Abstract
The objectives of the present work were in vitro propagation of Araucaria excelsa R. Br. var. glauca Carrière (Norfolk Island pine) with focus on the evaluation of the mean number of shoots per explant (MNS/E) and mean length of shoots per explants (MLS/E) produced by different parts of the orthotropic stem of A. excelsa R. Br. var. glauca in response to plant growth regulators. Norfolk Island pine axillary meristems responded very well to the 2-iso-pentenyl adenine (2iP) and thidiazuron (TDZ) levels. Explants taken from stem upper segments in the media containing 2iP had a higher MNS/E (3.47) and MLS/E (6.27 mm) in comparison to those taken from stem lower segments, which were 0.71 and 0.51 mm, respectively. Using 0.045 μM TDZ in the MS medium not only resulted in 4.60 MNS/E with 7.08 mm MLS/E but proliferated shoots showed a good performance as well. Investigating the best position of stem explant on mother plant as well as the best concentrations of growth regulators were performed which were useful for efficient micropropagation of this plant. Thirty three percent of explants were rooted in the MS medium containing 3 % sucrose, supplemented with 7.5 μM of both NAA and IBA for 2 weeks before transferring to a half strength MS medium without any growth regulator. Plantlets obtained were acclimatized and transferred to the greenhouse with less than 20 % mortality. This procedure considered the first successful report for regeneration and acclimatization of A. excelsa R. Br. var. glauca plantlet through main stem explants.
Keywords: Abnormality, Araucaria excelsa R. Br. var. glauca, Axillary meristems, Orthotropic stems, Proliferation, Topophysis
Introduction
Woody plant species comprise nearly 50 % of the earth biodiversity and have a major role in production of biomass and providing human oxygen. There is an immense need to protect tree ecosystems for both their ecological and aesthetic values. However, today research progress in forest trees has been hindered by their long life cycles, self-incompatibility, high heterozygosity and large genome. Progress has been made in forest tree breeding and genomics, for example; sequencing of Picea glauca and Pinus taeda genomes are in progress which would be highly valuable for breeding and biotechnological approaches (Campbell et al. 2003; Neale and Kremer 2011). Plant tissue culture and genetic transformation methods offer an important option for effective multiplication and improvement of trees within a limited time frame. Generally, regeneration of woody trees under in vitro conditions is difficult (Giri et al. 2004). As in other species of the Araucariaceae family, Araucaria excelsa R. Br. var. glauca has a highly determined orthotropic and plagiotropic shoot growths and is well known as a good specimen with symmetrical branches. Its common name, Norfolk Island pine implies, the tree is endemic to Norfolk Island, a small island in the Pacific Ocean. In genus Araucaria, the conventional propagation methods are slow in most species. Vegetative propagation by cutting is difficult due to topophysis and being difficult-to-root. Incompatibility is also a problem when grafting is used (Hanes and de Fossard 1977; Sarmast et al. 2009). In some species of Araucaria, propagation by seed is limited and not only depends on its recalcitrant behavior but is insufficient to produce large uniform progenies as well (Hartmann et al. 2011). Use of embryonic explants has been common in gymnosperm micropropagation. Recent reports on the Araucaria species focused on Araucaria angustifolia (Bert.) O. Kuntze specially via somatic embryogenesis (Astarita et al. 2003; Steiner et al. 2007). First report on in vitro culture of the Araucariaceae family using 4 mm pieces of orthotropic stem cultured in the medium supplemented with the mean level of each of the vitamins, minerals and sucrose, that had a highly significant effect on the proliferation rate of the 18 month old Araucaria cunninghamii Ait. was based on the work of Hanes and de Fossard (1977). Another work carried out on Araucaria cunninghamii Ait, using the main stem of 2 years old segments and their coppice shoots that were collected from the stumps of 20 years old trees (Burrows et al. 1988). Their experiment obviously indicated that 0.001–0.1 μM of BAP is unlikely to influence bud formation and development. However 1.0 μM BAP or 2iP not only bring about formation of distorted buds but concentration of 10 μM completely ceased bud formation. Although they did not mention the proliferation rate but they indicated up to 80 % rooting in MS modified medium supplemented with 10 μM IBA, with less than 5 % mortality after transferring the plantlet to the field. Sehgal et al. (1989) indicated that explants taken from secondary and tertiary branches of Araucaria columnaris Hook. in MS media supplemented with different concentration of BAP, Kin and thiourea showed normal orthotropic growth in culture media, with no report on rooting. In most reports on Araucaria species the upper parts of the main stem were used to micropropagate, except in Sehgal et al. (1989) that used plagiotropic stems of A. columnaris Hook. To our best knowledge, there is no successful report on the micropropagation of Araucaria excelsa R. Br. var. glauca. The aim of the present work was in vitro propagation of Araucaria excelsa R. Br. var. glauca with emphasis on the effects of the 2iP and TDZ on the proliferation rate of this species.
