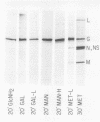
Abstract
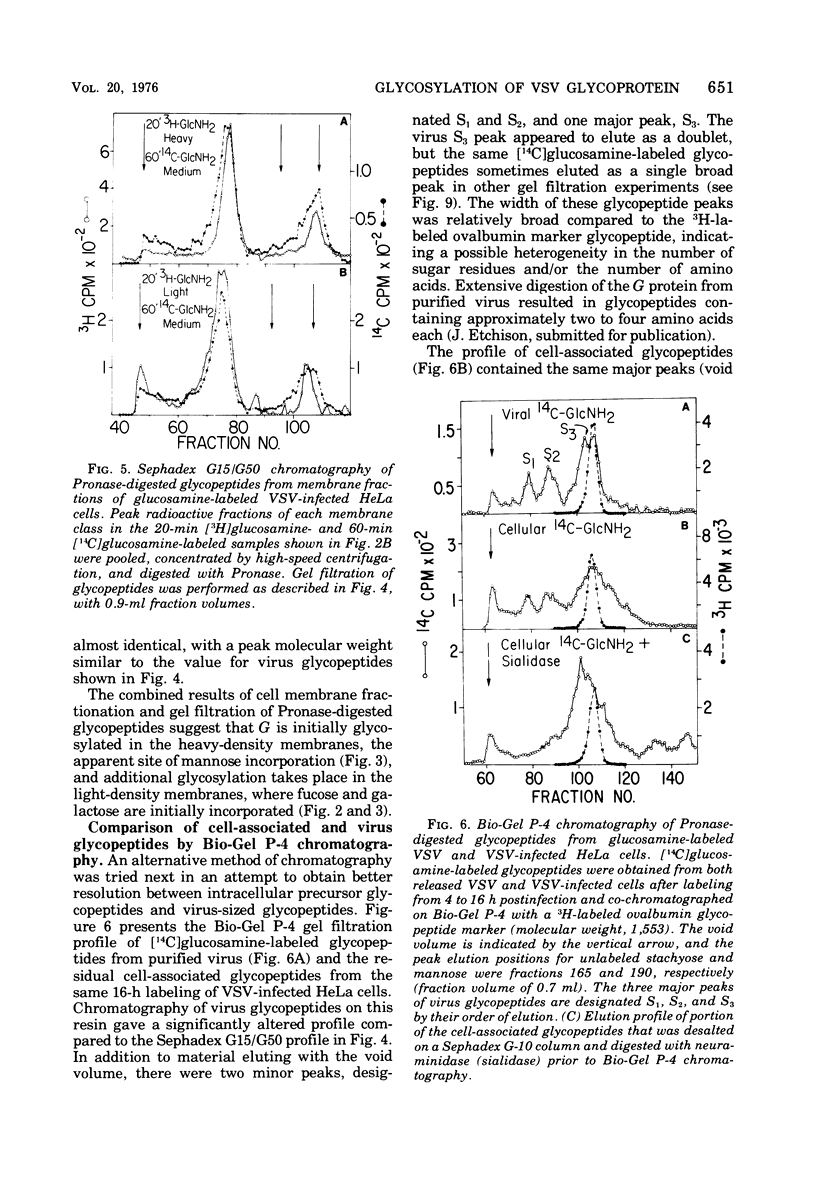
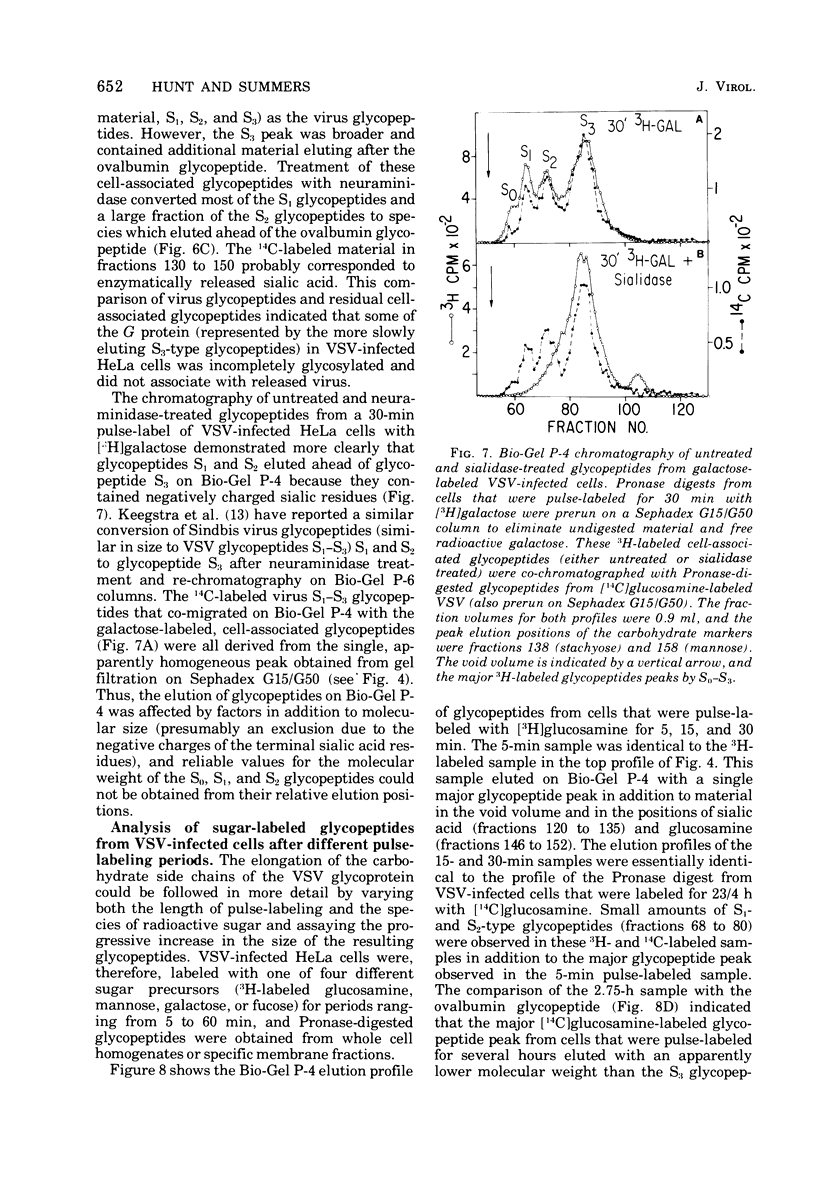
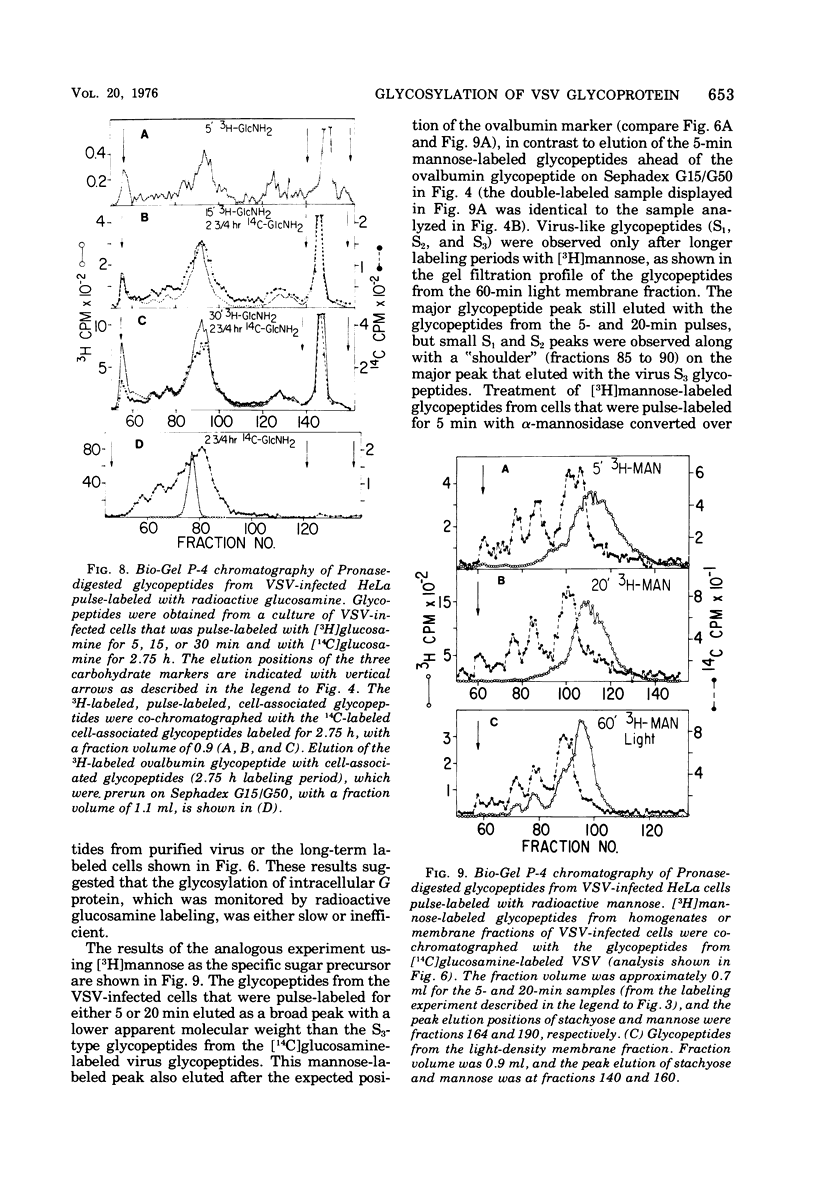
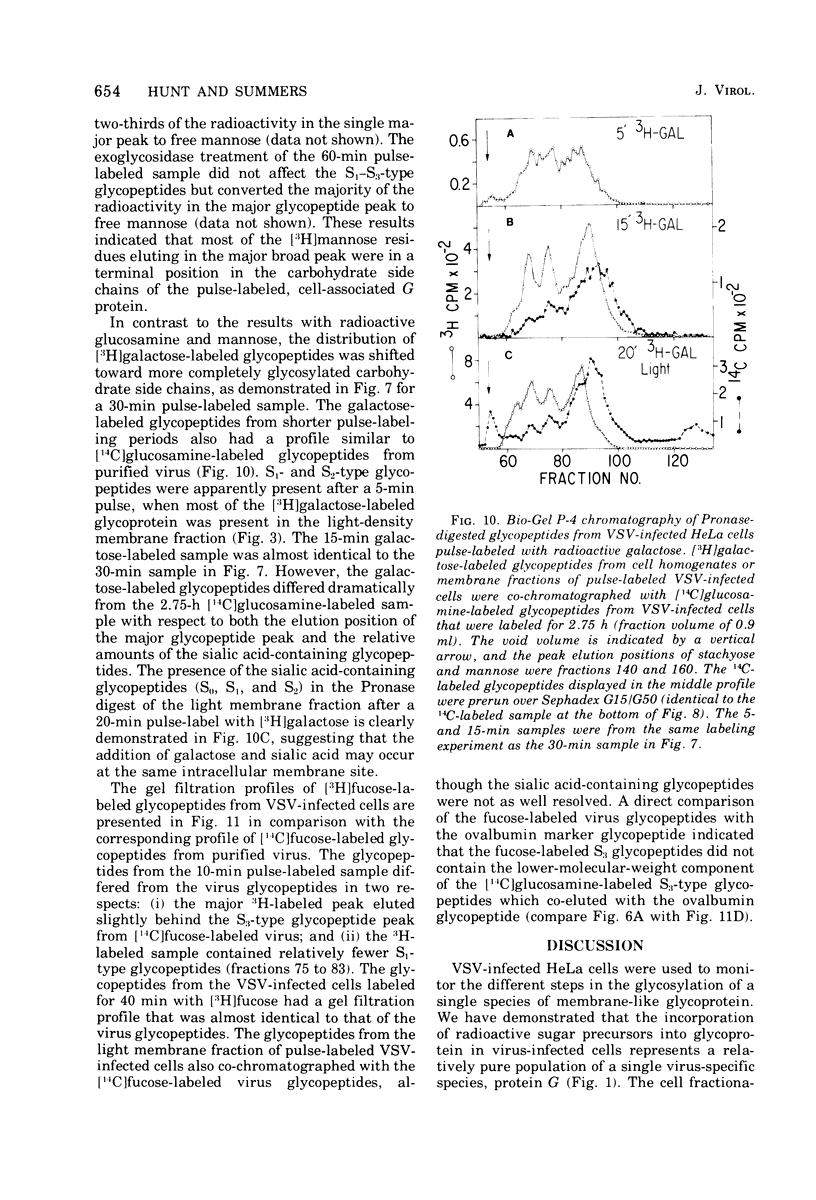
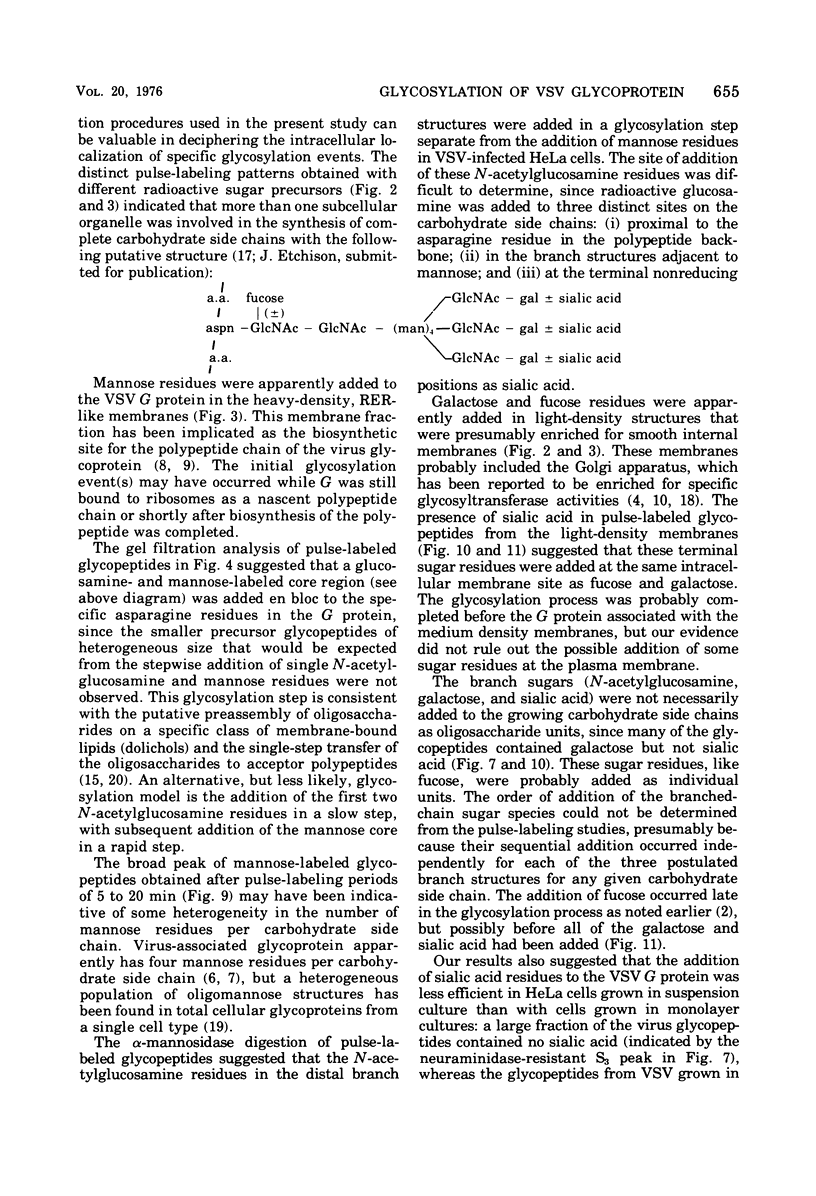
