Abstract
Objective
The purpose of this study is to evaluate neuroprotective effect of sacral neuromodulation in rat spinal cord injury (SCI) model in the histological and functional aspects.
Methods
Twenty-one female Sprague Dawley rats were randomly divided into 3 groups : the normal control group (CTL, n=7), the SCI with sham stimulation group (SCI, n=7), and the SCI with electrical stimulation (SCI+ES, n=7). Spinal cord was injured by dropping an impactor from 25 mm height. Sacral nerve electrical stimulation was performed by the following protocol : pulse duration, 0.1 ms; frequency, 20 Hz; stimulation time, 30 minutes; and stimulation duration, 4 weeks. Both locomotor function and histological examination were evaluated as scheduled.
Results
The number of anterior horn cell was 12.3±5.7 cells/high power field (HPF) in the CTL group, 7.8±4.9 cells/HPF in the SCI group, and 6.9±5.5 cells/HPF in the SCI+ES group, respectively. Both the SCI and the SCI+ES groups showed severe loss of anterior horn cells and myelin fibers compared with the CTL group. Cavitation and demyelinization of the nerve fibers has no significant difference between the SCI group and the SCI+ES group. Cavitation of dorsal column was more evident in only two rats of SCI group than the SCI+ES group. The locomotor function of all rats improved over time but there was no significant difference at any point in time between the SCI and the SCI+ES group.
Conclusion
In a rat thoracic spinal cord contusion model, we observed that sacral neuromodulation did not prevent SCI-induced myelin loss and apoptosis.
Keywords: Electrical stimulation, Spinal cord injury, Neuroprotection, Sacral nerve, Neuromodulation
INTRODUCTION
Traumatic spinal cord injury (SCI) often leads to serious neurological sequelae and medical complications. The secondary damage following SCI is induced by multiple pathophysiological mechanisms, including vascular perturbation, metabolic failure, ionic dysregulation, and celluar excitotoxicity7,10). These mechanisms increase blood-spinal cord barrier permeability, tissue edema, free radical formation, peroxidation of lipid membranes, cytokines release, and inflammation. A few investigations were introduced in order to minimize secondary damage using electrical stimulation, aminoacids, and drugs3,14,15,18).
Electrostimulation of sacral nerve roots is currently being used with promising results as a treatment for a wide spectrum of voiding dysfunctions. Also, some investigators reported additional effects of the sacral nerve stimulation such as change of spasticity and spasm, increased motility of intestinal tract and defecation, and improvement of leg motor in experimental and clinical studies3,6,13,17). There are some case reports about additional improvement of lower extremity motor function6). Prior investigators revealed that one patient with a long history of progressive spinal multiple sclerosis was able to stand and transfer, but not walking, before the sacral nerve stimulation6). Another paraplegic patient was showed improvement of motor power slowly, and after 2 years the patient could stand as confident as before6). The purpose of this study was to evaluate neuroprotective effect of sacral neuromodulation in rat thoracic spinal cord injury model in histological and functional aspects.
MATERIALS AND METHODS
Rats and treatment groups
Twenty-one Sprague Dawley of 6 months old female, weighing 200 to 250 gm, were randomly divided into 3 groups : a normal control group (CTL, n=7), SCI with sham stimulation group (SCI, n=7), and SCI with electrical stimulation (SCI+ES, n=7). The CTL included rats that were both uninjured and unstimulated. The SCI group included rats that were injured with electrodes implanted but did not receive stimulation. The SCI+ES group included rats that received electrical stimulation at the S2 or S3 nerve root using needle electrode. Rats in the SCI and SCI+ES groups were kept in separate cages under the same living conditions for a week before receiving SCIs.
