Abstract
Coelogyne nervosa is an epiphytic orchid endemic to Western Ghats, South India. The mature seeds of C. nervosa were cultured on ½ MS (Murashige and Skoog), MS, Kn (Knudson) and VW (Vacin and Went) media to evaluate the seed germination response. Of the four basal media used, MS medium supported maximum seed germination. Further experiments to enhance seed germination were done on MS medium supplemented with various concentrations (10, 20, 30 and 40 %) of coconut water (CW). Thirty percent CW gave the highest response in terms of percent seed germination (96), fresh weight (7.2 mg/seedling) and protocorm length (15.2 mm). Since CW containing medium did not support further seedling growth, each seedling was isolated and cultured on MS medium supplemented with either BA (6-benzylaminopurine) or Kin (kinetin) alone (1.0–4.0 mg/l each) or in combination with NAA (1-naphthaleneacetic acid; 0.2–1.0 mg/l). Maximum growth was observed on MS medium supplemented with BA (3.0 mg/l) and NAA (0.5 mg/l). On this medium, the seedlings reached an average length of 3.6 cm with 2.8 well expanded green leaves per seedling. Similarly optimum, healthy, white root induction (3.3 roots/seedlings) was also observed on the same medium. The rooted seedlings were successfully transplanted to pots with 91 % success. The 2-year-old tissue culture derived plants produced normal flowers and fruits.
Keywords: Coelogyne nervosa, Epiphytic orchid, Micropropagation, Seedlings, Tissue culture
Introduction
Orchidaceae is one of the largest and most diverse families of the flowering plants, consisting of about 35,000 species under 800 genera (Singh et al. 2007). Nearly 1,300 species are estimated to occur in India (Kumar and Sasidharan 1985). The orchids constitute an advanced and taxonomically complex group of plants, highly specialized in many ways. They are well known for their exquisite and perpetual flowers which made them doyen among ornamentals. Recently, there is an increase in world floriculture trade and orchids constitute the second most popular cut flowers as well as potted floriculture crop with wholesale prices estimated at $126 million (Anonymous 2008). Majority of orchids have become an object of concern to conservationists due to its high sensitivity to alterations in its environment (Rasmussen 1995). Since orchids are horticulturally important, it is collected indiscriminately from nature coupled with continuous habitat destruction for land reclamation, unauthorized trade and collection of orchids by orchid lovers, majority of rare and precious orchid species in nature are disappearing at an alarming rate.
As orchid seeds do not possess endosperm, their natural germination is limited and need a symbiotic association with specific mycorrhizal fungus. Orchids also propagate vegetatively. But the conventional methods of propagation of orchids are very slow and laborious. For these reasons the price of orchids is very high.
To save the diverse orchid species from extinction, in vitro mass propagation technique is being utilized to raise plants by growing them in nurseries (Hey and Hey 1966). Ever since the development of a protocol for asymbiotic seed germination of orchids by Knudson (1922), asymbiotic seed germination has become an important and favored method for orchid propagation and this method has been routinely used by many researchers for developing individual nutrient formulations for seed germination and mass propagation of different terrestrial as well as epiphytic orchid species (Yamazaki and Miyoshi 2006; Stewart and Kane 2006). The use of exogenous growth hormones stimulates the zygotic embryo to initiate protocorms that develop into plantlets (Pant and Gurung 2005). Furthermore, high demand from commercial sector has undoubtedly led to an increased emphasis on in vitro mass propagation and conservation of important orchid species (Stenberg and Kane 1998). Major problems associated with micropropagation of economically valuable orchids for commercial purposes include the lack of effective and reliable protocols for seed germination, obligate mycorrhizal association for natural seed germination and high mortality rate of seedlings during the early stages of culture.