Materials and methods
Plant materials
Three years old same-sized seedlings of A. excelsa R. Br. var. glauca were purchased from a commercial grower in Mazandran province in the North of Iran, and were transferred to the greenhouse of Department of Horticultural Science, College of Agriculture, Shiraz University, Shiraz, Iran.
Disinfection
Orthotropic stems of approximately 17 cm long were removed from the mother plants and after removing the apical bud, were divided into three parts (including upper, middle and lower segments of semihardwood orthotropic stems each 5 cm long) and were kept under running tap-water for 2 h. Because of high contamination, nano silver was used to eradicate the internal bacteria at concentration of 500 μg/ml under reduced pressure (300 mm Hg in 5 min) (Sarmast et al. 2011). Then the explants were treated with 70 % ethanol for 3 min and 15 % Clorox (containing 5.25 % sodium hypochlorite) with 0.2 % detergent for at least 10–20 min for surface sterilization, and then rinsed six times with sterilized distilled water. After surface sterilization, each segment (upper, middle and lower) was cut into 8–10 mm long pieces and put vertically on the MS (Murashige and Skoog 1962) basal medium with 3.0 % sucrose and 0.8 % agar supplemented with different concentrations of plant growth regulators and activated charcoal 1 %.
Plant growth regulator treatments
Various concentrations of 2iP (0.00, 0.10, 0.20, 0.30, 0.40, 0.50, 0.75, 1.00 and 1.25 μM) were evaluated for rapid multiple shoot production from upper, middle and lower segment of semihardwood orthotropic stems. In the other experiment, TDZ (0.00, 0.0045, 0.045, 0.45 and 4.50 μM) and 2iP (0.00–1.25 μM) were used only for upper segments of A. excelsa R. Br. main stems. The pH of the media was adjusted to 5.8 by 0.1 N HCl before autoclaving for 15 min at 121 °C. Cultures were kept at 25 °C under cool- white fluorescent light (30 μmol m−2 s−1) with 16/8 h day/night photoperiod.
Rooting of in vitro-derived shoots
Propagated shoots were kept under dark conditions for approximately 7 days and then were cultured on the MS liquid or solid medium containing 7.5 μM IBA and/or NAA for 2 weeks before moving to the half-strength MS medium supplemented with 0.0, 0.3 to 1 % AC. Some shoots with 1.5 cm length were quick-dipped (3–5 s) in IBA solutions with different concentrations (0, 10, 20, 30, 40, and 50 mM) and then were cultured on the half-strength MS solid medium without any growth regulator. Effects of ancillary compounds such as salicylic acid and putrescine (0.1 to 10 μM) on rooting were also investigated in addition to 7.5 μM IBA and NAA. Rooted explants maintained under high relative humidity (95 %) in a mixture of vermiculate/perlite (50/50 v/v) and plantlets were transferred to normal greenhouse conditions.
Data recording and statistical analysis
Mean number of shoots per explant (MNS/E), mean length of shoots per explants (MLS/E) and visual quality of axillary shoots were recorded after nearly 55 days (after 2 subcultures). For visual quality test, the proliferated shoots were ranked according to their color and abnormality [yellowing of the leaves, formation of malformed leaves and suppression of growth, ranking from 1 (lowest abnormality) to 3 (highest abnormality)]. Each experiment was carried out as a completely randomized design with at least 10 replications for each treatment. Data were analyzed using Tukey's test (P ≤ 0.05) of significance was applied to separate treatment means. The statistical analysis was done using SPSS version 16.0 (SPSS Inc., Chicago IL, USA).