Spinal cord injury and electrical stimulation
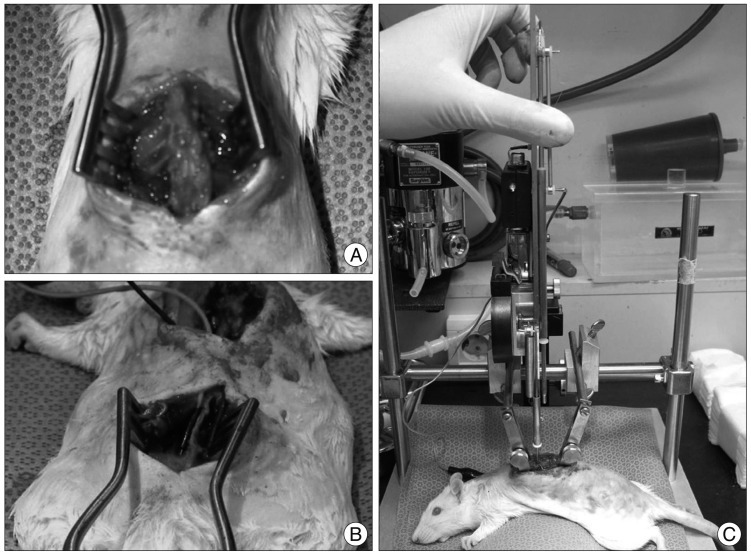
Rats were deprived of food and water for 12 hours before SCI. The animals were anesthetized with an intramuscular injection of Zoletil 15 mg and Xylazine 3 mg. Under general anesthesia, rats were placed in a prone position. After routine disinfection, a surgical incision was made through the skin, subcutaneous tissue, and the T8-12 vertebral lamina to the spinal canal (Fig. 1A). Total laminectomy of T9 or T10 was performed. Rostral and caudal spinous processes were fixed by clamp. Severe grade SCIs were induced in rats using an New York University-Multicenter Animal Spinal Cord Injury Study impactor. The impactor weighed 10 gm was dropped once from 25 mm height (Fig. 1B). The impact height of 25 mm was considered for severe cord injury based on a prior investigation1).
Fig. 1.
A : Exposure of spinal cord at T10-11 by laminectomy. B : Fine needle electrodes (0.5×27 G) are implanted into S2 foramen. C : Severe grade of crushing injury is made using New York University spinal cord impactor.
At the S1-3 level, a surgical incision was made through the skin, subcutaneous tissue, and the S2-3 vertebral lamina (Fig. 1C). Needle electrodes (0.5×27 G) were implanted at bilateral S2 or S3 neural foramina. Wounds were cleaned and sutured with wires inserted through the subcutaneous tissue extruding from the neck. The SCI+ES group received electrical stimulation 7 days after SCI with the following protocol : stimulation time 30 minutes; pulse duration, 0.1 ms; and frequency, 20 Hz. Epidural low frequency electric stimulation was applied 30 minutes per day for 4 weeks. The electrical stimulation treatment protocol was similar to that previously reported8,11,19).
Functional and histopathological outcome
Our institutional animal care and use committee permitted this study (SNUBH IACUC No. BA1003-058/013-01). Post-injury motor behavior is assessed by the Basso, Beattie, and Bresnahan (BBB) locomotor scale method4,5). The BBB score is used for functional recovery and locomotors testing in SCI study. The scale (0-21) represents sequential recovery stages and categorizes combinations of rat joint movement, hindlimb movements, stepping, forelimb and hindlimb coordination, trunk position and stability, paw placement, and tail position.
All rats were sacrificed at 4 weeks after SCI. Under general anesthesia, the spinal cord was dissected and embedded in formalin solution, cut into 5 µm-thick axial sections, and stained with hematoxylin and eosin (H&E) and Luxol fast blue to identify structural defects, motor neuron cell injury and myelin loss. Motor neuron cells were counted in the anterior horns of 1-2 mm rostral from the epicenter. Only complete neuronal cells with a clearly defined cell body and nucleus were counted.
RESULTS
Histopathologic examination
The SCI group tissue obtained 4 weeks after trauma revealed diffuse hemorrhage, widespread edema, and focal necrosis in both gray-matter and white-matter (Fig. 2). The specimens showed cystic vacuolar degeneration and dispersion of the myelin sheaths. Edema and an accumulation of fibrin were also observed throughout the spinal cord. The traumatized spinal cord was infiltrated with polymorphonuclear leucocytes, erythrocytes, and macrophages in H&E staining. The number of anterior horn cell was 12.3±5.7 cells/high power field (HPF) in the CTL group, 7.8±4.9 cells/HPF in the SCI group, and 6.9±5.5 cells/HPF in the SCI+ES group, respectively. Between SCI and SCI+ES group, the difference has no statistical significance.