C. nervosa is a threatened endemic orchid from Southern India which grows as an epiphyte and is important for its beautiful flowers (Khasim and Ramudu 2011). The plant has a pseudobulb and the terminal inflorescence originates from the top of it. The inflorescence droops with weight of the flowers and flowers are 3 cm across. Flower lip is white outside and marked with veins of mustard colour inside (Abraham and Vatsala 1981). There is no previous report on the micropropagation of C. nervosa. However, micropropagation of two related species C. cristata and C. mossiae was reported (Naing et al. 2011; Sebastianraj et al. 2006). The uncontrolled collection of this orchid from wild and habitat destruction made this plant to disappear from natural habitat. Therefore, it is necessary to develop a micropropagation protocol for this commercially valuable orchid. Hence, the present study was undertaken with a view to establish an efficient protocol for mass propagation, study the effects of various media, coconut water and plant growth regulators (PGRs) on seed germination and growth of seedlings in Coelogyne nervosa, a floriculturally important endemic orchid.
Materials and methods
Plant material and sterilization
C. nervosa is an epiphytic orchid bearing large white flowers which is endemic to South India. The plant flowers in August and September. The mature green capsules (about 210 days-old) post pollination have a size of about 3.5–5 cm long and 0.5 cm width. Such capsules collected from healthy individual plant were used for seed culture experiments. After collection, the fruits were stored in a small bottle containing silica gel and immediately brought to lab for in vitro culture experiments. The capsules were washed in tap water and then dipped in sodium hypochlorite (NaOCl, 0.6 % w/v) solution for 10 min. The capsules were then submerged in 95 % ethanol and flamed rapidly for few seconds followed by washing in autoclaved distilled water.
Culture initiation and media
Half strength MS (Murashige and Skoog 1962), MS, KC (Knudson 1922) and VW (Vacin and Went 1949) media were used for preliminary experiment to evaluate suitable medium for seed germination. After preliminary experiment, MS medium was found most suitable and was used for all further experiments. The surface sterilized capsules were then splitted longitudinally with a sterile scalpel and the seeds were scooped out from sterilized capsules and placed as a thin layer over the surface of the culture medium in glass test tubes with the help of a sterilized forceps. For getting maximum seed germination, MS medium was supplemented with four different concentrations (10, 20, 30 and 40 %) of coconut water (CW). The seedling elongation and further growth was obtained on MS medium supplemented with various concentrations (1.0–4.0 mg/l) of BA (6-benzylaminopurine) or Kin (kinetin) alone or in combination with NAA (1-naphthaleneacetic acid; 0.2–1.0 mg/l).
Culture conditions
The pH of all media was adjusted to 5.8 with 0.1 N NaOH or HCl before autoclaving at 120 °C and 105 kPa for 15 min. The culture tubes were capped with cotton plugs before autoclaving. The average number of green healthy seedlings, fresh weight of seedling (in mg), and average number of green and white protocorm-like bodies (PLB) developed per treatment were scored at appropriate culture period at 25 ± 2 °C under cool white fluorescent light at 40 μmol m−2 s−1 with a 16-h photoperiod per day. Each treatment consisted of at least 12 cultures and all experiments were repeated three times. Analysis of variance and Duncan’s multiple range test were used for comparison among treatment means (Duncan 1955).
Transplantation
Well rooted fully developed plantlets were removed from culture tubes, washed thoroughly in running tap water to remove agar, and transplanted to pots (15 cm diameter) containing sand, brick and soil (4:4:2) as substrates. The pots were initially covered with a plastic bag to maintain high humidity for about 20 days. The pots were incubated at 25 ± 2 °C under cool white fluorescent light (35 μmol m−2 s−1) with a 16-h photoperiod. Pots were maintained under 30–40 % natural light for 3 months, sprayed with water twice a day. Plant survival was determined by growth and development of normal seedlings into plants, which were transplanted to greenhouse. The acclimatized plants were eventually transferred to their natural habitat.
