Abstract
Recent findings have implicated the role of polyamines (putrescine, spermidine and spermine) in stress tolerance. Therefore, the present work was carried out with the goal of generating transgenic tomato plants with human S-adenosylmethionine decarboxylase (samdc) gene, a key gene involved in biosynthesis of polyamines, viz. spermidine and spermine and evaluating the transgenic plants for tolerance to both biotic and abiotic stresses. Several putative transgenic tomato plants with normal phenotype were obtained, and the transgene integration and expression was validated by PCR, Southern blot analysis and RT-PCR analysis, respectively. The transgenic plants exhibited high levels of polyamines as compared to the untransformed control plants. They also showed increased resistance against two important fungal pathogens of tomato, the wilt causing Fusarium oxysporum and the early blight causing Alternaria solani and tolerance to multiple abiotic stresses such as salinity, drought, cold and high temperature. These results suggest that engineering polyamine accumulation can confer tolerance to both biotic and abiotic stresses in plants.
Keywords: Solanum lycopersicum, Transgenic plants, Polyamines, Spermidine, S-adenosylmethionine decarboxylase, Biotic stress, Abiotic stress
Introduction
Crop productivity, which is of paramount significance to mankind get adversely affected by a plethora of biotic stresses like insects, fungi, bacteria and viruses and abiotic stresses like salinity, drought, flooding, heavy-metal toxicity, extreme temperatures and oxidative stresses (Rao et al. 1999; Punja 2001; Bhatnagar-Mathur et al. 2008). It has been estimated that annually about 42 % of the crop productivity is lost owing to various biotic and abiotic stress factors (Oerke et al. 1994). Among the biotic stresses, fungal pathogens are responsible for large scale damage to many cultivated species. In some instances, fungal diseases can completely destroy fields. In this backdrop, transgenic approaches have gained relevance for crop improvement and have added a new thrust in agriculture especially in recent times (Rao et al. 1999; Babu et al. 2003; Islam 2006; Bhatnagar-Mathur et al. 2008) and would continue to do so. Several novel molecules such as pathogenesis-related proteins (PR), chitinases, glucanases, osmotin, phytoalexins (Punja 2001) and polyamines (PAs) (Rajam 1997; Walters 2003a) have been studied for their role in defense mechanism against fungal pathogens and are potential source for genetic manipulation (Rao et al. 1999; Punja 2001; Islam 2006). Similarly, in case of abiotic stresses, manipulation of the expression or production of the identified genes (e.g. regulatory and transporter genes), osmoprotectants like proline, glycinebetaine, sugars and sugar alcohols (e.g. trehalose, mannitol etc.), proteins (e.g. LEA, heat shock proteins, etc.), enzymes (e.g. pyruvate decarboxylase, alcohol dehydrogenase, etc.) or compounds like PAs through transgenic approaches have been carried out (Bhattacharya and Rajam 2006; Kumar et al. 2006; Bhatnagar-Mathur et al. 2008).
PAs, putrescine (Put), spermidine (Spd) and spermine (Spm) are polycationic amines that are ubiquitously present in all living organisms. They play a critical role in several cellular and developmental processes, including the stabilization of essential biomolecules such as nucleic acids, membrane phospholipids and proteins, and the regulation of cell division, growth and differentiation, and senescence (Martin-Tanguy 2001; Kumar et al. 2006; Kusano et al. 2007) and various stress responses (Walters 2003a, b; Kumar et al. 2006; Groppa and Benavides 2008). The protective role of PAs under various stress conditions has been attributed to their function in maintaining ionic balance, cellular osmoticum and membrane integrity, prevention of chlorophyll loss and chromatin damage (Kumar et al. 2006). Besides, they are key free- radical scavengers owing to their positive charges (Ha et al. 1998). However, stress tolerance of genetically engineered plants for PA biosynthesis has not been extensively studied so far. Over-expression of PA pathway genes like adc (Capell et al. 2004; Roy and Wu 2001; Prabhavathi and Rajam 2007), odc (Kumria and Rajam 2002), samdc (Roy and Wu 2002; Waie and Rajam 2003; Wi et al.2006) and Spd Syn (Kasukabe et al. 2004, 2006; He et al. 2008; Wen et al. 2008) in plants like rice, tobacco, pear, eggplant, Arabidopsis and sweet potato has demonstrated tolerance to one or more abiotic stresses. Current reports of fungal resistance by over-expressing PA pathway gene are few; samdc in tobacco (Waie and Rajam 2003) and adc in eggplant (Prabhavathi and Rajam 2007). It is noteworthy that till date there are no reports of stress tolerance by manipulation of PA pathway in an important economic crop plant, tomato.
Therefore, the present study was undertaken with the objective of generating transgenic tomato plants with human S-adenosylmethionine decarboxylase (samdc; EC 4.1.1.50) gene, a key gene involved in PA biosynthesis and to evaluate the transgenic plants for PA levels and tolerance to both biotic and abiotic stresses under in vitro and in vivo growth conditions.
Materials and methods
Plant material, plasmids and culture conditions
The seeds of tomato (Solanum lycopersicum, cv Pusa Ruby) were procured from the National Seeds Corporation, New Delhi. The axenic seedlings were raised by germinating the soaked seeds (1–2 h in tap water) in egg trays containing soil and vermiculite (1:1) under controlled growth conditions (26 ± 0.5 °C, 16 h photoperiod with irradiance of 40 μEmolm−2s−1). The binary vector pMVR1SAMDC harboring samdc gene under the control of constitutive CaMV 35S promoter with hygromycin phosphotransferase (hpt) as the plant selection marker gene was available in the laboratory (Waie and Rajam 2003) and utilized for tomato transformation.
Tomato transformation and regeneration
Fully expanded cotyledons from 10 to 12 days old seedlings were used for transformation as per the protocol described by Madhulatha et al. (2007). The bacterial culture grown to an OD (A600) of 0.1–0.2 was used for infection (10 min in presence of 3 % sorbitol and 100 μM acetosyringone) using pre-cultured explants on shoot regeneration medium as (SRM) i.e. MS medium supplemented with 2.5 mg/l benzylaminopurine (BAP), 0.5 mg/l indole-3-acetic acid (IAA), 3 % maltose (in place of sucrose) and 0.5 mM putrescine for 2–3 days (Madhulatha et al. 2006) After infection, the explants were co-cultured on SRM supplemented with 3 % sorbitol and 100 μM acetosyringone for 2 days and then transferred to selection medium, i.e. SRM containing 10 mg/l hygromycin (selection agent) and 300 mg/l augmentin (bacteriostatic agent) and cultured for about 6 weeks with fresh subculture every fortnight. The small shoots obtained on selection medium were subjected to proliferation on MS medium fortified with 0.5 mg/l BAP. The well-grown shoots were excised and transferred to the rooting medium (½ MS + 300 mg/l augmentin). The rooted plants were transferred to pots containing vermiculite: soil (1:1) and covered with polythene bags for about a week to maintain high humidity for hardening in the tissue culture room and later transferred to green-house.
Polymerase chain reaction (PCR)
Genomic DNA extracted from the leaf tissue by CTAB method (Doyle and Doyle 1990) was utilized for PCR using primers specific to hpt and samdc to determine the integration of the transgene. About 100 ng of DNA were taken and mixed with 100 nM of forward and reverse primers (for hpt and samdc, seperately), 1 X PCR buffer, 2 mM MgCl2, 100 μM dNTP mix and 0.5 U of Taq polymerase (BIOTOOLS, India), the volume was made up to 25 μl with sterile double distilled water. The samples were denatured initially at 94 °C for 5 min, followed by 30 cycles of denaturation for 1 min at 94 °C, primer annealing for 1 min at appropriate temperature (52 °C for hpt and 53 °C for samdc) and synthesis for 2 min at 72 °C, with the final extension for 10 min at 72 °C. The PCR products were analyzed on 1 % agarose gel. The primer pairs used were—5′ CGC ATG AAA AAG CCT GAA CTC ACC GCG 3′ and 5′ GCA GGC TCC CGT TTC CTT ATC GAT 3′ for hpt gene (to amplify 500 bp) and 5′ GCT GCA CAT TTT TTC GAA G 3′ and 5′AGG TTT GAT CTG GCT GAC 3′ for samdc gene (to amplify 560 bp).