Fig. 2.
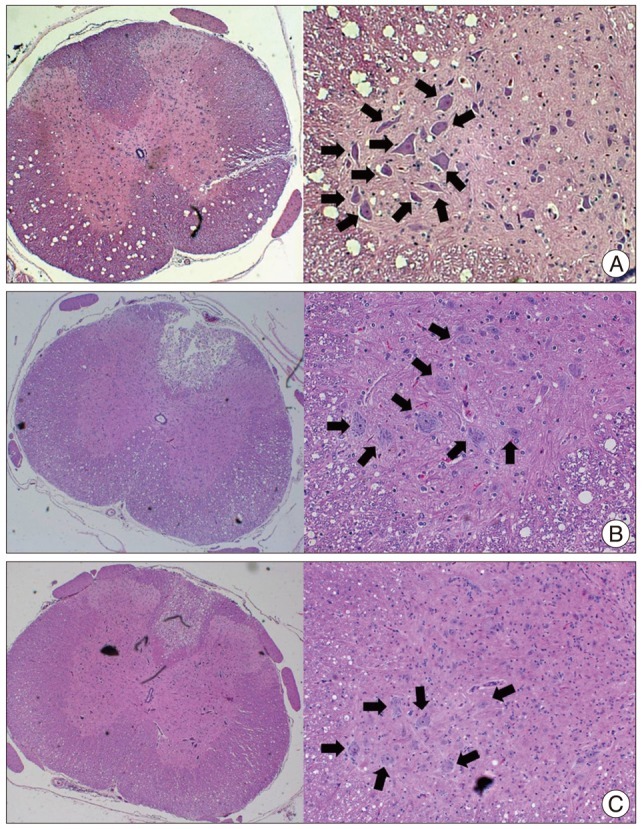
A : Hematoxylin and eosin staining of control group. Dorsal column is intact. There are many ant horn cells (arrows) in gray matter. B : The spinal cord injury (SCI) group reveals a cavitation of dorsal column and a loss of anterior horn cell (arrows) were observed. C : The SCI+ES group shows no structural defect, but loss of anterior horn cell (arrows) is similar with the SCI group.
A significant loss of myelin in the white matter was also observed in the SCI and SCI+ES groups compared with the CTL group (Fig. 3). Cavitation and demyelinization of the nerve fibers usually has no significant difference between the SCI group and the SCI+ES group. In only 2 specimens of the SCI group, dorsal column was cavitated and showed vacuoles (Fig. 3B). Loss of neurons and demyelinization were also evident.
Fig. 3.
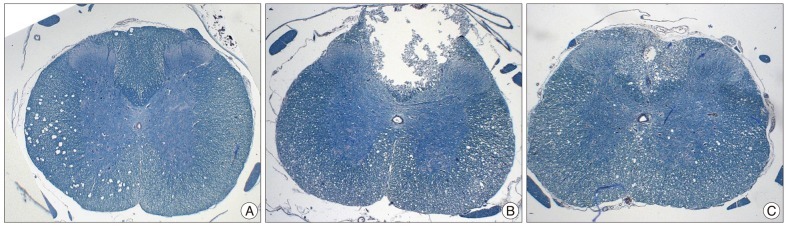
A : Luxol fast blue staining of control group. Myelin fibers are stained. B : The spinal cord injury (SCI) group shows structural defect and scant myelin fibers. C : The SCI+ES group also reveals cavitation of dorsal column with vacuolar change and loss of myelin fibers. Structural defect and cavitation of dorsal column is more evident in SCI group.