Results and discussion
In the present study, we used four media (½ MS, MS, KC and VW) to evaluate the seed germination capacity. Of the four media, full strength MS medium was found most effective for seed germination as compared to other media (Table 1). On MS basal medium, an average number of 49 seeds germinated as compared to 34, 31 and 36 on ½ MS, KC and VW basal media respectively. Similarly, days taken for seed germination, protocorm formation and plantlet development were also evaluated. On MS medium, an average number of 67, 44, 75 days were taken for seed germination, protocorm formation and plantlet development respectively. Whereas in KC to medium, 67, 68, 98 days were taken for seed germination, protocorm formation and plantlet development respectively (Table 1). Based on data, it was clear that full strength MS medium produced optimum response in C. nervosa as compared to ½ MS, Kn and VW media hence, MS medium was employed for all further experiments. Several researchers investigated the effect of various media on orchid seed germination. Based on previous reports it is clear that various orchid systems responded variously to different media. In Vanda teres, of the three media tried for seed germination, optimum response was observed on VW medium followed by MS and KN medium (Sinha and Roy 2004). In Geodorum densiflorum, MS and Phytamax (PM) media were tried for seed germination and MS medium produced optimum response as compared to PM medium. The authors concluded that the difference in response of MS and PM media was mainly due to the difference in the nutrient content (Bhadra and Hossain 2003). In Cymbidium findlaysonianum, of the three media (MS, ½ MS and VW) used for protocorm induction from seeds, MS medium produced optimum protocorm induction when used individually. However, a combination of MS and VW medium produced highest response (Tawaro et al. 2008).
Table 1.
Effect of various culture media on seed germination in C. nervosa
| Media | Average number of green seedlings per tubea | Days taken for | ||
|---|---|---|---|---|
| Seed germinationa | Protocorm formationa | Plantlet developmenta | ||
| ½ MS | 34 ± 4.3b | 78 ± 2.5a | 62 ± 6.5a | 82 ± 4.9b |
| MS | 49 ± 3.2a | 67 ± 4.3b | 44 ± 7.8b | 75 ± 6.2c |
| KC | 31 ± 5.3b | 71 ± 6.7a | 68 ± 6.7a | 98 ± 6.9a |
| VW | 36 ± 6.4b | 79 ± 5.9a | 66 ± 7.1a | 92 ± 7.3a |
aThe values represent the means (±SE) of three independent experiments. At least 12 cultures were raised for each experiment. Mean values within a column followed by the same letter are not significantly different by Duncan’s multiple range test (P ≥ 0.05)
Although optimum seed germination was observed on MS basal medium in the present study (Table 1), the percent seed germination was poor (Table 2). Hence various concentrations of coconut water (CW) was added to MS medium (10, 20, 30 and 40 %) to improve the response further. The addition of CW further enhanced the response both in terms of percent seed germination and days taken for development. The observations were taken in two stages i.e. 60 days and 100 days after seed culture. On MS basal medium only about 35% and 41 % seeds germinated 60 and 100 days after culture re-spectively, Whereas it was enhanced to 92% and 96 % in 30 % CW after 60 and 100 days of culture respectively (Table 2). The same medium supported highest average fresh weight (2.8 and 7.2 mg, 60 and 100 days after culture respectively) per protocorm and optimum proto- corm length of 4.5 mm and 15.2 mm after 60 and 100 days of culture respectively (Table 2). The same medium supported highest average fresh weight (2.8 and 7.2 mg, 60 and 100 days after culture respectively) per a single protocorm and optimum protocorm length of 4.5 mm and 15.2 mm after 60 and 100 days of culture respectively (Table 2). In orchid seed germination depends not only on the type of medium employed but also on the natural additives like CW, banana extract, pineapple juice and boiled potato present in the medium. CW has also been reported to play a critical role in seed germination in Rhynchostylis retusa. Of the 4 different concentrations (5, 10, 15 and 20 %) used, 15 % gave optimum response with 93 % seed germination (Thomas and Michael 2007). Similarly, CW has been reported to induce seed germination, protocorm and callus regeneration in several orchid species (Kitsaki et al. 2004; Roy and Banerjee 2003; Sheelavanthmath et al. 2005).
Table 2.