Results
Data obtained in this investigation showed that explants of Araucaria excelsa R. Br. var. glauca produce axillary shoots in the growth regulator-free medium. The axillary meristem growth from concealed bud traces in A. excelsa R. Br. was observed after nearly 13 days of culture. However, growth regulator treatments and AC could accelerate the growth of axillary shoots. Axillary shoots produced from some explants showed malformation characteristics, especially on media with more than 0.50 μM 2iP (0.75, 1 and 1.25 μM) and using lower segments of orthotropic semihardwood stems.
Comparison between upper, middle and lower parts of main stem
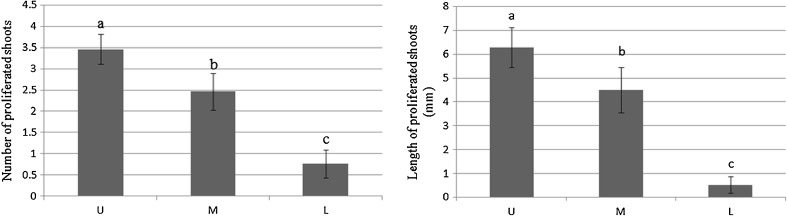
The results showed that explants taken from upper segments had a higher MNS/E and MLS/E as compared with explants taken from lower segments of semihardwood stems (Fig. 1). The concentration over 0.5 μM 2iP, irrespective of the position of explants, resulted in the formation of distorted buds and suppressed their growth (Table 1). Using the lower segments of semihardwood orthotropic stems with higher concentration of 2iP resulted in severe abnormality in growing axillary shoots (Fig. 2, a–d). In some cases, proliferated shoots reached a length of 20 mm within 55 days of culture, and regardless of the position on stem, the growth of plagiotropic shoots started earlier than orthotropic shoots (Fig. 2, e–f).
Fig. 1.
Comparison between mean shoot proliferation rate and length in upper, middle and lower segments of orthotropic stems in Araucaria excelsa R. Br. var. glauca in the medium supplemented with 0.2 μM 2iP. (U = Upper, M = Middle and L = Lower segments of orthotropic main stem). Bars are significantly different using Tukey's test at P ≤ 0.05
Table 1.
Comparison between upper, middle and lower segments of semihardwood orthotropic stems of Araucaria excelsa R. Br. var. glauca in response to 2iP in the culture medium after 55 days. MNS/E represent mean number of shoots per explant and MLS/E represent mean length of shoots per explants
| Treatment | MNS/E (SD)a | MLS/E (mm) (SD) | Abnormal shoots* (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2iP (μM) | U | M | L | U | M | L | U | M | L |
| 0.00 | 2.33 cd† (0.33) | 0.66 e(0.07) | 0.00 e(0.00) | 8.33 ab(0.03) | 3.66 d–f(0.07) | 0.00 h(0.00) | 0.00 f(0.00) | 0.00 f(0.00) | 0.00 f(0.00) |
| 0.10 | 3.00 b–d(0.00) | 2.66 cd(0.14) | 2.33 cd(0.33) | 7.00 bc(1.00) | 3.41 d–g(0.39) | 1.70 e–h(0.70) | 0.00 f(0.00) | 0.00 f(0.00) | 0.00 f(0.00) |
| 0.20 | 3.33 bc(0.02) | 2.33 cd(0.33) | 0.00 e(0.00) | 9.00 ab(1.00) | 8.33 ab(0.03) | 0.00 h(0.00) | 0.00 f(0.00) | 0.00 f(0.00) | 0.00 f(0.00) |
| 0.30 | 3.66 bc(1.94) | 2.00 cd(1.00) | 0.00 e(0.00) | 6.50 bc(0.05) | 4.83 cd(0.03) | 0.00 h(0.00) | 0.00 f(0.00) | 0.00 f(0.00) | 0.00 f(0.00) |
| 0.40 | 2.66 cd(0.14) | 0.66 c(0.07) | 0.00 e(0.00) | 8.00 ab(1.00) | 3.33 d–g(0.33) | 0.00 h(0.00) | 0.00 f(0.00) | 0.00 f(0.00) | 0.00 f(0.00) |
| 0.50 | 3.33 bc(0.34) | 4.66 ab(0.08) | 2.33 cd(1.27) | 6.75 bc(3.90) | 9.83 a(1.00) | 2.60 d–h(0.50) | 0.00 f(0.00) | 0.00 f(0.00) | 0.00 f(0.00) |