Southern analysis
Southern analysis was performed to confirm the integration and the copy number of the transgene in the PCR positive plants using the standard protocol (Sambrook et al. 1989). 15 μg of genomic DNA was restricted with BamHI and blot was prepared on Nylon (+vely charged) membrane (Sigma, USA). The hpt gene probe was prepared by using the Random priming kit (Takara, Japan) following as per the manufacturer’s guidelines. The probe was denatured before adding to the pre-hybridization buffer and the hybridization was carried out for 18–24 h at 42 °C. The membrane was washed and then exposed to X-ray film (Kodak).
RNA isolation and RT-PCR
Total RNA from leaf tissue weighing 100 mg were isolated according to the instructions provide by the manufacturer using iRIS kit (IHBT, Palampur). The pellet was dissolved in 10–20 μl RNase free water. The RNA samples (treated with RNase-free DNase) at the concentration of 250 ng was used as a template for the one-step RT-PCR reaction. The template RNA was mixed with 1X RT-PCR buffer, 10 mM of dNTP mix, 5 μM forward and reverse primers, 40 U of RNase inhibitor, and 2.5 U of Reverse Transcriptase and Taq Polymerase each. The reaction volume was made up to 25 μl. The reaction mixture was incubated at 48 °C for 30 min. The thermal cycler was preheated before placing the samples in it. After reverse transcription by omniscript and sensiscript reverse transcriptases, reaction mixture was heated to 94 °C for 5 min (to activate Hot Star Taq DNA polymerase and to simultaneously inactivate the reverse transcriptases). 35 cycles of 30 s denaturation at 94 °C, primer annealing at appropriate temperature for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 10 min were performed. The PCR product was analyzed on 1.2 % agarose gel.
Polyamine analysis
Leaf samples corresponding to 0 h (control), 48 h, 72 h and 144 h of fungal inoculated T1 seedlings (all of the same age group ~1 month old) along with untransformed control seedlings were taken for PA analysis. The extraction, dansylation, separation (using Thin Layer Chromatography) and estimation of PAs were carried out as per the protocol (Bajaj and Rajam 1995). Individual PA bands were scrapped off the TLC plate, resuspended in 3 ml ethylacetate and quantified spectrofluorometrically using Bio-Rad dual wavelength spectrofluorometer at an excitation wavelength of 350 nm and emission wavelength of 495 nm. Concentrations of PAs were calculated by extrapolating from the standard curve of known concentration standard PAs. PA analysis was repeated thrice using tissue from three different clones of each line.
Fungal resistance assays
The wilt causing fungus Fusarium oxysporum and the early blight causing Alternaria solani were considered for fungal resistance assays. All the assays were carried out on one-month-old T1 seedlings raised on ½ strength MS basal medium. Each assay was performed at least thrice using three replicates of each transgenic line as well as untransformed control. PCR-positive and/or segregated seedlings (6–8) were taken for each transgenic lines tested. Fungal spores were harvested from freshly grown cultures of F. oxysporum and A. solani on potato dextrose agar (PDA) plates for 10–15 days.
For in vitro assay, one-month-old seedlings of transgenic lines and untransformed (UT) control were inoculated by immersing the root system in freshly prepared F. oxysporum spore suspension (106 spores/ml) in 1/10th liquid MS basal medium for 20 min and were observed for 3–4 weeks time. Disease severity index (DI) was scored on a standard graduated scale by the time wild-type plant had developed severe wilting and necrosis (7–15 days post-inoculation). The disease symptoms were estimated by a five-class disease severity scale (Lin and Xiao 1995); the five classes being, class 0 = no disease symptoms-same size as uninfected controls; class 1 = no/few symptoms-only first true leaf necrotic or curled; class 2 = clear symptoms-first three leaves developed symptoms; class 3 = severe symptoms, only newest true leaves remain healthy, older ones being necrotic and curled, defoliation, growth retardation; class 4 = all developed leaves had fallen off, plant having only one new-formed leaf; class 5 = macerated/rotted or dead. Disease severity index (DI) was calculated by the formula 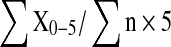 ; where X = no. of plants per class and n = total no. of plants tested. A highly susceptible plant score 1 on the disease severity index, while the tolerant plants has disease severity index below 1 and approaching towards zero. In case of in vivo assay, 20 ml of fungal spore suspension (106 spores/ml) was used to dip the roots of T1 transgenic and untransformed control seedlings for 20 min and then inserted in the plastic pots containing soil: vermiculite mix (1:1) as described by Waie and Rajam (2003). The pots containing these inoculated seedlings were then transferred into the plastic trays containing water and were covered by using polythene bags with small holes to maintain higher humidity. The seedlings were kept at optimum temperature conditions and degree of resistance to the infection was recorded and compared with the wild-type plants.
; where X = no. of plants per class and n = total no. of plants tested. A highly susceptible plant score 1 on the disease severity index, while the tolerant plants has disease severity index below 1 and approaching towards zero. In case of in vivo assay, 20 ml of fungal spore suspension (106 spores/ml) was used to dip the roots of T1 transgenic and untransformed control seedlings for 20 min and then inserted in the plastic pots containing soil: vermiculite mix (1:1) as described by Waie and Rajam (2003). The pots containing these inoculated seedlings were then transferred into the plastic trays containing water and were covered by using polythene bags with small holes to maintain higher humidity. The seedlings were kept at optimum temperature conditions and degree of resistance to the infection was recorded and compared with the wild-type plants.
In case of A. solani, surface-sterilized and fully expanded leaves were used for the in vitro detached leaf assay, where leaves were transferred to petri-plates containing wet blotting sheet. Leaves were inoculated by puncturing them with a sterile needle (one wound/leaf) and then inoculated 10 μl of the A. solani spore suspension (106 spores/ml). Severity of infection was scored on a comparative scale as compared to the response in the wild-plant (Rajam et al. 2007):—(no infection), + (20 % infection), + + (40 % infection), + + + (60 % infection), + + + + (80 % infection), + + + + + (100 % infection). In case of in vivo assay, the leaves of segregated T1 seedlings of different transgenic lines and untransformed control of same age (two-month-old) were inoculated with freshly harvested spore suspension culture (106 spore/ml) of A. solani. The second leaf of each line was punctured with a sterile needle and spore suspension was inoculated on the punctured area with the help of micropipette. Pots containing infected seedlings were transferred to a plastic tray containing water and then properly covered with plastic sheet with small holes to maintain high humidity. The challenged plants were then kept in humidity chamber room maintaining high humidity conditions.
Abiotic stress tolerance assays
The tolerance of the T1 transgenic lines to salinity (NaCl), drought (PEG-mediated), and low and high temperature stress was tested. The experiments were repeated at least thrice.
