood and tissues, Cd stimulates the formation of metallothioneins and reactive oxygen species (ROS), thus causing oxidative damage in erythrocytes and in various tissues. This produces a loss of membrane functions [7].
Oxidative stress is a condition associated with an increased rate of cellular damage induced by oxygen and oxygen-derived oxidants commonly known as reactive oxygen species [8]. The cellular damage in the gonads may be due to an improper balance between ROS (reactive oxygen species) generation and scavenging activities [9]. The scavenging potential in the gonad is normally maintained by adequate levels of antioxidant superoxide dismutase (SOD), catalase and glutathione [10]. Long-term exposure to Cd increases lipid peroxidation and causes inhibition of SOD activity, indicating oxidative damage [11]. The increase in lipid peroxidation (LPO) may be attributed to the alteration in the antioxidant defense system. This defense system includes the enzymes SOD, catalase, glutathione peroxidase (GPx), glutathione-s-transferase (GST) as well as glutathione, which normally protect against radical toxicity.
A number of plant compounds had been reported to exhibit a protective role against ROS and lipid peroxidation [12,13]. In southern India, several herbal products had been reported to fortify the reproductive systems of women and to mitigate oxidative stress due to ROS in the gonads after parturition [14]. The herbal products are collected from different parts of the plants, shade dried and finely powdered, mixed well and given to women soon after parturition. This herbal mixture (“Perukala rasayanam”) has been a household remedy used by many women.
This study tests the effect of Perukala rasayanam (PR) on the oxidative stress on ovarian tissue in rats that were affected by Cd toxicity. The influence of the herbal mixture on the oxidative stress in the ovary is studied after suppressing the antioxidant status in the rat with Cd exposure.
Materials and Methods
Thiobarbituric acid, dithio-bis-nitrobenzoic acid, sodium azide and bovine serum albumin were purchased from Sigma-Aldrich Co, St. Louis, USA. Sulphuric acid, hydrogen peroxide, chloroform and sodium hydroxide were procured from Qualigens Fine Chemicals, Mumbai, India. Pyrogallol and petroleum ether were purchased from E-Merck (India) Limited, Mumbai, India. All other chemicals were purchased from Sisco Research Laboratories Private Limited, Mumbai, India.
For toxicity evaluations, Cd (in the form of cadmium chloride) was selected. After preliminary toxicity evaluation, two test doses of 50 ppm and 200 ppm were selected. PR plant products were collected from a local Ayurvedic shop. All plant materials were identified by the Department of Botany, Rani Anna Govt. College for Women, Tirunelveli, Tamil Nadu, India. Then, the plant materials were shade dried.
Preparation of Crude Plant Extract
The preparation of extract was carried out following the procedure of Uboh et al. [15]. The plant materials viz., Zingiber officinale (rhizome), Piper nigrum (fruits), Trachyspermum ammi (seeds), Brassica nigra (seeds), Ferula asafietida (resin from rhizome), Curcuma longa (rhizome), Anethum graveolens (seeds), Hemidesmus indicus (root), Vernomia anthelmintica (seeds), P. longum (fruits and root), Crataeva religiosa (bark), Allium sativa (bulb), Spilanthes calva (flowers), Alphinia calcarata Roscoe (rhizome), Nigella sativa (seeds), Acorus calamus (rhizome) and Trianthema decandra (root) were collected and shade dried. Two hundred grams of the ground powder was soaked in 1.0 l of distilled water for 48 h at room temperature. The mixture was filtered into a 500-ml conical flask with a Whatman filter paper (No. 1). The filtrate was dried at a temperature of 30°C for 10 h to produce a gel-like extract, which weighed 20.5 g. Appropriate concentration of the extract was then subsequently made by dilution with distilled water into 200 mg/kg body weight and administered to the animals.
