Abstract
An efficient and reproducible protocol has been developed for in vitro propagation of Pithecellobium dulce (Roxb.) Benth (a multipurpose leguminous tree) from field grown nodal segments (axillary bud). Shoot bud induction occurred from nodal explants of 15-years-old tree on Murashige and Skoog (MS) basal medium supplemented with 4.4 μM 6-benzyladenine (BA) and multiplication was achieved on MS medium supplemented with 4.4 μM BA + 0.73 μM phenylacetic acid (PAA) i.e. up to 7 shoot buds in the period of 5–6 weeks. Addition of adenine sulphate (AdS) to this medium further enhanced the number of shoot buds up to 10. Proliferating shoot cultures were established by repeatedly subculturing primary culture on fresh medium (MS + 4.4 μM BA + 0.73 μM PAA) after every 25 days. In vitro rooting was achieved on MS medium supplemented with 2.46 μM Indole-3-butyric acid (IBA) + 41.63 μM activated charcoal (AC). The micropropagated shoots with well developed roots were acclimatized in green house in pots containing sand, soil and manure (1:1:1). Genetic stability of micropropagated clones was evaluated using Random amplified polymorphic DNA (RAPD) and Inter simple sequence repeat (ISSR) markers. The amplification products were monomorphic in micropropagated plants and similar to those of mother plant. No polymorphism was detected revealing the genetic uniformity of micropropagated plants. This is the first report of an efficient protocol for regeneration of P. dulce through organogenesis, which can be used for further genetic transformation and pharmaceutical purposes.
Keywords: Legume tree; Nodal segments; Activated charcoal; Adenine sulphate; RAPD, ISSR marker
Introduction
Pithecellobium dulce (Roxb.) Benth (Family Leguminosae, subfamily Mimosoidae) popularly known as Jungle jalebi or Inga dulce is a rare forest leguminous tree that originated from Mexico and has been introduced in India as a hedge plant (Khatra et al. 1994). Nitrogen fixing ability of this plant is the reason of its great economic and ecological significance. It is a multipurpose tree with many uses as food (sweet pods), firewood, honey, fodder, soap oil, tannin, hedges, and shade. It has many ethno medical uses like decoction of leaves is used to allay pain from venereal sores, cure indigestion, convulsions, and diabetes, arils are used in preparation of beverage, saline extract of seeds showed haemolytic agglutinating reaction with human blood (Sivakumar and Murgesan 2005). It has also been reported to act as emollient, anodyne and larvicidal in folk medicines and as folk remedy for earache, leprosy, peptic ulcers, toothache etc. (Rajasab and Isaq 2004). Pithedulosides of the plant are found to be abortifacient, anti-inflammatory, antivenom, protease inhibitory, spermicidal, antimicrobial and antitubercular (Barrera et al. 2003; Pithayanukul et al. 2005). Manna et al. (2011) analyzed the beneficial role of the aqueous extract of fruits of P. dulce against carbon tetrachloride induced hepatic injury using a murine model. This valuable raw material is an important source of income for the local tribal communities who harvest the pods every year from the wild.
Despite its great economic importance, natural stand of the species is decreasing day by day due to indiscriminate exploitation and deforestation. One of the main problems faced by people involved with reforestation is lack of good quality planting material. Because of commercial interest or insufficient experience, such situations have led to the use of seeds with poor quality genetics.
In vitro technique can contribute to the propagation of large numbers of superior individuals or so called ‘Plus trees’ (Valverde-Cerdas et al. 1997). Micropropagation has many advantages over conventional propagation of plants. Tissue culture plantlets are superior, uniform, season independent and require smaller space as compared to seed grown saplings (Vengadesan et al. 2002). Tissue culture of tree species offers a rapid means of producing clonal planting stock for afforestation, woody biomass production and conservation of elite germplasm. In recent years, in vitro culture techniques have been extensively and effectively used as a tool for mass multiplication and germplasm conservation of leguminous as well as several woody tree species including Swartzia madegascarensis, Dalbergia sissoo, Acacia sinuata, Sesbania rostrata, Melia azedarach and Hagenia abyssinica (Berger and Schaffner 1995; Pradhan et al. 1998; Feyissa et al. 2005; Husain and Anis 2006). So far there is no report on in vitro regeneration of P. dulce. We are introducing for the first time a highly efficient protocol for micropropagation of this important leguminous tree from nodal explants of a mature tree and determined the genetic stability of the micropropagated plants through RAPD and ISSR markers. It is hoped that this study would be helpful in the domestication and ex situ conservation of this economically and environmentally important tree species.
Material and method
Plant material
Fresh and mature branches with 10–15 nodes were collected from field grown trees (~15 yrs old) with large trunk, well spread canopy, regular flowering and fruiting characteristics, growing in Grass Farm Nursery, Jaipur, India. After excision of leaflets and thorns branches were cut into smaller segments (4–5 nodes long) and rinsed in 20 % (v/v) ‘Extran’ (liquid detergent; Merck, India) for 10 min then treated with 0.5 % (w/v) bavistin (Carnbandazin powder), a broad spectrum fungicide for 8–10 min and finally washed in running tap water for 30 min. These segments were then aseptically surface sterilized with 0.1 % mercuric chloride (w/v) for 3 min followed by 4–5 rinses with sterile distilled water. Since the use of sodium hypochlorite did not prevent contamination, mercuric chloride was used as sterilizing agent throughout the experiment. Segments having 4–5 nodes were recut into segments of about 1 cm having single node in each explant and cultured aseptically in vertical position in the culture flask (100 ml, Borosil) containing nutrient medium.
Culture media and culture conditions
The nutrient medium consisting of full strength MS (Murashige and Skoog 1962) and WPM (Lloyd and McCown 1980) containing mineral salts, vitamins, 3 % (w/v) sucrose (Merck, India) and 0.8 % (w/v) agar (bacteriological grade; Merck, India) were compared to identify the suitable medium formulation for in vitro growth of nodal explants. The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C and 1.05 kg/cm2 pressure for 20 min. All experiments were conducted in growth chamber at 26 ± 1 °C and a 16-h photoperiod with 25 μmol m−2 s−1 illumination provided by cool white fluorescent lamps (Philips, India, 36 W).
