Abstract
In waterlogged soil, deficiency of oxygen triggers development of aerenchyma in roots which facilitates gas diffusion between roots and the aerial environment. However, in contrast to other monocots, roots of rice (Oryza sativa L.) constitutively form aerenchyma even in aerobic conditions. The formation of cortical aerenchyma in roots is thought to occur by either lysigeny or schizogeny. Schizogenous aerenchyma is developed without cortical cell death. However, lysigenous gas-spaces are formed as a consequence of senescence of specific cells in primary cortex followed by their death due to autolysis. In the last stage of aerenchyma formation, a ‘spoked wheel’ arrangement is observed in the cortical region of root. Ultrastructural studies show that cell death is constitutive and no characteristic cell structural differentiation takes place in the dying cells with respect to surrounding cells. Cell collapse initiation occurs in the center of the cortical tissues which are characterized by shorter with radically enlarged diameter. Then, cell death proceeds by acidification of cytoplasm followed by rupturing of plasma membrane, loss of cellular contents and cell wall degradation, while cells nuclei remain intact. Dying cells releases a signal through symplast which initiates cell death in neighboring cells. During early stages, middle lamella-degenerating enzymes are synthesized in the rough endoplasmic reticulum which are transported through dictyosome and discharged through plasmalemma beneath the cell wall. In rice several features of root aerenchyma formation are analogous to a gene regulated developmental process called programmed cell death (PCD), for instance, specific cortical cell death, obligate production of aerenchyma under environmental stresses and early changes in nuclear structure which includes clumping of chromatin, fragmentation, disruption of nuclear membrane and apparent engulfment by the vacuole. These processes are followed by crenulation of plasma membrane, formation of electron-lucent regions in the cytoplasm, tonoplast disintegration, organellar swelling and disruption, loss of cytoplasmic contents, and collapse of cell. Many processes in lysing cells are structural features of apoptosis, but certain characteristics of apoptosis i.e., pycnosis of the nucleus, plasma membrane blebbing, and apoptotic bodies formation are still lacking and thus classified as non-apoptotic PCD. This review article, describes most recent observations alike to PCD involved in aerenchyma formation and their systematic distributions in rice roots.
Keywords: Apoptosis, Cell death, Hypoxia, Lysigeny, Programmed cell death, Regulation, Rice
Introduction
During flooding or waterlogging, diffusion of O2 from air into the soil is effectively blocked and the respiratory consumption by plant roots, soil fauna and microorganisms totally depletes the oxygen. Long term oxygen deficiency triggers functional and developmental responses to promote acclimation to hypoxic or anoxic conditions. These conditions lead to long-term anatomical adaptations (Kawase 1981; Justin and Armstrong 1987; Geigenberger 2003) such as development of aerenchyma in roots (Drew et al.2000; Gunawardena et al.2001a; Colmer 2003a; Malik et al.2003). Aerenchyma is the term given to plant tissues containing enlarged gas spaces exceeding those commonly found as intracellular spaces. It is formed in the roots and shoots of plants adapted to aquatic or semi-aquatic conditions and some dryland species under adverse conditions, either constitutively or because of abiotic stress i.e., flooding (Jackson 1990), nutrient deficiency (Konings and Lambers 1991), soil compaction (He et al. 1996), hypoxia (Sairam et al. 2009), high temperature and drought (Visser and Bogemann 2006). It was first considered 160 years ago that enlarged air spaces or lacunae are formed primarily in the root cortex of flowering plants that grow in aquatic or wetland environments under hypoxic conditions. Such ‘receptacles of air’ were depicted to be formed by the destruction of a mass of parenchyma (Schleiden 1849). Thirty years later Sachs and De Bary identified that enlarged air chambers are formed as either lysogenously or schizogenously (Sachs 1882; De Bary 1884). The term ‘aerenchym’, or aerenchyma was used initially by Schenck for these enlarged spaces particularly in peridermal and cortical tissues (Schenck 1890).
Root aerenchyma is a tissue extending to the shoot and acts as gas transport pathway either due to simple diffusion or by pressure flow in a waterlogged, O2-deficient environment (Armstrong 1971; Das and Jat 1977; Kawase and Whitmoyer 1980; Raven 1996; Jackson and Armstrong 1999) and maintains strength with the least tissue. It transports oxygen to the root tip and rhizosphere besides the reverse diffusion of reduced volatile compounds from the roots and soils, including carbon dioxide, ethylene, organic acids and methane (Neue et al. 1990; Shannon et al. 1996; Colmer 2003b). Methane diffusing out from anaerobic soils of rice fields into the atmosphere through aerenchyma is a major source of greenhouse gas causing global warming. Secondly, aerenchyma formation decreases oxygen demand affected by the removal of cortical cells (Armstrong 1979).
Soil waterlogging and aerenchyma development
Prominent gas spaces or lacunae form in the roots of several monocotyledonous and dicotyledonous species of plants which either habitually grows under hypoxia as in aquatic plants and rice or are subjected to such conditions by poor soil drainage and subsequent waterlogging (Hook and Scholtens 1978; Armstrong 1979; Kawase 1981). In general, formation of aerenchyma is accelerated in response to waterlogged environments during hypoxia e.g. in barley (John 1977) and maize (Jackson et al. 1985a; Drew et al. 2000). However, in root of rice, aerenchyma formation is constitutive type which takes place even in aerobic conditions (John 1977; Clark and Harris 1981), although the extent of aerenchyma formation is triggered by soil waterlogging (Das and Jat 1977; Justin and Armstrong 1991). Furthermore, studies on root aerenchyma formation in rice have also shown enhanced formation of aerenchyma, when O2 deficiency was imposed in hydroponics (Colmer et al. 1998; Colmer 2003a). Ethylene signalling had previously been implicated in enhanced aerenchyma formation in rice (Jackson et al. 1985a) however, further studies cleared that aerenchyma formation in adventitious roots is not controlled by ethylene or hypoxia (Jackson et al. 1985b).