| 0.75 | 3.00 b–d(1.00) | 3.00 b–d(1.00) | 0.33 e(0.03) | 6.60 bc(3.03) | 0.90 gh(0.15) | 0.00 h(0.00) | 0.00 f(0.00) | 2.66 b(0.55) | 1.33 e(0.00) |
| 1.00 | 6.00 a(1.00) | 2.66 cd(0.14) | 1.33 de(0.33) | 1.50 f–h(0.10) | 4.33 c–e(0.33) | 0.03 h(0.01) | 2.33c(0.03) | 1.33 e(0.00) | 1.33 e(0.00) |
| 1.25 | 4.00 bc(1.00) | 3.66 bc(0.34) | 0.66 e(0.07) | 2.83 d–h(1.06) | 1.90 e–h(1.00) | 0.33 h(0.00) | 2.33 c(0.00) | 4.33 a(0.33) | 1.66 d(0.00) |
†In each parameter, means followed by the same letters are not significantly different using Tukey's test at P ≤ 0.05. (U = Upper, M = Middle and L = Lower segments of semihardwood orthotropic stems). *Ranking from 1 (lowest abnormality) to 3 (highest abnormality). a Standard Deviation (SD)
Fig. 2.
Shoots derived from main stem of A. excelsa R. Br. var. glauca. a and b: Abnormal shoots produced through upper segments in the medium supplemented with 1 and 1.25 μM 2iP, respectively. c and d: Abnormal shoots produced through lower segments in the medium supplemented with 1 and 1.25 μM 2iP, respectively. e and f: Axillary shoots produced after near to 55 days in the medium supplemented with 0.045 μM TDZ (e) and 0.5 μM 2iP (F). g: Rooted plantlet, 85 days after culturing the explants. h: Acclimatized A. excelsa R. Br. derived from orthotropic stem of 3 yr old mother plant. i: Acclimatized A. excelsa R. Br. after 12 month
Effects of 2iP and TDZ
Some differences were observed in the number of shoots produced by control explants vs. explants cultured on the medium supplemented with 1 and 1.25 μM 2iP (Table 2).
Table 2.
Effects of 2iP on mean number of shoots per explant (MNS/E) and mean length of shoots per explants (MLS/E) in A. excelsa R. Br. var. glauca after 55 days
| 2iP (μM) | MNS/E (SD) a | MLS/E (mm) (SD) | Abnormal shoots* (SD) |
|---|---|---|---|
| 0.00 | 1.25 d† (0.05) | 9.00 a (2.00) | 0.00 c (0.00) |
| 0.10 | 3.25 bc (1.17) | 6.56 b (0.00) | 0.02 c (0.00) |
| 0.20 | 3.75 bc (0.25) | 10.00 a (1.00) | 0.04 c (0.00) |
| 0.30 | 3.25 bc (1.16) | 6.63 b (0.00) | 0.06 c (0.02) |
| 0.40 | 2.25 cb (0.00) | 7.00 b (0.00) | 0.08 c (0.47) |
| 0.50 | 3.00 bc (1.00) | 7.56 b (0.07) | 0.00 c (0.00) |
| 0.75 | 3.25 bc (1.17) | 5.00 c (0.00) | 1.71b (0.01) |
| 1.00 | 5.25 a (0.25) | 2.60 d (0.60) | 1.71b (1.13) |
| 1.25 | 4.00 ab (1.00) | 2.50 d (0.00) | 3.57 a (1.26) |
†In each column, means followed by the same letters are not significantly different using Tukey's test at P ≤ 0.05. *Ranking from 1 (lowest abnormality) to 3 (highest abnormality). a Standard Deviation (SD)
Based on sensitivity of explants to cytokinin, 0.2 μM 2iP was considered as a suitable concentration for shoot production which resulted in 3.75 MNS/E with 10.00 mm MLS/E after 55 days. A highly significant difference in the response of 0.045 mg L−1 TDZ was observed on upper segments of semihardwood orthotropic stems of A. excelsa R. Br. in comparison to control explants (Table 3). Explants were grown on MS medium supplemented with TDZ produced vigorous axillary shoots than other plant growth regulators and did not show any abnormal axillary shoot within tested concentrations of TDZ.