Shoot induction and multiplication
Nodal explants were collected through out the year to study the influence of different seasons on in vitro shoot bud sprouting. Nodal segments were placed vertically on both Murashige and Skoog (MS) medium and Woody Plant Medium (WPM) supplemented with different concentrations of 6-benzyladenine (BA; 4.4, 8.8, 13.3 μM). In the next step, explants were placed on MS medium supplemented with BA (4.4, 8.8, 13.3 μM) in combination with auxins indole-3-acetic acid (IAA; 0.57, 2.8, 5.7 μM), indole-3-butyric acid (IBA; 0.49, 2.4, 4.9 μM), phenylacetic acid (PAA; 0.73, 3.67, 7.35 μM) and α-naphthaleneacetic acid (NAA; 0.54, 2.7, 5.4 μM) at different concentrations. Effect of adenine sulphate (AdS; 12.36 μM–123.64 μM) was studied in combination with optimal cytokinin and auxin concentration to analyze the combined effect and to standardize the best combination of plant growth regulators on shoot bud induction. After 6 weeks of culture, shoot bud induction and number of shoots per explants were recorded. Effect of position of nodes was studied in an independent experiment in which apical (1–5), medial (5–20) and basal nodes (20–35) were used. Subcultures were done at 25 days interval to study the effect of culture passages on shoot bud induction and shoot proliferation.
In vitro rooting and acclimatization
In vitro raised shoots (2–3 cm) were excised and cultured on full strength MS medium supplemented with IBA (2.46 μM) in combination with two concentrations of activated charcoal (41.63 μM, 83.26 μM). In vitro grown rooted plants were removed from adhering gel, transplanted to plastic pots containing mixture of sand, soil and manure (1:1:1) and placed in green house for acclimatization.
DNA extraction
DNA was extracted from fresh leaves of 11 randomly selected regenerated plants and from the leaves of mother plant (Mo). The method involves a modified CTAB extraction, employing high salt concentrations to remove polysaccharides, the use of polyvinyl pyrrolidone (PVP) to remove polyphenols, an extended RNase treatment and a phenol-chloroform extraction (Porebski et al. 1997). The leaf samples were powdered in liquid nitrogen and stored at −80 °C until used for extraction.
DNA amplification
RAPD Amplification was carried out in 20 μl reaction volume containing 25 ng DNA (2 μl), 2.0 μl of 10× PCR buffer (Taq buffer A including MgCl2), 0.5 μl of 100 μM dNTP, 2.0 μl of RAPD primer, 0.3 μl of Taq DNA polymerase (Bangalore Genei, India) and MilliQ water to make up the volume. The PCR was performed at an initial denaturation at 94 °C for 4 min followed by 40 cycles of 1 min denaturation at 94 °C, 45 s annealing at 37 °C and 2 min extension at 72 °C with a final extension of 72 °C for 10 min using a thermal cycler (BioRad, UK). The PCR products obtained were separated on 1.2 % agarose (Himedia, India) gel through electrophoresis using100 bp and 1 kb ladder as the band size standard and photographed using Gel Documentation System (Bio-Rad, Germany).
In case of ISSR primers, optimal annealing temperature was found to vary according to base composition of the primers. Eight ISSR primers (UBC primer set no. 9, University of British Columbia, Canada) were used in the study. Amplification was carried out in 20 μl reaction volume containing 25 ng genomic DNA (2 μl) as template, 1.5 μl MgCl2, 0.5 μl of 100 μM dNTP, 2.0 μl of 10× PCR buffer (Taq buffer B devoid of MgCl2), 2.0 μl of ISSR primers (10 pM), 0.3 μl of Taq DNA polymerase (Bangalore Genei, India) and MilliQ water to make up the volume. PCR amplifications were performed with initial denaturation at 94 °C for 4 min followed by 35 cycles of 30 s denaturation at 92 °C, 1 min at the annealing temperature (depending on the primer), 2 min extension at 72 °C with a final extension at 72 °C for 7 min using thermal cycler (BioRad, UK). The PCR products were separated on 2 % agarose gel (Himedia, India) using 100 bp and 1 kb markers as the band size standard and photographed in a gel documentation system ((Bio-Rad, Germany).
Data analysis
The data on shoot bud formation and rooting were collected after 6 weeks. All the experiments were repeated twice. Data were subjected to analysis of variance (ANOVA) for a factorial experiment. Critical differences (CD) were calculated to determine the statistical significance of difference among means which were compared using Duncan’s (1955) multiple range test at a significance of P = 0.05 according to Gomez and Gomez (1984). Data are presented as mean ± standard error (SE).
Results
Initially both shoot tips and nodal segments were used as explants but shoot tips swelled after 4 weeks and did not show any kind of organogenesis so further experiments were conducted by using nodal stem segments as explants in this study. The nodal explants cultured on MS medium and WPM showed a different response. MS medium was found better as compared to WPM both in terms of shoot number and caullogenic percentage (Table 1).
Table 1.