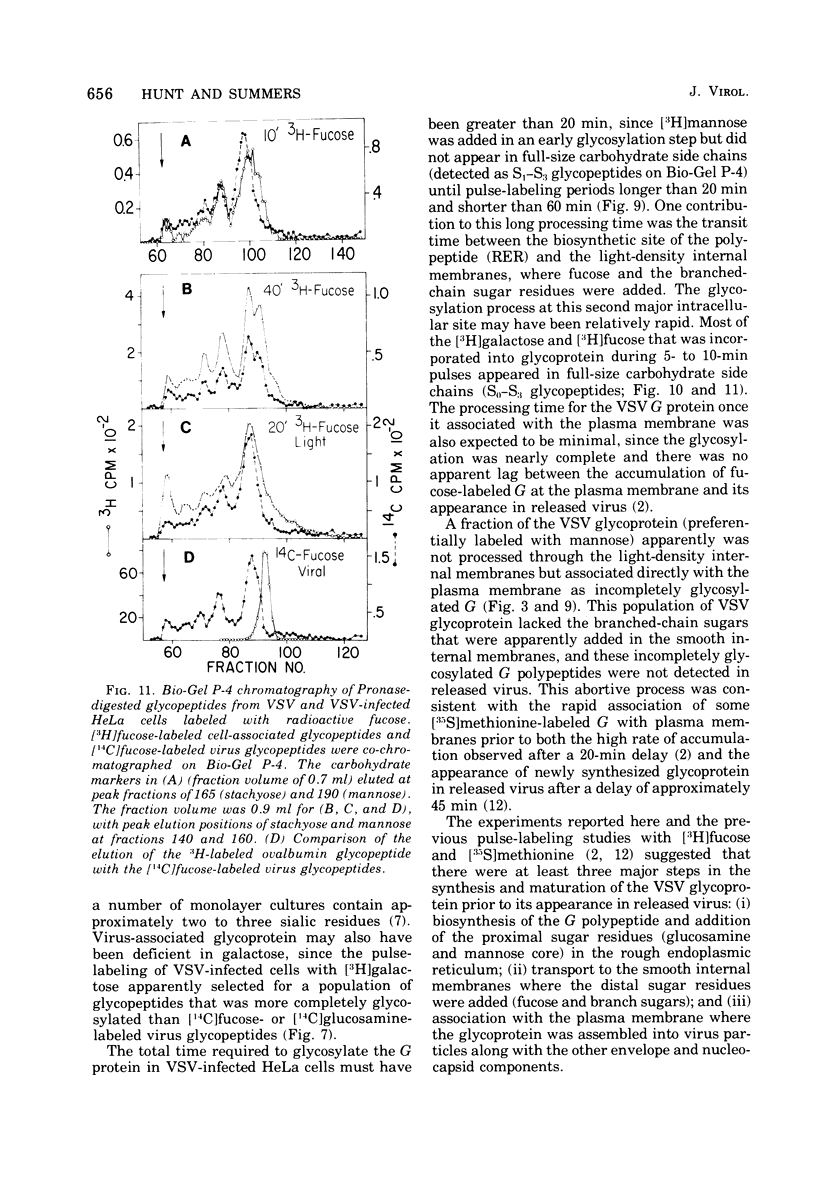
Glycosylation of the envelope glycoprotein of vesicular stomatitis virus was examined using virus-infected HeLa cells that were pulse-labeled with radioactive sugar precursors. The intracellular sites of glycosylation and the stepwise elongation of the carbohydrate side chains of the G protein were monitored by membrane fractionation and gel filtration of Pronase-digested glycopeptides. The results with short pulses of sugar label (5 to 10 mtein linkage (glucosamine and mannose) are added to G which was associated with the rough endoplasmic reticulum-enriched membrane fraction, whereas the more distal sugars (galactose, sialic acid, fucose, and possibly more glucosamine) are added in the light-density internal membrane fraction. Accumulation of mature G was observed in the plasma membrane-enriched fraction. The gel filtration studies indicated that the initial glycosylation event may be the en bloc addition of a mannose and glucosamine oligomer, followed by the stepwise addition of the more distal sugars.