Influence of different concentrations of coconut water on development of protocorm like bodies (PLBs) from seed cultures of C. nervosa 60 days and 100 days after culture. Medium: MS
| CW Concentration (%) | % seed germination | Fresh weight of protocorm (mg) | Protocorm length (mm) | |||
|---|---|---|---|---|---|---|
| 60 days | 100 days | 60 daysa | 100 daysa | 60 daysa | 100 daysa | |
| 0.0 | 35 | 41 | 0.9 ± 0.3c | 4.3 ± 0.8c | 1.9 ± 0.5c | 9.2 ± 1.1c |
| 10 | 48 | 53 | 1.3 ± 0.4c | 5.4 ± 0.7b | 3.1 ± 0.4b | 10.9 ± 0.9b |
| 20 | 67 | 69 | 1.6 ± 0.5c | 5.8 ± 0.9b | 3.4 ± 0.6b | 12.3 ± 1.4b |
| 30 | 92 | 96 | 2.8 ± 0.4a | 7.2 ± 0.7a | 4.5 ± 0.5a | 15.2 ± 1.0a |
| 40 | 78 | 81 | 1.9 ± 0.5b | 5.9 ± 0.8b | 3.8 ± 0.6b | 13.4 ± 1.6b |
aThe values represent the means (±SE) of three independent experiments. At least 12 cultures were raised for each experiment. Mean values within a column followed by the same letter are not significantly different by Duncan’s multiple range test (P ≥ 0.05)
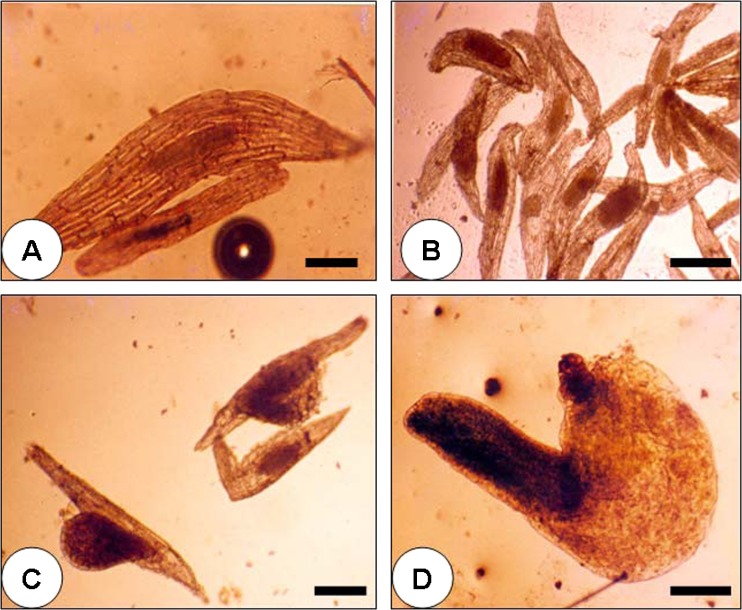
The cultured seeds puffed up slowly during the initial stages of development. Microscopic observation showed that the seeds contained an oval shaped embryo inside it at the time of culture and this shape was retained for about 1 week of culture (Fig. 1a and b). After about 2 weeks the shape of the embryo gradually changed to globose. This is followed by enlargement of the embryo after absorbing nourishment from medium which resulted in the rupturing of thin layer of seed coat by the globular shaped embryo (Fig. 1c). A close observation on the upper side of the globular embryo showed cells with rapid mitotic activity. Further, the protocorm elongated and developed a papilla like projection at the upper side that can be called as the first prophyll (Fig. 1d). After breaking the seed coat the globular embryo enlarged and elongated further in about 60 days. At this stage the embryo was visible to the naked eye as small green propagule (Fig. 2a). In about 20 days, the green seedlings elongated further and small leaves were initiated from it (Fig. 2b). The further development of seedlings to a length of about 15.2 mm with elongated green leaves was observed in another 20 days (Fig. 2c).
Fig. 1.
Initial stages of seed germination in C. nervosa. a An enlarged view of a seed at the time of culture. A small sized embryo is present in the seed. b The seeds 1 week after culture on MS medium supplemented with 30 % CW. The embryo inside the seed enlarged further. c The seeds 3 weeks after culture on MS medium supplemented with 30 % CW. The seed coat has ruptured and the globular shaped embryo came out of the seed. d The elongated embryo 5 weeks after culture. Scale bar = 0.1 mm (a–d)
Fig. 2.