Shoot bud induction from nodal segments of Pithecellobium dulce cultured on MS/WPM with different concentrations of BAP
| BAP(μM) | % response (Mean ± SE) | Shoot buds (Mean ± SE) | ||
|---|---|---|---|---|
| MS | WPM | MS | WPM | |
| 4.4 | 80b ± 2.5 | 48b ± 4.7 | 2.5c ± 0.2 | 1.5c ± 0.2 |
| 8.8 | 62b ± 3.3 | 55b ± 4.7 | 2.2bc ± 0.4 | 1.4bc ± 0.2 |
| 13.3 | 60b ± 2.8 | 30a ± 4.0 | 1.5ab ± 0.2 | 0.5a ± 0.3 |
| 17.8 | 27a ± 2.5 | 56b ± 2.8 | 1.2a ± 0.2 | 0.2a ± 0.5 |
Each treatment consisted of 5 replicates, each with 3 explants and experiment was repeated twice. Mean with the same letter(s) within the same column are not significantly different at the 5 % level according to Duncan’s multiple range test
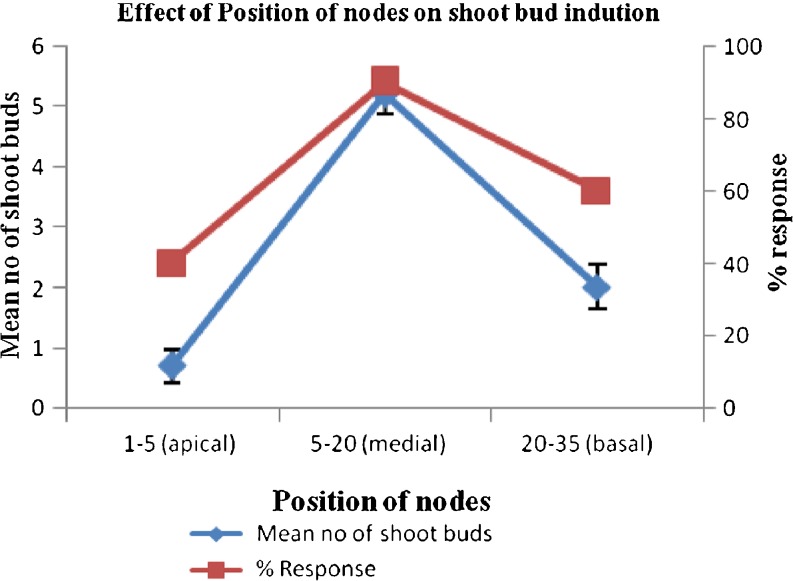
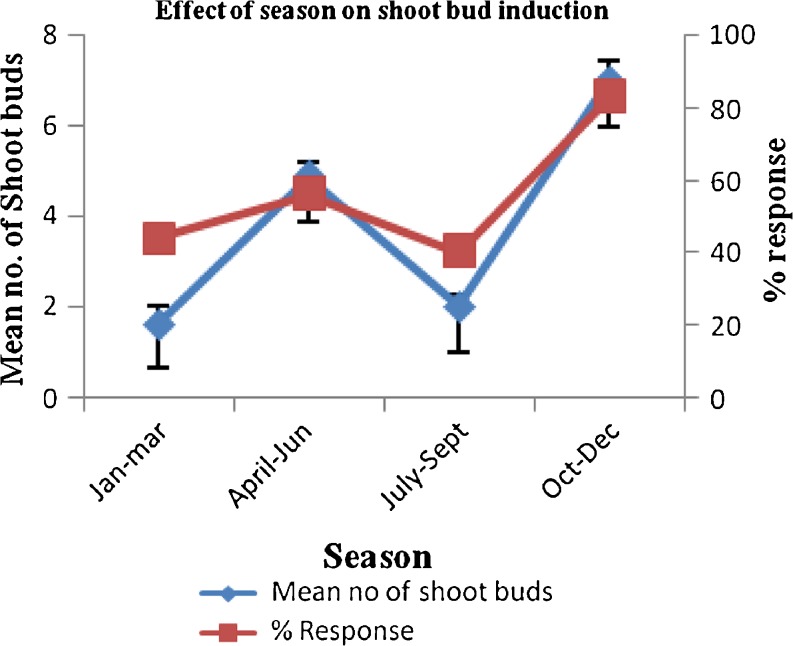
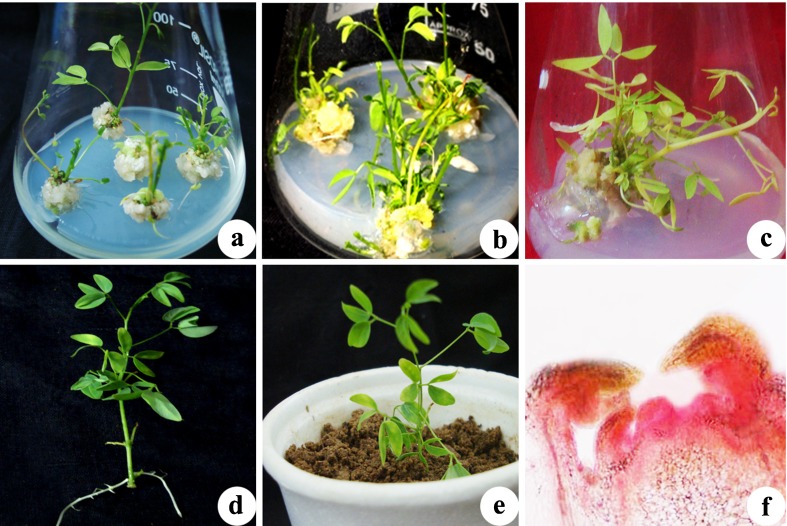
Position of explants play an important role in morphogenesis, nodal explants of medial position showed better response as compared to apical and basal nodes. (Fig. 1) The % response (number of explants responded per total explants placed in the flask) and average number of shoots formed per explants varied considerably with season and type of growth regulator used at different concentrations. Explants taken in season from October to December showed best morphogenic response than others. (Fig. 2). Among different cytokinins tested BA (4.4 μM) was found responsive as 1–2 shoots regenerated from the axils of each explant (Fig. 4a). Further studies were conducted with MS medium supplemented with BA and different concentrations of auxins for multiplication (Table 2). BA (13.3 μM) in combination with IAA (0.57 μM) produced profuse callus and only 2–3 yellow stunted shoots, BA (4.4 μM) with NAA (0.54 μM) resulted in powdery pale yellow callus along with 3–4 shoots, BA (8.8 μM) and IBA (0.49 μM) induced 4 stout leafy shoots with negligible callus at the base. Addition of PAA (0.73 μM) in the medium containing BA (4.4 μM) significantly enhanced the shoot regeneration capacity and found to be best in inducing morphogenic response as 6–8 axillary shoots (healthy) were obtained from the explants.
Fig. 1.
Effect of position of nodes on shoot bud induction
Fig. 2.
Effect of season on shoot bud induction
Fig. 4.
Micropropagation of Pithecellobium dulce using field grown nodal explants. a Shoot bud induction on MS medium containing 4.4 μM BA after 5 weeks of culture. b Multiple shoot formation on MS medium containing 4.4 μM BA, 0.73 μM PAA and 49.45 μM AdS. c Multiple shoot induction in mother explant after second stage subculture on MS medium containing 4.4 μM BA and 0.73 μM PAA. d Rooting of microshoot cultured on full strength MS medium containing 2.46 μM IBA and 41.63 μM AC. e Plantlet acclimatized and transferred to plastic pots containing a mixture of sand, soil and manure (1:1:1). f Longitudinal section of nodal segments after 15 days of culture showing direct organogenesis
Table 2.
