Abstract
The phytotoxicity and oxidative damage in response to different concentrations of Hg (0.0, 2.5, 5.0, 10 and 25 μM) were evaluated in wheat plants. The root and shoot growth, content of chlorophyll and total soluble protein declined at 10 and 25 μM Hg. Roots of the plant were more affected as compared to the shoot. The malondialdehyde (MDA) quantity enhanced in the roots of wheat plants treated with 10 and 25 μM Hg and in the leaves of plants treated with 25 μM Hg. The concentration of H2O2 decreased at low concentration and increased at high concentration of Hg. The induction of enzymatic antioxidants (catalase, CAT; ascorbate peroxidase, APX; peroxidase, POX and superoxide dismutase, SOD) was found in the roots and leaves of plants with increased concentration of Hg up to 10 μM and low activities of these enzymes were observed at 25 μM Hg. Also, the level of K, Ca and Mg declined in leaf tissues of Hg treated plants. Thus wheat plants exposed to lower concentrations of Hg did not experience any oxidative stress. However, on treatment with 10 μM Hg, the roots and leaves responded differently. Both the leaves and roots of plants treated with higher concentration of Hg were subjected to comparatively greater oxidative damage and demonstrated that the antioxidative components were not able to remove the stress due to higher concentration of Hg and thus might affect the productivity in wheat plants.
Keywords: Mercury, Phytotoxicity, Oxidative damage, Antioxidants, Triticum aestivum L.
Introduction
Mercury (Hg) constitutes a major pollutant in soils because of the annual import of toxic Hg into the agricultural lands (Patra and Sharma 2000). It is a highly toxic non-essential element and its dispersion in the environment is considered to be a serious environmental problem for its persistent character (Liu et al. 2010). The uptake of Hg leading to impairment of various biochemical and physiological processes has been observed in a variety of plants. The toxic effects of Hg on seed germination, growth and yield have been studied in different plant species (Mukherjee and De 1996; Varshney 1991; Vizarova et al. 1985). Long term exposure of Hg into the agricultural field results into the accumulation of toxic level of the element in the vegetables (Shriapanahi and Anderson 1986). The interaction between Hg and plant systems is very important because Hg has largely been employed in seed disinfectants, in fertilizers and in herbicides (McLaughlin et al. 1996).
Mercuric ions are able to induce oxidative stress by triggering generation of reactive oxygen species (ROS), e.g. superoxide radical (·O2−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH) in plants (Israr et al. 2006). Hg and other heavy metals are also known to generate an antioxidant protective mechanism including the enzymatic and non enzymatic constituents to resist oxidative damage in stressed plant tissue (Zhou et al. 2009). Different concentrations of Hg have been used in different plants and are known to induce the generation of antioxidative defenses. However, the effect of Hg in the generation of ROS remains to be thoroughly understood in various plant species.
The present study has been undertaken to determine the effect of different concentrations of Hg on the growth and biomass of wheat (Triticum aestivum L. Var. Sharbati C306) plants. To find out the Hg induced oxidative damage the electrolytic leakage, H2O2 and malondialdehyde (MDA) content has been measured. Also the amount of chlorophyll and non enzymatic antioxidants such as carotenoid and ascorbate (ASC) has been estimated. Furthermore, the level of total soluble protein and the major enzymatic antioxidants such as catalase (CAT), guaiacol peroxidase (POX), asorbate peroxidae (APX) and superoxide dismutase (SOD) have been quantitatively estimated under the influence of Hg. The above parameters have been studied to evaluate the oxidative damage and phytotoxic effect of different concentrations of Hg on wheat plants and to find out a possible defense mechanism mediated through antioxidants.
Materials and methods
Experimental design and plant growth
Wheat (Triticum aestivum L. Var. Sharbati C306) seeds were obtained from the local market. Selected healthy seeds were sterilized with 1 % sodium hypochlorite solution (w/v) for 10 min, thoroughly washed with double distilled water and were soaked in sterile distilled water for 1 h. Soaked seeds were geminated on moist cotton pad in Petri dishes in dark at 25 °C for overnight. Hoagland nutrient medium (Hoagland and Arnon 1950) was used throughout the study for growth of the seedlings. The pH of the medium was maintained at 6.5. The germinated seeds were uniformly kept on nylon mesh fixed at the mouth of plastic glasses containing one-fourth strength of Hoagland nutrient medium with different concentrations of HgCl2 (2.5, 5, 10 and 25 μM). A set of plants grown in the medium without HgCl2 was taken as control. The seedlings were allowed to grow in the plant growth chamber under an 8-h photoperiod at 25 ± 2 °C. The light intensity was approximately 200 μmol m−2 s−1 provided from a bank of fluorescence tubes. Primary leaves and roots of 7-d old seedlings were used for experimental purposes.
Growth analysis
Five seedlings (7-d old) along with roots were randomly taken from each set of plants. Length of root and shoot, fresh and dry weight were measured for growth analysis.
Pigment estimation
Fresh leaf tissues were homogenized in absolute cold methanol in dark and the extract was centrifuged. The absorbance of the supernatant was taken at 470, 652.4, 653, 665.2, and 666 nm. The chlorophyll concentration was calculated using the formula as described by Porra et al. (1989). The formula described by Wellburn (1994) was used to determine carotenoid concentration.
Measurement of electrolyte leakage
Fresh roots were cut into pieces of 1 cm length and placed in test tubes containing deionised water. The tubes were incubated in a water bath at 30 °C for 2 h and the initial electrical conductivity of the medium was measured. The samples were boiled at 100 °C for 15 min to release all the electrolytes, cooled and the final electrical conductivity was measured. The leakage percentage of electrolytes was calculated by using the formula: (EC1/EC2) × 100, where EC1 and EC2 are the initial and final electrical conductivity (Gong et al. 1998).
Lipid peroxidation
Lipid peroxidation was measured according to Heath and Packer 1968. Fresh leaf tissue was homogenized with 5 % (w/v) TCA (Trichloroacetic acid) and the homogenate was used for estimation of MDA. Assay mixture consisted of 0.5 % TBA prepared in 20 % TCA and tissue homogenate. The homogenate and 20 % TCA were used for correction blank. The assay mixture was heated at 95 °C in the water bath for 30 min, cooled and centrifuged at 12,000 × g for 10 min. The absorbance of the cooled supernatant was measured at 532 nm and was corrected for nonspecific absorbance at 600 nm. The MDA concentration was calculated and expressed as nmol g−1 fresh weight.
Hydrogen peroxide content measurement
H2O2 content was measured according to Loreto and Velikova (2001). Fresh leaf and root tissues were cut into pieces and homogenized in cold 0.1 % (w/v) TCA. The homogenate was centrifuged. Assay mixture contains supernatant, 10 mM potassium phosphate buffer (pH 7.0) and 1 M KI. The absorbency of the mixture was read at 390 nm. The content of H2O2 was determined from a standard curve prepared with known concentrations of H2O2 and expressed as mmol g−1 FW.
Estimation of ascorbate
Determination of ASC was performed as described by Jacques-Silva et al. (2001). Both roots and shoots were homogenized separately in a solution containing 50 mM Tris–HCl and 10 ml l−1 Triton X-100 (pH 7.5). The homogenate was centrifuged. To the supernatant, 10 % TCA was added at a proportion of 1:1 (v/v) followed by centrifugation to remove protein. An aliquot of the sample (300 μl) was incubated at 37 °C in a medium containing 13.3 % TCA, deionised water and 2,4-Dinitrophenylhydrazine (DNPH). The DNPH solution contained 2 % DNPH, 0.23 % thiourea, 0.27 % CuSO4 diluted in 49 % H2SO4. After 3 h, 65 % H2SO4 was added and the samples were read at 520 nm. The concentration of ASC was determined from the graph prepared by using L-ascorbate as a standard.
Extraction of protein and enzyme
For the extraction of proteins and enzymes, fresh tissues were homogenised in 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA (Ethylene diamine tetra acetate and 1 % polyvinylpyrrolidone (PVP). The buffer contained 1 mM ASC in case of APX extraction. The homogenate was centrifuged at 15,000 × g for 15 min at 4 °C and the supernatant was used for estimation of protein and enzyme assays. The total soluble protein was estimated according to the method of Bradford (1976) with bovine serum albumin (BSA) as a standard.