Seedling development, isolation and field transfer of C. nervosa. a Small green globular shaped propagules appeared 60 days after culture. Scale bar = 12 mm. b Green seedlings with initiated leaves 80 days after culture on MS medium supplemented with 30 % CW. Scale bar = 18 mm. c Same as in Fig. 2b, 100 days after culture. The seedlings have attained more size. Scale bar = 10 mm. d An isolated single seedling on MS medium supplemented with BA (3 mg/l) and NAA, 0.5 mg/l). Scale bar = 0.8 cm. e An in vitro seedling derived plant about 2 years after field transfer. The plant has two flowers on it. Scale bar = 4.0 cm
The seedlings developed on MS medium supplemented with CW reached a length ranging from 9.2 mm to 15.2 mm in 100 days (Table 2). However, further growth and root initiation was not observed after prolonged incubation on the same medium. Therefore, MS medium supplemented with either a cytokinin alone (BA or Kin in the range of 1–4 mg/l) or in combination with 0.2–1.0 mg/l NAA was used for further development of seedlings. Of the two cytokinins used, BA was comparatively better than Kin in terms of seedling length. However, the number of leaves did not show any significant difference on both cytokinin containing media. Optimum growth was observed on MS medium supplemented with 3 mg/l BA when used individually. Here the seedlings reached an average height of 1.8 cm with 1.6 leaves in about 90 days (Table 3). Similarly Kin at 2.0 mg/l produced optimum response of 1.8 cm seedling height with 1.4 leaves. There was no root development when cytokinin was used individually. The addition of NAA (0.2–1.0 mg/l) along with BA (3 mg/l) and Kin (2.0 mg/l) significantly promoted the seedling growth and development. Highest response was observed on MS medium supplemented with 3.0 mg/l BA and 0.5 mg/l NAA. On this medium, the seedlings showed prolific growth and developed to a size of 3.6 cm with 2.8 leaves per seedling in 90 days (Fig. 2d). Moreover, the synergistic action of auxin-cytokinin combination induced roots which were not observed when cytokinin alone was used. The optimum rooting response was observed on MS medium supplemented with 3.0 mg/l BA and 0.5 mg/l NAA. Here an average number of 3.3 roots were observed per seedling (Table 3; Fig. 2d). Cytokinins either alone or in combination with an auxin is routinely employed for orchid propagation from various explants (Chugh et al. 2009). For maximum seedling growth in Rhynchostylis retusa, a combination of BA and NAA was sufficient. The individual seedlings reached a size of about 2.3 cm in about 1 month on this medium (Thomas and Michael 2007). The role of BA either alone or in combination with NAA in inducing seedling growth was reported in several orchid species like Grammatophylum speciosum (Khampa et al. 2010), Cymbidium elegans (Pradhan and Pant 2009), Epidendrum ibaguense (Hossain 2008). The prolifically growing seedlings with well developed long roots when transplanted to pots containing sand, brick and soil (4:4:2) acclimatized well. Of the 56 plants transplanted to pots, 51 (91 %) survived. The two-year-old tissue culture derived transplanted plants produced normal flowers (Fig. 2e).
Table 3.