Influence of different concentrations of BAP and Auxins on shoot bud induction and multiple shoot bud proliferation from cultured nodal segments of Pithecellobium dulce
| PGR(μM) | % Response (Mean ± SE) | No. of shoot buds (Mean ± SE) | |
|---|---|---|---|
| BAP | IAA | ||
| 4.4 | 0.57 | 36ef ± 4.0 | 1.6de ± 0.4 |
| 8.8 | 0.57 | 36ef ± 4.0 | 1.8ef ± 0.4 |
| 13.3 | 0.57 | 44gh ± 4.0 | 2.2gh ± 0.2 |
| 17.8 | 0.57 | 28cd ± 3.7 | 1.4cd ± 0.2 |
| 4.4 | 2.8 | 20ab ± 3.1 | 0.6a ± 0.2 |
| 8.8 | 2.8 | 24bc ± 4.0 | 1.0b ± 0.3 |
| 13.3 | 2.8 | 36ef ± 2.4 | 1.8ef ± 0.2 |
| 17.8 | 2.8 | 16a ± 4.0 | 0.8ab ± 0.4 |
| 4.4 | 5.7 | 28cd ± 2.0 | 1.4cd ± 0.6 |
| 8.8 | 5.7 | 32de ± 4.9 | 0.8ab ± 0.2 |
| 13.3 | 5.7 | 48h ± 4.9 | 2.4h ± 0.4 |
| 17.8 | 5.7 | 40fg ± 4.9 | 2.0f ± 0.2 |
| 4.4 | 11.4 | 28cd ± 3.1 | 1.4cd ± 0.4 |
| 8.8 | 11.4 | 28cd ± 4.9 | 1.6de ± 0.4 |
| 13.3 | 11.4 | 32de ± 4.9 | 1.4cd ± 0.4 |
| 17.8 | 11.4 | 36ef ± 4.0 | 0.6a ± 0.2 |
| BAP | IBA | ||
| 4.4 | 0.49 | 28b ± 4.9 | 1.6ab ± 0.2 |
| 8.8 | 0.49 | 88d ± 4.9 | 4.2cd ± 0.3 |
| 13.3 | 0.49 | 16b ± 2.4 | 1.4ab ± 0.2 |
| 17.8 | 0.49 | 32bc ± 4.9 | 1.6ab ± 0.4 |
| 4.4 | 2.4 | 28b ± 4.9 | 1.4ab ± 0.2 |
| 8.8 | 2.4 | 92e ± 4.9 | 4.2cd ± 0.2 |
| 13.3 | 2.4 | 32bc ± 4.9 | 1.6ab ± 0.4 |
| 17.8 | 2.4 | 28b ± 4.9 | 1.4ab ± 0.2 |
| 4.4 | 4.9 | 0.0a ± 0.0 | 0.4a ± 0.2 |
| 8.8 | 4.9 | 0.0a ± 0.0 | 0.4a ± 0.2 |
| 13.3 | 4.9 | 56c ± 4.0 | 2.8bc ± 0.3 |
| 17.8 | 4.9 | 44c ± 4.0 | 2.2b ± 0.3 |
| 4.4 | 9.8 | 24b ± 4.0 | 0.6a ± 0.2 |
| 8.8 | 9.8 | 28b ± 4.9 | 1.4ab ± 0.3 |
| 13.3 | 9.8 | 28b ± 4.9 | 1.2ab ± 0.4 |
| 17.8 | 9.8 | 16b ± 2.4 | 0.6a ± 0.2 |
| BAP | NAA | ||
| 4.4 | 0.54 | 64f ± 7.4 | 3.2e ± 0.2 |
| 8.8 | 0.54 | 24ab ± 4.0 | 1.4bc ± 0.4 |
| 13.3 | 0.54 | 40d ± 3.7 | 1.4bc ± 0.2 |
| 17.8 | 0.54 | 40d ± 6.3 | 1.2ab ± 0.3 |
| 4.4 | 2.7 | 24ab ± 4.0 | 1.2ab ± 0.2 |
| 8.8 | 2.7 | 56e ± 4.0 | 2.8d ± 0.5 |
| 13.3 | 2.7 | 24ab ± 2.4 | 1.2ab ± 0.2 |
| 17.8 | 2.7 | 28ab ± 4.9 | 1.4bc ± 0.5 |
| 4.4 | 5.4 | 24ab ± 6.6 | 1.2ab ± 0.2 |
| 8.8 | 5.4 | 28ab ± 4.9 | 1.4bc ± 0.2 |
| 13.3 | 5.4 | 24ab ± 4.0 | 1.2ab ± 0.2 |
| 17.8 | 5.4 | 32c ± 2.0 | 1.6c ± 0.4 |
| 4.4 | 10.7 | 24ab ± 4.0 | 1.2ab ± 0.2 |
| 8.8 | 10.7 | 32c ± 2.0 | 1.6c ± 0.2 |
| 13.3 | 10.7 | 20a ± 3.1 | 1.0a ± 0.3 |
| 17.8 | 10.7 | 20a ± 3.1 | 1.2ab ± 0.3 |
| BAP | PAA | ||
| 4.4 | 0.73 | 96f ± 4.0 | 6.8f ± 0.4 |
| 8.8 | 0.73 | 24ab ± 2.4 | 1.2ab ± 0.2 |
| 13.3 | 0.73 | 72e ± 8.0 | 4.0e ± 0.3 |
| 17.8 | 0.73 | 52d ± 4.0 | 2.6cd ± 0.2 |
| 4.4 | 3.67 | 32bc ± 4.8 | 1.6bc ± 0.4 |
| 8.8 | 3.67 | 72e ± 3.7 | 3.4de ± 0.2 |
| 13.3 | 3.67 | 52d ± 2.4 | 2.6cd ± 0.6 |
| 17.8 | 3.67 | 36bc ± 4.0 | 1.6bc ± 0.2 |
| 4.4 | 7.35 | 56d ± 2.4 | 2.8cd ± 0.5 |
| 8.8 | 7.35 | 28ab ± 3.7 | 1.4ab ± 0.2 |
| 13.3 | 7.35 | 36bc ± 2.4 | 1.6bc ± 0.2 |
| 17.8 | 7.35 | 44cd ± 4.0 | 2.2c ± 0.2 |
| 4.4 | 14.7 | 16a ± 4.0 | 0.6a ± 0.2 |
| 8.8 | 14.7 | 32bc ± 4.8 | 1.6bc ± 0.3 |
| 13.3 | 14.7 | 56d ± 4.8 | 2.8cd ± 0.5 |
| 17.8 | 14.7 | 16a ± 4.0 | 0.8ab ± 0.2 |
Each treatment consisted of 5 replicates, each with 3 explants and experiment was repeated twice. Mean with the same letter(s) within the same column are not significantly different at the 5 % level according to Duncan’s multiple range test
Effect of adenine sulphate for further improved response
The addition of adenine sulphate at a concentration of (49.45 μM) along with BA (4.4 μM) and PAA (0.73 μM) on MS medium improved the establishment of nodal explants (Fig. 4b). Green healthy shoots (8–10 in number) were obtained on MS medium fortified with 49.45 μM AdS, BA (4.4 μM) and PAA (0.73 μM). This combination gave maximum response (90 %) with the highest no. of shoots (9.6 ± 0.24) after 6–7 weeks of culture (Table 3).