Table 3.
Effects of TDZ on mean number of shoots per explant (MNS/E) and mean length of shoots per explants (MLS/E) in Araucaria excelsa R. Br. var. glauca after 55 days
| TDZ (μM) | MNS/E (SD) | MLS/E (mm) (SD) |
|---|---|---|
| 0 | 1.66 b (1.66) † | 1.83 b (1.02) |
| 0.0045 | 3.57 ab (1.56) | 5.18 a (4.01) |
| 0.045 | 4.60 a (0.08) | 7.08 a (0.65) |
| 0.45 | 3.83 a (1.29) | 4.83 ab (0.33) |
| 4.5 | 3.25 ab (0.95) | 5.43 a (0.00) |
† In each column, means followed by the same letters are not significantly different using Tukey's test at P ≤ 0.05
Means are followed by Standard Deviation (SD) in parenthesis
Rooting of in vitro-derived shoots
Use of ancillary compounds did not noticeably influence root induction or development. Quick-dip in 0, 10, 20, 30, 40 and 50 mM IBA was not successful in rooting of in vitro-derived shoots in the species used. Only 33 % of explants were rooted in the MS medium containing 3.0 % sucrose, supplemented with 7.5 μM of both NAA and IBA for 2 weeks before transferring to half strength MS medium without any growth regulators (data not shown). For subsequent root development, the treated shoots were subcultured onto 1/4 strength of the MS medium without any growth regulators. Up to 2 roots were observed in each in vitro-derived shoot (Fig. 2, g). Acclimatized plantlets were transferred to the greenhouse with less than 20 % mortality and subsequent growth was very slow in survived plants (Fig. 2, h–i).
Discussion
In the present study, axillary bud formation was induced directly from in vitro cultured main stem explants of Araucaria excelsa R. Br. var. glauca. In the leaf axils of most dicotyledonous trees, well developed buds are present. However, in most conifers, few axillary buds will form relative to the large number of leaves that either lack, or gradually lose them. In contrast, species of Agathis, Araucaria and Wollemia (all belong to family Araucariaceae) possess an apparently unique axillary structure consisting of undifferentiated axillary meristems that have neither leaf primordial nor vascular connections. These meristems are initially formed in an exogenous site but are transferred to an endogenous site through the activity of a localized phellogen (Burrows et al. 2003). In contrast to Burrows (1987) results, Holthusen (1940) observed no meristem or bud primordium in the apparently blank axils of 22 species of conifers in 11 genera, including Araucaria. Our previous study demonstrated that plagiotropic stems of A. excelsa R. Br. can produce axillary meristems in the MS medium supplemented with more than 3 μM BA (Sarmast et al. 2009). However, removing the leaves of explants induced axillary meristems in the MS growth regulator-free medium after nearly 40–50 days. In contrast, axillary meristems were induced on the orthotropic stem segment in the MS growth regulator- free medium supplemented with 0.3 % AC after 12–14 days. Some problems were encountered with woody species, including systemic infections, episodic growth, and production of polyphenols, tannins and volatile substances, and hyperhydricity of cultures. Active charcoal could be used to absorb the polyphenols and tannins. Observations showed that, not only early subculturing (in 20 day time-intervals) significantly increased the proliferation rate, but the addition of 0.3 to 1 % AC to the medium also accelerated the rate of growth (data not shown). Similar results have been reported for Araucaria spp. and Pinus spp. (Burrows et al. 1988; Sehgal et al. 1989; De Diego et al. 2008). The A. excelsa R. Br. explants were highly sensitive to concentration over 0.5 μM 2iP. Abnormality in axillary shoots was increased according to higher concentration of 2iP used. Abnormal explants had yellow shoot and their growth was suppressed.