Assay of enzymatic antioxidants
The enzyme CAT (EC 1.11.16) was assayed following the method as described by Aebi (1983). The reaction mixture contained 50 mM potassium phosphate buffer (pH 6.5), enzyme extract equivalent to 20 μg protein and 10 mM H2O2. The decrease in absorbance at 240 nm due to the consumption of H2O2 was recorded using a UV-Visible spectrophotometer. The activity of CAT was calculated using the extinction co-efficient of 40 mM−1 cm−1 for H2O2.
The activity of APX (EC 1.11.1.11) was determined following the procedure as described by Rao et al. (1997). The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.5), 0.5 mM ASC, 0.2 mM H2O2 and the enzyme extract equivalent to 30 μg protein. The decrease in absorbance at 290 nm (decrease in ASC concentration) for 3 min was recorded using a UV-Visible spectrophotometer. The activity of the enzyme was calculated using the extinction co-efficient of 2.8 mM−1 cm−1 for ASC.
The enzyme POX (guaiacol specific) was assayed as described by Rao et al. (1997). The reaction mixture of 3 ml consisted 100 mM potassium phosphate buffer (pH 6.5), 16 mM guaiacol and 10 μl of 10 % H2O2 and enzyme extract equivalent to 10 μg protein. The activity was determined by the increase in absorbance at 470 nm (tetraguaiacol formation) for 5 min using a UV-Visible spectrophotometer. The POX activity was calculated using the extinction coefficient of 26.6 mM−1 cm−1 for tetraguaiacol formation at 470 nm.
The activity of CAT, APX and POX was expressed as nkatal mg−1 protein. One katal is defined as 1 mol of substrate transformed by the enzyme in 1 s.
The activity of SOD (EC 1.15.1.1) was assayed following the method of Marklund and Marklund (1974). 3 ml of reaction mixture consisted 2.850 ml of 50 mM Tris–HCl buffer (pH 8.2), 0.1 ml enzyme extract and 50 μl of 20 mM pyrogallol (prepared in 10 mM HCl). The reaction was initiated with addition of pyrogallol and the change in absorbance at 420 nm was recorded up to 3 min. For the auto-oxidation of pyrogallol, the reaction mixture contained 2.950 ml of 50 mM Tris–HCl buffer (pH 8.2) and 50 μl of 20 mM pyrogallol. The activity of SOD was expressed in unit SOD mg−1 protein. One unit of SOD activity is equal to 50 % inhibition of pyrogallol auto-oxidation.
Measurement of mercury and nutrient content
For measurement of Hg and mineral nutrients, shoots and roots were separated and thoroughly washed in deionised water. The samples were dried at 70 °C for 48 h. The dried tissues were ground in to a fine powder. 10 ml of deionized water, 3 ml of HNO3 and 2 ml of H2O2 were added to 0.5 g of the dried powder sample. The mixture was digested at 1.5 kPa and 125 °C in an autoclave (Lozano-Rodriguez et al. 1995). The solution was filtered and diluted to 25 ml. Measurement of Hg, K, Ca and Mg was carried out by atomic absorption spectrophotometer (AA 6200 Shimadzu).
Statistical analysis
All the data presented are the arithmetic mean of at least three independent experiments. The data were statistically evaluated using standard deviation (SD) and Student’s t-test methods.
Results
Growth response and biomass analysis
A drastic decrease in shoot length (15.4 to 8.31 cm) was observed with 25 μM HgCl2 as compared to the control plants. Though the root growth slightly increased at low concentration of HgCl2 (2.5 μM) a significant decline in root length was noticed with elevated concentrations. Treatment with HgCl2 for 7 days caused a decline in fresh weight of the plants. The reduction in weight of plants was concentration dependent. The maximum reduction was 30 % for fresh weight with 25 μM HgCl2 treatment as compared to control. The dry weight of the treated plants increased in a dose dependent manner with increased concentration of HgCl2. The effect of HgCl2 on plant growth and biomass of 7-d old wheat plants has been shown in Table 1.
Table 1.
The fresh and dry weights of 7-d old wheat plants treated with different concentrations of HgCl2. Mean values with ± SD are presented. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
| HgCl2 (μM) | Root length (cm) | Shoot length (cm) | FW plant−1 (g) | DW plant−1 (g) | DW:FW |
|---|---|---|---|---|---|
| 0 | 15.48 ± 0.507 | 12.04 ± 0.183 | 0.291 ± 0.0036 | 0.0256 ±0.00013 | 0.0879 |
| 2.5 | 15.26 ± 0.244 | 13.97 ± 0.31 | 0.295 ± 0.0045 | 0.0261 ±0.00013 | 0.0884 |
| 5 | 15.12 ± 0.30 | 10.10* ± 0.19 | 0.279 ± 0.0036 | 0.0270 ±0.00088 | 0.096 |
| 10 | 15.05 ± 0.155 | 8.6* ± 0.401 | 0.252* ± 0.00264 | 0.0291* ±0.00021 | 0.1154 |
| 25 | 8.38* ± 0.339 | 3.65* ± 0.39 | 0.206* ±0.00132 | 0.0312* ±0.0002 | 0.1456 |
Total protein, chlorophyll and carotenoid content
The treatment with different concentrations of HgCl2 affected the chlorophyll and carotenoid content. Treatment of plants with 2.5 μM HgCl2 enhanced the content of chlorophyll. However with further increase in concentration of HgCl2, the chlorophyll amount decreased significantly. The content of carotenoid increased with 2.5, 5 and 10 μM HgCl2 and decreased in content with treatment 25 μM Hg. The total protein content of wheat plants grown with HgCl2 decreased in a concentration dependent manner except at low concentration (2.5 μM). At this concentration, the level of protein in leaves and roots was enhanced by 10 % and 16 % respectively over control plants. A maximum decrease of total protein by 19 % in leaves and 24 % in roots was noticed with 25 μM HgCl2. Table 2 presents the content of chlorophyll, carotenoid and total soluble protein in wheat plants under the influence of HgCl2.
Table 2.
Effect of different concentrations of HgCl2 on chlorophyll, carotenoid and total protein content of 7-d old wheat plants. Mean values with ± SD are presented. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
| HgCl2 (μM) | Chlorophyll a + b (μg g−1 FW) | Carotenoid (μg g−1 FW) | Percentage of total protein (relative to control) | |
|---|---|---|---|---|
| Leaf | Root | |||
| 0 | 1404.163 ± 3.33 | 312.99 ± 2.01 | 100 ± 0.0 | 100 ± 0.0 |
| 2.5 | 1541.034 ± 3.09 | 328.55 ± 2.70 | 116 ± 1.52 | 110 ± 2.21 |
| 5 | 1356.894* ± 2.38 | 340.41* ± 0.95 | 87 ± 3.24 | 92 ± 3.06 |
| 10 | 1248.843* ± 2.85 | 356.95* ± 1.07 | 80* ± 4.12 | 88 ± 2.64 |
| 25 | 1221.843* ± 1.24 | 301.38* ± 1.46 | 81* ± 2.09 | 76* ± 1.83 |
Content of nutrients
The content of K, Ca and Mg was strongly affected in wheat plants grown with different Hg concentrations. The treatment of wheat plants with 2.5 and 5 μM HgCl2 caused about 17 % and 23 % decrease in K content of leaves compared to control plants. The level of K further decreased by 38 % and 49 % in plants grown with 10 and 25 μM Hg respectively. The concentration of Ca and Mg was slightly stimulated (by 11 %) in plants treated with low dose of Hg (2.5 μM). On the other hand, the decrease in the content of Ca and Mg was almost similar to that of K in plants treated with 5, 10 and 25 μM HgCl2. The content of the major nutrients in control and Hg treated plants has been presented in Table 3.
Table 3.