Table 3.
Effect of adenine sulphate in combination with BAP (4.4μM) and PAA (0.73μM) on shoot bud regeneration from cultured nodal segments Pithecellobium dulce
| AdS(μM) | % Response (Mean ± SE) | No of shoot buds (Mean ± SE) |
|---|---|---|
| 12.36 | 40ab ± 6.3 | 1.8a ± 0.5 |
| 24.72 | 44ab ± 7.4 | 2.0a ± 0.5 |
| 49.45 | 90c ± 4.8 | 9.6b ± 0.2 |
| 74.18 | 58bc ± 6.3 | 3.0a ± 0.4 |
| 123.64 | 12a ± 3.7 | 0.6a ± 0.0 |
Each treatment consisted of 5 replicates, each with 3 explants and experiment was repeated twice. Mean with the same letter(s) within the same column are not significantly different at the 5 % level according to Duncan’s multiple range test
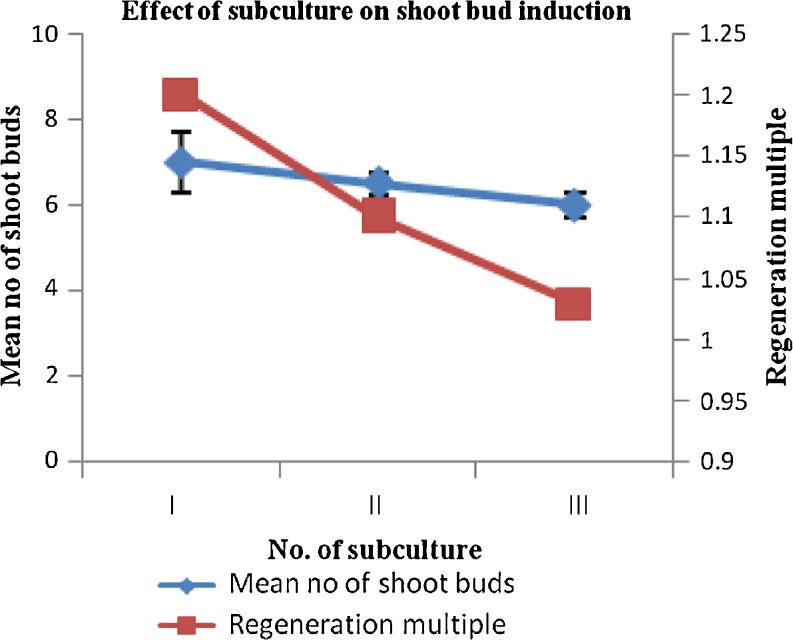
Subculture
The frequency of shoot formation and number of shoots per node increased during first two culture passages; afterwards a gradual decline in multiplication rate was observed (Fig. 3). Multiplied shoots were sectored in clumps of 4 and transferred on subculture medium consisting of BA (4.4 μM) and PAA (0.73 μM) after 25 days of culture. A prolific shoot culture free from basal callusing was established by repeated subculturing of nodal segments from newly formed shoots (Fig. 4c).
Fig. 3.
Effect of subculture stages on shoot proliferation
In vitro rooting and acclimatization
Auxin alone failed to induce rooting of in vitro raised shoots in the present study, so 3–4 cm long healthy shoots were rooted on full strength MS medium supplemented with IBA (2.46 μM) and two different concentrations of activated charcoal (41.63 μM, 83.26 μM). 80 % roots were obtained on MS medium supplemented with 2.46 μM IBA and 2.46 μM AC with an average root length of 5.2 cm and root number 3.2 ± 0.20 (Fig. 4d). In contrast, on media without AC, rooting efficiency was significantly lower (40–60 %) (Table 4) and callus was formed at the shoot base. Increased concentration of activated charcoal decreased the number and length of roots. Similarly at higher concentration (4.4, 8.8 μM) of IBA the % of rooting decreased and callus formation occurred at basal cut end. Well rooted plantlets were transferred to plastic pots containing a mixture of sand, soil and manure (1:1:1). These were then covered with polythene bags and kept in green house. Covers were removed upon the appearance of new leaves (Fig. 4e). Histological study (hand cut section) revealed that in the axil of each leaf, a distinct meristematic zone of small densely stained cells was present over a differentiated zone. Shoot primordia could be observed arising directly from base of cultured nodal explant. Although callusing was observed at the cut end but shoot buds differentiated directly from the nodal explants (Fig. 4f).
Table 4.