It is shown that hoop pine (Araucaria cunninghamii Ait.) explants were highly sensitive to cytokinin; 1 and 10 μM 6-benzylaminopurine led into the formation of distorted buds and total inhibition of bud development, respectively (Burrows et al. 1988). The same results of positive effects of low cytokinin in culture media reported by Traore et al. (2005) on vegetative buds of Pseudotsuga menziesii Mirb. Franco and in organogenesis of Pinus sylvestris L. (De Diego et al. 2010). The findings of this experiment showed that up to 0.5 μM 2iP had a significant effect on MNS/E and MLS/E in comparison to control without noticeable abnormality and in most cases, the upper segment was better than other segments. It can be concluded that low concentration of 2iP could serve as a provocative for production of axillary buds. Regarding to PGRs, MNS/E was not always correlated to MLS/E. It is shown that higher concentration of PGRs especially cytokinins results in somaclonal variation in plants (Hartmann et al. 2011; Sarmast et al. 2012). The highest axillary shoots formation ability occurred on MS medium supplemented with 0.045 μM TDZ that not only was able to induce more MNS/E and MLS/E but also proliferated shoots showed a good performance. Our attempt to root the shoots was not quite successful, due to secretion of phenolic compounds into the culture medium from microcuttings. With regards of hard-to-root characteristic in these species other biotechnological approach such as application of Agrobacterium rhizogenes might be used, and for reducing mortality of transferred plantlets to the field, they can be inoculated with mycorrhizal fungi (Zhu et al. 2010). It has been reported that A. rhizogenes increases plant cellular sensitivity to auxin. This, in turn, enhances rooting percentage in many conifers and fruit trees (Holefors et al. 1998; Damiano and Momticelli, 1998; Villalobos-Amador et al. 2002; Zdravkovic-Korac et al. 2004). Furthermore, other research results indicate that the utilization of A. rhizogenes increases the root:shoot ratio (Casanova et al. 2005), which may yield plantlets that are more tolerant to the stressful conditions in the field.
Abbreviations
- 2iP
2-iso-pentenyl adenine
- AC
Activated charcoal
- BAP
6-benzyl-amino-purine
- IBA
Indole-3-butyric acid
- Kin
Kinetin
- MLS/E
Mean length of shoots per explant
- MNS/E
Mean number of shoots per explant
- MS
Murashige and Skoog
- NAA
α-naphthaleneacetic acid
- PGRs
Plant growth regulators
- TDZ
Thidiazuron
References
- Astarita LV, Floh IS, Handro W. Changing in IAA, tryptophan and activity of soluble peroxidase associated with zygotic embryogenesis in Araucaria angustifolia (Brazilian pine) Plant Growth Regul. 2003;39:113–118. doi: 10.1023/A:1022542618945. [DOI] [Google Scholar]
- Burrows GE. Leaf axil anatomy in the Araucariaceae. Aust J Bot. 1987;35:631–640. doi: 10.1071/BT9870631. [DOI] [Google Scholar]
- Burrows GE, Doley DD, Haines RJ, Nikles DG. In vitro propagation of Araucaria cunninghamii and other species of the Araucariaceae via axillary meristems. Aust J Bot. 1988;36:665–676. doi: 10.1071/BT9880665. [DOI] [Google Scholar]
- Burrows GE, Offord CA, Meagher PF, Ashton K. Axillary meristems and the development of epicormic buds in Wollemi Pine (Wollemia nobilis) Ann Bot. 2003;92:835–844. doi: 10.1093/aob/mcg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Brunner AM, Jones H, Strauss SH (2003) Forestry’s fertile crescent: the application of biotechnology to forest trees. Plant Biotechnol J 1:141–154 [DOI] [PubMed]
- Casanova E, Trillas MI, Moysset L, Vainston A. Infuence of rol gene in floriculture. Biotechnol Advances. 2005;23:3–39. doi: 10.1016/j.biotechadv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Damiano C, Momticelli S. In vitro fruit trees rooting by Agrobacterium rhizogenes wild type infection. Elect J Biotechnol. 1998;2:1–7. [Google Scholar]