Experimental Design
Animals
Ninety-day-old female albino rats of Wistar strain (Rattus norvegicus) obtained from the National Institute of Nutrition, Hyderabad, weighing 140 ± 10 g were used for the present investigation. The rats were maintained in a temperature controlled animals’ quarter with 12 h dark:12 h light schedule and were fed standard rat pellet diet (Broke Bond, Lipton India Ltd., India) and drinking water ad libitum. The animals were dewormed with albendazole (Bendex-400, Protec Cipala Ltd., India) (10 mg/kg body weight, orally), before the initiation of the experiment. The females were mated with males at a ratio of 2:1. Cohabitation began at approximately 16.30 h on each mating day. On the following morning the females were removed from the mating cages and smeared individually for the presence of sperm in the vaginal lavage. The presence of sperm in the vaginal lavage is indicative that the females mated, and those were selected for further studies. The pregnant animals were then allowed to give birth. The mother animals with female pups were divided into the following groups:
control
50 ppm
200 ppm
The minimum (50 ppm) and maximum effective doses (200 ppm) of Cd were selected [16], and the mother rats along with female pups were treated with Cd in the form of cadmium chloride and the crude plant extract mixed in drinking water from 0 day post-parturition (pp) to 65 days pp.
45-day-old rat—puberty occurred
65-day-old rat—full growth of ovary
At the end of the experimental period, animals were killed by cervical decapitation, and the uteri were dissected and washed with ice-cold saline. A 10% homogenate (100 mg in 1 ml buffer) of washed tissue was prepared in 0.1 M Tris–HCl buffer, pH 7.4, and used for the assay of the following biochemical parameters.
Protein
The protein content was determined by the method of Lowry et al. [17]. The total protein content was expressed as gram/100 g tissue.
Tissue Anti-oxidizing Enzymes
Superoxide Dismutase
The enzyme was assayed according to the method of Marklund and Marklund [18]. The enzyme activity was expressed as units/milligram protein.
Catalase (CAT)
The activity of catalase was assayed by the method of Sinha [19]. The activity of catalase was expressed in units/milligram protein (1 unit is the amount of enzyme that utilizes 1 μmol of hydrogen peroxide/min).
Glutathione Peroxidase
The activity of GPx was determined by the method of Rotruck et al. [20]. The enzyme activity was expressed as units/protein (1 unit is the amount of enzyme that converts 1 μmol GSH to GSSG in the presence of hydrogen peroxide/min).
Glutathione-s-transferase
This enzyme was assayed by the method of Habig et al. [21]. The enzyme activity was expressed as units/milligram protein (1 unit is the amount of enzyme that converts 1 μmol GSH to GSSG in the presence of hydrogen peroxide/min).
Statistical Analysis
All data were presented as means ± standard error of the mean (SEM). Statistical significance was calculated using ANOVA to test the significance of individual variations. The value of probability was obtained from the degree of freedom by using standard table value, given by Fischer and Yates [22]. The level of significance was assessed at p < 0.05.
Results
Antioxidant Enzymes
Superoxide Dismutase
Significant duration-dependent decrease in SOD activity was noted in animals treated with Cd when compared to controls. SOD levels were enormously reduced in the ovary of 50 ppm and 200 ppm Cd-exposed rats during 45 and 65 days PND. The present study was undertaken to evaluate the antioxidant activity of a selected herbal extract. The SOD level was significantly (p < 0.05) increased in herbal administered group in both age groups (45 and 65 days) (Fig. 1).
Fig. 1.
Effect of herbal extract and cadmium exposure on ovarian SOD in developing female rats. Each bar represents the mean and SEM (n = 4). Statistical significance of difference among groups at p < 0.05; control versus 50 ppm cadmium treatment; 50 ppm cadmium treatment versus 50 ppm cadmium treatment along with herbal administration (both 45 and 65 days)
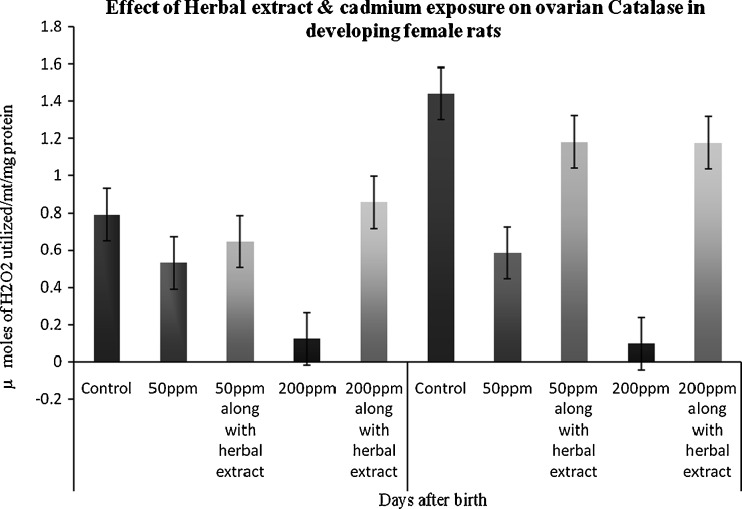
Catalase (CAT)
The activity of catalase was significantly decreased in the 45th and 65th days of the animals’ exposure to Cd when compared to control. But in those treated with herbal extract and Cd there was a significant increase in the catalase levels (Fig. 2).