Rice roots are more sensitive to anoxia than other non-tolerant crops (Vartapetian et al. 1970). Aerobic cultivars maintain many semi aquatic adaptations i.e., aerenchyma development in roots and large amount of non-stomatal water loss from leaves (Lafitte and Bennett 2002). However, mitochondrial destruction is observed in anaerobic conditions, which is found to be absent under aerobic conditions in rice roots (Vartapetian and Andreeva 1986). Aerenchyma formation in rice occur due to separation of cell walls from adjacent cells so that the radial walls from the collapsing cells aggregate together, forming “forks”, leaving a large gas-filled space or lacuna between them (Clark and Harris 1981).
Oxygen diffuses from shoots to root tips within the aerenchyma, and radial oxygen loss from roots to the anaerobic root medium is relatively small, so that the outer parts of root have relatively lower permeability to oxygen than to water. Besides this, there should be a sufficient hydraulic permeability for water uptake. Thus in rice, water uptake is hydraulic in nature and oxygen losses are diffusive (Colmer et al. 1998). Some rice cultivars were found to allow oxygen diffusion from aerenchyma to outer surface of roots in order to keep the rhizosphere aerobic during anoxia (Briones et al. 2002). Lack of induction of the barrier to radial oxygen loss is also a positive response of aerenchyma formation to hypoxia and ethylene. This implies that these two root aeration traits, considered to act synergistically to enhance O2 diffusion to the root apex are differentially regulated (Armstrong 1971; Colmer 2003b).
In rice, as compared to root age, probably root-length is the key factor that determines root porosity i.e., aerenchyma during flooding (Justin and Armstrong 1991). However, further studies cleared that roots of plants under waterlogged conditions are relatively shorter and more aerenchymatous than non-waterlogged conditions (Visser et al. 2000) because of slower root growth in the tissue localized near the root apex (Rost 1994). Root aerenchyma does not form during anoxia (Roberts et al.1984). True tolerance to anoxia is found only in few species in the plant kingdom, e.g. rice seeds have the ability to germinate under anoxic conditions (Sauter 2000). The formation of cortical aerenchyma in stems and roots is thought to occur either by lysigeny or schizogeny (Evans 2003; Visser and Voesenek 2004; Seago et al. 2005). Schizogenous aerenchyma occurs when intercellular gas spaces form during tissue development without cell death taking place. Spaces are formed by differential growth, with adjacent cells separating from one another at the middle lamella. It is therefore, a normal developmental process involving the formation of specialized cortical cells that divide and enlarge differentially to create ordered gas spaces by cell separation (Evans 2003). Lysigeny results in the formation of an aerenchyma with conspicuous gas spaces, often with a less regular or ‘ordered’ structure than that seen for an aerenchyma formed by schizogeny. Lysigeny is developed through cell disintegration in the primary cortex of adventitious roots (Shimamura et al. 2007) involving programmed cell death. This review focuses on the programmed cell death during lysigenous aerenchyma formation.
Lysigenous aerenchyma formation
Lysigenous aerenchyma (or lysigeny) is created via programmed cell death (Jackson and Armstrong 1999; Evans 2004). Lysigenous gas spaces form as a consequence of senescence of specific cells followed by their autolysis and cell disintegration (death) in the primary cortex of adventitious roots of rice (Justin and Armstrong 1991; Colmer 2003a; Colmer et al. 2006). Lysigenous aerenchyma develops behind the root tip, in the cortical region having the complete cell expansion (Ranathunge et al.2003; Jung et al.2008). Both schizogenous and lysigenous aerenchyma are fascinating developmental systems as both produce the same end result (aerenchyma) and lysigeny results in the formation of an aerenchyma with conspicuous gas spaces, with a less ordered process. Transverse section of rice root in which lysigenous aerenchyma has been induced shows strands of surviving cells separated through gas spaces in the cortex. This forms a tissue structure resembling a ‘spoked wheel’ arrangement (Joshi et al.2010). The summary of lysigenous aerenchyma development in flooded and aerobic conditions has been depicted in Fig. 1.
Fig. 1.
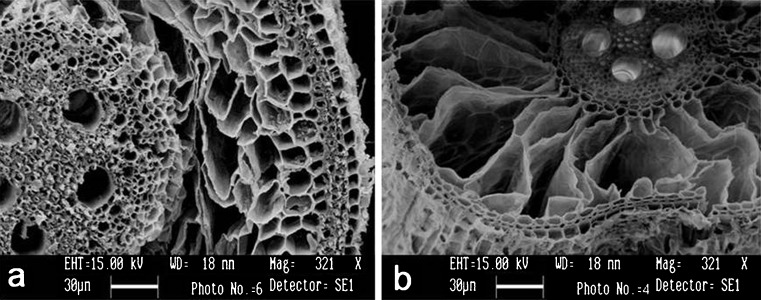
Aerenchyma formation in rice roots under hypoxia. a Cross section of roots that emerged under aerobic conditions (15–20 cm from the root base) showing less aerenchyma. b Cross section of roots that emerged under flooded conitions for 2 weeks (0.5 cm from the root-shoot junction) showing fully developed aerenchyma
Ultrastructural changes during lysigenous aerenchyma formation
In recent decades, response of plant cells during hypoxia was studied principally through modern physical and chemical methods and their conceptualized interpretations were exemplified using electron microscopy. Electron microscopy further reveals the static and dynamic rearrangements of cellular membranes provoked during adaptive or degenerative changes induced by stress. In aerated conditions, rate of elongation of rice is quiet fast (c. 40 mm d−1) and after 6 h, rice cortical cells have been reported to reach the vacuolation stage (Webb and Jackson 1986). However, during hypoxia, rice tissue starts degeneration only 6 h later and shows the marked cell wall degeneration by 12 h. In rice cell wall, middle lamella dissolution occurs in cortical tissue and simultaneously the tonoplast integrity is lost which leads to lysigenous cavitation by 24 h (Webb and Jackson 1986). Meanwhile, the cytoplasm becomes denser and appears like a thin lining between the plasmalemma and the tonoplast (Jackson et al.1985a, b).