Effect of different concentrations of HgCl2 on the content of major nutrients (K, Ca and Mg) in leaves of wheat plants. Mean values with ± SD are presented. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
| Hg treatment (μM) | K (mmol g−1 DW) | Ca (mmol g−1 DW) | Mg (mmol g−1 DW) |
|---|---|---|---|
| 0 | 0.391 ± 0.028 | 0.623 ± 0.189 | 0.723 ± 0.0985 |
| 2.5 | 0.332 ± 0.096 | 0.693 ± 0.236 | 0.803 ± 0.0236 |
| 5 | 0.301* ± 0.040 | 0.472* ± 0.085 | 0.624 ± 0.154 |
| 10 | 0.241* ± 0.082 | 0.412* ± 0.051 | 0.439* ± 0.065 |
| 25 | 0.201* ± 0.102 | 0.342* ± 0.035 | 0.346* ± 0.098 |
Membrane leakage and lipid peroxidation
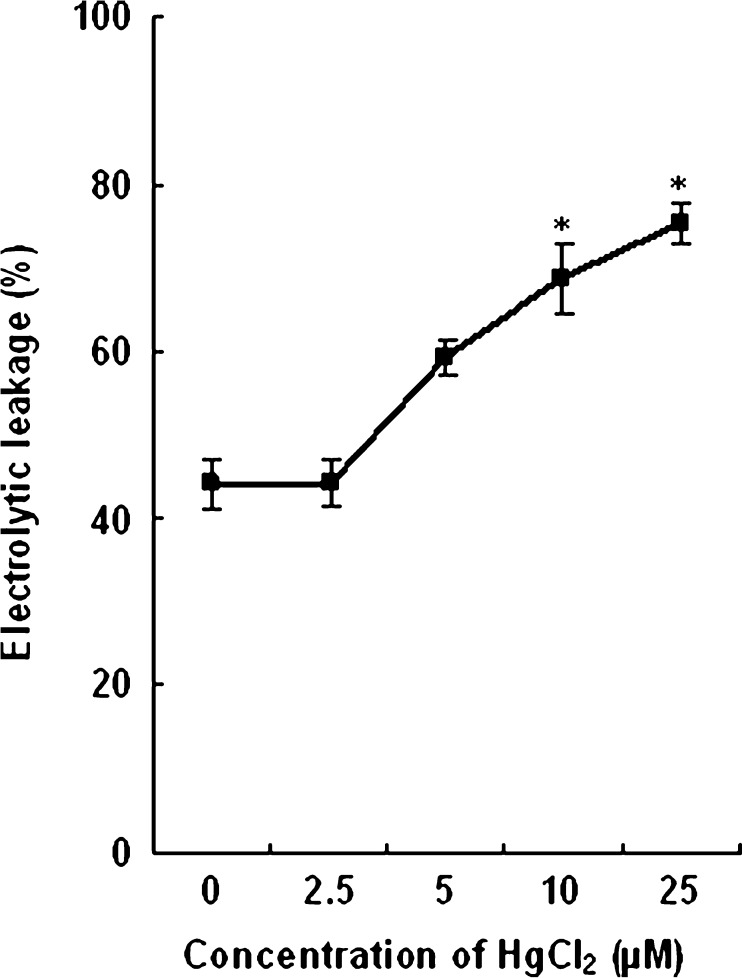
The effect of Hg on plasma membrane leakage of root tissues of wheat seedlings is shown in Fig. 1. No marked damage to the membranes (electrolytic leakage%) with low and moderate concentration (2.5 and 5 μM) was observed. However, compared to control (43.99 %) the leakage percentage increased in root tissues of the plants treated with 10 and 25 μM HgCl2 (68.69 % and 75.36 % respectively).
Fig. 1.
Effect of HgCl2 on membrane leakage (electrolytic leakage%) in roots of wheat plants. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
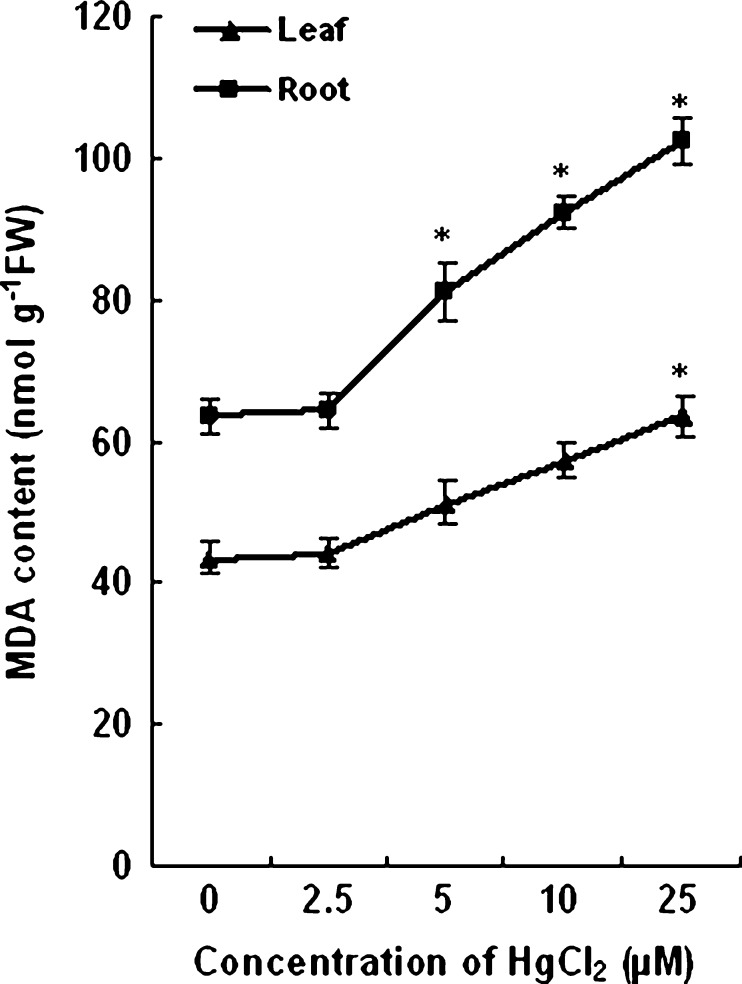
No significant change in MDA content was observed in roots and leaves of 2.5 and 5 μM HgCl2 treated plants as compared to the control. With further increase in concentration of HgCl2, an elevation of MDA content was recorded. The MDA content in roots increased by 45 % and 61 % with 10 and 25 μM HgCl2 respectively. In leaves of plants grown with 25 μM HgCl2 the MDA content increased by 58 %. The changes in MDA content have been shown in Fig. 2.
Fig. 2.
Changes in MDA content of roots and leaves of wheat plants grown with and without HgCl2. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
Hydrogen peroxide content
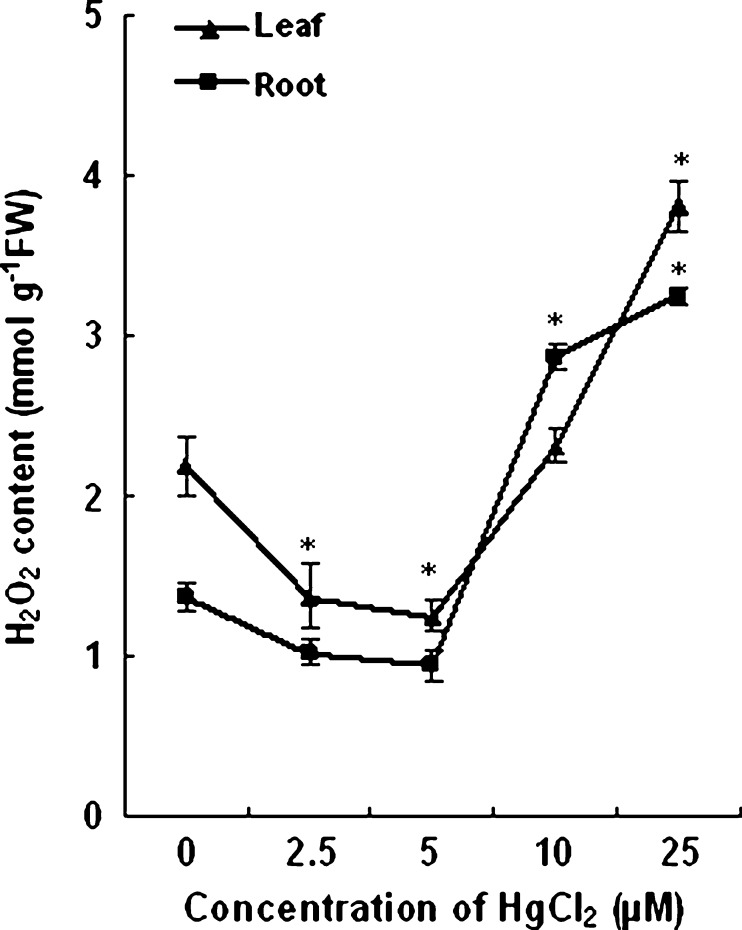
HgCl2 induced changes in H2O2 content are shown in Fig. 3. The level of H2O2 with 2.5 and 5 μM HgCl2 treated plants decreased by 40–45 % in leaves and about 30 % in roots as compared to that of untreated plants. The H2O2 content in root and leaf of control plants were estimated to be 1.4 and 2.2 μmolg−1 fresh weight respectively. A twofold increase in H2O2 level as compared to control was observed in the root tissues of wheat plants treated with 25 μM HgCl2. The concentration of H2O2 in leaves of plants grown with 25 μM HgCl2 increased by 75 % over control.