Influence of IBA and activated charcoal (AC) on rooting response from in vitro regenerated shoots of Pithecellobium dulce
| PGR (μM) | % Response | Root Length (cm) | No of roots | ||
|---|---|---|---|---|---|
| MS | IBA | AC | (Mean ± SE) | (Mean ± SE) | (Mean ± SE) |
| MS | 2.46 | 41.63 | 80c ± 6.3 | 5.2b ± 0.3 | 3.4b ± 0.2 |
| MS | 2.46 | 83.26 | 60bc ± 5.0 | 3.2ab ± 0.3 | 1.4a ± 0.2 |
| 1/2MS | 2.46 | 41.63 | 32ab ± 6.6 | 1.6a ± 0.4 | 1.2a ± 0.2 |
| 1/2MS | 2.46 | 83.26 | 20a ± 6.3 | 1.4a ± 0.2 | 1.0a ± 0.0 |
Each treatment consisted of 6 replicates and experiment was repeated twice. Mean with the same letter(s) within the same column are not significantly different at the 5 % level according to Duncan’s multiple range test
Assessment of genetic fidelity of micropropagated plants
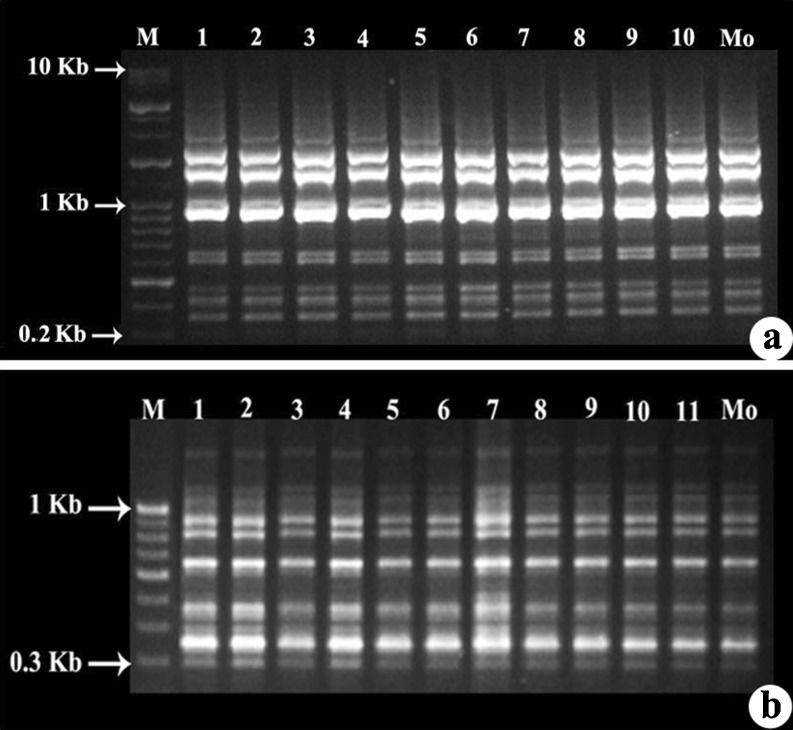
The regenerated plants were subjected to RAPD and ISSR analysis to check their genetic stability. Twenty random RAPD primers (OPF 01–10, OPT 05–10, OPA 01–05) were used, of which 12 produced distinct and scorable bands. A total of 1,116 amplicons were obtained and primer OPF-08 generated highest number of bands (10) bands (Fig. 5a). Fourteen ISSR primers (UBC 801–803, 805, 807, 808, 811–813, 815–818, 820) were used, of which 7 produced distinct and scorable bands. A total of 550 amplicons were obtained and primer UBC 811 produced highly reproducible banding pattern with 12 bands (Fig. 5b). DNA fingerprinting profiles of regenerants revealed that there were no polymorphic DNA fragments and no variation among mother and tissue culture-raised plants. RAPD and ISSR profile obtained through amplification of genomic DNA of the in vitro grown plants and that of the Mo was similar in all respects.
Fig. 5.
RAPD and ISSR profiles of mother plant and in vitro regenerated plants. a PCR amplification products obtained with RAPD primer OPF 08. b PCR amplification products obtained with ISSR primer UBC 811. M—Molecular Marker, Mo—Mother Plant, 1 to 10 and 1 to 11-Regenerated plants
Discussion
In the present study nodal segments (from field grown trees) were used as explants. Nodal segment has protected axillary buds, which do not get damaged during surface sterilization and this makes it better responsive explant than other ones. Position and season of collection of explant played an important role in the establishment and growth of in vitro cultures (Bhatt and Dhar 2004). In this study 5th–20th nodes on twigs, taken in season of October–December were found to be more responsive than other nodes taken in any other season. This period not only showed maximum bud break but also low fungal contamination. MS medium was found better than WPM as also reported earlier in many woody tree species including Swartzia medegascariaensis (Berger and Schaffner 1995) and Lagerstromia parviflora (Tiwari et al. 2002). In the present study, a strong influence of BA in shoot bud regeneration was observed. Role of BA for regeneration of shoot buds has also been reported earlier in many leguminous trees including Dalbergia sissoo (Mukhopadhayay and Mohanram Ram 1981), Albizzia lebbeck (Upadhayay and Chandra 1983), Leucaena leucocephala (Dhawan and Bhojwani 1985), and Prosopis laevegata (Gonzalez et al. 2007).
However a combination of BA and PAA was found crucial for direct shoot regeneration in P. dulce. The synergetic effect of auxin along with cytokinins on shoot bud induction and shoot multiplication has been reported by several workers (Chand and Singh 2004). BA (4.4 μM) along with PAA (0.73 μM) gave best response (96 %) as 6–8 healthy shoots were obtained which further increased in number up to 9.6 ± 0.2 by addition of adenine sulphate (49.45 μM). Adenine in the form of adenine sulphate can stimulate cell growth and greatly enhance shoot formation (Murashige 1974). It provides an available source of nitrogen to the cell which can generally be taken up more rapidly than the inorganic nitrogen. The promotive role of AdS in shoot induction and multiplication has been emphasized in different woody species namely Tectona grandis (Devi et al. 1994), and Bauhinia vahlii (Dhar and Upreti 1999). Subculture up to three passages was successful after that morphogenic capability of explant dwindled. Increase in shoot number may be due to suppression of apical dominance during subculture that induces basal dormant meristematic cells to form new shoots (Shukla et al. 2009).