For salt and drought stress tolerance assays, the T1 seeds of the transgenic lines as well as untransformed control were surface-sterilized and inoculated on to ½ MS basal medium supplemented with 100 and 200 mM NaCl (for salt stress) or 10 and 15 % PEG (MW 8000) (for drought) for salt and drought tolerance assays, respectively. Three-weeks-old segregated T1 seedlings of the transgenic lines as well as UT control seedlings were grown in plastic pots containing vermiculite: soil (1:1) mix. 15 ml of 1/10th MS solution supplemented with 150 and 200 mM NaCl and 10 % and 15 % PEG was poured per pot once a day for 14 days. Data on seedlings height, fresh weight, and dry weight was scored after a period of 1 month for control and transgenic seedlings grown under stress conditions. For floating leaf assays, the leaves of transgenic lines and UT control were cut into small pieces using cork borer and were floated in salt solutions of 150 mM and 200 mM NaCl. Data of the leaf discs was scored after 1 week of inoculation. For desiccation tolerance assays, the detached leaves of the transgenic lines as well as the controls were placed on dry Whatman no.1 filter paper and incubated in the dark for 24 h, and the leaves were scored for water loss (dehydration) as compared to the controls (Prabhavathi and Rajam 2007).
In case of low temperature stress tolerance assays, the T1 seeds of the transgenic lines as well as UT control inoculated on plastic pots were incubated at 6–8 °C for 10 days and then were kept under normal conditions for a month. The germination percentage and seedling growth parameters were scored after a period of 1 month.
In case of high temperature stress tolerance assays, the T1 seeds of the transgenic lines and control inoculated on plastic pots were incubated at room temperature (RT), 40 °C and 45 °C for 48 h and then were kept under normal conditions for a month. The germination percentage and seedling growth parameters were scored after a period of 1 month.
Data analysis
All the experiments were repeated minimum of three times and the data presented are the average (mean) with the standard error from all the experiments and the data was analysed statistically using student’s t-test.
Results and discussion
Transformation and regeneration
The co-cultivated explants transformed using the binary vector pMVR1SAMDC exhibited the appearance of shoot buds within 15 days of transfer to selection medium and developed into plantlets after their transfer to proliferation and rooting medium. The transformation frequency, which was based on the number of explants surviving and regenerating on selection medium upon the total number of co-cultivated explants, was in the range of 16–38 % (Data not shown). These putative transgenic plants named as SAM, no abnormalities were seen in plant morphology, flowering, pollen viability and fruit setting when assessed alongside the UT control plants. In previous studies on genetic manipulation of PA biosynthetic pathway, broadly two lines of observations have been reported. One category of reports pertained to abnormal phenotypes seen in transgenic plants (Capell et al. 2004; Kumria and Rajam 2002; Waie and Rajam 2003), while others demonstrated transgenic plants with normal phenotype (Roy and Wu 2001, 2002; Kasukabe et al. 2004, 2006; Prabhavathi and Rajam 2007; He et al. 2008). Our results and the published reports denote that the phenotypic abnormalities in transgenic plants over-expressing PA genes depend upon the constitutive levels of PAs, and also enhanced levels of PAs due to the transgene expression.
Transgene integration and expression
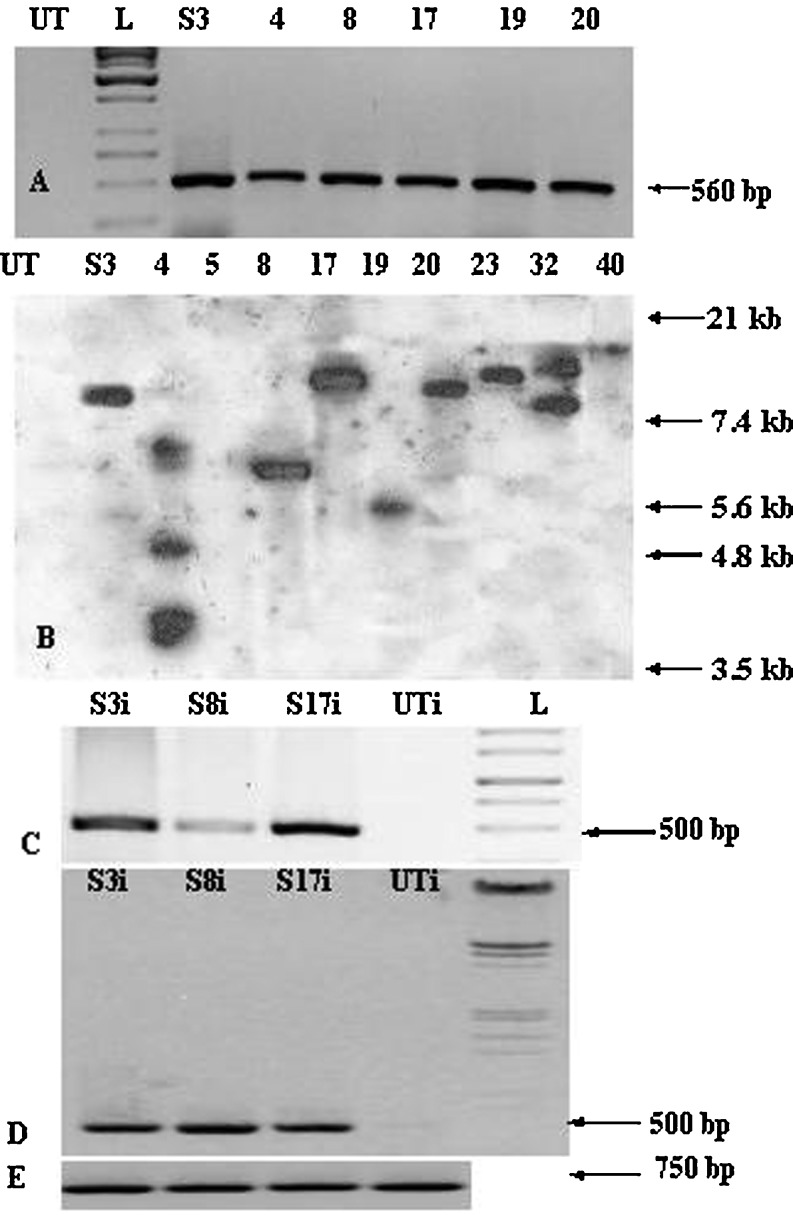
PCR analysis was performed at primary transformant as well as at T1 stage to confirm the transgene integration (Fig. 1A), utilizing primers specific to hpt and samdc transgene. PCR analysis of genomic DNA from putative transgenic SAM lines revealed 500 bp (for hpt specific primers) and 560 bp (for samdc gene specific primers) respectively as expected. Majority of the T1 progenies (~70 %) showed the integration of the transgene, whereas the remaining segregated as null homozygotes without any amplification (data not shown). The PCR positive SAM primary transformants as well as T1 transgenic progenies were further employed for Southern analysis (Fig. 1B). In this case, SAM3, SAM8, SAM17, SAM19, SAM20, SAM23 and SAM40 displayed single copy integration whereas SAM4 and SAM32 displayed multiple copy integration. The transgene expression was analyzed in few SAM transgenic lines by RT-PCR using samdc gene-specific primers. In case of SAM lines (SAM3 and SAM17) induced with F. oxysporum, the transcript levels appear to be more or less of similar level, whereas SAM8 showed lower level. The disparity in the transcript levels among the different SAM lines can be attributed to the positional effect. It is well known that the transgene expression can be strongly influenced by site of integration (Kooter et al.1999). Moreover, variabilities in the levels of gene expression from independent transformants are very common in plant transformation systems (He et al.2008). Certain other factors such as transgene copy number and construct fidelity of the transgene also influence gene expression (Kooter et al. 1999). In case of induction with A. solani for the three lines tested SAM3, SAM8, and SAM17, the transcript levels were higher as compared to the F. oxysporum induction set (Fig. 1C, D). This may be due to the difference in virulence and degree of induction by the two pathogens for the same line itself.
Fig. 1.