Fig. 3.
Effect of different concentrations of HgCl2 on H2O2 content of roots and leaves of wheat plants. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
Ascorbate content
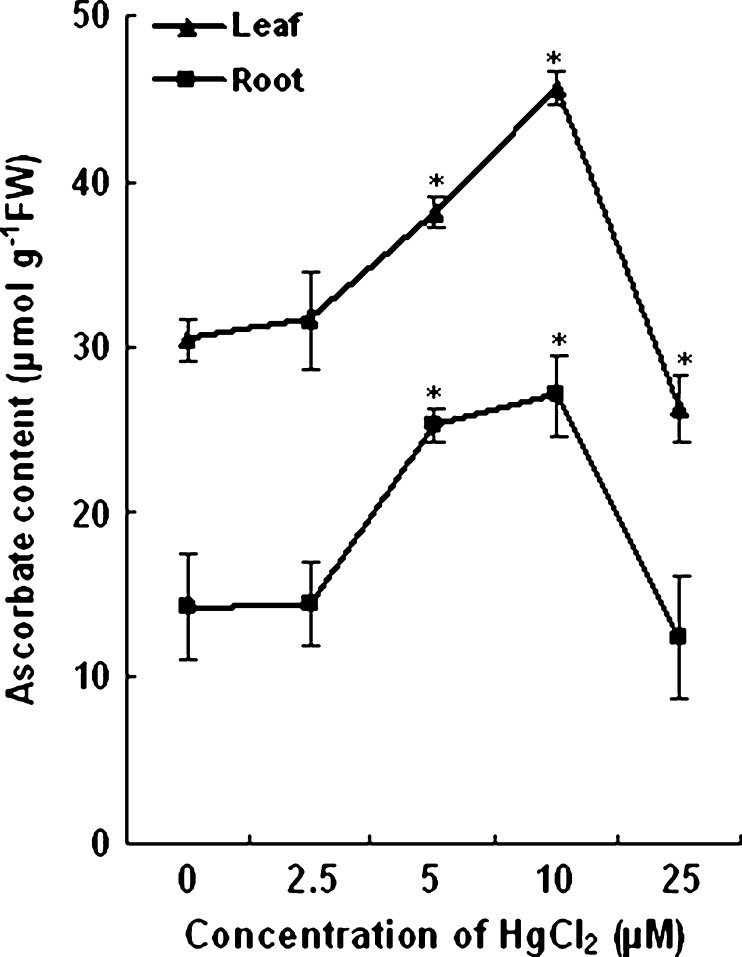
The ASC content of both root and leaf tissues of plants treated with 2.5 μM HgCl2 remained almost same as that of the control plants. Further increase in concentration to 5 and 10 μM the ASC content in leaf tissues increased by 25 % and 49 % and in root tissues by 78 % and 91 % respectively as compared to plants grown without HgCl2. On the other hand a decrease in ASC content approximately by 15 % as compared to control plants was noticed in plants treated with high concentration of HgCl2 (25 μM). The change in ASC content in the roots and leaves of treated and untreated plants is shown in Fig. 4.
Fig. 4.
Changes in ASC content of control and HgCl2 treated wheat plants. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
Activity of enzymatic antioxidants
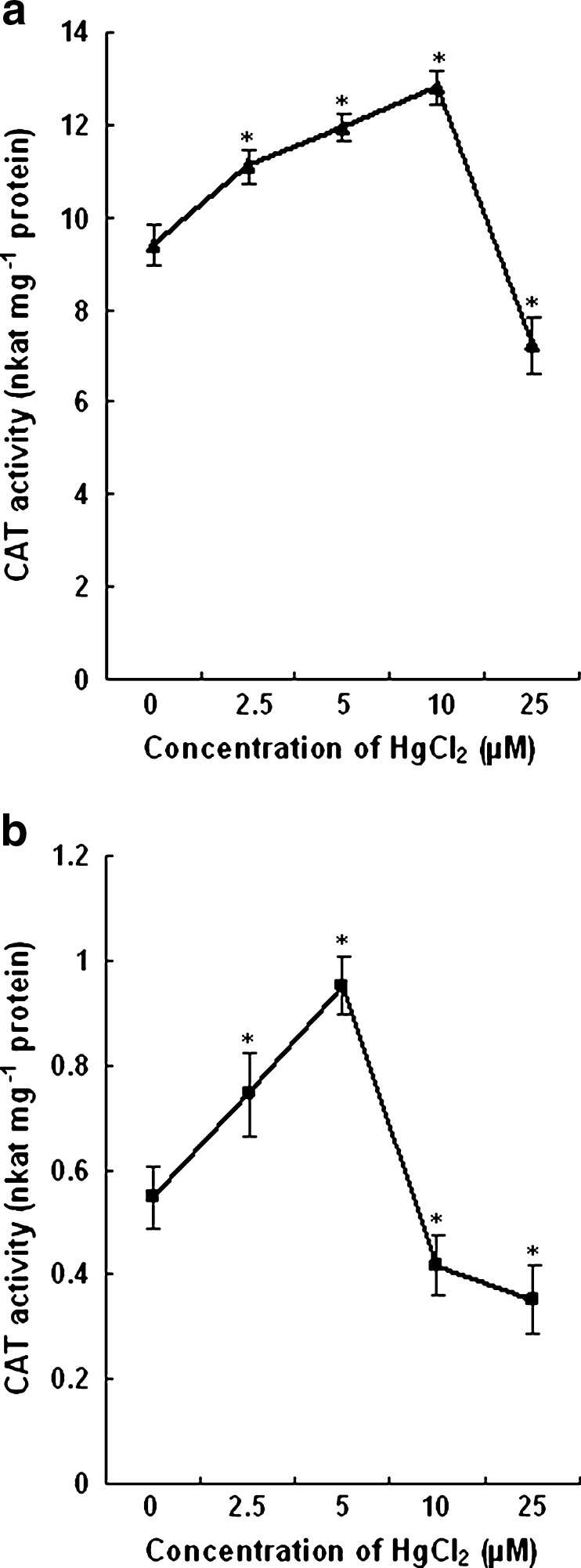
The activity of CAT increased in roots of plants grown with 2.5 and 5 μM HgCl2. Also, the enhancement of activity of the enzyme in leaves of plants grown with 2.5, 5 and 10 μM HgCl2 was recorded as compared to control plants. In the leaves of plants treated with 2.5, 5 and 10 μM HgCl2, the CAT activity increased by 18 %, 27 % and 36 % respectively. The CAT activity decreased by 23 % in the leaves of plants grown with 25 μM HgCl2. In roots, the activity was elevated by 36 % and 74 % with 2.5 and 5 μM HgCl2 while it decreased by 24 % and 36 % respectively with 10 and 25 μM HgCl2. The change in CAT activity has been shown in Fig. 5.