Formation of lysigenous aerenchyma in rice is initiated by the death of cortical cells. Since in rice roots, cell death is constitutive type, therefore it is very much difficult to establish chronological pattern. There are many differences between the descriptions of events in aerenchyma formation in rice, likely due to cultivar differences or the problems of working with a non-inducible system. Cell wall breakdown precedes the lysis of the vacuole and after 12 h apparent loss of cell turgour takes place. Aerenchyma formation is observed in cortical tissue 8–10 mm above the root tip (Kawai et al. 1998) and fully developed aerenchyma is observed at a distance of about 100 mm away from tip. However, no appreciable aerenchyma has been found in the apical 1–2 mm of rice roots, even under waterlogging (John 1977). In rice even no characteristic cell morphology differentiation has been observed in the dying cells from surrounding cells (Inada et al.2002). Cell death precedes acidification of cytoplasm, plasma membrane rupture, loss of cellular contents and wall degradation, while cell nuclei remains intact to the late stage (Kawai et al.1998).
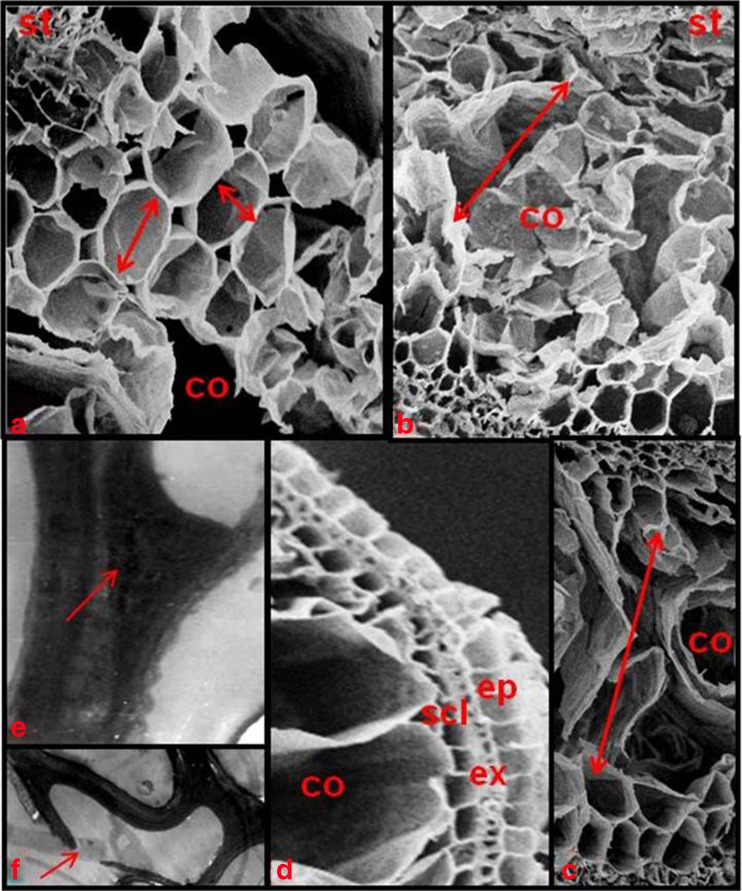
The cells start collapsing from the center of the cortical tissue towards periphery (Kawai et al. 1998). In this position, cells are characterized by being shorter and radially enlarged diameter than other cortical cells (Fig. 2a). This indicates a well defined targeting mechanism for initiating the first cell death. Well-defined outer part of root contains four cell layers after aerenchyma formation (Fig. 2d). Outermost rhizodermis surrounding exodermis is single layered dead sclerenchyma fibre below exodermis and innermost unmodified cortical cell layer adjacent to aerenchyma are separated from each other by radial, monolayered walls, which appeared as spokes in cross-sections (Clark and Harris 1981).
Fig. 2.