Molecular characterization of SAM tomato transgenic plants (A) PCR analysis of putative primary transformants with primers specific to human samdc gene: DNA from untransformed control (UT), 1 kb ladder (L), DNA from putative primary transformants (SAM3, SAM4, SAM8, SAM17, SAM19 and SAM20); (B) Southern blot analysis of T1 progenies of SAM lines using hpt gene probe: genomic DNA of untransformed control (UT), and SAM lines, SAM3, SAM4, SAM5, SAM8, SAM17, SAM19, SAM20, SAM23, SAM32 and SAM40, respectively, restricted with Bam HI enzyme; (C) RT-PCR with primers specific to samdc gene for SAM3i, SAM8i, SAM17i & UTi, all samples induced with F. oxysporum; (D) RT-PCR with primers specific to samdc gene for SAM3i, SAM8i, SAM17i & UTi, all samples induced with A. solani; (E) Panel shows internal control of same samples: SAM3i, SAM8i, SAM17i and UTi with tomato actin primers
Polyamine content in transgenic lines
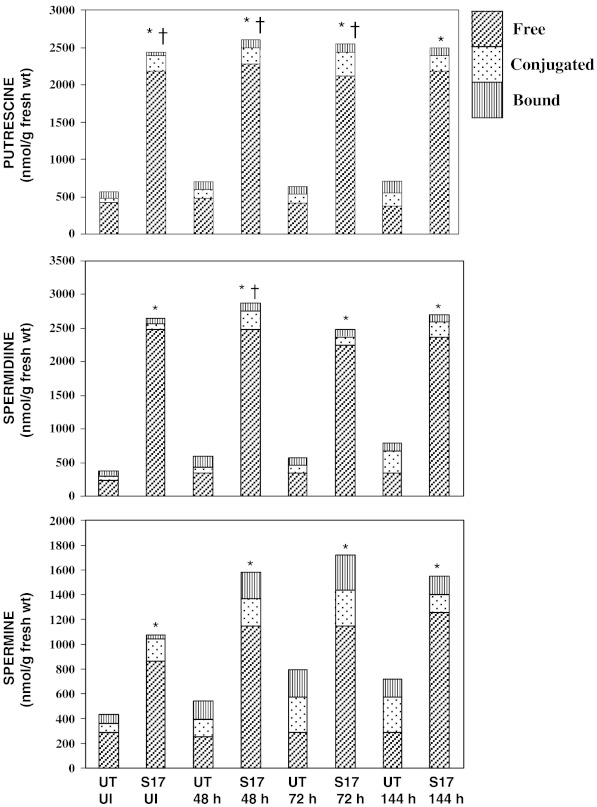
PA levels were assessed in UT control as well as in selected SAM lines (SAM3, SAM8 and SAM17) at T1 stage. In UT control tomato seedlings, out of the three PAs, Put and Spd levels were found to be more or less similar level, followed by Spm. In case of UT control seedlings induced by F. oxysporum, total Put content rose as 1.26 fold (48 h) to 1.2 fold (72 h) up to 1.3 fold (144 h). This rise can be attributed to the increase in conjugated (C) fraction of Put over the time period i.e. 2.6 fold (48 h), 2.5 fold (72 h), 3.7 fold (144 h) and a marginal increase in bound form (B) (Fig. 2). But the free (F) form was more or less maintained over the induction time period. In case of Spd the rise in total Spd is mainly attributable to rise in C fraction i.e. 1.2 to 3.6 fold from 48 h to 144 h, whereas there is no increase in F fraction over the same time period. Again, a similar trend was found in Spm where major increase was reflected in C form (3-fold in 144 h) followed by B form though no increase or rather a marginal dip was seen in F fraction. This indicates that on induction by the pathogen, the system tries to prepare itself for defense against the invading pathogen by an increase in total PA levels, but the main emphasis is on re-adjustment of the available PA pool via inter-conversions among the fractions via a possible conversion of F to C fraction. This signifies that C fraction may have possible vital role to play during plant defense mechanism against fungal pathogens and therefore we come across the inter-conversions and re-channelizing of the PA fractions. In literature, inter-conversions between free PAs and C ones have been shown, as an important element for regulation of cellular free PAs and this possibility cannot be ruled out (Martin-Tanguy 1985; Bagni and Tassoni 2001).
Fig. 2.
Polyamine levels in SAM17 line of un-induced sample and samples collected at time periods (48, 72, 144 h) in comparison to untransformed (UT) control; *, †, and ‡ denote significant differences in free, conjugated and bound fractions respectively from respective control at 5 % level
In case of SAM17 T1 seedlings, the levels of all the three PAs checked were found high and there was not much increase on induction as compared to un-induced stage (Fig. 2). This was expected as SAM lines are driven by a constitutive (CaMV-35 S) promoter. A major increase in C fraction (3.3 fold, 2.6 fold in Spd at 48 h, 144 h respectively) was seen besides that of B fraction of Spm (6–8 fold). The rise in C fraction was complimented by a marginal decline in F fractions especially in Put and Spd. Overall, we found PAs in this increasing order as Spd>Put>Spm which is possibly due to the gene samdc which is being up-regulated. This shows that the SAM line behaves similar to UT control to the extent that there is a possible inter-conversion of F to C fraction during pathogenic induction and attack, which denotes that C fraction, may be more crucial than other fractions during stress conditions. This has been well documented in the recent literature (Martin-Tanguy 1985; Rajam 1997; Walters 2003a, b). When the PA levels in SAM17 were compared in relation to UT, the following trend is evident. The total Put level of SAM17 was 4.3 fold as compared to UT at un-induced stages, which was found later to be 3.6 fold at 48 h induction stage. In case of Spd it was 7 fold at un-induced stage compared to UT and was 4.8 fold at 48 h for SAM17 compared to UT. In case of Spm, the levels are 2.0–2.4 fold for SAM17 compared to UT. The maximum difference was observed in Spd and that is implicit. In a nutshell, the F fractions of Put, Spd and Spm at un-induced and at all the induced time-points are significant. Also, C fraction of Put (at un-induced, 48 h, 72 h) and of Spd (at 48 h) is statistically significant. The trend was more or less similar in other SAM lines and hence the details are not included here to avoid repetition.
It is quite evident from the above data that F and C fractions are significant for the SAM lines examined allowing us to comprehend a crucial role for both these fractions under stress conditions. However, we also need to analyze and interpret the trend, which is that initial rise, is noticeable in the F fractions for more or less all the three PAs but as the induction time stir ahead; there is a progressive increment of the C fraction coupled with a slight drop seen in all the lines in the F fractions. This points out towards a readjustment in the system on account of the pathogen induction and there seems to be inter-conversion of different PA fractions mainly F form to C form. We can contemplate a credible role for the C fraction though role of F cannot be ruled out. It is well established that C forms of PAs, especially hydroxycinnamic acid amides (HCA amides) and coumaroylagmatine have been known to have anti-microbial properties (Martin-Tanguy 1985; Rajam 1997; Walters 2003a, b). These amides are thought to contribute to the formation of a phenolic barrier, making the cell wall more resistant to enzymatic hydrolysis (Walters 2003a, b). A role for hydrocinnamic acid amide conjugates has also been proposed in defense mechanisms against both abiotic and biotic stress, mainly acting as radical scavengers (Bors et al. 1989). In a work related to HR, Cowley and Walters (2002a) reported that infection of powdery mildew fungus on barley leaves revealed an increase in the F and C forms of PAs (Put, Spd and Spm) levels in 1–4 days of infection and accompanied by the enhanced activity of the PA biosynthetic and catabolic enzymes (DAO and PAO). Subsequently, in an incompatible interaction between barley and powdery mildew where the resistance was penetration based, levels of C form of Put, and F and C forms of Spd and catabolic enzyme activity was found to increase after 1–3 days of inoculation (Cowley and Walters 2002b). Witzell et al. (2005) also reported relative abundance of F and C forms of individual PAs associated with parasite infection in Vaccinium myrtillus. We also find a similar increment in F and C fractions of Put and Spd and of F forms of Spm. In fact, we found another interesting trend where initially C form remains low but F fraction was high but on induction C fraction increases progressively possibly due to conversion of F form to C form. This is very evident for UT as well as SAM lines. Recently, significant rise in PAs (Put and Spd) especially of C form has shown to confer multiple stresses, including against Fusarium wilt in eggplant transgenics (Prabhavathi and Rajam 2007).
