Abstract
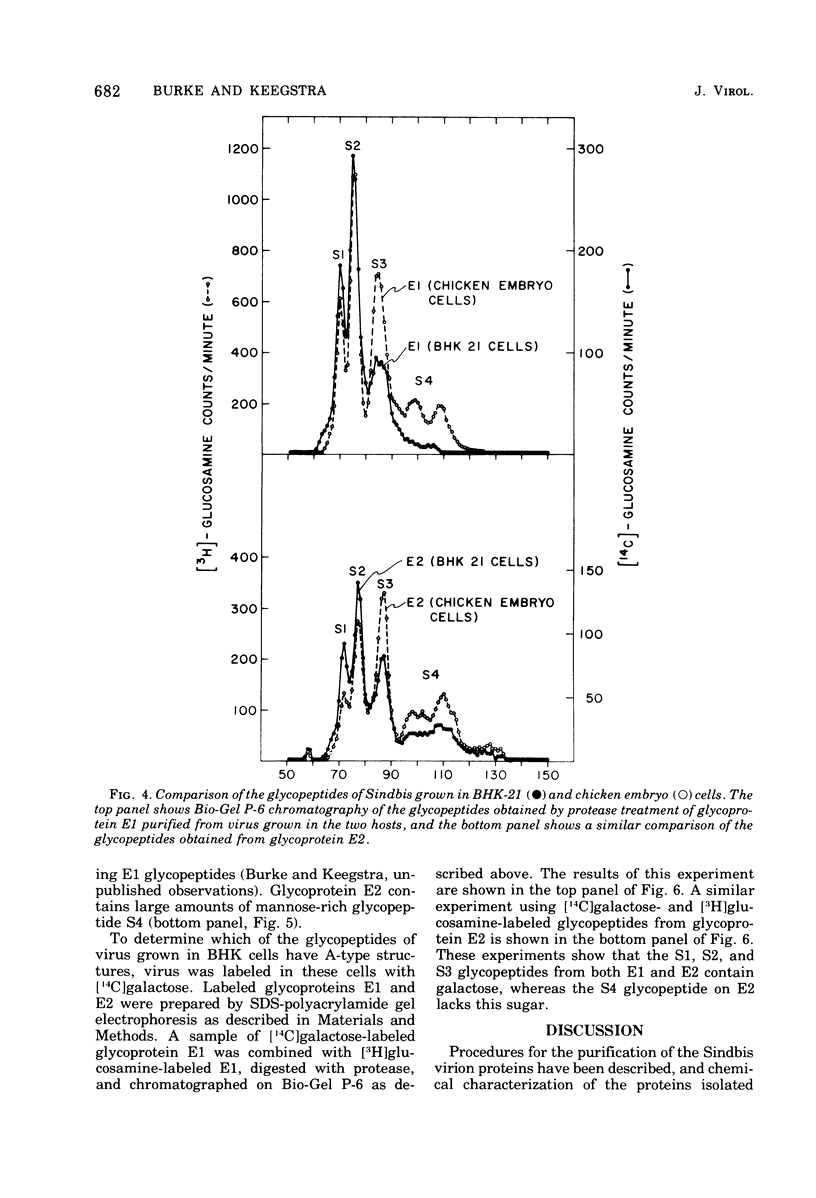
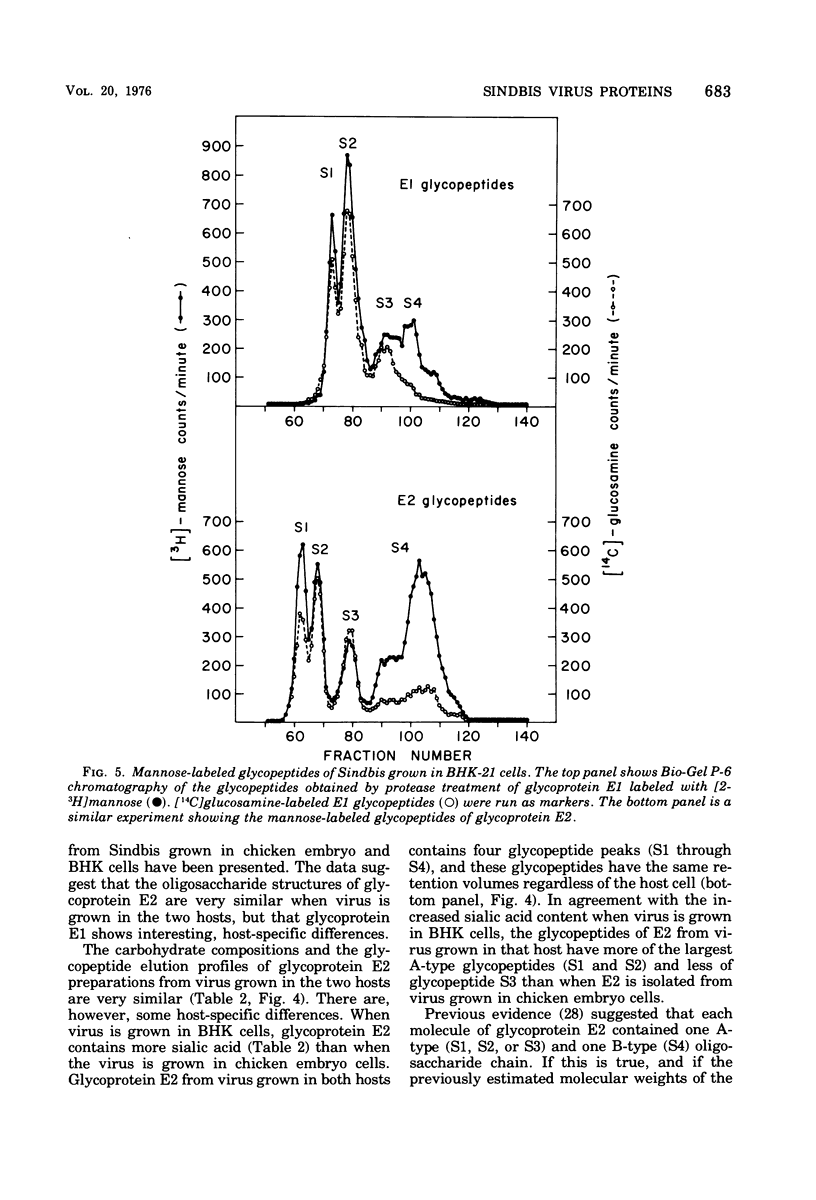
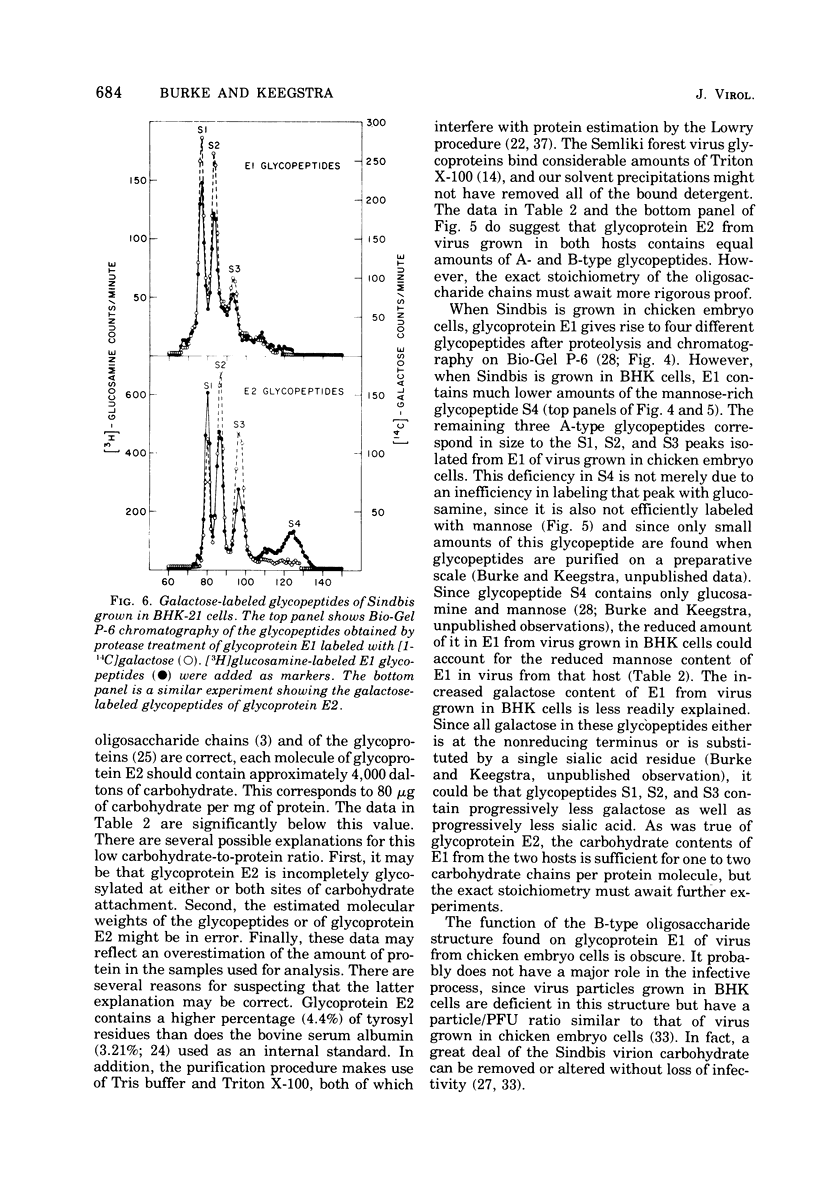
Procedures are described for the purification of the Sindbis virus structural proteins. The amino acid and carbohydrate compositions of the purified proteins are presented for virus grown in BHK-21/13 and chicken embryo cells. Glycoprotein E1 from virus grown in BHK cells is deficient in a mannose-rich glycopeptide found on that glycoprotein when virus is grown in chicken embryo cells. The complex glactose-containing glycopeptides appear similar for virus grown in both hosts. However, when virus is grown in BHK cells, both glycoproteins are enriched in those glycopeptides containing more sialic acid. Since the two viral glycoproteins are difficult to separate cleanly during purification, it is suggested that there may be strong, but noncovalent, interactions between glycoproteins E1 and E2. It is also suggested that there may be an interaction between glycoprotein E2 and a component of the nucleocapsid.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971 Jan 27;229(4):114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- David A. E. Lipid composition of Sindbis virus. Virology. 1971 Dec;46(3):711–720. doi: 10.1016/0042-6822(71)90073-0. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus. Virology. 1974 Jul;60(1):217–229. doi: 10.1016/0042-6822(74)90379-1. