Abstract
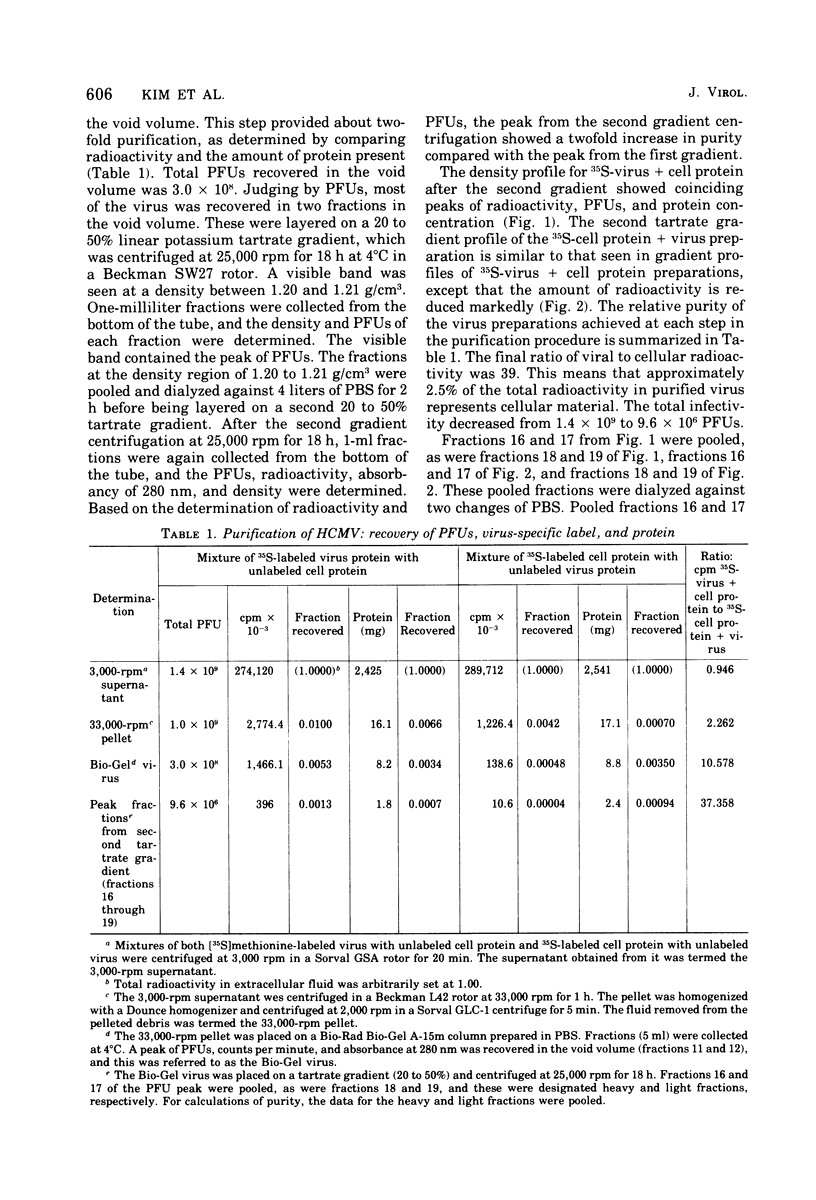
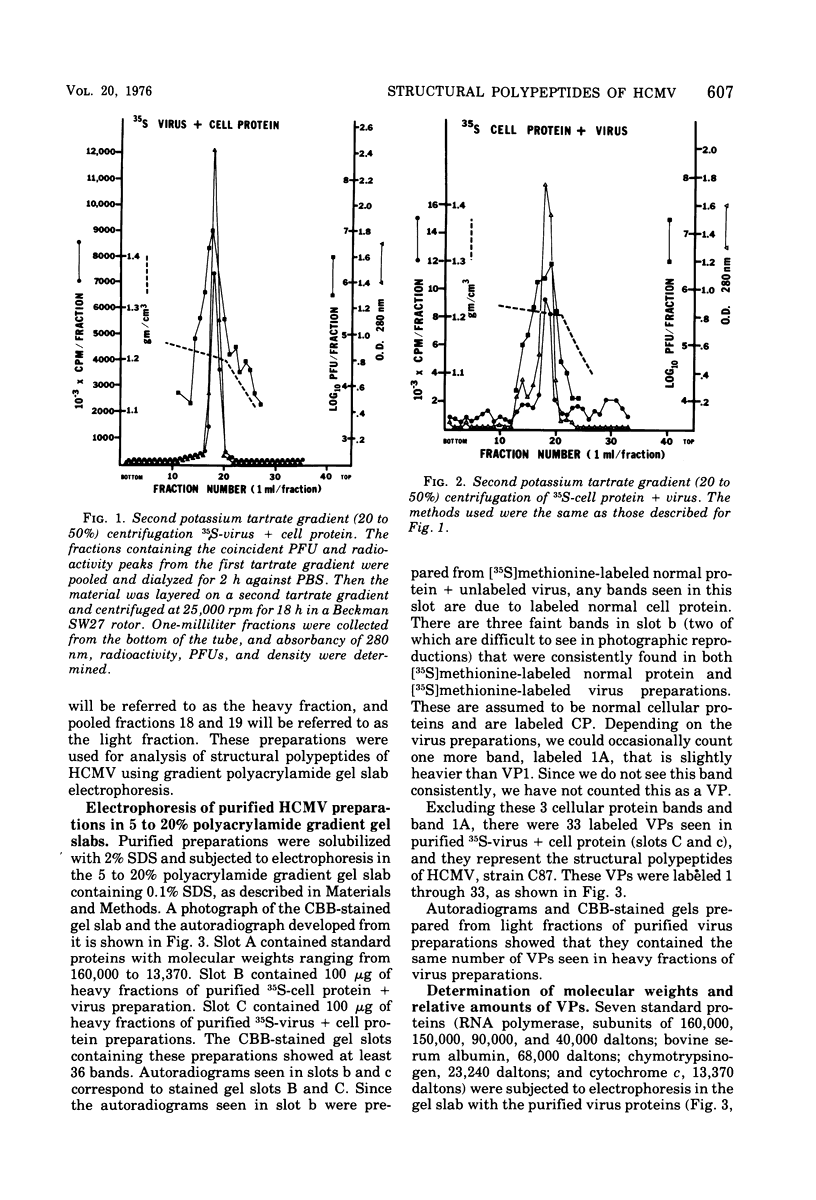
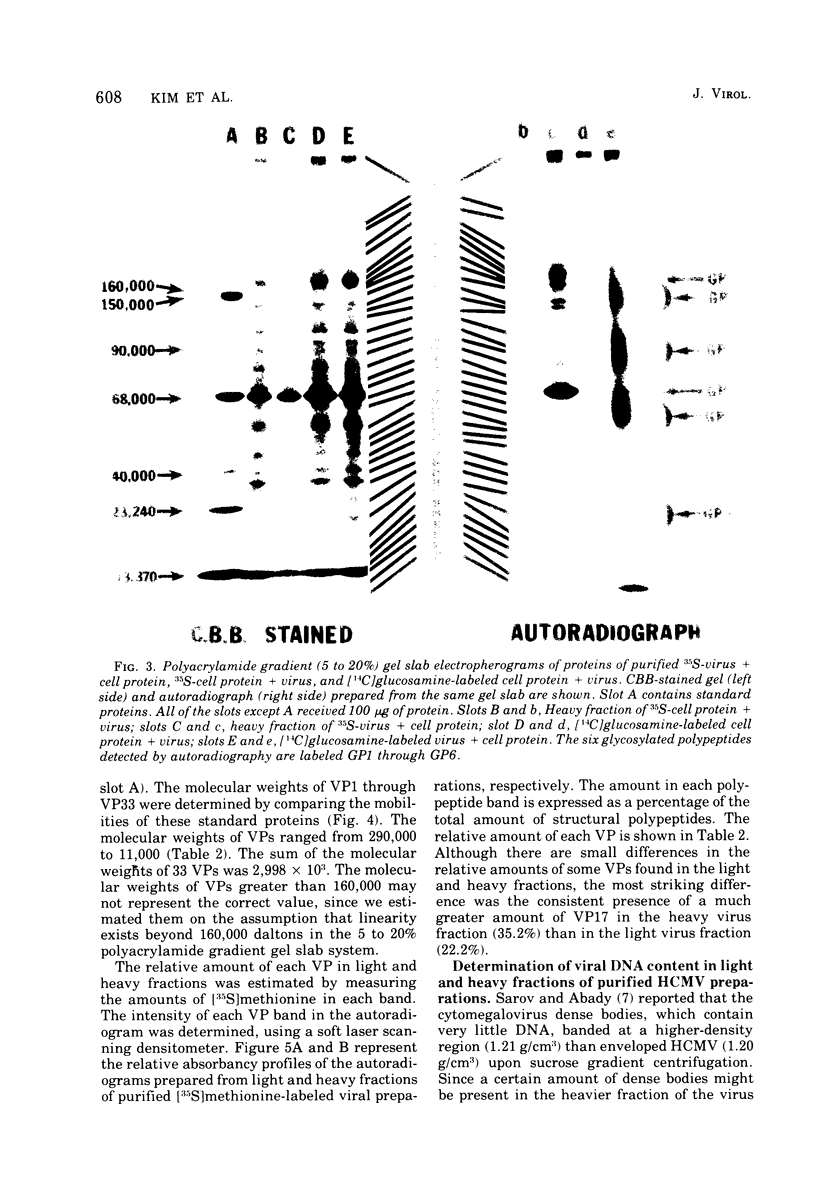
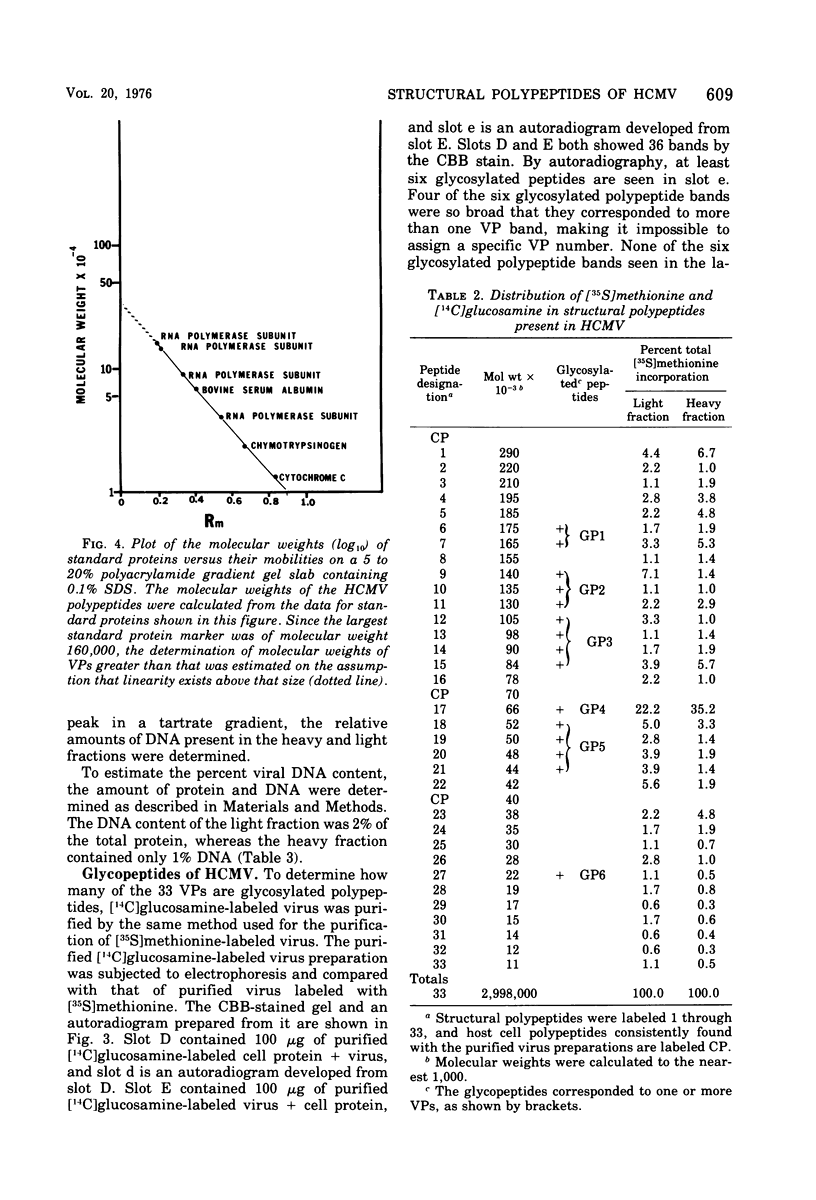
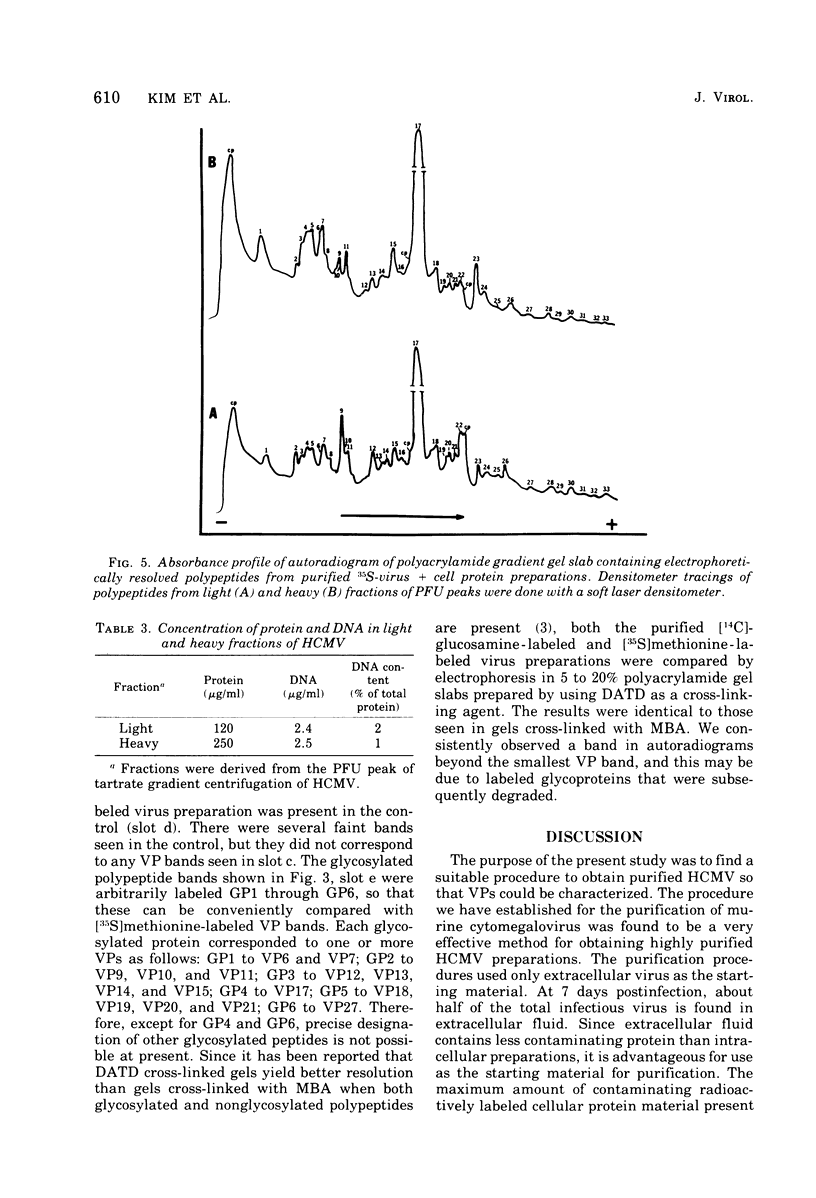
Human cytomegalovirus strain C87 was purified by the following procedures. (i) Extracellular virus was concentrated by centrifugation at 100,000 X g for 90 min and passed through a Bio-Rad Bio-Gel A-15m column. Most of the virus was recovered in the void volume. (ii) After two consecutive isopycnic potassium tartrate gradient centrifugations (20 to 50%), coinciding peaks of plaque titer, protein, and radioactivity were found at a density of from 1.20 to 1.21 