Full text
PDF

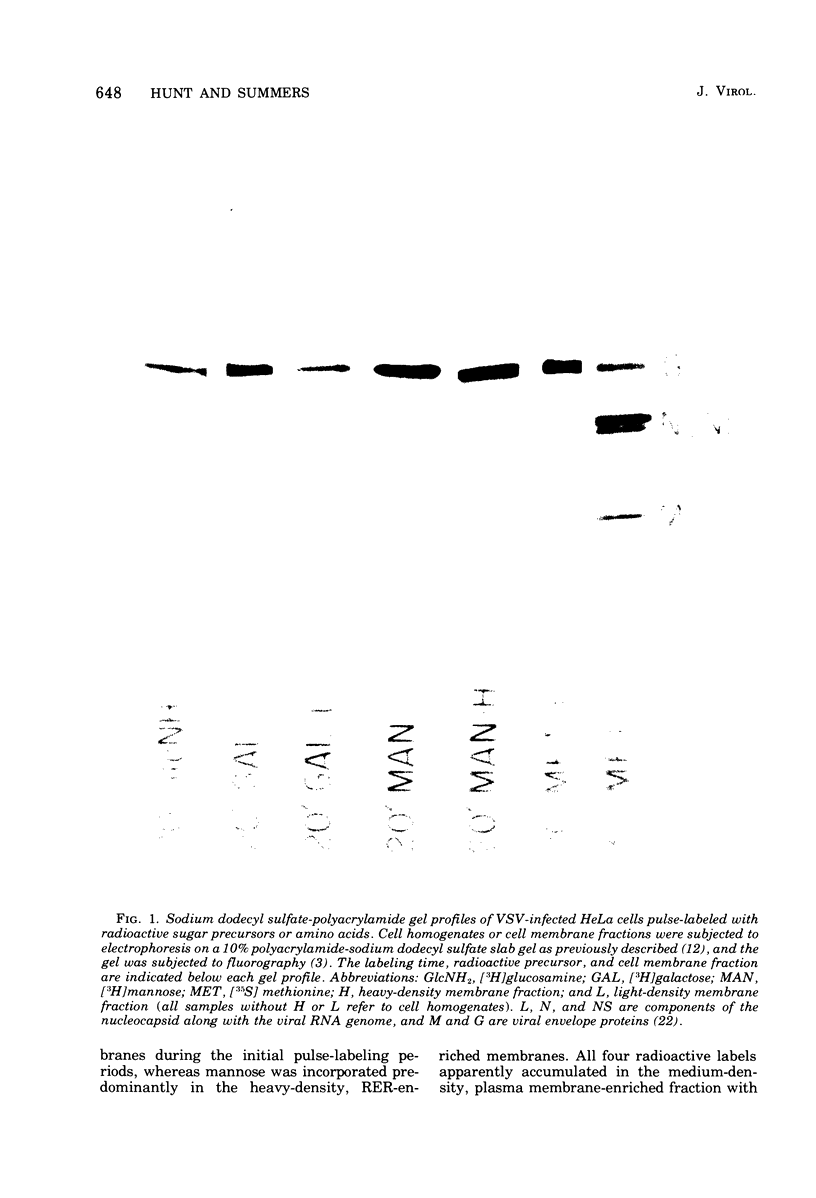
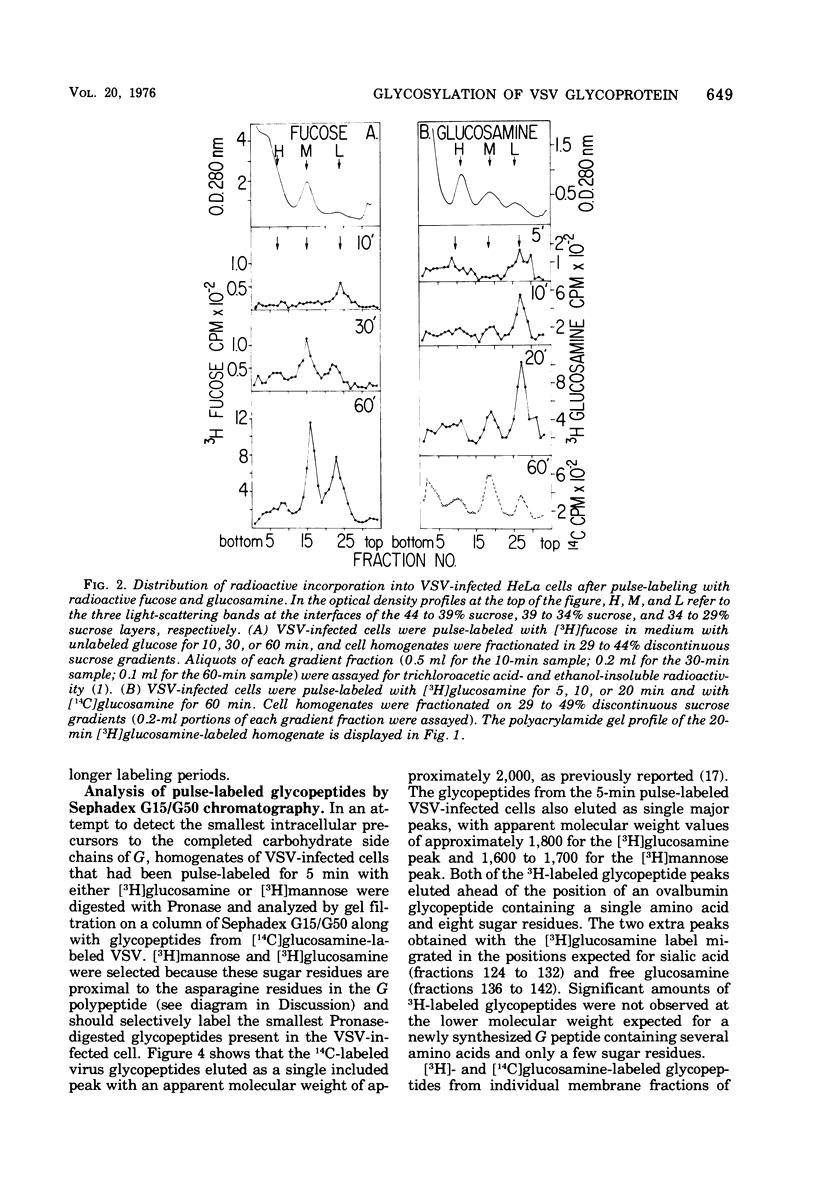
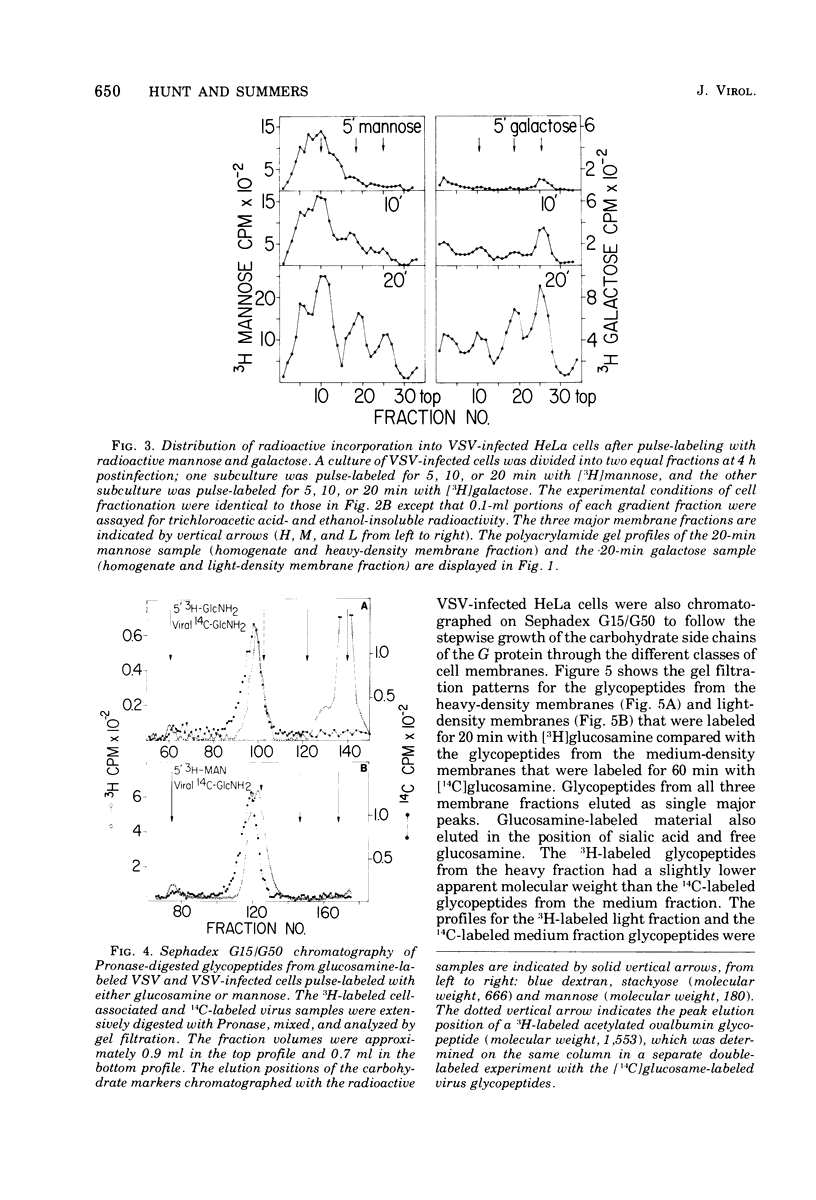

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Moyer S. A., Summers D. F. Assembly of vesicular stomatitis virus glycoprotein and matrix protein into HeLa cell plasma membranes. J Mol Biol. 1976 Apr 15;102(3):613–631. doi: 10.1016/0022-2836(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H. Synthesis and assembly of HeLa cell plasma membrane glycoproteins and proteins. J Biol Chem. 1975 Mar 25;250(6):2123–2134. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Glycoprotein biosynthesis: the characterization of two glycoprotein:frucosyl transferases in HeLa cells. Arch Biochem Biophys. 1968 Nov;128(2):470–481. doi: 10.1016/0003-9861(68)90053-2. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus. Virology. 1974 Jul;60(1):217–229. doi: 10.1016/0042-6822(74)90379-1. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Ehrenfeld E., Summers D. F. In vitro synthesis of proteins by membrane-bound polyribosomes from vesicular stomatitis virus-infected HeLa cells. J Virol. 1974 Sep;14(3):560–571. doi: 10.1128/jvi.14.3.560-571.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M. J., Moyer S. A., Banerjee A. K., Ehrenfeld E. Sub-cellular localization of vesicular stomatitis virus messenger RNAs. Biochem Biophys Res Commun. 