Fig. 5.
Changes in CAT activity in the leaves (a) and roots (b) of control and HgCl2 treated wheat plants. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
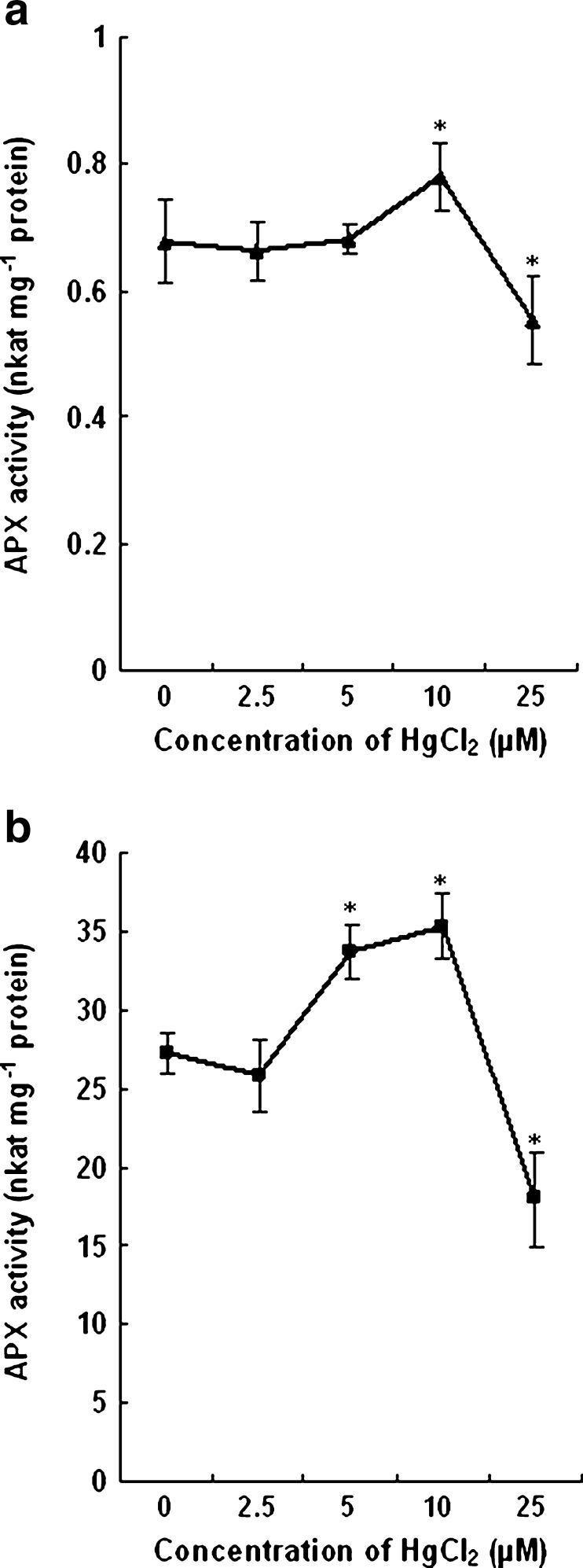
The activity of the enzyme APX in leaves did not show any appreciable change when treated with 2.5 and 5 μM HgCl2. However, an increase of 15 % APX activity as compared to control was observed in leaves of plants treated with 10 μM HgCl2. With further increase of HgCl2 to 25 μM, the APX activity declined by 20 % in leaf tissues (Fig. 6a). The activity of APX decreased slightly in roots of plants treated with low concentration of Hg. With increase in HgCl2 concentration to 5 and 10 μM, the APX activity increased over control by 23 % and 29 % respectively. However, with 25 μM, the enzyme activity declined significantly. The decrease in activity was about 35 % when compared to that of the roots of control plants (Fig. 6b).
Fig. 6.
Activity of APX in leaves (a) and roots (b) of wheat plants treated with different concentrations of HgCl2. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
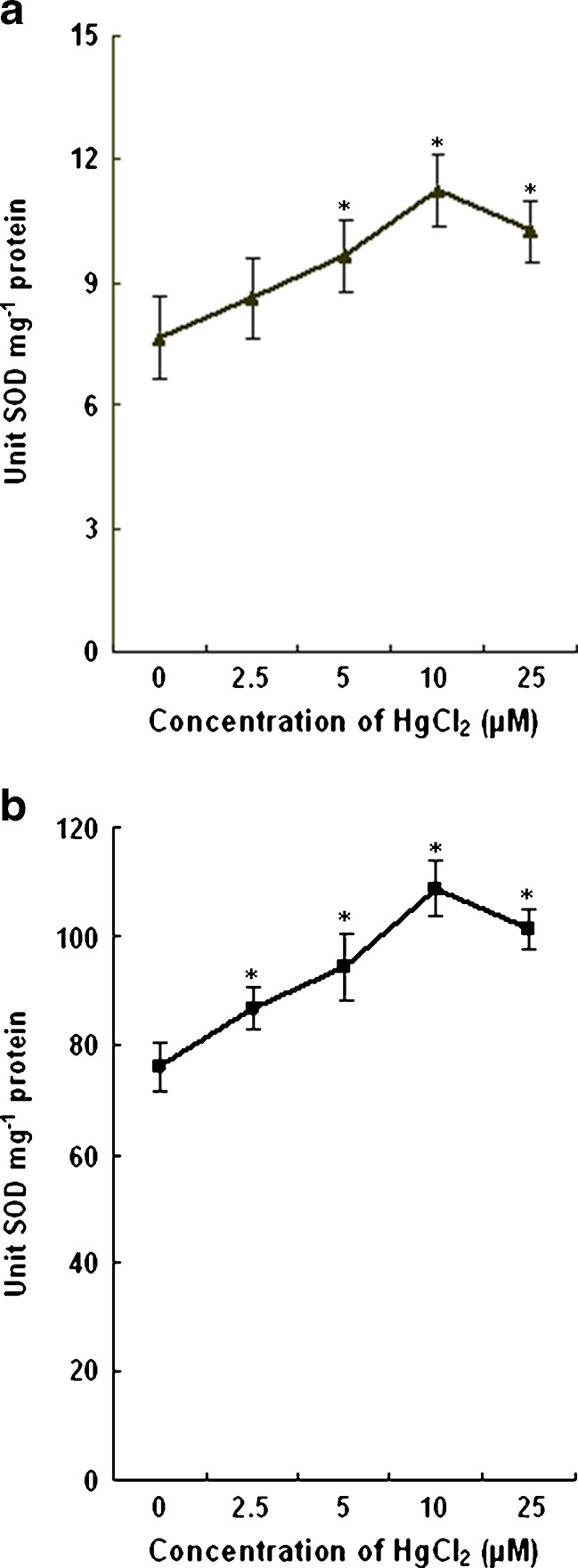
In the present study, the activity of SOD in the leaves increased by 13 %, 26 %, 46 % and 34 % with 2.5, 5, 10 and 25 μM HgCl2 (7.65 unit SOD mg−1 protein in control to 8.65, 9.65, 11.23 and 10.25 unit SOD mg−1 protein in HgCl2 treated plants) respectively. In comparison to control plants, the SOD activity in roots increased by 14 %, 24 %, 43 %, and 33 % respectively with 2.5, 5,10 and 25 μM HgCl2. The increase in SOD activity in leaves and roots were progressive with Hg concentration up to 10 μM. The effects of HgCl2 on SOD activity in wheat seedlings are shown in Fig. 7.
Fig. 7.