g/cm3. To characterize the structural polypeptides of human cytomegalovirus and to establish relative purification criteria, virus was purified from two mixtures: (i) [35S]methionine-labeled extracellular virus mixed with an equal volume of unlabeled normal culture fluid; (ii) unlabeled extracellular virus mixed with an equal volume of [357a1methionine-labeled normal culture fluid. The extent of purification, as judged by the ratio of cellular to viral radioactivity, was 39-fold; i.e. about 2.5% of the protein in the purified virus preparation could be accounted for by host protein contamination. Electrophoresis of purified [35S]methionine-labeled virus on a polyacrylamide gel slab showed that there were at least 33 viral structural polypeptides (VPs), and their molecular weights ranged from 11,000 to 290,000. Autoradiograms obtained from electropherograms of purified [14C]glucosamine labeled virus showed six bands. Four of these were so broad that several VPs corresponded to each of the glycosylated bands. When heavy (two fractions close to 1.21 g/cm3) and light (two fractions close to 1.20 g/cm3) fractions of the PFU peak from the second potassium tartrate gradient were analyzed separately, the number of polypeptides observed was the same, but the relative amounts of some polypeptides differed. The major polypeptide, VP17, was found in greater amounts in the heavy fraction (35%) than in the light fraction (22%). The amount of DNA as a percentage of the weight of protein was 2% for the light fraction and 1% for the heavy fraction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural proteins of purified murine cytomegalovirus. J Virol. 1976 Mar;17(3):906–915. doi: 10.1128/jvi.17.3.906-915.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sarov I., Abady I. The morphogenesis of human cytomegalovirus. Isolation and polypeptide characterization of cytomegalovirions and dense bodies. Virology. 1975 Aug;66(2):464–473. doi: 10.1016/0042-6822(75)90218-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]