Fig. 2.
Effect of herbal extract and cadmium exposure on ovarian catalase in developing female rats. Each bar represents the mean and SEM (n = 4). Statistical significance of difference among groups at p < 0.05; control versus 50 ppm cadmium treatment; 50 ppm cadmium treatment versus 50 ppm cadmium treatment along with herbal administration (both 45 and 65 days)
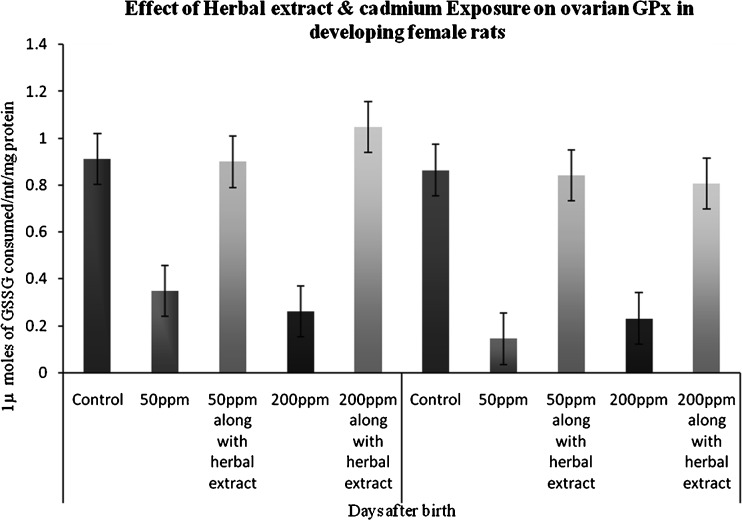
Glutathione Peroxidase
A duration-dependent response was observed in exposed rats. Animals which were persistently exposed to Cd showed a significant suppression of ovarian GPx activity in the 45th PND when compared to control. A marked increase in activities of GPx antioxidant enzymes was observed in herbal extract administered group (Fig. 3).
Fig. 3.
Effect of herbal extract and cadmium exposure on ovarian GPx in developing female rats. Each bar represents the mean and SEM (n = 4). Statistical significance of difference among groups at p < 0.05; control versus 50 ppm cadmium treatment; 50 ppm cadmium treatment versus 50 ppm cadmium treatment along with herbal administration (both 45 and 65 days)
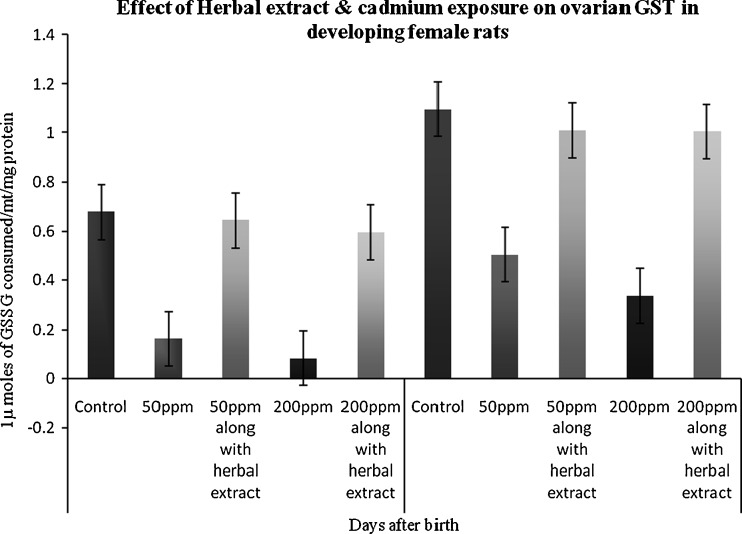
Glutathione-s-transferase
A low level of GST activity was observed in both the Cd-exposed groups of rat ovaries when compared to control. The depletion of GST activity was greater in the ovary of 45th-day treated animals. The GST activity was found to be significantly (p < 0.05) increased in herbal administered group when compared to control and Cd-only treated group (Fig. 4).