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Utermann G., Simons K. The membrane proteins of Semliki Forest virus have a hydrophobic part attached to the viral membrane. FEBS Lett. 1972 Dec 1;28(2):179–182. doi: 10.1016/0014-5793(72)80706-3. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Grimes W. J., Burge B. W. Modification of Sindbis virus glycoprotein by host-specified glycosyl transferases. J Virol. 1971 Mar;7(3):309–313. doi: 10.1128/jvi.7.3.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- Hirschberg C. B., Robbins P. W. The glycolipids and phospholipids of Sindbis virus and their relation to the lipids of the host cell plasma membrane. Virology. 1974 Oct;61(2):602–608. doi: 10.1016/0042-6822(74)90295-5. [DOI] [PubMed] [Google Scholar]
- Johnson I., Clamp J. R. The oligosaccharide units of a human type L immunoglobulin M (Macroglobulin). Biochem J. 1971 Aug;123(5):739–745. doi: 10.1042/bj1230739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Pedersen C. E., Jr, Marker S. C., Eddy G. A. Comparative electrophoretic studies on the structural proteins of selected group A arboviruses. Virology. 1974 Jul;60(1):312–314. doi: 10.1016/0042-6822(74)90392-4. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Hawn C. Isolation of two large peptide fragments from the amino- and carboxyl-terminal positions of bovine serum albumin. J Biol Chem. 1967 Apr 10;242(7):1566–1573. [PubMed] [Google Scholar]