In all the transgenic lines, Put levels were higher as compared to UT, although it is not expected as samdc gene was introduced, which should lead to increased levels of higher PAs, Spd and Spm. This can be due to back-conversion of higher PAs to Put as higher PAs generally are toxic beyond a threshold level (De Agazio et al.1995). It is known that Put is a regulator of samdc gene, so Put up-regulates the samdc gene leading to production and consequently abundance of higher PAs i.e. Spd and Spm, which again due to their threshold maxima are reverted back to their precursor molecule Put. Studies to date reveal that increased Spd and Spm biosynthesis in animal cells is often accompanied by their increased catabolic breakdown through Spd/Spm acetyltransferase and PAO activities (Cohen 1998). Whether or not in plants also, Spd and Spm levels are managed via a similar compensatory mechanisms involving their catabolic turnover by PAO is still unclear, although there are reports of increased DAO activity in PA transgenic plants (Waie and Rajam 2003; Prabhavathi and Rajam 2007).
Testing of transgenic lines for fungal resistance
The SAM transgenic lines were assessed for their resistance to F. oxysporum (wilt causing fungus) and A. solani (early blight causing fungus) under both in vitro and in vivo growth conditions.
In vitro and in vivo assays for resistance against F. oxysporum
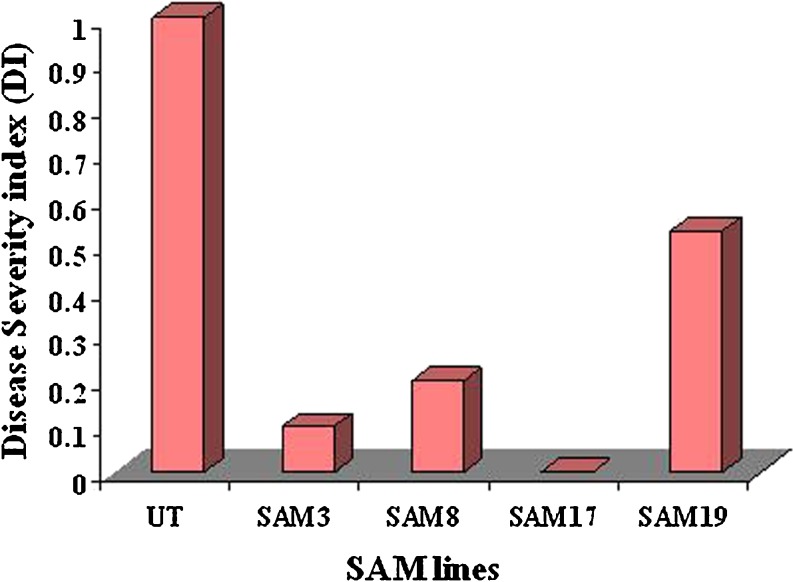
SAM transgenic lines, SAM3, SAM8, SAM17 and SAM19 were tested for fungal resistance against Fusarium wilt (Fig. 3A, B). Early symptoms like leaf yellowing started appearing, after 4 days of inoculation, on the lower leaves of the UT while the transgenic lines were healthy. Within the subsequent week, the wilting symptoms on the UT seedlings spread towards upper leaves. Most of the lower leaves had got defoliated. Yellowing and wilting of upper leaves and complete defoliation of lower leaves was observed in these seedlings after 15 days after inoculation, which eventually led to their death by third week. The transgenic seedlings exhibited delayed onset of disease symptoms and those were confined to the older leaves only. The response of different transgenic lines towards the fungal infection was variable (Fig. 4). Among all the tested transgenic lines, SAM3, SAM8, SAM17 can be categorized as highly resistant and SAM19 as moderately resistant based on the severity and time gap of appearance of disease symptoms and disease index (DI) as compared to the wild-type UT seedlings. For in vivo study of the fungal resistance assay, the development of disease symptoms was monitored by visual observation, based on wilting symptoms. The disease symptoms started appearing on the lower leaves of the control plants within 10 days of inoculation. The lower leaves started wilting and defoliated within 15 days of inoculation. Following 3 weeks of inoculation, all the leaves from control had fallen and stem developed necrosis, while in the transgenic seedlings; there was no occurrence of any disease symptom after 10 days of fungal spore’s inoculation, though slight necrosis was observed after 15 days of inoculation. In subsequent week, necrosis and yellowing of the older leaves was observed in the transgenic lines SAM19 which, is categorized as moderately resistant. SAM3, SAM8 and SAM17 showed high level of resistance with minimal symptoms even after 20 days of inoculation.
Fig. 3.
Biotic stress assay of T1 progenies of SAM transgenic lines against infection by F. oxysporum and A. solani (A) In vitro assay of T1 progenies of SAM transgenic lines against infection by F. oxysporum (a) Untransformed control seedling un-inoculated (b) Untransformed seedling inoculated by the pathogen (c) A representative SAM (SAM17 T1 progeny seedling) un-inoculated (d, e, f) SAM3, SAM8 & SAM17 lines inoculated by the pathogen; (B) In vivo assay (a) Untransformed control plant inoculated with the pathogen (b) A representative SAM (SAM17) inoculated by the pathogen; (C) In vitro detached leaf assay of SAM transgenic lines against infection caused by A. solani (a) Untransformed control plant inoculated (b) A representative SAM (SAM8) line inoculated with A. solani spores; (D) In vivo assay (a) Untransformed control seedling inoculated by A. solani (b) A representative SAM (SAM17 T1 progeny shown) inoculated by A. solani. 6–8 T1 progeny seedlings per line tested for all the experiments
Fig. 4.
Disease severity index (DI) values against Fusarium wilt of SAM transgenic lines and untransformed control (UT) based on in vitro root dip assay
In vitro detached leaf and in vivo assays for resistance against A. solani
Fully expanded leaves of T0 as well as T1 generation of SAM transgenics lines, SAM3, SAM8, SAM17 and SAM19 were tested for resistance against A. solani by using the detached leaf assay (Fig. 3C, D). Disease severity and degree of resistance against the fungus was recorded on the basis of typical symptoms as mentioned in literature (Rajam et al. 2007; Scott and Gardner 2007). Transgenic leaves showed increased resistance to Alternaria blight as compared to the control leaves. The typical symptoms of leaf blight, i.e. small black spots and yellowing of leaves around the infection site started appearing 4 days post-inoculation on the control, whereas leaves of the transgenic lines were healthy and green. 8 days after inoculation, dark brown to black concentric lesions were observed on control leaves and whereas very small black spots with yellowing around it could be seen in transgenic lines. However, after 12 days of inoculation, the UT leaves got completely infected, whereas SAM19 leaves developed dark concentric rings and yellowing indicating they were low in resistance compared to other transgenic lines. Transgenic lines SAM8 and SAM17 been categorized as highly resistant as evident from the healthy leaves even 12 days after inoculation, whereas SAM3 is categorized as resistant based on the disease symptoms (Table 1). In case of in vivo study, disease symptoms were not noticeable till 7 days post-inoculation both on control as well as on transgenic plants but after 10th day post-inoculation, symptoms could be observed on control plant leaves. The leaves of control were severely damaged and large dark brown-black spots were visible on the leaf surface. However, the transgenic line SAM8 and SAM17 were highly resistant to the fungal infection even after 3 weeks post–inoculation, as only minimal yellowing on the surface of infected leaves was observed. Though mild infection symptoms were observed on the leaf surface of SAM3 but the disease progress was restricted to few small brown spots.