Cross section of a root showing programmed cell death during aerenchyma formation in rice roots. a Spatial distribution of cortical cell expansion during later stages. Arrow indicates the radial and tangential cell diameters. b-c Initiation of PCD and radial expansion during aerenchyma development. Arrows indicate the direction of PCD from centre of cortical tissue towards periphery. d Later stages of aerenchyma development showing outer part of roots contained four cell layers (rhizodermis, exodermis, sclerenchyma and one cortical cell layer). e Condensation of the cytoplasm against the edges of the cortical cell during later stages (arrows). f Membrane degaradation before the collapse of the cells during later stages. co cortical cells, ep epidermis, ex exodermis, scl sclerenchyma, st stele
Physiological events leading to lysigenous cell death
Jackson et al. (1985b) reported that aerenchyma formation in adventitious roots of rice is not controlled by ethylene or by low partial pressure of oxygen because neither inhibitors of ethylene action and biosynthesis prevent PCD nor exogenous ethylene concentrations increase root porosity. Later on further studies using different cultivars contradicted these results (Justin and Armstrong 1991; He et al. 1996) and role of ethylene as a regulator of lysigenous aerenchyma formation in inducible systems was confirmed (Drew et al.2000; Gunawardena et al.2001a; Colmer et al.2006). Exogenous ethylene increases aerenchyma formation in rice plants as seen in hypoxia (Colmer et al.2006). Similarly, aerenchyma formation is inhibited when endogenous ethylene synthesis is blocked in rice (Justin and Armstrong 1991). Once released, ethylene is detected by an ethylene receptor in the cortical cells that die to form aerenchyma. However, other reports suggested that unlike maize, rice always forms a certain degree of aerenchyma in its roots (Colmer 2003a) and fails to synthesize more ethylene in hypoxia. Intervarietal differences in sensitivity and ethylene mediated promotion of gas space formation have been reported in rice (Justin and Armstrong 1991).
Thus different mechanisms are involved during cell wall breakdown process in rice. During the early stages of growth and maturation, middle lamella-degenerating enzymes are synthesized in the rough endoplasmic reticulum. These are further transported via dictyosome or in individual vesicles and discharge through plasmalemma either directly or deposite accumulations in the vicinity of plasma membrane. In addition to changes in esterified and de-esterified pectins (Gunawardena et al.2001b), the role of other wall degrading enzymes including expansins, cellulases, xyloglucan endo-transglycosylase and pectinases has also been reported (Jackson and Armstrong 1999). It is likely that there are differences in the sensitivity of cells to the stimuli initiating cell death, whether hypoxia or ethylene or in subsequent response pathways. Recently, three different mechanisms have been reported for sensing hypoxia or anoxia: non-symbiotic haemoglobin and nitric oxide gene expression linked to alternative type of respiration besides mitochondrial electron transport (Sairam et al.2009), changes in cytosolic Ca2+ concentration (Drew 1997) and ethylene (Colmer 2003b; Evans 2003; Voesenek et al. 2006). Sensing also occur if only a part of root is exposed to hypoxic conditions leading to a response along the whole root (Malik et al. 2003).
Regulation of lysigenous aerenchyma formation
To date, a plenty of research work is available in literature on the regulation of lysigenous aerenchyma formation in rice roots. Since in rice the lysigenous aerenchyma formation is constitutive type, therefore research work on aerenchyma development is available under both normal as well as hypoxia conditions (Jackson et al. 1985b). Hypoxia conditions induce lysigenous aerenchyma, despite the fact that aerenchyma formation is constitutive type in rice (Das and Jat 1977, Joshi et al.2010). Therefore, it is obvious that cell death during aerenchyma formation is governed endogenously by internal factors. Induced lysigenous aerenchyma formation is initiated by internal and external stimuli. The key initiator of lysigeny in hypoxia is ethylene, which accumulates within the tissue (Jackson et al.1985a). Moreover, cellular changes are not merely a direct consequence of hypoxia, which are initiated by programmed cell death (Gunawardena et al.2001a). Sequential spread of programmed cell death takes place due to H2O2 produced as an oxidative burst. In this context, the higher doses of H2O2 have been reported to induce cell death in higher plants (Tenhaken et al.1995). Interactions of H2O2 signals in conjunction with ethylene further stimulate aerenchyma formation (Colmer et al.2006).
At present using modern tools, the cells that are going to die at an early stage during constitutive lysigenous aerenchyma formation can be studied. In rice roots, aerenchyma formation is initiated in the mid cortex and these cells are shorter and larger in radial diameter than other cortical cells (Fig. 2a) (Kawai et al.1998). During cell collapsing, the cells loose contact with tangential cells, and are sequentially degraded in a radial fashion in cortical parenchyma tissues (Fig. 2b–d). Dying cells release a message for initiating PCD in neighboring cells through a cytoplasmic syncytium termed the symplast, which initiates the process of cell death in neighboring cells (Baron-Epel et al.1988). Further investigations have suggested that symplastic transport is mediated by specialized trans-cell wall structures called plasmodesmata. It was also reported that oxidative stress-activated MAP triple-kinase-1 (OMTK1) which is a specific MAPK kinase kinase can be activated only by H2O2 and not by abiotic stresses or hormones. Consequently, OMTK1 activates downstream MAP kinase MMK3, which results in cell death. MMK3 can also be activated by ethylene and elicitors, thus serving as a convergence point of the cell death pathway (Nakagami et al.2004).
Non-apoptotic programmed cell death during aerenchyma formation
Programmed cell death (PCD) is gene regulated process which occurs during development and in response to environmental cues (Greenberg 1996; Jones and Dangl 1996; Collazo et al. 2006). This is energy dependent asynchronic process characterized by loss of cellular connections, cytoplasmic shrinkage, membrane blebbing, DNA fragmentation, nuclear disassembly and apoptotic body formation (Collazo et al.2006). Other changes in cell structure depends upon the type of cell death examined (Greenberg 1996). Various methods are now available to identify programmed cell death and now it can be implicated with more confidence. In animal cells, apoptosis is characterized by development of characteristic cell morphology (Kitanaka and Kuchino 1999), including cell shrinkage, chromatin condensation (pycnosis), nuclear fragmentation, and exocytosis of cell contents in membrane-bound “apoptotic” bodies by macrophages (Kerr et al.1972). However, a second form of PCD occurs in both animal as well as plant, known as cytoplasmic cell death (CCD) (Clarke 1990) in which organelles are degraded in specific sequence, before chromatin condensation but nuclear changes do not occur until vesiculation and the formation of autophagic vacuoles. In contrast, necrotic cell death is an unregulated process of traumatic destruction, characterized by initial changes in mitochondria and cell swelling prior to death (Kerr et al. 1972) without the active participation of the cell (Okada and Mak 2004).