Effect of various plant growth regulators on seedling development in C. nervosa. Culture medium: MS, Culture period: 90 days
| Plant growth regulators (mg/l) | Seedling length (cm)a | Leaf numbera | Root numbera | ||
|---|---|---|---|---|---|
| BA | Kn | NAA | |||
| 0.0 | 0.0 | 0.0 | 0.7 ± 0.2d | 1.2 ± 0.2b | 0.0 |
| 1.0 | – | – | 1.3 ± 0.4c | 1.4 ± 0.4b | 0.0 |
| 2.0 | – | – | 1.6 ± 0.5c | 1.5 ± 0.5b | 0.0 |
| 3.0 | – | – | 1.8 ± 0.4c | 1.6 ± 0.3b | 0.0 |
| 4.0 | – | – | 1.5 ± 0.6c | 1.6 ± 0.4b | 0.0 |
| 1.0 | – | 0.8 ± 0.4d | 1.4 ± 0.3b | 0.0 | |
| 2.0 | – | 1.0 ± 0.3d | 1.4 ± 0.6b | 0.0 | |
| 3.0 | – | 1.3 ± 0.4c | 1.6 ± 0.5b | 0.0 | |
| 4.0 | – | 1.6 ± 0.5c | 1.5 ± 0.7b | 0.0 | |
| 3.0 | 0.2 | 2.3 ± 0.5b | 2.4 ± 0.6a | 1.9 ± 0.6c | |
| 3.0 | 0.5 | 3.6 ± 0.4a | 2.8 ± 0.5a | 3.3 ± 0.5a | |
| 3.0 | 1.0 | 2.2 ± 0.3b | 2.6 ± 0.6a | 2.1 ± 0.6b | |
| 2.0 | 0.2 | 1.4 ± 0.5c | 1.6 ± 0.5b | 1.1 ± 0.4c | |
| 2.0 | 0.5 | 1.7 ± 0.4c | 1.9 ± 0.6b | 1.2 ± 0.6c | |
| 2.0 | 1.0 | 1.8 ± 0.6c | 1.7 ± 0.6b | 1.6 ± 0.5c | |
aThe values represent the means (±SE) of three independent experiments. At least 12 cultures were raised for each experiment. Mean values within a column followed by the same letter are not significantly different by Duncan’s multiple range test (P ≥ 0.05)
In conclusion, an efficient protocol for the micropropagation of C. nervosa has been developed. The procedure described here is relatively simple and reliable where the seeds germinated and seedlings developed into normal and healthy plants. Our results indicated that MS medium is best for seed germination and CW plays a crucial role in promoting seedling growth. Further synergistic action of BA and NAA promoted individual seedling elongation and root formation. Plant tissue culture technique described here is an ideal tool for rapid mass propagation and conservation of this rare and threatened orchid.
Acknowledgements
TDT acknowledges the financial assistance from UGC in the form of a major research project (Project no. 38-233/2009).
Abbreviations
- BA
6-benzylaminopurine
- CW
Coconut water
- KN
Knudson medium
- Kin
Kinetin
- MS
Murashige and Skoog medium
- NAA
1-naphthalene acetic acid
- PGR
Plant growth regulator
- VW
Vacin and Went medium
References
- Abraham A, Vatsala P (1981) Introduction to orchids with illustrations and descriptions of 150 South Indian orchids. Tropical botanical graden and research Institute, Palode, Thiruvanthapuram, Kerala, India, pp 285–287
- Anonymous (2008) US department of agriculture. Floriculture crops 2007 summary. Agr Stat Board, Washington, DC
- Bhadra SK, Hossain MM. In vitro germination and micropropagation of Geodorum densiflorum (Lam.) Schltr., an endangered orchid species. Plant Tissue Cult. 2003;13:65–171. [Google Scholar]
- Chugh S, Guha S, Rao UI. Micropropagation of orchids: a review on the potential of different explants. Sci Hortic. 2009;122:507–520. doi: 10.1016/j.scienta.2009.07.016. [DOI] [Google Scholar]
- Duncan DB. Multiple range and multiple F test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Hey GL, Hey MG (1966) Raising rare orchids from seeds. In: De Garmo LR (ed) Proc. 5th World Orchid Conf, Long Beach, USA, pp 35–38
- Hossain MM. Asymbiotic seed germination and in vitro seedling development of Epidendrum ibaguense Kunth. (Orchidaceae) Afr J Biotechnol. 2008;7:3614–3619. [Google Scholar]
- Khampa S, Wangsomnuk P, Wangsomnuk P. Factors affecting seed germination of Grammatophylum speciosum cultured in vitro. AsPac J Mol Biol Biotechnol. 2010;18:193–197. [Google Scholar]
- Khasim SM, Ramudu J (2011) Genetic diversity in Coelogyne nervosa R. Rich., an endemic orchid from Southern India. In: Plant Canada, 2011, Proceedings of the joint meeting of the Canadian society of agronomy, Canadian society of horticultural science, Canadian society of plant physiologists, Canadian botanical association, Canadian phytopathological society, Canadian weed science society, Saint Mary’s University, Halifax nova scotia agricultural college, Truro, pp 106