Table 1.
Detached leaf assay for testing SAM transgenic lines for resistance against infections caused by Alternaria solani
| Transgenic lines | 4-DAI | 8-DAI | 12-DAI | Degree of resistance |
|---|---|---|---|---|
| UT | ++++ | ++++ | +++++ | SUSCEPTIBLE |
| SAM3 | -- | + | ++ | RESISTANT |
| SAM8 | -- | -- | + | HIGHLY RESISTANT |
| SAM17 | -- | -- | + | HIGHLY RESISTANT |
Severity of infection was scored on a comparative scale as compared to the response in the wild plant: -- no infection, + − 20 % infection, + + − 40 % infection, + + + − 60 % infection, + + + + − 80 % infection, + + + + + − 100 % infection. UT-untransformed control leaves, SAM3-17-SAM transgenic lines. DAI - days after inoculation.
These results are in agreement with those observed in earlier studies. Waie and Rajam (2003) have reported the enhanced resistance to fungal wilt caused by F. oxysporum and V. dahliae in samdc tobacco transgenics, where they found enhanced titers of Put and Spd, especially C forms. In addition, adc eggplant transgenics showing increased PA levels (especially in C forms) displayed resistance to F. oxysporum (Prabhavathi and Rajam 2007). Changes in the level of F and C forms of PAs upon infection by various pathogens have been observed in other studies also (Reviewed by Martin–Tanguy 1985; Rajam 1997) with the C forms of PAs being well known for anti–microbial properties (Rajam 1997; Walters 2003a, b). The enhanced resistance seen in the SAM transgenic lines may be attributed to the increased levels of F and C fraction of PAs. The accumulated PAs might contribute to the protection by strengthening the cell wall via lignification and suberization, thereby preventing the entry of different pathogens. Further, Spm has been shown to be an endogenous inducer for pathogenesis-related (PR) proteins, PR-1, PR-2, PR-3 and PR-5 (Yamakawa et al. 1998) and is also probably involved in triggering the caspase activity and hence hypersensitive response (Walters 2003a, b).
Testing transgenic tomato lines for abiotic stress tolerance
T1 and/or T2 seeds and T1 seedlings of SAM transgenic lines were also considered for various abiotic stress assays such as salinity, drought, cold, and high temperature.
Salt stress assays
In the medium without NaCl, there was no noticeable difference in the seed germination percentage and seedling growth between the UT and transgenic lines. 200 mM NaCl supplemented medium was lethal as control seeds could not germinate at all, whereas the transgenic seeds exhibited low percent seed germination with a delay in initiation of germination response by about 8–12 days in comparison to medium devoid of NaCl. The germination frequency in the transgenic lines varied from 35 % to 88 % (Table 2). One-month old T1 seedlings (raised in soil: vermiculite 1:1 mix) from UT and transgenic lines SAM3, SAM8, SAM17 were screened for their tolerance to salt stress using 1/10th MS liquid medium containing 150 and 200 mM NaCl (Fig. 5A, C). The transgenic seedlings were green, healthy and exhibited low degree of necrosis. All the tested transgenic lines survived stress caused by 150 mM NaCl, but SAM3 survived even under 200 mM NaCl stress though they did suffer some growth penalty. On the contrary, UT seedlings suffered from some growth penalty in 150 mM NaCl. The UT seedlings in 200 mM NaCl suffered from extensive necrosis and died within 7–10 days, showing that this concentration is lethal for wild-type. The growth of the transgenic seedlings in terms of seedling height, fresh, and dry weight could be distinguished from that of the untransformed control seedlings, as demonstrated by improved growth upon exposure to salt (Table 3). The enhanced growth was reflected by a significant increase in seedling height in SAM transgenic lines (19–60 %) over the control. The transgenic SAM line seedlings showed a significant increase in fresh and dry weight content than the UT control seedlings (Table 3). Salt stress inhibited the growth of wild-type seedlings (as reflected by the growth parameters), but the seedlings of all the transgenic lines performed better and maintained consistent growth. Overall, SAM3 responded best among the SAM lines.
Table 2.
Germination of untransformed control and T1 SAM transgenics on ½ MS medium without stress, 200 mM NaCl (salinity), 15 % PEG (drought), 45 °C (high temperature) and 4–8 °C (low temperature) stress conditions
| Abiotic stress | Control | Transgenic line | ||||||
|---|---|---|---|---|---|---|---|---|
| UT | SAM3 | SAM8 | SAM17 | |||||
| No. of seeds inoculated | No. of seeds germinated | No. of seeds inoculated | No. of seeds germinated | No. of seeds inoculated | No. of seeds germinated | No. of seeds inoculated | No. of seeds germinated | |
| Without stress | 60 | 54 (90) | 50 | 45 (90) | 60 | 57 (95) | 60 | 54 (90) |
| Salinity | 60 | 0 (0) | 50 | 44 (88) | 60 | 21 (35) | 60 | 30 (50) |
| Drought | 60 | 0 (0) | 60 | 36 (60) | 60 | 33 (55) | 60 | 60 (100) |
| High temperature | 60 | 0 (0) | 60 | 42 (70) | NT | NT | 60 | 45 (75) |
| Low temperature | 60 | 8 (14) | 60 | 50 (83) | 60 | 24 (40) | 60 | 32 (54) |
Data is based on three independent experiments and the data was collected after 2 weeks of seed inoculation.
Values in parentheses represent percent seed germination; NT not tested
Fig. 5.
Salt and drought stress assays of T1 or T2 progenies of SAM transgenic lines (A) In vivo assay on soil: vermiculite (1:1) mix watered with 150 mM NaCl (a) UT control seedlings (b, c, d) SAM3, SAM8 & SAM17 T1 progeny seedlings; (B) In vitro floating leaf assay (water + 200 mM NaCl) (a) UT control leaves (b) A representative SAM (SAM8) leaves (C) In vivo assay on soil: vermiculite (1:1) mix watered with 200 mM NaCl (d) UT control seedlings (a, b, c, d, e, f) Transgenic lines SAM3, SAM8 SAM17, SAM115 & SAM143 T1 progeny seedlings; (D) In vitro seed germination assay (a, b) UT control seed germination on 10 and 15 % PEG respectively, (b, d) SAM17.2 T2 seed germination on 10 and 15 % PEG respectively; (e) In vivo assay on soil: vermiculite (1:1) mix watered with 10 % PEG, (a) UT control seedlings, (b, c) SAM3 & SAM17 T1 progeny seedlings; (f) In vivo assay on soil: vermiculite (1:1) mix watered with 15 % PEG (a) UT control seedlings, (b, c) SAM3 & SAM17 T1 progeny seedlings
Table 3.