Effect of HgCl2 on SOD activity of leaves (a) and roots (b) of wheat plants. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
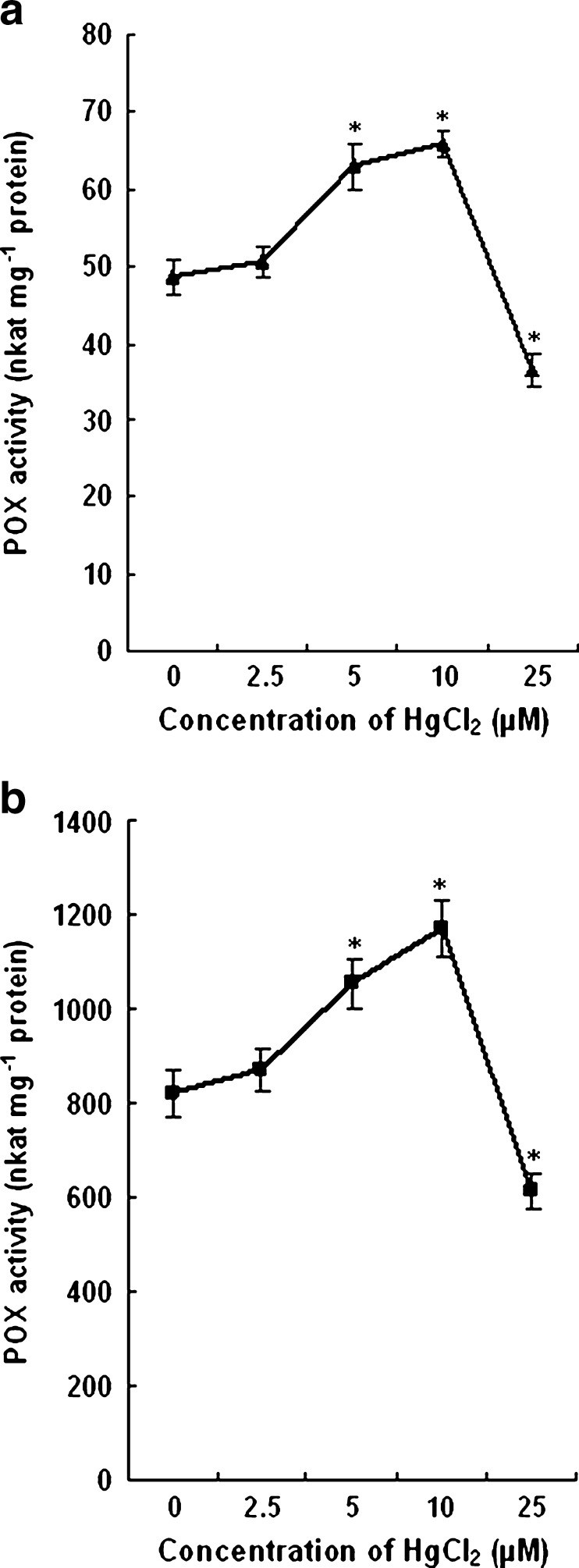
The POX activity of control plants remained almost same with that of 2.5 μM HgCl2 treated plants. With 5 and 10 μM HgCl2 the enzyme activity increased significantly both in leaves and roots as compared to control plants. A decline in activity approximately by 25 % was noticed in the plants treated with high concentration of HgCl2. The change in POX activity under the influence of Hg stress has been shown in Fig. 8.
Fig. 8.
Effect of HgCl2 on POX activities in leaves (a) and roots (b) of wheat seedlings. The values are the mean of three independent measurements. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
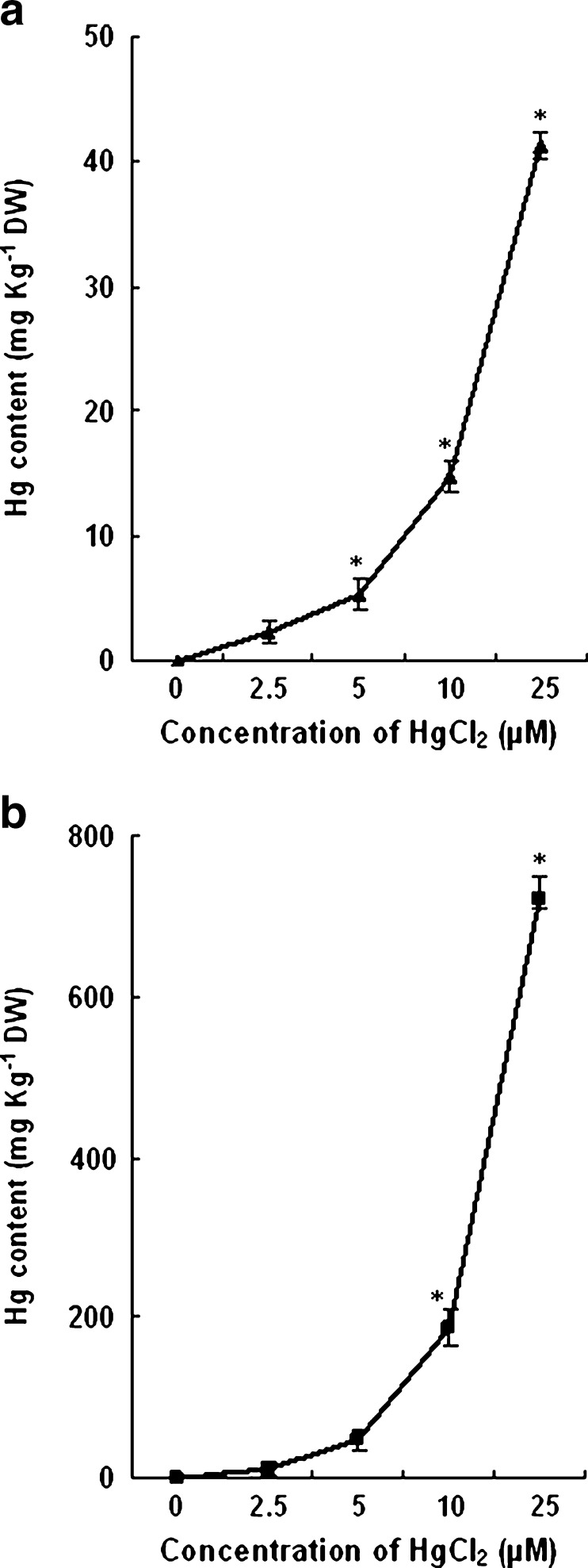
Mercury accumulation
The Hg accumulation both in leaves and roots of the treated plants increased with the increased concentration of HgCl2. The content of Hg in roots was comparatively more than the leaves. The level of Hg found in 7 d old leaves of 2.5, 5, 10 and 25 μM HgCl2 treated plants were 2.32, 5.39, 14.72 and 41.32 mg Kg−1 DW respectively. In roots of these treated plants the concentration of Hg measured were 11.26, 47.25, 187.18 and 721.54 mg Kg−1 DW respectively. The concentration of Hg in leaves and roots of wheat plants grown with different concentrations of Hg is shown in Fig. 9.
Fig. 9.
Mercury concentration in leaves (a) and roots (b) of 7-d old wheat plants treated with different concentrations of HgCl2. Vertical bars represent ± SD. Asterisks indicate the significance of difference at P < 0.05 level by Student’s t-test when compared to control
Discussion
Reduction in growth, biomass and pigment content of the plants is an indicator for evaluation of phytoyoxicity in response to heavy metal stress. The growth of roots of the plant being in direct contact with the soil are more affected compared to the shoot which was clearly observed in this work. In the present study, the root and shoot length, plant fresh weight, amount of total chlorophyll, soluble protein, Ca and Mg content were induced marginally at low concentration of Hg (2.5 μM) and declined slightly at moderate concentration of Hg (5 μM). The induction of chlorophyll at low concentration of Hg in wheat plants is already reported (Liu et al. 2010). The stimulating effects of low concentrations of heavy metal on several plant growth parameters is normally related to the hormetic effect, which probably represents an overcompensation response to a disruption in the homeostasis of the organism (Aina et al. 2007). Skrebsky et al. (2008) reported that there was an increase in both root and shoot biomass of Pfaffia glomerata plantlets at low Cd levels. On the other hand, the reduction in root growth, content of chlorophyll and total soluble protein caused by increased concentration of Hg to 10 and 25 μM has been observed in our study. Earlier studies have indicated that Hg at higher concentration is highly phytotoxic to cells and induce visible injuries and physiological alterations (Ortega-Villasante et al. 2005; Zhou et al. 2007). The reduction of plant growth caused by mercury as observed for Triticum aestivum in this study has also been reported in other plant species such as tomato (Cho and Park 2000) and tobacco (Suszcynsky and Shann 1995). Reduced growth is also indicated by a high dry weight to fresh weight ratio in plants grown with10 and 25 μM Hg. An increased dry weight to fresh weight ratio may be a sign of reduced water uptake, which in turn causes inhibited elongation and enlargement of cells leading to reduced growth (Mukherji and Mukherji 1979). The decrease in chlorophyll concentration in the present investigation due to Hg is similar to the findings in tomato plants grown with 10 μM Hg (Cho and Park 2000). In plants, mercury ions may substitute metal ions in photosynthetic pigments causing a decrease in photosynthesis rates (Xylander et al. 1996; Kupper et al. 1998). Many organic compounds including chlorophylls have been used as biomarkers for the early detection of metal toxicity in plants (Prasad 2003). The biosynthesis of chlorophyll is known to be inhibited by Hg that interacts with δ-aminolevulenicacid dehydratase (Prasad and Prasad 1987).