Functional outcome
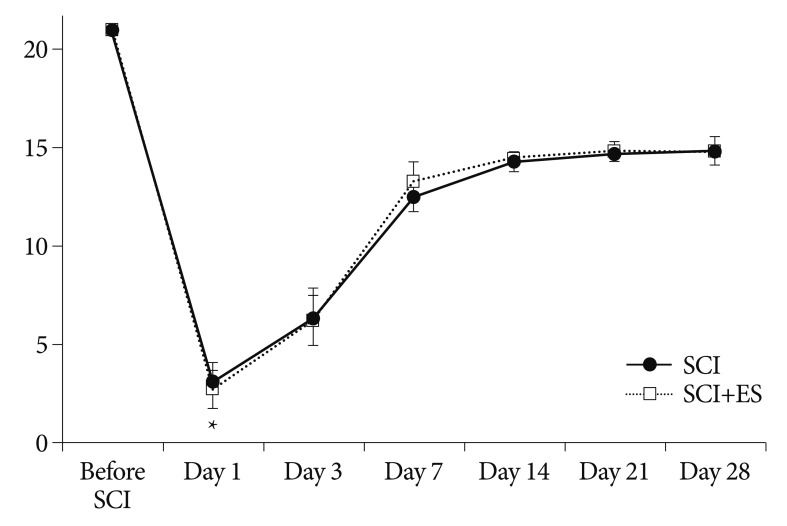
The open field locomotor scores (BBB test) of the corresponding groups are presented in Fig. 4. Before the injury, all rats showed normal function on the BBB score. As demonstrated, all traumatized rats exhibited a severe BBB score at 1 day after SCI. At day 1 of SCI, the BBB scores of the SCI and the ES group were 3.0±1.0 and 2.7±1.0, respectively. The difference between two groups was not significant. During subsequent scoring periods, BBB scores increased and plateaued around 14 days after SCI. They improved significantly at days 3, 7, and 14 after SCI (3 days : 6.4±1.5 and 6.2±1.3, 7 days : 12.5±0.8 and 13.3±1.0, and 14 days : 14.3±0.5 and 14.5±0.3). The animals did improve over time but there was no significant different at any point in time between SCI and SCI+ES groups.
Fig. 4.
The time course of Basso, Beattie, and Bresnahan (BBB) scores in the spinal cord injury (SCI) and SCI+ES group. All rats show a low BBB score at 1 day after SCI then recovered slowly. The difference between the groups is not significant.
DISCUSSION
Electrical stimulation has been reported to show neurobiological effects, such as relieving pain in the region of head and face and restoring movement and function in individuals with spinal cord injury6,9). It has also been indicated that electrical stimulation can directly exert effects on regenerating neural tissues observed under a well-controlled experimental environment9). Several investigators have reported that a low-frequency electrical stimulation is a promise approach to accelerate nerve regeneration after injury2,12). There are some studies on neuroprotective effects of sacral nerve stimulation in the SCI patients2,3,6,11,17). The additional effect of sacral neuromodulation was improvement of neurogenic bladder, intestinal motility, penile erection, pain discrimination, and motor power of leg6).
However, the current study showed that sacral neuromodulation to rat injured thoracic spinal cord did not prevent SCI-induced myelin loss and cavitation. The number of anterior horn cell of the SCI and SCI+ES groups did not show significant differences. Moreover, no significant differences at any point in time between the SCI and SCI+ES groups were found in terms of locomotor function as assessed by the BBB test. Our hypothesis was that sacral neuromodulation might prevent loss of myelin and promote regeneration of myelinated fiber. Only two subjects were concordant with our hypothesis.
No behavioral improvement was observed in the present study. We suggest that it could be caused by an improper protocol of stimulation. Several investigators have reported that a low-frequency electrical stimulation is a promise approach to accelerate nerve regeneration after injury2,12). However, a high frequency of electrical stimulation may increase failure of nerve regeneration16). Hence, these results cannot exclude potential beneficial effects of sacral nerve stimulation in other models of SCI with different treatment protocols, the disappointing outcomes in this study were limited by the treatment protocol.
CONCLUSION
In a rat thoracic spinal cord contusional model, we observed that sacral neuromodulation does not prevent SCI-induced myelin loss and cavitation. Further experimental and clinical studies are needed to shed light on the therapeutic potentials of sacral neuromodulation and to determine the most effective protocol of stimulation.