- Kitsaki CK, Zygouraki S, Ziobora M, Kintzios S. In vitro germination, protocorm formation and plantlet development of mature versus immature seeds from several Ophrys species (Orchidaceae) Plant Cell Rep. 2004;23:284–290. doi: 10.1007/s00299-004-0841-8. [DOI] [PubMed] [Google Scholar]
- Knudson L. Non-symbiotic germination of orchid seeds. Bot Gaz. 1922;73:1–25. doi: 10.1086/332956. [DOI] [Google Scholar]
- Kumar M, Sasidharan S. Orchids of Kerala and their conservation. In: Vij SP, editor. Biology, conservation and culture of orchids. New Delhi: Affiliated East—West Press; 1985. pp. 267–272. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Naing AH, Chung JD, Park IS, Lim KB. Efficient plant regeneration of the endangered medicinal orchid. Coelogyne cristata using protocorm-like bodies. Acta Physiol Plant. 2011;33:659–666. doi: 10.1007/s11738-010-0586-7. [DOI] [Google Scholar]
- Pant B, Gurung R. In vitro seed germination and seedling development in Aerides odorata Lour. J Orchid Soc Ind. 2005;19:51–55. [Google Scholar]
- Pradhan S, Pant B. In vitro seed germination in Cymbidium elegans Lindl. and Dendrobium densiflorum Lindl. ex Wall. (Orchidaceae) Botanica Orien. 2009;6:100–102. [Google Scholar]
- Rasmussen HN. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Roy J, Banerjee N. Induction of callus and plant regeneration from shoot tip explants of Dendrobium fimbriatum Lindl. var. oculatum Hk.f. Sci Hortic. 2003;97:333–340. doi: 10.1016/S0304-4238(02)00156-5. [DOI] [Google Scholar]
- Sebastianraj J, Britto JS, Robinson PJ, Kumar VD, Kumar SS. In vitro seed germination and plantlet regeneration of Coelogyne mossiae Rolfe. J Biol Res. 2006;5:79–84. [Google Scholar]
- Sheelavanthmath SS, Murthy HN, Hema BP, Hahn EJ, Paek KY. High frequency of protocorm like bodies (PLBs) induction and plant regeneration from protocorm and leaf sections of Aerides crispum. Sci Hortic. 2005;106:395–401. doi: 10.1016/j.scienta.2005.04.012. [DOI] [Google Scholar]
- Singh MK, Sherpa AR, Hallan V, Zaidi AA. A poty virus in Cymbidium spp. in northern India. Aust Plant Dis Notes. 2007;2:11–13. doi: 10.1071/DN07005. [DOI] [Google Scholar]
- Sinha P, Roy SK. Regeneration of an indigenous orchid, Vanda teres (Roxb.) Lindl. through in vitro culture. Plant Tissue Cult. 2004;14:55–61. [Google Scholar]
- Stenberg ML, Kane ME. In vitro seed germination and greenhouse cultivation of Encyclia boothiana var. erythronioides, an endangered Florida Orchid. Lindleyana. 1998;13:101–112. [Google Scholar]
- Stewart SL, Kane ME. Asymbiotic seed germination and in vitro seedling development of Habenaria macroceratitis (Orchidaceae), a rare Florida terrestrial orchid. Plant Cell Tiss Org Cult. 2006;86:147–158. doi: 10.1007/s11240-006-9098-y. [DOI] [Google Scholar]
- Tawaro S, Suraninpong P, Chanprame S. Germination and regeneration of Cymbidium findlaysonianum Lindl. on a medium supplemented with some organic sources. Walailak J Sci Technol. 2008;5:125–135. [Google Scholar]
- Thomas TD, Michael A. High frequency plantlet regeneration and multiple shoot formation from cultured immature seeds of Rhynchostylis retusa Blume., an exquisite orchid. Plant Biotech Rep. 2007;1:243–249. doi: 10.1007/s11816-007-0038-z. [DOI] [Google Scholar]
- Vacin E, Went FW. Some pH changes in nutrient solutions. Bot Gaz. 1949;110:605–613. doi: 10.1086/335561. [DOI] [Google Scholar]
- Yamazaki J, Miyoshi K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae) Ann Bot. 2006;98:1197–1206. doi: 10.1093/aob/mcl223. [DOI] [PMC free article] [PubMed] [Google Scholar]