1975 Feb 3;62(3):531–538. doi: 10.1016/0006-291x(75)90431-3. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., WHITMORE G. F. The development and structure of vesicular stomatitis virus. Virology. 1962 Apr;16:466–478. doi: 10.1016/0042-6822(62)90228-3. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Bosmann H. B., Eylar E. H. Glycoprotein biosynthesis: the localization of polypeptidyl: N-acetylgalactosaminyl, collagen: glucosyl, and glycoprotein:galactosyl transferases in HeLa cell membrane fractions. Arch Biochem Biophys. 1968 Nov;128(2):387–396. doi: 10.1016/0003-9861(68)90045-3. [DOI] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Association of vesicular stomatitis virus proteins with HeLa cell membranes and released virus. J Virol. 1976 Dec;20(3):637–645. doi: 10.1128/jvi.20.3.637-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycolipid content of vesicular stomatitis virus grown in baby hamster kidney cells. J Virol. 1971 Mar;7(3):416–417. doi: 10.1128/jvi.7.3.416-417.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Vesicular stomatitis virus envelope glycoprotein alterations induced by host cell transformation. Cell. 1974 May;2(1):63–70. doi: 10.1016/0092-8674(74)90009-9. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Tsang J. M., Atkinson P. H., Summers D. F. Oligosaccharide moieties of the glycoprotein of vesicular stomatitis virus. J Virol. 