Fig. 4.
Effect of herbal extract and cadmium exposure on ovarian GST in developing female rats. Each bar represents the mean and SEM (n = 4). Statistical significance of difference among groups at p < 0.05; control versus 50 ppm cadmium treatment; 50 ppm cadmium treatment versus 50 ppm cadmium treatment along with herbal administration (both 45 and 65 days)
Discussion
The role of antioxidants is to neutralize the excess of free radicals, to protect the cells from toxic effects and to contribute to disease prevention. [23]. In the present study, a significant decrease in SOD activity was observed in the ovaries of the 45-day and 65-day age group animals (Fig. 1). The activity of another antioxidant enzyme, catalase, was also lowered in the ovaries of 45-day and 65-day age groups of Cd-exposed animals (Fig. 2). This is in accordance with the findings of previous studies. Studies by some authors have shown that Cd inhibits the activity of the majority of enzymes involved in AOS [24,25], inducing an increased production of free radicals, lipid peroxidation and destruction of cell membranes [26,27]. SOD has proven a useful probe for studying the free radicals in reactions involving oxygen, since it acts as a defense against oxidative tissue damage by dismutation of superoxide radicals [28]. SOD also plays an important role in the regulation of the luteal function during pregnancy [9]. Long-term exposure to Cd increases lipid peroxidation and causes inhibition of SOD activity, resulting in oxidative damage to liver, kidney and testes [11]. Also in the Cd-treated group, hepatic LPO increased and SOD and CAT activities were decreased, as observed in earlier studies [29,30]. The increased LPO may be due to decreased activities of SOD and CAT, the free radical scavenging enzymes [31].
Antioxidant-based drug formulations are used for the prevention and treatment of complex diseases like atherosclerosis, stroke, diabetes, Alzheimer’s disease and cancer [32]. There is evidence that indigenous antioxidants may be useful in preventing the deleterious consequences of oxidative stress, and there is increasing interest in the protective biochemical functions of natural antioxidants contained in spices, herbs and medicinal plants [33]. In the present study a significant increase in SOD and catalase activity was observed in the ovaries of 45-day and 65-day age group animals (Figs. 1 and 2). Piper species, commonly used in diet and traditional medicine, were assessed for their antioxidant potential. P. cubeba Linn increased antioxidants like SOD and catalase activities [13]. Catalase activity was predominant in P. longum [14]. There are more than 50 antioxidants isolated from rhizomes of ginger [34]. Z. officinale (ginger) significantly lowers lipid peroxidation by maintaining the activities of the antioxidant enzymes: SOD, catalase and glutathione peroxides in rats [12]. C. nurvala shows the highest SOD activity (122.53 units/min/mg) [35]. H. indicus root bark has also been reported to possess antioxidant property [36].
The decreases in GPx and GST activities in the ovaries of 45-day and 65-day age group animals are given in Figs. 3 and 4. A drastic reduction in GPx activity was noticed in the ovary of 45-day age group rats treated with 50 ppm Cd when compared to control. A similar trend was also noticed in GPx activity in the ovary of 65-day-old rats treated with 50 ppm Cd when compared to control. Glutathione, the cofactor for the lipid hydrogen-peroxide detoxifying enzyme, GPx, is also present in high concentrations in ovarian tissue [37]. Numerous reports in animal models have shown that Cd intoxication significantly increases the malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) [38,39]. Free radicals generated by Cd are scavenged by GSH directly or via the GSH peroxidase/GSH system [28]. The GST enzyme has an important role in the detoxification of xenobiotics, drugs and carcinogens, and thus protects the cells against redox cycling and oxidative stress [40,41].