Programmed cell death in higher plants is characterized by developing tracheary cells (Mittler and Lam 1995), root cap cells (Wang et al.1996), tapetum cell degradation for pollen development (Davies et al.1992), sexual organ formation (Delong et al.1993) and several other processes. Aerenchyma development under hypoxia is an example of PCD in which rice root cortical cells are induced to die and form larger airspaces (Drew et al.2000). However PCD is not purely identical to apoptosis as well as cytoplasmic cell death, though its some properties resemble to both. Due to this perhaps it is termed ‘lysogenetic cell death’ (De Bary 1884). The predictable lysis of root cortex cells suggests aerenchyma production in roots depends on a genetically controlled program of cell death (Jones and Dangl 1996; Drew 1997; Kawai et al.1998). In the view of above facts, the question arises that whether PCD in rice roots resembles apoptosis or CCD? Previous reports yet do not show any evidence for membrane inclusions resembling apoptotic bodies and staining nuclei using TUNEL assay shows negative results (Kawai and Uchimiya 2000). This in turn suggests that the formation of constitutive aerenchyma in rice is surprisingly unique type. Further, ultrastructural study reveals chromatin condensation and its redistribution to the periphery of the nucleus in cells of the mid cortex at an early stage which is a characteristic of apoptosis in animal cells, which validates TUNEL data. However, there are marked differences in the later stages of PCD between plants and animals.
We hypothesize that aerenchyma development in rice is nonapoptotic based on some previous studies (Campbell and Drew 1983; Webb and Jackson 1986; Joshi et al.2010). In the early phase of aerenchyma formation in rice roots, nuclei remain intact. In later stages middle lamella dissolution precedes breakdown of the tonoplast in O. sativa. Concentric circles of membranes are observed in lysing cells. These membranes are hypothesized to be endoplasmic reticulum which was reorganized at energy charge values (Davies et al.1992). In O. sativa several features of root aerenchyma formation are consistent with PCD, i.e., specific cortical cell death (Schussler and Longstreth 1996), obligate production of aerenchyma under various environmental conditions (Ranathunge et al.2003; Vartapetian et al.2003; Jung et al.2008), and early changes in nuclear structure including clumping of chromatin, fragmentation, disruption of nuclear membrane and apparent engulfment by the vacuole followed by crenulation of plasma membrane, formation of electron-lucent regions in the cytoplasm, tonoplast disintegration, organellar swelling and disruption, loss of cytoplasmic contents, and collapse of cell (Fig. 2e–f) (Borras et al.2006; Joshi et al.2010). These observations are not consistent with cell necrosis (Kerr et al.1972) and characteristics of apoptosis i.e., pycnosis of the nucleus, plasma membrane blebbing, and subsequent production of apoptotic bodies have also not been observed in lysing cells. Thus PCD during aerenchyma formation in rice (O. sativa) roots indicates nonapoptotic degradation.
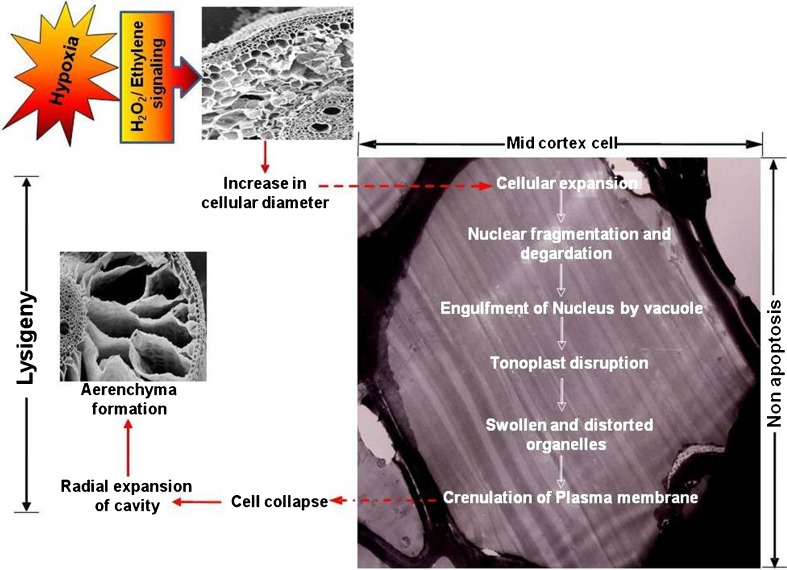
A proposed model of nonapoptotic cortical cell death leading to lysigenous aerenchyma formation in rice roots presented in Fig. 3. During hypoxia, signaling events, which may include endogenous ethylene or H2O2 leads to prominent cellular expansion in mid cortex. After these events, nuclear degradation and tonoplast breakdown occur, followed by cell death, which coincides with degradation of organelles and crenulation of plasmamembrane. Cells lose contact with neighboring (tangential) cell followed by rapid expansion of cavity in radial direction and at last formation of aerenchyma.
Fig. 3.