For any micropropagation protocol, successful rooting of microshoots is a prerequisite to facilitate their establishment in soil. IBA is the most commonly used auxin for root formation from shoots of woody trees (Nandwani and Ramawat 1991; Krieken et al. 1993). In our study use of IBA or any other cytokinin alone in MS medium was insufficient for rooting, therefore, AC was added to the MS medium along with IBA for successful rooting. The maximum frequency of root formation (80 %) was obtained (~5.2 cm root length and 3.2 ± 0.2 root number) on medium containing 2.46 μM IBA and 2.46 μM AC. Use of AC has a remarkable positive influence on the rooting efficiency (Siddique and Anis 2009) and is also reported earlier in some woody species and pineapple (Firoozabady et al. 2006). Normally, activated charcoal is included in the rooting medium after auxin treatment (Dumas and Monteuuis 1995) but in the present study activated charcoal was used along with auxin without any pretreatment. Roots were produced without an intervening callus phase. The promotary effects of AC on morphogenesis may be mainly due to its irreversible adsorption of inhibitory compounds in the culture medium and substantially decreasing the toxic metabolites, phenolic exudation and accumulation of brown exudate (Thomas 2008).
True-to-type clonal fidelity is one of the most important prerequisites in the micropropagation of any plant species. A major problem encountered with the in vitro culture is the presence of somaclonal variation amongst sub-clones of one parental line, arising as a direct consequence of in vitro culture of plant cells, tissues or organs (Lakshmanan et al. 2007). A better analysis of genetic stability of plantlets can be made by using a combination of two types of markers which amplify different regions of the genome (Martins et al. 2004). In the present study, 20 RAPD and 14 ISSR markers were used. Amongst them 12 RAPD and 7 ISSR markers produced scorable bands which are monomorphic and similar to the mother plant confirming the genetic uniformity of regenerants. The study of genetic integrity in micropropagated plants by using different molecular markers has been reported earlier in many plant species (Rani and Raina 2000; Tyagi et al. 2010; Jain et al. 2011).
To conclude, this is the first report of organogenesis in Pithecellobium dulce (Roxb.) Benth to the best of our knowledge. The present study describes an efficient protocol for in vitro propagation of P. dulce and the culture system produced masses of plant material that would be suitable for commercial applications. The protocol developed here is simple and cost-effective and can be used for the sustainable supply of plant materials to the pharmaceutical industries and for conservation of elite germplasm. As a part of domestication strategy, these plants can be grown and further cultivated in fields. The application of this protocol can help to minimize the pressure on wild populations, contribute to the conservation of the valuable flora of Rajasthan, India and serve as the basis for the micropropagation of other leguminous tree species. The protocol could also be applied for the improvement of this medicinally and pharmaceutically important tree species by genetic engineering.
Acknowledgements
Pooja Goyal thanks Council for Scientific and Industrial Research (CSIR), New Delhi for the award of SRF. Dr. Arunima Sinha thanks University Grants Comission (UGC) for financial support in the form of R&D project 33-154/2007(SR).
Abbreviations
- AC
Activated charcoal
- AdS
Adenine sulphate
- BA
6-benzyladenine
- IAA
Indole-3-acetic acid
- IBA
Indole-3-butyric acid
- ISSR
Inter-simple sequence repeats
- Mo
Mother plant
- MS
Murashige and Skoog
- NAA
α-naphthaleneacetic acid
- PAA
Phenylacetic acid
- RAPD
Random amplified polymorphic DNA
- WPM
Woody plant medium
Contributor Information
Pooja Goyal, Email: poojagoyal16@gmail.com.
Sumita Kachhwaha, Email: Kachhwahasumita@rediffmail.com.
S. L. Kothari, Phone: +91-141-2703439, Email: slkothari@mailcity.com
References
- Barrera NL, Bautista BS, Bravo LL, Bermudez TK, Garcia SF, Jimenez EM, Reyes CR. Antifungal activity against postharvest fungi by extracts and compounds of Pithecellobium dulce seeds (huamuchil) Acta Hort. 2003;628:761–766. [Google Scholar]
- Berger K, Schaffner W. In vitro propagation of the leguminous tree Swartzia madegascariensis. Plant Cell Tiss Org Cult. 1995;40:289–291. doi: 10.1007/BF00048136. [DOI] [Google Scholar]
- Bhatt ID, Dhar U. Factors controlling micropropagation of Myrica esculenta buch-Ham. ExD. Don: a high value wild edible of Kumaun Himalaya. Afr J Biotech. 2004;3:534–540. [Google Scholar]
- Chand S, Singh AK. In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree Pterocarpus marsupium Roxb. In vitro Cell Dev Biol-Plant. 2004;40:167–170. doi: 10.1079/IVP2003488. [DOI] [Google Scholar]
- Devi SY, Mukherrjee BB, Gupta S. Rapid cloning of elite teak (Tectona grandis Linn.) by in vitro multiple shoot production. Indian J Exp Biol. 1994;32:668–671. [Google Scholar]
- Dhar U, Upreti J. In vitro regeneration of a mature leguminous liana (Bauhinia vahlii Wight and Arnott.) Plant Cell Rep. 1999;18:664–669. doi: 10.1007/s002990050639. [DOI] [Google Scholar]
- Dhawan V, Bhojwani SS. In vitro vegetative propagation of Leucaena leucocephala (Lam.) de wit. Plant Cell Report. 1985;4:315–318. doi: 10.1007/BF00269887. [DOI] [PubMed] [Google Scholar]
- Dumas E, Monteuuis O. In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Cell Tiss Org Cult. 1995;40:231–235. doi: 10.1007/BF00048128. [DOI] [Google Scholar]
- Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
- Feyissa T, Welander M, Negash L. Micropropagation of Hagenia abyssinica (Bruce) J.F. Gmel.: a multipurpose tree. Plant Cell Tiss Org Cult. 2005;80:119–127. doi: 10.1007/s11240-004-9157-1. [DOI] [Google Scholar]