The increases in GPx and GST activities in the ovaries of 45-day and 65-day age group animals are given in Figs. 3 and 4. This was consistent with the previous findings that N. sativa oil increases glutathione (GSH) and SOD [42]. C. longa protects against ischemia-induced changes by increasing the antioxidant defense mechanisms [43]. B. juncea administered to animal groups increases activity of SOD and catalase. It also sharply increased the activity of glutathione reductase (GSH), GPx and GST in the experimental group compared to the controls [44]. The N. sativa oil produced an increase in glutathione level in the stomach [45]. N. sativa oil has been reported in other publications to possess antioxidant activity [46].
Conclusion
In the present study, Cd treatment to female Wistar rats inhibited the action of enzymes involved in the scavenging of ROS. However, the inhibited antioxidant enzyme levels were elevated significantly due to the action of the bioactive principles present in the herbal antioxidant restoration mixture. The protective role of the extracts of 17 plant products in the reproductive functioning had been well known to traditional healers and needs further phytochemical analysis.
References
- 1.Morrow H (2001) Cadmium and cadmium alloys. In. Kirk–Othmer encyclopedia of chemical technology. Wiley, New York, 471–507. Nutrition Reviews 52: 253–265
- 2.Herron N. Cadmium compounds. In: Kirk–Othmer encyclopedia of chemical technology, vol. 4. New York: Wiley; 2003. pp. 507–523. [Google Scholar]
- 3.Massanyi P, Uhrin V, Toman R, Pivko J, Lukac N, Zs F, Somosy Z, Fabis M, Danko J. Ultrastructural changes of ovaries in rabbits following cadmium administration. Acta Vet Brno. 2005;74:29–35. doi: 10.2754/avb200574010029. [DOI] [Google Scholar]
- 4.Kjellstrom T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environ Health Perspect. 1979;28:169–197. doi: 10.1289/ehp.28-1637502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massanyi P, Uhrin V, Sirotkin AV, Paksy K, Forgacs ZS, Tomom R, Kovacik J. Effects of cadmium on ultrastructure and steroidogenesis in cultured porcine ovarian granulose cells. Acta vet Brno. 2000;69:101–106. doi: 10.2754/avb200069020101. [DOI] [Google Scholar]
- 6.Toman R, Massamyi P, Uhrin V. Changes in the testis and epididymis of rabbits after an inliapcitoneal and peroral administration of cadmium. Trace Elem Electrolytes. 2002;19:114–117. [Google Scholar]
- 7.Sarkar S, Yadov P, Bhatnagar D. Lipid peroxidative damage on cadmium exposure and alterations in antioxidant defence system in rat erythrocytes: a study with relation to time. Bio Metals. 1998;11:153–157. doi: 10.1023/a:1009286130324. [DOI] [PubMed] [Google Scholar]
- 8.Zikic RV, Stajn AS, Ognjanovic BI, Saicic ZS, Kostic MM, Pavlovic SZ, Petrovic VM. The effect of cadmium and selenium on the antioxidant enzyme activities in rat heart. J Environ Pathol Toxicol Oncol. 1998;17:259–264. [PubMed] [Google Scholar]
- 9.Pajavic SB, Saicic ZS. Modulation of antioxidant enzymes activities by sexual steroid hormone. Physio Res. 2008;57:801–811. doi: 10.33549/physiolres.931377. [DOI] [PubMed] [Google Scholar]
- 10.Shi W, Li CM, Tyler PC, Furneaux RH, Cahill SM, Girvin ME, Grubmeyer C, Schramm VL, Almo SC. The 2.0 Å structure of malarial purine phosphoribosyltransferase in complex with a transition-state analogue inhibitor. Biochemistry. 1999;3(38(31)):9872–9880. doi: 10.1021/bi990664p. [DOI] [PubMed] [Google Scholar]