Schematic model of programmed cell death during aerenchyma formation in rice roots. Aerenchyma in rice roots form by nonapoptotic lysigeny of cortical cells. Hypoxia induced signalling leads to cell enlargement in the mid cortex, followed by nuclear disruption and engulfment by vacuole. In later stages, tonoplast disruption and distortion of cellular organelles occur, followed by the loss of plasma-membrane integrity. After these events, cells lose contact with neighboring (tangential) cells, and collapse. Once cell collapse begins, the cavity then rapidly expands radially leading to aerenchyma formation
Conclusion and key questions to be unveiled
In flowering plants, long term oxygen deficiency during waterlogging lead to the development of aerenchyma. Rice roots are more sensitive to anoxia than other non-tolerant crops. This review article covers the static and dynamic rearrangements of cellular membranes provoked during adaptive or degenerative changes induced by stress. Though in rice the lysigenous aerenchyma formation is constitutive but hypoxia conditions accelerate initiation of aerenchyma showing induced lysigenous aerenchyma. The cells start collapsing from the center of the cortical tissue towards periphery. Dying cells release a message for initiating PCD in neighboring cells through symplast. Cell death precedes acidification of cytoplasm, plasma membrane rupture, loss of cellular contents and wall degradation, while cell nuclei remain intact till late stage. There are several features of root aerenchyma formation that are consistent with PCD, i.e., specific cortical cell death, early changes in nuclear structure and apparent engulfment by the vacuole followed by crenulation of plasma membrane, formation of electron-lucent regions in the cytoplasm, tonoplast disintegration, loss of cytoplasmic contents, and collapse of cell. But some characteristics of apoptosis like pycnosis of the nucleus, plasma membrane blebbing, and subsequent production of apoptotic bodies are not observed in lysing cells. In the view of above facts, it is concluded that PCD during aerenchyma formation is non-apoptotic type in rice roots.
However, there are few key questions to be unveiled in future. The first and foremost question arises that why only some cells within the cortical tissue die and not other? One possibility is that the cells die by necrosis, which is a localized and uncontrolled form of cell death that probably occurs when the cells are exposed to condition varying from optimum physiological conditions (Evans 2003). Necrotic cell death is triggered by acidification of the cytoplasm in oxygen scarcity (Vartapetian and Jackson 1997). To date, it is obscure why constitutive root aerenchyma benefits wetland plants more than inducible aerenchyma? When the plants are grown in conditions with fluctuating soil-water levels, roots become adapted rapidly to switch from a flooding system to drained conditions and vice-versa (Jansen et al.2005). It is also difficult to understand that roots containing aerenchyma are less well suited for growth under drained conditions.
In addition, there is further need to understand the basis of physiological, biochemical and genetic regulation during aerenchyma formation. Limiting factors affecting both induced and constitutive lysigenous aerenchyma development need to be studied. Another research goal for induced aerenchyma seems to be, analysis of the signal transduction pathways involving cytosolic calcium and protein phosphorylation in cell death. Although the working concepts of PCD was originated with plants, but the plant researchers still use animal paradigms for understanding plant PCD. Probably comparative study on PCD in plant and animal cells may give an insight into the primordial pathway in future.
References
- Armstrong W. Radial oxygen losses from rice roots as affected by distance from the apex, respiration and water logging. Physiol Plant. 1971;25:192–197. doi: 10.1111/j.1399-3054.1971.tb01427.x. [DOI] [Google Scholar]
- Armstrong W. Aeration in higher plants. In: Woolhouse HW, editor. Advances in botanical research. London: Academic; 1979. pp. 225–332. [Google Scholar]
- Baron-Epel D, Hernandez D, Jiang LW, Meiners S, Schindler M. Dynamic continuity of cytoplasmic and membrane compartments between plant cells. J Cell Biol. 1988;106:715–721. doi: 10.1083/jcb.106.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás O, Collazo C, Chacón O. Programmed cell death in plants resembles apoptosis of animals. Biotecnol Apl. 2006;23:1–10. [Google Scholar]
- Briones AM, Okabe S, Umemiya Y, Ramsing NB, Reichardt W, Okuyama H. Influence of different cultivars on population of ammonia-oxidizing bacteria in the root environment of rice. Appl Environ Microbiol. 2002;68:3067–3075. doi: 10.1128/AEM.68.6.3067-3075.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R, Drew MC. Electron microscopy of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to oxygen shortage. Planta. 1983;157:350–357. doi: 10.1007/BF00397407. [DOI] [PubMed] [Google Scholar]
- Clark LH, Harris WM. Observations on the root anatomy of rice (Oryza sativa L.) Am J Bot. 1981;68:154–161. doi: 10.2307/2442846. [DOI] [Google Scholar]
- Clarke P. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Collazo C, Chacon O, Borras O. Programmed cell death in plants resembles apoptosis of animals. Biotecnol Apl. 2006;23:1–10. [Google Scholar]
- Colmer TD. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.) Ann Bot. 2003;91:301–309. doi: 10.1093/aob/mcf114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. Long distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Pl Cell Environ. 2003;26:17–36. doi: 10.1046/j.1365-3040.2003.00846.x. [DOI] [Google Scholar]