- De Diego N, Montalbán IA, Fernández E, Moncaleán P. In vitro regeneration of Pinus pinaster adult trees. Can J For Res. 2008;38:2607–2615. doi: 10.1139/X08-102. [DOI] [Google Scholar]
- De Diego N, Montalbán IA, Moncaleán P. In vitro regeneration of adult Pinus sylvestris L. trees. South Afri J Bot. 2010;76:158–162. doi: 10.1016/j.sajb.2009.09.007. [DOI] [Google Scholar]
- Giri CC, Shyamkmar B, Anjaneylnu C. Progresses in tissue culture, genetic transformation and application of biotechnology to trees: An overview. Trees. 2004;18:115–135. doi: 10.1007/s00468-003-0287-6. [DOI] [Google Scholar]
- Hanes RJ, de Fossard RA. Propagation of hoop pine (Araucaria cunninghamii AIT.) Acta Hortic. 1977;78:297–302. doi: 10.17660/ActaHortic.1977.78.38. [DOI] [Google Scholar]
- Hartmann HT, Kester DE, Davies FT, Geneve RL (2011) Plant propagation: principles and practices. Prentice Hall, Upper Saddle River, NJ. USA. 928p
- Holefors A, Xue ZT, Welander M. Transformation of the apple rootstock M26 with the rolA gene and its influence on growth. Plant Sci. 1998;136:69–78. doi: 10.1016/S0168-9452(98)00106-X. [DOI] [Google Scholar]
- Holthusen K. Untersuchungen Über das Vorkommen and den Zustand der Achselknospen bei den höeren Pflanzen. Planta. 1940;30:590–635. doi: 10.1007/BF01917043. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Neale DB, Kremer A. Forest tree genomics: growing resources and applications. Nature Rev. 2011;12:111–122. doi: 10.1038/nrg2931. [DOI] [PubMed] [Google Scholar]
- Sarmast MK, Salehi H, Khosh-Khui M. Using plagiotropic shoot explant in tissue culture of Araucaria excelsa R. Br. var. glauca. Adv Environ Biol. 2009;3:191–194. [Google Scholar]
- Sarmast MK, Salehi H, Khosh-Khui M. Nano silver treatment is effective in reducing bacterial contamination of Araucaria excelsa R. Br. var. glauca explants. Acta Biol Hung. 2011;62:477–484. doi: 10.1556/ABiol.62.2011.4.12. [DOI] [PubMed] [Google Scholar]
- Sarmast MK, Salehi H, Ramzani A, Abolimoghadam AA, Niazi A, Khosh-Khui M. RAPD fingerprint to appraise the genetic fidelity of in vitro propagated Araucaria excelsa R. Br. var. glauca plantlets. Mol Biotechnol. 2012;50:181–188. doi: 10.1007/s12033-011-9421-7. [DOI] [PubMed] [Google Scholar]
- Sehgal L, Sehgal OP, Khosla PK. Micropropagation of Araucaria columnaris Hook. Ann Sci For. 1989;46:158–160. doi: 10.1051/forest:19890536. [DOI] [Google Scholar]
- Steiner N, Santa-Catarina C, Silveira V, Floh EIS, Guerra MP. Polyamine effects on growth and endogenous hormones levels in Araucaria angustifolia embryonic cultures. Plant Cell Tiss Org Cult. 2007;89:55–62. doi: 10.1007/s11240-007-9216-5. [DOI] [Google Scholar]
- Traore A, Xing Z, Bonser A, Carlson J. Optimizing a protocol for sterilization and in vitro stablishment of vegetative bud from mature Douglas fir trees. HortScience. 2005;40:1464–1468. [Google Scholar]
- Villalobos-Amador E, Rodríguez-Hernández G, Pérez-Molphe-Balch E. Organogenesis and Agrobacterium rhizogenes-induced rooting in Pinus maximartinezii Rzedowsky and P. pinceana Gordon. Plant Cell Rep. 2002;20:779–785. doi: 10.1007/s00299-001-0396-x. [DOI] [Google Scholar]
- Zdravkovic-Korac SY, Muhovski P, Druart DC, Radojevic AL. Agrobacterium rhizogenes-mediated DNA transfer to Aesculus hippocastanum L. and the regeneration of transformed plants. Plant Cell Rep. 2004;22:698–704. doi: 10.1007/s00299-004-0756-4. [DOI] [PubMed] [Google Scholar]
- Zhu LH, Wu XQ, Qu HY, Ji J, Ye JR. Micropropagation of Pinus massoniana and micorrhiza formation in vitro. Plant Cell Tiss Org Cult. 2010;102:121–128. doi: 10.1007/s11240-010-9711-y. [DOI] [Google Scholar]