- 11.Patra RC, Swarup D, Senapati SK. Effects of cadmium on lipid peroxides and superoxide dismutase in hepatic, renal and testicular tissue in rats. Vet Human Toxicol. 1999;41:65–67. [PubMed] [Google Scholar]
- 12.Ahmed RS, Seth V, Banerjee BD. Influence of dietary ginger (Zingiber officinale Rosa on antioxidant defense system in rat: comparison with ascorbic acid. Indian J Exp Biol. 2000;38:604–606. [PubMed] [Google Scholar]
- 13.Aqil F, Ahmed I, Mehmood Z. Anti-oxidants and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk J Biol. 2006;30:177–183. [Google Scholar]
- 14.Karthikeyan J, Rani P. Enzymatic and non-enzymatic antioxidants in selected Piper species. Indian J Exp Biol. 2003;41:135–140. [PubMed] [Google Scholar]
- 15.Uboh FE, Edet EE, Eteng MU, Eyong EU. Comparative effect of aqueous extract of P. guajava leaves and ascorbic acid on serum sex hormones levels in male and female rats. J Appl Sci Res. 2010;6(4):275–279. [Google Scholar]
- 16.Samuel BJ (2001) Reproductive toxicity of chromium on ovarian development in female Wistar rats–an endocrine and a biochemical approach, Chapter I & III, Ph.D. Thesis
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Marklund S, Marklund G. Involvement of superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Chem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinha AK. Colorimetric assay of catalase. Analalytical Biochemistry. 1972;47:389–395. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 20.Rotruck JT, Pope AC, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidases. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase. The first enzymatic step in mercapturic formation. J Biol Chem. 1973;249:7130–7139. [PubMed] [Google Scholar]
- 22.Fischer RA, Yates F. In: Statistical tables for biological, agricultural and medical Research. London: Oliver and Boyd; 1948. [Google Scholar]
- 23.Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the Cochrane. Database Syst Rev. 2000;2:10000980. doi: 10.1002/14651858.CD000980. [DOI] [PubMed] [Google Scholar]
- 24.Casalino E, Calzaretli G, Sblano C, Landriscina C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology. 2002;179:37–50. doi: 10.1016/S0300-483X(02)00245-7. [DOI] [PubMed] [Google Scholar]
- 25.Jamall IS, Sprowls JJ. Effects of cadmium and dietary selenium on cytoplasmic and mitochondrial antioxidant defense systems in the heart of rats fed high dietary copper. Toxicol Appl Pharmacol. 1987;87:102–110. doi: 10.1016/0041-008X(87)90088-3. [DOI] [PubMed] [Google Scholar]
- 26.Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys. 1997;346:171–179. doi: 10.1006/abbi.1997.0197. [DOI] [PubMed] [Google Scholar]
- 27.Kostic MM, Ognijanovic B, Dimitrijevic S, Zikic RV, Stajn A, Risic GL. Cadmium-induced changes of antioxidant and metabolic status in red blood cells of rats: in vivo effects. Eur J Haematol. 1993;51:86–92. doi: 10.1111/j.1600-0609.1993.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 28.Ognijanovic BI, Pavlovic SZ, Maletic SD, Zikic RV, Stajn AS, Radijicic RM, et al. Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res. 2003;52:563–570. [PubMed] [Google Scholar]
- 29.Jamall IS, Smith C. Effects of cadmium on glutathione peroxidase, superoxide dismutase and lipid peroxidation in the rat heart: possible mechanisms of cadmium cardio toxicity. Toxicol Appl Pharmacol. 1965;80:33–42. doi: 10.1016/0041-008X(85)90098-5. [DOI] [PubMed] [Google Scholar]
- 30.Stracey H, Louris R, Cantilena JR, Klaassen CD. Cadmium toxicity and lipid peroxidation in isolated rat hepatocytes. Toxicol Appl Pharmacol. 1980;53:470–480. doi: 10.1016/0041-008X(80)90359-2. [DOI] [PubMed] [Google Scholar]