Abiotic stress assay based on growth parameters of one-month old T1 seedlings of SAM lines
| Abiotic stress | UT | SAM3 | SAM8 | SAM17 |
|---|---|---|---|---|
| Without stress | ||||
| Shoot length (cm) | 12.316 ± 1.10 (100) | 8.750 ± 0.333 (71)* | NT | 7.983 ± 0.671 (65)a |
| Fresh weight (g) | 0.213 ± 0.03 (100) | 0.156 ± 0.016 (74) | NT | 0.126 ± 0.019 (60)a |
| Dry weight (g) | 0.0282 ± 0.01 (100) | 0.016 ± 0.002 (57) | NT | 0.009 ± 0.001 (4) |
| Salt | ||||
| (150 mM NaCl) | ||||
| Shoot length (cm) | 7.400 ± 0.288 (100) | 8.800 ± 0.352 (119)a | 10.280 ± 1.312 (138)a | 11.830 ± 0.520 (160)a |
| Fresh weight (g) | 0.238 ± 0.050 (100) | 0.600 ± 0.063 (252)a | 0.665 ± 0.193 (279)a | 1.83 ± 0.080 (768)a |
| Dry weight (g) | 0.010 ± 0.002 (100) | 0.041 ± 0.006 (410)a | 0.045 ± 0.013 (450)a | 0.097 ± 0.020 (970)a |
| (200 mM NaCl) | ||||
| Shoot length (cm) | NA | 6.333 ± 0.258 | NA | NA |
| Fresh weight (g) | NA | 0.553 ± 0.069 | NA | NA |
| Dry weight (g) | NA | 0.038 ± 0.005 | NA | NA |
| Drought | ||||
| (10 % PEG) | ||||
| Shoot length (cm) | 10.00 ± 0.417 (100) | 14.81 ± 1.161 (148)a | 14.44 ± 0.570 (144)a | 14.040 ± 0.863 (140)a |
| Fresh weight (g) | 0.467 ± 0.054 (100) | 2.174 ± 0.284 (465)a | 1.324 ± 0.200 (283)a | 1.704 ± 0.145 (364)a |
| Dry weight (g) | 0.040 ± 0.006 (100) | 0.1684 ± 0.037 (419)a | 0.112 ± 0.015 (279)a | 0.096 ± 0.027 (240) |
| (15 % PEG) | ||||
| Shoot length (cm) | NA | 11.06 ± 1.049 | 10.96 ± 1.01 | 11.56 ± 0.385 |
| Fresh weight (g) | NA | 1.0408 ± 0.218 | 0.789 ± 0.157 | 0.90 ± 0.07 |
| Dry weight (g) | NA | 0.0826 ± 0.021 | 0.058 ± 0.016 | 0.10 ± 0.016 |
| High temperature | ||||
| (40 °C) | ||||
| Shoot length (cm) | 10.566 ± 0.77 (100) | 9.860 ± 0.460 (94) | NT | 9.133 ± 0.251 (87) |
| Fresh weight (g) | 0.235 ± 0.03 (100) | 0.223 ± 0.033 (96) | NT | 0.183 ± 0.012 (78) |
| Dry weight (g) | 0.012 ± 0.01 (100) | 0.0270 ± 0.007 (225)a | NT | 0.010 ± 0.002 (84) |
| (45 °C) | ||||
| Shoot length (cm) | 5.283 ± 0.37 (100) | 8.183 ± 0.574 (155)a | NT | 8.066 ± 0.503 (153)a |
| Fresh weight (g) | 0.061 ± 0.01 (100) | 0.165 ± 0.014 (270)a | NT | 0.195 ± 0.015 (320)a |
| Dry weight (g) | 0.004 ± 0.00 (100) | 0.013 ± 0.000 (325)a | NT | 0.024 ± 0.002 (600)a |
| Low temperature | ||||
| (4–8 °C) | ||||
| Shoot length (cm) | 7.860 ± 0.385 (100) | 9.280 ± 0.475 (118)a | 8.680 ± 0.576 (110) | 8.960 ± 0.110 (114)a |
| Fresh weight (g) | 0.200 ± 0.03 (100) | 0.180 ± 0.019 (90) | 0.448 ± 0.075 (224)a | 0.265 ± 0.018 (132)a |
| Dry weight (g) | 0.008 ± 0.001 (100) | 0.022 ± 0.007 (275)a | 0.014 ± 0.002 (175)a | 0.013 ± 0.001 (175)a |
adenotes significant difference from respective control at 5 % level. NT not tested, NA not available due to lethality
Floating leaf assay
The floating leaf assay with 150 mM NaCl did not show any significant difference between UT and transgenic lines, but in case of 200 mM NaCl, the UT leaf discs exhibited bleaching unlike the transgenic lines, which showed the retention of chlorophyll (Fig. 5B).
Drought stress assays
When checked for drought stress tolerance by germinating the T2 seeds on ½ MS medium supplemented with 10 and 15 % PEG (MW 8000), there was neither any visible variation in the germination percentage nor in growth of the seedlings of the UT control and transgenic lines in unstressed conditions. In both the supplemented medium containing 10 and 15 % PEG, the seed germination percentage ranged from 65 % to 100 % in 10 % PEG and 55–100 % in 15 % PEG. The UT control seeds failed to germinate in 15 % PEG and very few germinated in 10 % PEG (Fig. 5D, Table 2). The response of the T1 seedlings was also examined under in vivo stress conditions by pouring 10 and 15 % PEG (MW 8000) in 1/10th MS medium for 14 days (Fig. 5E, F). The transgenic SAM seedlings exhibited a significant increase (40–48 %) in shoot length under stress conditions. SAM seedlings showed a significant improvement in fresh (183–365 %) and in dry (140–319 %) weight (Table 3). The transgenic seedlings exhibited delay in appearance of drought symptoms in comparison to the UT control seedlings. Withholding water resulted in leaf drying and wilting and complete collapse in the UT control within 4 to 5 days, while in the transgenic seedlings these symptoms were delayed until the seventh day. During recovery, the transgenic seedlings recovered after watering (rehydration) and displayed vigorous growth and survived. Conversely, the UT control seedlings could not revive at all. In case of 15 % PEG, all the tested transgenics could still survive the stress though they did suffer from some growth penalty (Table 3) but UT just could not survive and collapsed completely within 4–5 days after withdrawal of stress.
Desiccation stress assay
The leaves from the transgenic lines remained fresh even after 24 h of desiccation as evident from 44 % to 58 % water retention relative to the control leaves (data not shown).
High temperature stress assays
The T1 seeds from the transgenic lines SAM3 and SAM17 along with UT were evaluated for their germination ability upon exposure to high temperature (40 °C and 45 °C) for 48 h interval along with RT. The transgenic lines along with the UT control reported 75–90 % germination at unstressed condition as well as at 40 °C; however the heat stress at 45 °C was obvious for UT seeds as they failed to germinate at all. The germination ability got affected only marginally for transgenics (70–75 %) in the same condition (Table 2). Heat treatment had less effect on the growth of the transgenic seedlings, while the UT control seedlings developed severe symptoms of heat damage (Fig. 6A). They displayed shriveled leaves, smaller than normal leaves, and death of shoots. In terms of growth parameters, the transgenic SAM seedlings displayed significant increment in height (53–55 %), fresh weight (170–220 %) and dry weight (225 %) over the control (Table 3). During the recovery period, when transferred back to room temperature growth conditions, the transgenic seedlings resumed normal growth and continued to grow, while the UT control did not show any signs of recovery and consequently died. This emphasizes that the transgenic lines were significantly more thermo-tolerant than UT control plants. Transgenic fruits of SAM3, SAM8, SAM17 and SAM19 at primary transformants stage were evaluated for their tolerance at high temperature for 48 h. The UT and transgenic fruits did not show any difference at room temperature (Fig. 6B, C). But the difference was visible at elevated temperatures (40 °C and 45 °C). The UT fruits started showing signs of deterioration after 12 h itself and got severely degraded after 48 h at both the elevated temperatures. The UT fruits became very soft and tender and some even got burst open. In contrast, transgenic fruits remained almost fit throughout the period. However, the transgenic fruits kept at 45 °C after 48 h did become tender compared to those kept at room temperature, but they still remained intact unlike UT fruits which got spoiled completely. This denote that majority of the tested transgenic fruits could withstand elevated temperature stress thereby showing that PAs may have role to play in their tolerance capability, which ultimately led to their increased shelf life.