Membrane damage caused by different abiotic stress including heavy metals is largely mediated through membrane lipid peroxidation (Chaudhuri and Chaudhuri 1993). Increase in the level of MDA in response to Hg has been observed in roots and leaves of Medicago sativa (Ortega-Villasante et al. 2005) and several other plants. We observed the enhancement of MDA quantity in the roots of wheat plants treated with 10 and 25 μM Hg and the leaves of plants treated with only 25 μM Hg. These results indicate the oxidative damage to the membranes when wheat plants are exposed to high concentrations of Hg. We have also observed an increase of electrolytic leakage percentage in the roots of wheat plants with high concentration of mercury. Occurrence of lipid peroxidation under the influence of Hg has been reported in many plants such as tomato (Cho and Park 2000), mustard (Shiyab et al. 2008), alfalfa (Zhou et al. 2007) etc. The degree of oxidative stress was relatively more in roots as compared to leaves. This might happen due to high Hg accumulation in roots and/or non induction of the antioxidant components for the scavenge of the active oxygen species. Mishra and Choudhuri (1999) have noticed the increase of MDA content as well as electrical conductivity in roots and shoots of two varieties of Oryza sativa treated with 10 μM Hg. Our experimental results are in agreement of the above study.
The content of mineral nutrients in leaves of wheat plants were greatly affected by Hg treatment. The supply of Hg both at moderate and high concentration reduced the concentrations of K, Ca and Mg. Reduction of K, Ca and other mineral nutrients under the influence of Hg have been reported in M. Vulgare, R. induratus (Moreno-Jimenz et al. 2007) and V. spiralis (Gupta and Chandra 1998). Increased electrolytic leakage in the root tissues of Hg treated plants as observed in our study might have reduced the uptake of nutrients from the growth medium. Mercury is known to cause oxidative stress in plant tissues (Cho and Park 2000) leading to membrane damage and thus affecting the alteration of nutrient uptake and translocation. Low dose of Hg induced the content of only Ca and Mg. Stimulation of nutrient uptake under the influence of low dose of heavy metals is known in various plants (Carbonell-Barrachina et al. 1998; Zornoza et al. 2002).
The generation of ROS particularly H2O2 has been reported to serve as signal molecule in response to various abiotic stress including heavy metals (Mittler 2002). The heavy metal Hg is known to induce oxidative stress in plants resulting in the alteration of antioxidant enzyme activities. The increase of H2O2 under mercury stress as noticed in this study could be due to the reduced activity of H2O2 scavenging enzymes such as CAT and POX. The concentration of H2O2 was noticed to decrease in the roots and shoots of 2.5 and 5 μM Hg treatment corresponds to proportional increase of CAT in these tissues. The enzyme CAT is known to mediate the decomposition of H2O2 to water and oxygen. (Scandalios 1993). The level of H2O2 in response to Hg exposure might be comparable to the CAT activities found in plants (Srivastava et al. 2005). An inverse relationship between the concentrations of H2O2 and CAT as clearly observed in the roots and leaves of wheat plants has also been reported in the shoots of Brassica juncea (Shiyab et al. 2008). These authors have measured an increased H2O2 level with mercury treatment time that declined significantly with increase of Hg concentration. In contrast, the content of H2O2 in tomato decreased in comparison to control without any such inverse relationship (Cho and Park 1999). Thus the relation between H2O2 and CAT activity under Hg stress seems to depend on the species, treatment concentration and duration of exposure.
The enzymatic antioxidant SOD is the first enzyme in the detoxifying process that catalyses the dismutation of ·O2− to H2O2 and H2O (Fridovich 1986) and is considered to be an essential component of antioxidant defense system in plants. A marked increase in SOD activity in response to 10 μM Hg has been noticed in tomato seedlings (Cho and park 2000). In their study they have observed a parallel increase in SOD activity and H2O2 content. In our work, the SOD activity was found to increase progressively in the roots and leaves of plants with increased concentration of Hg up to 10 μM. The induction of SOD and the H2O2 detoxifying enzymes (CAT, POX) at lower Hg concentration indicates that wheat plants are subjected to oxidative stress at low Hg treatment and are protected by the generation of antioxidant system. Meanwhile, lower activity of SOD at 25 μM Hg compared to 10 μM Hg and simultaneous decline of CAT, POX and APX activity at this concentration points to the generation of oxidative stress in wheat plants. The activity of SOD is possibly not enough for the dismutation of generated ·O2− that induces oxidative stress at higher concentration of Hg and may have caused the significant membrane damage and increase in MDA content thus supporting the reduction in biomass.
The changes in activity of POX have been observed under varied concentrations of heavy metals (Schutzendubel and Polle 2002). The present study has observed an increase in POX activity in the roots and leaves of plants treated with 5 and 10 μM Hg. Further increase of Hg to 25 μM, the activity of POX declined sharply. This report is in agreement with the observation of Zhou et al. 2007. These authors have observed progressive enhancement of POX activity in alfalfa with increasing concentration of Hg up to 20 μM. A very similar observation has also been noticed by Shiyab et al. 2008 in Brassica juncea. The enzyme APX has a high affinity for H2O2 and is able to detoxify low concentrations of H2O2. It catalyzes the reduction of H2O2 to water by using ASC as specific electron donor (Foyer and Halliwell 1976). In our study, both roots and leaves registered a significant decrease in APX activity at 25 μM Hg treatment. The decline in POX, CAT, SOD and APX activities in stressed plants exposed to higher concentration of Hg might be due to inhibition of enzyme synthesis or due to a change in the assembly of enzyme subunits under such conditions (Ushimaru et al. 1999).
Non enzymatic antioxidants also play a significant role in the regulation of ROS level under stressful condition in plants. In this study, two of these components ASC and carotenoid have been measured under Hg stress. The content of ASC and carotenoid had no changes at low concentration of Hg (2.5 μM) but increased in plants treated with 5 and 10 μM Hg. The content showed a decrease in level with 25 μM Hg as compared to control. This further provides evidence that at higher Hg concentration the leaves and roots of wheat plants are subjected to severe oxidative stress and the antioxidative system of the plant was unable to remove the stress.
In conclusion, wheat plants exposed to lower concentrations of Hg (2.5 and 5 μM) did not experience any oxidative stress. However, on treatment with 10 μM Hg, the roots and leaves responded differently. The roots of these plants though could not induce the antioxidant defense system against the oxidative stress, the leaves effectively generated the defense system mediated by antioxidants (CAT and POX) to scavenge H2O2. Both the leaves and roots of plants treated with higher concentration of Hg (25 μM) were subjected to comparatively greater oxidative damage and demonstrated that the antioxidative components were not able to remove the Hg stress at higher concentration. Thus the study of growth response, nutrient uptake and biochemical analysis of antioxidants and other parameters reveal that Hg (≥ 10 μM) may severely affect the overall productivity in wheat plants.
Acknowledgements
We wish our sincere thanks to the authorities of MATS University for providing us laboratory facilities to carry out this work.