- Colmer TD, Gibbered MR, Wiengweera A, Tinh TK. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J Expt Bot. 1998;49:1431–1436. doi: 10.1093/jexbot/49.325.1431. [DOI] [Google Scholar]
- Colmer TD, Cox MCH, Voesenek LACJ. Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide and ethylene signals for acclimation of roots in waterlogged soils. New Phytol. 2006;170:767–778. doi: 10.1111/j.1469-8137.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- Das DK, Jat RL. Influence of three soil water regimes on root porosity and growth of four rice varieties. Agron J. 1977;69:197–210. doi: 10.2134/agronj1977.00021962006900020001x. [DOI] [Google Scholar]
- Davies RA, Singh MB, Knox RB. Identification and in situ localization of pollen-specific genes. Int Rev Cytol. 1992;140:19–34. doi: 10.1016/S0074-7696(08)61092-X. [DOI] [Google Scholar]
- De Bary A. Comparative anatomy of the vegetative organs of phanerogams and ferns. UK: Oxford University Press; 1884. [Google Scholar]
- Delong A, Calderon-Urrea A, Dellaporta SL. Sex determination genes TASSLESEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage-specific floral organ abortion. Cell. 1993;74:757–768. doi: 10.1016/0092-8674(93)90522-R. [DOI] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/S1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Evans DE. Aerencyma formation. Transley review. New Phytol. 2003;161:35–49. doi: 10.1046/j.1469-8137.2003.00907.x. [DOI] [Google Scholar]
- Evans DE. Aerenchyma formation. New Phytol. 2004;161:35–49. doi: 10.1046/j.1469-8137.2003.00907.x. [DOI] [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Curr Opin Plant Biol. 2003;6:247–256. doi: 10.1016/S1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE. Characterisation of programmed cell death during aerenhyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.) Planta. 2001;212:205–214. doi: 10.1007/s004250000381. [DOI] [PubMed] [Google Scholar]
- Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE. Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant Cell Environ. 2001;24:1369–1375. doi: 10.1046/j.1365-3040.2001.00774.x. [DOI] [Google Scholar]
- He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol. 1996;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook DD, Scholtens JR. Adaptations and flood tolerance of tree species. In: Hook DD, Crawford RMM, editors. Plant life in anaerobic environments. Ann Arbor: Ann Arbor Science Publishers; 1978. pp. 299–331. [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T (2002) Three-dimensional progression of programmed death in the rice coleoptile. Int Rev Cytol—a Survey of Cell Biol 218 [DOI] [PubMed]
- Jackson MB. Hormones and developmental change in plants subjected to submergence or soil waterlogging. Aquat Bot. 1990;38:49–72. doi: 10.1016/0304-3770(90)90098-6. [DOI] [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999;1:274–287. doi: 10.1111/j.1438-8677.1999.tb00253.x. [DOI] [Google Scholar]
- Jackson MB, Fenning TM, Drew MC, Saker LR. Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta. 1985;165:486–492. doi: 10.1007/BF00398093. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Fenning TM, Jenkins W. Aerenchyma (gas space) formation in adventitious roots of rice (Oryza sativa L.) is not controlled by ethylene or small partial pressures of oxygen. J Exp Bot. 1985;36:1566–1572. doi: 10.1093/jxb/36.10.1566. [DOI] [Google Scholar]
- Jansen C, van de Steeg HM, de Kroon H. Investigating a trade-off in root morphological responses to a heterogeneous nutrient supply and to flooding. Funct Ecol. 2005;19:952–960. doi: 10.1111/j.1365-2435.2005.01049.x. [DOI] [Google Scholar]
- John CD. The structure of rice roots grown in aerobic and anaerobic environments. Plant Soil. 1977;47:269–274. doi: 10.1007/BF00010390. [DOI] [Google Scholar]
- Jones AM, Dangl JL. Logjam at the styx: programmed cell death in plants. Trends Plant Sci. 1996;1:114–119. doi: 10.1016/S1360-1385(96)90005-9. [DOI] [Google Scholar]
- Joshi R, Shukla A, Mani SC, Kumar P. Hypoxia induced non-apoptotic cellular changes during aerenchyma formation in rice (Oryza sativa L.) roots. Physiol Mol Biol Plants. 2010;16:99–106. doi: 10.1007/s12298-010-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Lee SC, Choi HK. Anatomical patterns of aerenchyma in aquatic and wetland plants. J Plant Biol. 2008;51:428–439. doi: 10.1007/BF03036065. [DOI] [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytol. 1987;106:465–495. doi: 10.1111/j.1469-8137.1987.tb00153.x. [DOI] [Google Scholar]
- Justin SHFW, Armstrong W. Evidence for the involvement of ethane in aerenchyma formation in adventitious roots of rice (Oryza sativa L.) New Phytol. 1991;118:49–62. doi: 10.1111/j.1469-8137.1991.tb00564.x. [DOI] [Google Scholar]
- Kawai M, Uchimiya H. Coleoptile senescence in rice (Oryza sativa L.) Ann Bot. 2000;86:405–414. doi: 10.1006/anbo.2000.1199. [DOI] [Google Scholar]
- Kawai M, Samarajeewa PK, Barrero RA, Nishiguchi M, Uchimiya H. Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta. 1998;204:277–287. doi: 10.1007/s004250050257. [DOI] [Google Scholar]
- Kawase M. Anatomical and morphological adaptation of plants to waterlogging. Hort Sci. 1981;16:30–34. [Google Scholar]
- Kawase M, Whitmoyer RE. Aerenchyma development in waterlogged plants. Am J Bot. 1980;67:18–22. doi: 10.2307/2442533. [DOI] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Brit J Canc. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka C, Kuchino Y. Caspase-independent cell death with necrotic morphology. Cell Death Diff. 1999;6:508–515. doi: 10.1038/sj.cdd.4400526. [DOI] [PubMed] [Google Scholar]