- 31.Jawahar B, Samuel JA, Stanley DP, Roopha GV, Anbalagan J, Banu SK, Arul das MM. Lactational hexavalent chromium exposure-induced oxidative stress in rat uterus is associated with delayed puberty and improved gonadotropin levels. Hum Exp Toxicol. 2010;000(00):1–11. doi: 10.1177/0960327110364638. [DOI] [PubMed] [Google Scholar]
- 32.Devasagayam TPA, Tilak JC, Boloor KK, et al. Review: free radicals and anti-oxidants in human health: Carr stat fut prosp. JAPL. 2004;52:794–804. [PubMed] [Google Scholar]
- 33.Noda Y, Metal AK. Hyduoxyl and superoxide anion radical scavenging activities of natural souuce anti-oxidants using the computerized JES-FR 30 ESR spectrophotometer system. Bioch Mol Biol Inter. 1997;42:35–44. doi: 10.1080/15216549700202411. [DOI] [PubMed] [Google Scholar]
- 34.Masuda Y, Kikuzaki H, Hisamoto M, Nakatani N. Antioxidant properties of gingerol related compounds from ginger. Biofactors. 2004;21:293–296. doi: 10.1002/biof.552210157. [DOI] [PubMed] [Google Scholar]
- 35.Archana kumari, Poonam kakkar. Screening of antioxidant potential of selected barks of Indian medicinal plants by multiple in vitro assays. Biomed Environ Sci. 2008;21:24–29. doi: 10.1016/S0895-3988(08)60003-3. [DOI] [PubMed] [Google Scholar]
- 36.Ravi shankara MN, Shrivastava N, Padh HZ, Rajani M. Evaluation of antioxidant properties of root barks H. Indicus (anantmul) Phytomedicine. 2002;9:153. doi: 10.1078/0944-7113-00104. [DOI] [PubMed] [Google Scholar]
- 37.Mattison DR, Shiromizu K, Pendergrass JA, Thorgeirsson SS. Ontogeny of ovarian glutathione and sensitivity to primordial oocytes destruction by cyclophosphamide. Pediat Pharmacol. 1983;3:49–55. [PubMed] [Google Scholar]
- 38.Cosic DD, Bulat ZP, Ninkovic M, Malicevic Z, Matovic V. Effect of subacute cadmium intoxication iron and lipid peroxidation in mouse liver. Toxicol Lett. 2007;172:s209. doi: 10.1016/j.toxlet.2007.05.526. [DOI] [Google Scholar]
- 39.Yang JM, Arnush M, Chen QY, Wu XD, Pang B, Jiang XZ. Cadmium induced damage to primary cultures of rat Leydig cells. Reprod Toxicol. 2003;17:553–560. doi: 10.1016/S0890-6238(03)00100-X. [DOI] [PubMed] [Google Scholar]
- 40.Caslino E, Sblano C, Landriscina V, Calzaretti G, Landriscina C. Rat liver glutathione-s-transferase activity stimulation following acute cadmium or manganese intoxication. Toxicology. 2004;200:29–38. doi: 10.1016/j.tox.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Mates M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/S0300-483X(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 42.El-Abhar HS, Abdallah DM, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischemia/reperfusion in rats. J Ethnopharmacol. 2003;84(2–3):251–258. doi: 10.1016/S0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 43.Dikshit M, Rastogi L, Shukla R, Srimal RC. Prevention of ischemia-induced biochemical changes by curcumin and guanidine in cat heart. Indian I Med Res. 1995;101:31–35. [PubMed] [Google Scholar]
- 44.Khan BA, Abraham A, Leelamma S. Role of Murrays koenijii (curry leaf) and Brassica juncea (mustard) in lipid peroxidation. Indian J Physiol Pharmacol. 1996;40:155–158. [PubMed] [Google Scholar]
- 45.El-Dakhakhny M, Barakat M, El-Halim MA, Aly SM. Effect of Nigella sativa oil or gastric secretion and ethanol misused celcerin rats. J Ethnopharmacol. 2000;72:299–304. doi: 10.1016/S0378-8741(00)00235-X. [DOI] [PubMed] [Google Scholar]
- 46.Bruits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother – Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]