References
- Aebi HE. Catalase. In: Bergemeyer HU, editor. Methods of enzymatic analysis. Verlag Chemie: Weinheim; 1983. pp. 273–285. [Google Scholar]
- Aina R, Labra M, Fumagalli P, Vannini C, Marsoni M. Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environ Exp Bot. 2007;59:381–392. doi: 10.1016/j.envexpbot.2006.04.010. [DOI] [Google Scholar]
- Bradford M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Arabi MA, Delaune RD, Gambrell RP, Patrick WH. Arsenic in wetland vegetation: availability, phytotoxicity, uptake and effects on plant growth and nutrition. Sci Total Environ. 1998;217:189–199. doi: 10.1016/S0048-9697(98)00195-8. [DOI] [Google Scholar]
- Chaudhuri K, Chaudhuri MA. Effects of short term NaCl salinity stress on free radicals mediated membrane damage in two jute species. Indian J Exp Biol. 1993;3:327–331. [Google Scholar]
- Cho U, Park J. Changes in hydrogen peroxide content and activities of antioxidant enzymes in tomato seedlings exposed to mercury. J Plant Biol. 1999;42:41–48. doi: 10.1007/BF03031145. [DOI] [Google Scholar]
- Cho U, Park J. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000;156:1–9. doi: 10.1016/S0168-9452(00)00227-2. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986;24:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Gong M, Li YJ, Chen SZ. Abscisic acid induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant system. J Plant Physiol. 1998;153:488–496. doi: 10.1016/S0176-1617(98)80179-X. [DOI] [Google Scholar]
- Gupta M, Chandra P. Bioaccumulation and toxicity of mercury in rooted-submerged macrophyte Vallisneria spiralis. Environ Pollut. 1998;103:327–332. doi: 10.1016/S0269-7491(98)00102-X. [DOI] [Google Scholar]
- Heath RL, Packer L. Photooxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–194. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method of growing plant without soil. Calif Agr Expt Stat Circ. 1950;374:1–32. [Google Scholar]
- Israr M, Sahi S, Datta R, Sarkar D. Bioaccumulation and physiological effects of mercury in Sesbania drummonii. Chemosphere. 2006;65:591–598. doi: 10.1016/j.chemosphere.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol. 2001;88:119–125. doi: 10.1034/j.1600-0773.2001.d01-92.x. [DOI] [PubMed] [Google Scholar]
- Kupper H, Kupper F, Spiller M. In situ detection of heavy metal substituted chlorophylls in water plants. Photosyn Res. 1998;58:123–133. doi: 10.1023/A:1006132608181. [DOI] [Google Scholar]
- Liu D, Wang X, Chen Z, Xu H, Wang Y. Influence of mercury on chlorophyll content in winter wheat and mercury bioaccumulation. Plant Soil Environ. 2010;56:139–143. [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. doi: 10.1104/pp.010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Rodriguez E, Luguera M, Lucena JJ, Carpena-Ruiz RO. Evaluation of two different acid digestion methods in closed systems of trace element determination in plants. Quim Anal. 1995;14:27–30. [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin MJ, Tiller KG, Naidu R, Stevens DP. The behaviour and environmental impact of contaminants in fertilizers. Aust J Soil Res. 1996;34:1–54. doi: 10.1071/SR9960001. [DOI] [Google Scholar]
- Mishra A, Choudhuri MA. Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant. 1999;42:409–415. doi: 10.1023/A:1002469303670. [DOI] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Moreno-Jimenz E, Penalosa JM, Esteban E, Carpena Ruiz RO. Mercury accumulation and resistance to mercury stress in Rumex induratus Marrubium vulgare grown in perlite. J Plant Nutr Soil Sci. 2007;170:485–494. doi: 10.1002/jpln.200625238. [DOI] [Google Scholar]
- Mukherjee AK, De B. Hg induced metabolic changes in seedlings of cultivated cells of tomato. Geobios. 1996;23:83–88. [Google Scholar]
- Mukherji S, Mukherji C. Characterization of cadmium effects in different plant materials. Indian J Exp Biol. 1979;17:265–269. [Google Scholar]
- Ortega-Villasante C, Rellan-Alvarez R, del Campo FF, et al. Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot. 2005;56:2239–2251. doi: 10.1093/jxb/eri223. [DOI] [PubMed] [Google Scholar]
- Patra M, Sharma A. Mercury toxicity in plants. Bot Review. 2000;66:379–422. doi: 10.1007/BF02868923. [DOI] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- Prasad MNV. Biomarkers. In: Prasad MNV, Hagemeyer J, editors. Heavy metal stress in plants from molecules to ecosystem. Berlin: Springer Verlag; 2003. pp. 445–448. [Google Scholar]
- Prasad DDK, Prasad ARK. Altered δ-aminolevulenicacid metabolism by lead and mercury in germinating seedlings of bajra (Pennisetum typhoideum) J Plant Physiol. 1987;127:241–249. doi: 10.1016/S0176-1617(87)80143-8. [DOI] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Regulation and properties of plant catalases. In: Foyer CH, Mullineaux PM, editors. Causes of photooxidative stress and amelioration of defense systems in plants. Boca Raton: CRC Press; 1993. pp. 275–315. [Google Scholar]
- Schutzendubel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot. 2002;53:1351–1365. doi: 10.1093/jexbot/53.372.1351. [DOI] [PubMed] [Google Scholar]
- Shiyab S, Chen J, Fengxiang XH, David LM, Fank BM, Mengmeng G, Yi S, Motasim AM. Mercury-induced oxidative stress in Indian mustard (Brassica juncea L.) Environ Toxicol. 2008;24:462–471. doi: 10.1002/tox.20450. [DOI] [PubMed] [Google Scholar]
- Shriapanahi M, Anderson AC. Accumulation of Cd, Hg, Pb by vegetables following long-term land application of waste water. Sci Total Environ. 1986;52:41–48. doi: 10.1016/0048-9697(86)90103-8. [DOI] [PubMed] [Google Scholar]
- Skrebsky EC, Tabaldi LA, Pereira LB, Rauber R, Maldaner J, Cargnelutti D, Gonc¸alves JF, Castro GY, Schetinger MRC, Nicoloso FT. Effect of cadmium on growth, micronutrient concentration, and d-aminolevulinic acid dehydratase and acid phosphatase activities in plants of Pfaffia glomerata. Braz J Plant Physiol. 2008;20:285–294. doi: 10.1590/S1677-04202008000400004. [DOI] [Google Scholar]
- Srivastava M, Ma LQ, Singh N, Singh S. Antioxidant responses of hyperaccumulator and sensitive fern species to arsenic. J Exp Bot. 2005;56:1335–1342. doi: 10.1093/jxb/eri134. [DOI] [PubMed] [Google Scholar]
- Suszcynsky EM, Shann JR. Phytotoxity and accumulation of mercury in tobacco subjected to different exposure routes. Environ Toxicol Chem. 1995;14:61–67. doi: 10.1002/etc.5620140108. [DOI] [Google Scholar]
- Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H. Effect of hypoxia on antioxidant enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant. 1999;107:181–187. doi: 10.1034/j.1399-3054.1999.100205.x. [DOI] [Google Scholar]
- Varshney AK. Phytotoxic effects of mercuric acetate on the development of chlorophyll in excised cotyledons of two cucurbits. Geobios. 1991;18:119–124. [Google Scholar]
- Vizarova G, Zatkalikova T, Zelenakoua E. Effects of Hg on some physiological processes in barley. Biologia. 1985;50:573–576. [Google Scholar]
- Wellburn AR. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Xylander M, Hagen C, Braune W. Mercury increases light susceptibility in the green alga Haematococcus lacustris. Botanica Acta. 1996;109:222–228. [Google Scholar]
- Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM. Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. Inorg Biochem. 2007;101:1–9. doi: 10.1016/j.jinorgbio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Zhou ZS, Guo K, Elbaz AA, Yang ZM. Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot. 2009;65:27–34. doi: 10.1016/j.envexpbot.2008.06.001. [DOI] [Google Scholar]
- Zornoza P, Vazquez S, Esteban E, Fernandez-Pascual M, Carpena R. Cadmium-stress in nodulated white lupin: strategies to avoid toxicity. Plant Physiol Biochem. 2002;40:1003–1009. doi: 10.1016/S0981-9428(02)01464-X. [DOI] [Google Scholar]