1976 Apr;18(1):167–175. doi: 10.1128/jvi.18.1.167-175.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. R., Narasimhan S., Wetmore S., Riordan J. R., Schachter H. Intracellular localization of GDP-L-fucose:glycoprotein and CMP-sialic acid: apolipoprotein glycosyltransferases in rat and pork livers. Arch Biochem Biophys. 1975 Jul;169(1):269–277. doi: 10.1016/0003-9861(75)90341-0. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Koide N., Ogata-Arakawa M. Analysis of oligomannosyl cores of cellular glycopeptides by digestion with endo-beta-N-acetylglucosaminidases. Biochem Biophys Res Commun. 1975 Oct 6;66(3):881–888. doi: 10.1016/0006-291x(75)90722-6. [DOI] [PubMed] [Google Scholar]
- Oliver G. J., Harrison J., Hemming F. W. The mannosylation of dolichol-diphosphate oligosaccharides in relation to the formation of oligosaccharides and glycoproteins in pig-liver endoplasmic reticulum. Eur J Biochem. 1975 Oct 1;58(1):223–229. doi: 10.1111/j.1432-1033.1975.tb02367.x. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. Studies on fetuin, a glycoprotein of fetal serum. II. Nature of the carbohydrate units. J Biol Chem. 1962 Feb;237:382–388. [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee Y. C., Hackett A. J., Talens L. Vesicular stomatitis virus maturation sites in six different host cells. J Gen Virol. 1970;7(2):95–102. doi: 10.1099/0022-1317-7-2-95. [DOI] [PubMed] [Google Scholar]