Acknowledgements
This study was supported by grant No. 11-2010-001 from the Seoul National University Bundang Hospital Research Fund. Seoul National University Bundang Hospital institutional animal care and use committee permitted this study (SNUBH IACUC No. BA1003-058/013-01).
References
- 1.Agrawal G, Kerr C, Thakor NV, All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine (Phila Pa 1976) 2010;35:1122–1127. doi: 10.1097/BRS.0b013e3181be5fa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai C, An H, Wang S, Jiang D, Fan W, Nie H. Treatment and prevention of bacterial translocation and endotoxemia with stimulation of the sacral nerve root in a rabbit model of spinal cord injury. Spine (Phila Pa 1976) 2011;36:363–371. doi: 10.1097/BRS.0b013e3181d25495. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 6.Brindley GS, Polkey CE, Rushton DN, Cardozo L. Sacral anterior root stimulators for bladder control in paraplegia : the first 50 cases. J Neurol Neurosurg Psychiatry. 1986;49:1104–1114. doi: 10.1136/jnnp.49.10.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, et al. Blood-spinal cord barrier permeability in experimental spinal cord injury : dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner A, Jiang C, Lindström S. Intravesical electrical stimulation--an experimental analysis of the mechanism of action. J Urol. 1992;148:920–924. doi: 10.1016/s0022-5347(17)36778-2. [DOI] [PubMed] [Google Scholar]
- 9.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 11.Hong CH, Lee HY, Jin MH, Noh JY, Lee BH, Han SW. The effect of intravesical electrical stimulation on bladder function and synaptic neurotransmission in the rat spinal cord after spinal cord injury. BJU Int. 2009;103:1136–1141. doi: 10.1111/j.1464-410X.2008.08189.x. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Hojo T, Yano T, Katsumi Y. The effects of electroacupuncture on peripheral nerve regeneration in rats. Acupunct Med. 2003;21:9–17. doi: 10.1136/aim.21.1-2.9. [DOI] [PubMed] [Google Scholar]
- 13.Ishigooka M, Suzuki Y, Hashimoto T, Sasagawa I, Nakada T, Handa Y. A new technique for sacral nerve stimulation : a percutaneous method for urinary incontinence caused by spinal cord injury. Br J Urol. 1998;81:315–318. doi: 10.1046/j.1464-410x.1998.00569.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim KT, Nam TK, Park YS, Kim YB, Park SW. Neuroprotective effect of anthocyanin on experimental traumatic spinal cord injury. J Korean Neurosurg Soc. 2011;49:205–211. doi: 10.3340/jkns.2011.49.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TT, Green BA, Dietrich WD, Yezierski RP. Neuroprotective effects of basic fibroblast growth factor following spinal cord contusion injury in the rat. J Neurotrauma. 1999;16:347–356. doi: 10.1089/neu.1999.16.347. [DOI] [PubMed] [Google Scholar]
- 16.O'Gara T, Urban W, Polishchuk D, Pierre-Louis A, Stewart M. Continuous stimulation of transected distal nerves fails to prolong action potential propagation. Clin Orthop Relat Res. 2006;447:209–213. doi: 10.1097/01.blo.0000203481.11797.0f. [DOI] [PubMed] [Google Scholar]
- 17.Rijkhoff NJ, Wijkstra H, van Kerrebroeck PE, Debruyne FM. Selective detrusor activation by electrical sacral nerve root stimulation in spinal cord injury. J Urol. 1997;157:1504–1508. [PubMed] [Google Scholar]
- 18.Sharma HS, Badgaiyan RD, Alm P, Mohanty S, Wiklund L. Neuroprotective effects of nitric oxide synthase inhibitors in spinal cord injury-induced pathophysiology and motor functions : an experimental study in the rat. Ann N Y Acad Sci. 2005;1053:422–434. doi: 10.1111/j.1749-6632.2005.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 19.Sharma N, Coughlin L, Porter RG, Tanzer L, Wurster RD, Marzo SJ, et al. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor Neurol Neurosci. 2009;27:633–644. doi: 10.3233/RNN-2009-0489. [DOI] [PubMed] [Google Scholar]