- Firoozabady E, Heckert M, Gutterson N. Transformation and regeneration of pineapple. Plant Cell Tiss Org Cult. 2006;84:1–16. doi: 10.1007/s11240-005-1371-y. [DOI] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures in agricultural research. 2. NY: Wiley; 1984. [Google Scholar]
- Gonzalez B, Orozco-Villafuerte J, Cruz-Sosa F, Chavez-Avilla VM, Vernon-Carter EJ. Conal propagation of mesquite tree (Prosopis laevigata Humb. and Bonpl. Ex willd. MC Johnston). I. via cotyledonary nodes. In Vitro Cell Dev Biol Plant. 2007;43:260–266. doi: 10.1007/s11627-007-9027-8. [DOI] [Google Scholar]
- Husain MK, Anis M. Rapid in vitro propagation of Eclipta alba (L.) Hassk. through high frequency axillary shoot proliferation. Acta Physiol Plant. 2006;28:325–330. doi: 10.1007/s11738-006-0028-8. [DOI] [Google Scholar]
- Jain R, Sinha A, Jain D, Kachhwaha S, Kothari SL. Adventitious shoot regeneration and in vitro biosynthesis of steroidal lactones in Withania coagulans (Stocks) Dunal. Plant Cell Tiss Org Cult. 2011;105:135–140. doi: 10.1007/s11240-010-9840-3. [DOI] [Google Scholar]
- Khatra LM, Nasir MKA, Saleem R, Valhari MU. The fatty acid composition of Pithecellobium dulce seed oil. Pak J Sci Ind Res. 1994;37:216. [Google Scholar]
- Krieken WM, Breteler H, Visser MHM, Mavridon D. The role of conversion of IBA into IAA on root regeneration in apple: introduction of a test system. Plant Cell Rep. 1993;12:203–206. doi: 10.1007/BF00237054. [DOI] [PubMed] [Google Scholar]
- Lakshmanan V, Venkataramareddy SR, Neelwarne B. Molecular analysis of genetic stability in long term micropropagated shoots of banana using RAPD and ISSR markers. E J Biotechnol. 2007;10:1–8. [Google Scholar]
- Lloyd G, McCown B. Commercially feasible micropropagation of mountain laurel Kalmia latifolia by use of shoot tip culture. Proc Int Comb Plant Prop Soc. 1980;30:421–427. [Google Scholar]
- Manna P, Bhattacharyya S, Das J, Ghosh J, Sil PC (2011) Phytomedical role of Pithecellobium dulce against CCl4-mediated hepatic oxidative impairments and necrotic cell death. Evid based Comp Alt Med. doi:10.1093/ecam/neq065 [DOI] [PMC free article] [PubMed] [Retracted]
- Martins M, Sermento D, Oliveire MM. Genetic stability of micropropagated almond plantlets as assessed by RAPD and ISSR markers. Plant Cell Rep. 2004;23:492–496. doi: 10.1007/s00299-004-0870-3. [DOI] [PubMed] [Google Scholar]
- Mukhopadhayay A, Mohanram Ram HY. Regeneration of plantlets from excised roots of Dalbergia sissoo. Indian J Exp Biol. 1981;19:113–119. [Google Scholar]
- Murashige T. Plant propagation through tissue culture. Ann Rev Plant Physiol. 1974;25:135–166. doi: 10.1146/annurev.pp.25.060174.001031. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nandwani D, Ramawat KG. Callus culture and plantlets formation from nodal explant of Prosopis juliflora (Swartz) DC. Indian J Exp Biol. 1991;29:523–527. [Google Scholar]
- Pithayanukul P, Ruenraroengsak P, Bavovada R, Pakmanee N, Suttisri R, Saenoon S. Inhibition of Naja kaouthia venom activities by plant polyphenols. J Ethano Pharmacol. 2005;97:527–533. doi: 10.1016/j.jep.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Porebski S, Bailey LG, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharides and polyphenol component. Plant Mol Biol Reporter. 1997;15:8–15. doi: 10.1007/BF02772108. [DOI] [Google Scholar]
- Pradhan C, Kar S, Pattnaik S, Chand PK. Propagation of Dalbergia sissoo Roxb. through in vitro shoot proliferation from cotyledonary nodes. Plant Cell Rep. 1998;18:122–126. doi: 10.1007/s002990050543. [DOI] [Google Scholar]
- Rajasab AH, Isaq M. Documentation of folk knowledge on edible wild plants of north Karnataka. Indian J Trad Knowled. 2004;4:419–429. [Google Scholar]
- Rani V, Raina SN. Genetic fidelity of organized meristem derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant. 2000;36:319–330. doi: 10.1007/s11627-000-0059-6. [DOI] [Google Scholar]
- Shukla S, Shukla SK, Mishra SK. In vitro plant regeneration from seedling explants of Stereospermum personatum DC: a medicinal tree. Trees. 2009;23:409–413. doi: 10.1007/s00468-008-0290-z. [DOI] [Google Scholar]
- Siddique I, Anis M. Direct plant regeneration from nodal explants of Balanites aegyptiaca L. (Del.): a valuable medicinal tree. New forest. 2009;37:53–62. doi: 10.1007/s11056-008-9110-y. [DOI] [Google Scholar]
- Sivakumar A, Murgesan M. Ethnabotanical studies on the wild edible plants used by the tribals of Anaimalai hills, the western ghats. Anc Sci Life. 2005;30:69–73. [PMC free article] [PubMed] [Google Scholar]
- Thomas TD. The role of activated charcoal in plant tissue culture. Biotechnol Adv. 2008;26:618–631. doi: 10.1016/j.biotechadv.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Tiwari SK, Kashyap MK, Ujjaini MM, Agrawal AP. In vitro propagation of Lagerstroemia parviflora Roxb. from adult tree. Indian J Exp Biol. 2002;40:212–215. [PubMed] [Google Scholar]
- Tyagi P, Khanduja S, Kothari SL. In vitro culture of Capparis decidua and assessment of clonal fidelity of the regenerated plants. Biol Plant. 2010;541:126–130. doi: 10.1007/s10535-010-0019-x. [DOI] [Google Scholar]
- Upadhayay S, Chandra N. Shoot and plantlets formation in organ and callus cultures of Albizzia lebbeck Benth. Ann Bot. 1983;52:421–424. [Google Scholar]
- Valverde-Cerdas L, Dufour M, Villalobos VM. In vitro propagation of Pithecellobium saman (Rain tree) In Vitro Cell Dev Biol Plant. 1997;33:38–42. doi: 10.1007/s11627-997-0038-2. [DOI] [Google Scholar]
- Vengadesan G, Ganapathi A, Prem Anand R, Anbazhagan RV. In vitro propagation of Acacia sinuata (Lour.) Merr. via cotyledonary nodes. Agrofor Syst. 2002;55:9–15. doi: 10.1023/A:1020269022363. [DOI] [Google Scholar]