- Konings H, Lambers H. Respiratory metabolism, oxygen transport and the induction of aerenchyma in roots. In: Jackson MB, Davies DD, Lambers H, editors. Plant Life Under Oxygen Deprivation. The Hague: SPB Academic Publishing; 1991. pp. 247–265. [Google Scholar]
- Lafitte HR, Bennett J (2002) Requirements for aerobic rice: physiological and molecular considerations. In: Bouman BAM, Hengsdijk H, Bindraban PS, Tuong TP, Hardy B, Ladha JK (eds) Water wise rice production. International workshop on water-wise rice production. Los Banos, Phillipines. 8–11 Apr. IRRI, pp. 259–274
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. Aerencyma formation and radial O2 loss along adventitious roots of wheat with only the apical root portion exposed to O2 deficiency. Plant Cell Environ. 2003;26:1713–1722. doi: 10.1046/j.1365-3040.2003.01089.x. [DOI] [Google Scholar]
- Mittler R, Lam E. In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiol. 1995;108:489–493. doi: 10.1104/pp.108.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Kiegerl S, Hirt H. OMTK1, a novel MAPKKK, channels oxidative stress signalling through direct MAPK interaction. J Biol Chem. 2004;279:26959–26966. doi: 10.1074/jbc.M312662200. [DOI] [PubMed] [Google Scholar]
- Neue HU, Becker-Heidmann P, Scharpenseel HW. Organic matter dynamics, soil properties, and cultural practices in rice lands and their relationship to methane production. In: Bouwman AF, editor. Soils and greenhouse effect. Chichester: Wiley; 1990. pp. 457–466. [Google Scholar]
- Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nature Rev. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta. 2003;217:193–205. doi: 10.1007/s00425-003-0984-9. [DOI] [PubMed] [Google Scholar]
- Raven JA. Into the voids: The distribution, function, development and maintenance of gas spaces in plants. Annals of Bot. 1996;78:137–142. doi: 10.1006/anbo.1996.0105. [DOI] [Google Scholar]
- Roberts AWCJ, Wemmer D, Walbot V, Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. PNAS USA. 1984;81:3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost TL. Root tip organization and spatial relationship of differentiation events. In: Iqbal M, editor. Growth patterns in vascular plants. Portland: Dioscorides Press; 1994. pp. 59–76. [Google Scholar]
- Sachs JA. A text book of botany. UK: Oxford University Press; 1882. [Google Scholar]
- Sairam RK, Kumutha D, Ezhilmathi K. Waterlogging tolerance: nonsymbiotic haemoglobin–nitric oxide homeostasis and antioxidants. Curr Sci. 2009;96:674–682. [Google Scholar]
- Sauter M. Rice in deep water: “How to take heed against a sea of troubles?”. Naturwissenschaften. 2000;87:289–303. doi: 10.1007/s001140050725. [DOI] [PubMed] [Google Scholar]
- Schenck H. Ueber das Aerenchym ein dem Kork homologes Gewebe bei Sumpfpflanzen. Jahrbucher fur Wissenschaftliche Botanik. 1890;20:526–574. [Google Scholar]
- Schleiden JM (1849) Principles of scientific botany. (Translation by Lankaster E, 1849). London: Longman, Brown, Green, and Longmans
- Schussler EE, Longstreth DJ. Aerenchyma develops by cell lysis in roots and cell separation in leaf petioles in Sagittaria lancifolia (Alismataceae) Am J Bot. 1996;83:1266–1273. doi: 10.2307/2446110. [DOI] [Google Scholar]
- Seago JL, Marsh LC, Stevens KJ, Soukup A, Votrubova O, Enstone DE. A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann Bot. 2005;96:565–579. doi: 10.1093/aob/mci211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RD, White JR, Lawson JE, Gilmour BS. Methane efflux from emergent vegetation in peatlands. J Ecol. 1996;84:239–246. doi: 10.2307/2261359. [DOI] [Google Scholar]
- Shimamura S, Yoshida S, Mochizuki T. Cortical aerenchyma formation in hypocotyl and adventitious roots of Luffa cylindrica subjected to soil flooding. Annals Bot. 2007;100:1431–1439. doi: 10.1093/aob/mcm239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. PNAS USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian BB, Andreeva IN. Mitochondrial ultrastructure of three hydrophyte species at anoxia and in anoxic glucose supplemented medium. J Expt Bot. 1986;37:685–692. doi: 10.1093/jxb/37.5.685. [DOI] [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptation to anaerobic stress. Ann Bot. 1997;79:3–20. doi: 10.1006/anbo.1996.0295. [DOI] [Google Scholar]
- Vartapetian BB, Andreeva IN, Maslova IP, Davtian NG. The oxygen and ultrastructure of root cells. Agrochimica. 1970;15:1–19. [Google Scholar]
- Vartapetian BB, Andreeva IN, Generozova IN, Polyakova LI, Maslova IP, Dolgikh YI, Stepanova AY. Functional electron microscopy in studies of plant response and adaptation to anaerobic stress. Ann Bot. 2003;91:155–172. doi: 10.1093/aob/mcf244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Bögemann GM. Aerenchyma formation in the wetland plant Juncus effuses is independent of ethylene. New Phytol. 2006;171:305–314. doi: 10.1111/j.1469-8137.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Voesenek LACJ. Acclimation to soil flooding sensing and signal transduction. Pl Soil. 2004;254:197–214. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ. 2000;23:1237–1245. doi: 10.1046/j.1365-3040.2000.00628.x. [DOI] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. How plants cope with complete submergence. New Phytol. 2006;170:213–226. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: a functional paradigm for programmed plant cell death induced by a host selective phytotoxin and invoked during development. Plant Cell. 1996;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J, Jackson MB. A transmission and cryoscanning electron microscopic study of the formation of aerenchyma (cortical gas-filled space) in adventitious roots of rice (Oryza sativa) J Expt Bot. 1986;37:832–841. doi: 10.1093/jxb/37.6.832. [DOI] [Google Scholar]