- Rej R., Richards A. H. Interference by Tris buffer in the estimation of protein by the Lowry procedure. Anal Biochem. 1974 Nov;62(1):240–247. doi: 10.1016/0003-2697(74)90383-2. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. THE CARBOHYDRATE UNITS OF THYROGLOBULIN. J Biol Chem. 1965 Apr;240:1603–1610. [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. W. New opportunities in biological research offered by arthropod cell cultures. I. some speculations on the possible role of arthropods in the evolution of arboviruses. Curr Top Microbiol Immunol. 1971;55:241–245. doi: 10.1007/978-3-642-65224-0_40. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb C., Feil P., Gelb N., Kornfeld S. Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J Virol. 1975 Jan;17(1):239–246. doi: 10.1128/jvi.17.1.239-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Burge B. W. Biosynthesis of the Sindbis virus carbohydrates. J Virol. 1973 Dec;12(6):1366–1374. doi: 10.1128/jvi.12.6.1366-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simons K., Käriäinen L. Characterization of the Semliki Forest virus core and envelope protein. Biochem Biophys Res Commun. 1970 Mar 12;38(5):981–988. doi: 10.1016/0006-291x(70)90818-1. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stollar B. D., Koo R., Harrap K. A., Schlesinger R. W. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976 Jan;69(1):104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J Virol. 1975 Jul;16(1):141–145. doi: 10.1128/jvi.16.1.141-145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]