Fig. 6.
High temperature stress assay of SAM transgenic lines (A) In vivo seedling assay on soil: vermiculite (1:1) mix for high temperature (RT—room temperature, 40 °C and 45 °C) (a, b, c) SAM8 T1 progeny seedlings at RT, 40 °C and 45 °C respectively (d, e, f) SAM17 T1 progeny seedlings at RT, 40 °C and 45 °C respectively (g, h, i) UT control seedlings at RT, 40 °C and 45 °C respectively (B) High temperature assay on UT control fruits (a) RT (b) 40 °C (c) 45 °C (C) High temperature assay on SAM17 fruits (a) RT (b) 40 °C (c) 45 °C
Cold stress assays
There was 3–4 days of delay in onset of seed germination as well as reduction in the final germination of the UT control and transgenic seeds at 4–8 °C as compared to the seed germination at normal room temperature. However, the seeds of transgenic lines were more tolerant to low temperature during germination as reflected by higher percentage of seed germination than UT control. The seed germination percentage following cold stress was about 40–83 % for SAM lines while UT control showed only 14 % germination (Table 2). The above results show that the germination capacity at normal temperature was not different between UT control and transgenic seeds, but the transgenic seeds displayed improved degree of cold tolerance than the UT control seeds. The seedlings from the above experiment were analyzed for growth after 1 month. Under unstressed conditions, the control and transgenic lines were able to grow normally. However, the transgenics showed faster and vigorous growth under cold stress conditions, whereas the UT controls showed growth inhibition. The transgenic SAM seedlings showed increase in height (10–18 %), fresh weight (32–124 %) and dry weight (75–175 %) in comparison to the UT control seedlings under stress conditions (Table 3, Fig. 7A). Transgenic fruits of SAM3, SAM8, SAM17 and SAM19 primary transformant lines were also evaluated for their tolerance at low temperature for 14, 21 and 28 days (Fig. 7B, C). The difference was visible at low temperature (4–8 °C) after 28 days. The UT fruits displayed signs of deterioration after 28 days, whereas transgenic fruits could withstand cold stress for beyond 28 days. This demonstrates that transgenic fruits could withstand long-term storage for about a month at low temperature.
Fig. 7.
Cold stress assay of SAM transgenic lines (A) In vivo seedling assay (soil: vermiculite (1:1) mix at 4–8 °C (a) UT control seedlings (b, c, d) SAM3, SAM8 & SAM17 T1 progeny seedlings; (B,C) Fruits tested at 4–8 °C (a) UT fruit (b) Fruit of SAM17 (b) Fruit of SAM19 lines
Our results for all the tested abiotic stresses can be fortified by recent reports seen in case of abiotic stress studies as reviewed recently (Bhattacharya and Rajam 2006; Groppa and Benavides 2008), which demonstrated that higher PAs (Spd and/or Spm) may play a crucial role during stress conditions. Shevyakova et al. (2006) reported the role of free and conjugated forms of PAs during stress induced under NaCl stress in Mesembryanthemum crystallinum. Tassoni et al. (2008) revealed the specific role of free forms of Spd and Spm in the response and tolerance to salt stress of Arabidopsis thaliana flowers. The promotion of stress tolerance by free and conjugated forms of Spd and Spm could be ascribed to direct role in ROS scavenging and membrane stabilization due to their polycationic properties as has been shown (Kubis 2008). The over-expression of SPDS gene in Arabidopisis induced the expressions of various stress-regulated genes such as DREB, low-temperature-induced protein 78 (LTI 78 or rd29A) in addition to many transcription factors such as WRKY, B-box zinc finger proteins, NAM proteins, and MYB and provided credence to Spd as a signaling molecule under abiotic stress (Kasukabe et al. 2004). Wen et al. (2008) suggested the possibility of SPDS triggering the signaling pathways for multiple environmental stresses. However, it is possible that, being cationic at physiological pH, the PAs interact with anionic molecules such as DNA, RNA, proteins and membrane lipids which, makes it possible for PAs to implicate in multiple stress tolerance. Spd could bind to these molecules and prevent them from degradation under stress conditions and affect their physiological impact (Wen et al. 2008). In a further extension of Wen et al. (2008) investigations, He et al. (2008) reported that the one of the transgenic pear line tested (line # 32) over-expressing MdSPDS1 as well as control reported accumulation of Spd with the former accumulating more (2.4–2.9-fold more in transgenic line # 32). They suggested that this difference in increase in Spd titers between control and transgenic line might be responsible for the stress tolerance to NaCl and mannitol in the transgenic line unlike in the control. The transgenic line also contained higher antioxidant enzyme activities, less H2O2 and malondialdehyde (MDA) than the wild control, implying it suffered from less injury.
In conclusion, the introduction of samdc gene in tomato resulted in PA accumulation and possible readjustments of the PA between the three major PAs as well between the three forms naturally available in the cells with an increment in conjugated fractions. This signifies that SAM lines happen to behave similar to wild-type to the extent that there is a possible inter-conversion of free to conjugated fraction during pathogenic induction and attack, which denotes that conjugated fraction, may be crucial during stress conditions. The SAM lines were able to tolerate an array of abiotic stresses and as well as two fungal pathogens due to increment in the polyamine levels especially that of Spd and Put and to certain extent in Spm followed by a re-adjustment in the system via a possible conversion of free to conjugated fractions fortifying the role of conjugated fractions on stress alleviation. Therefore, the genetic manipulation of PA biosynthetic pathway is very viable, promising and emerging as new way to obtain stress tolerant crop plants, especially in light of the current global environmental perturbations and natural stresses.
Acknowledgements
This study was generously supported by the Department of Science and Technology (DST—Grant no SP/SO/BB16/2002) to MVR, New Delhi. The authors are thankful to Dr. Bhawna Waie for making the binary vector pMVR1SAMDC used for this study. Senior research fellowship to PH by Council of Scientific and Industrial Research is also acknowledged. We also thank the University Grants Commission for providing ‘Special Assistant Program’, and DST for FIST program.
References
- Babu RM, Sajeena A, Seetharaman K, Reddy MS. Advances in genetically engineered (transgenic) plants in pest management- an overview. Crop Prot. 2003;22:1071–1086. doi: 10.1016/S0261-2194(03)00142-X. [DOI] [Google Scholar]
- Bagni N, Tassoni A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids. 2001;20:301–317. doi: 10.1007/s007260170046. [DOI] [PubMed] [Google Scholar]
- Bajaj S, Rajam MV. Efficient plant regeneration from long-term callus cultures of rice by spermidine. Plant Cell Rep. 1995;14:717–720. doi: 10.1007/BF00232654. [DOI] [PubMed] [Google Scholar]
- Bhatnagar-Mathur P, Vadez V, Sharma KK. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep. 2008;27:411–424. doi: 10.1007/s00299-007-0474-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharya E, Rajam MV. Polyamine biosynthesis as a novel target for engineering crop plants for abiotic stress tolerance. J Plant Biol. 2006;33:99–105. [Google Scholar]
- Bors W, Langebartels C, Michel C, Sandermann H., Jr Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry. 1989;28:1589–1595. doi: 10.1016/S0031-9422(00)97805-1. [DOI] [Google Scholar]
- Capell T, Bassie L, Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA. 2004;101:9909–9914. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS. A guide to the Polyamines. Oxford: Oxford University Press; 1998. [Google Scholar]
- Cowley T, Walters DR. Polyamine metabolism in an incompatible interaction between and the powdery mildew fungus Blumeria graminis f. sp. Hordei J Phytopathol. 2002;150:1–7. doi: 10.1046/j.1439-0434.2002.00707.x. [DOI] [Google Scholar]
- Cowley T, Walters DR. Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp. Hordei. Plant Cell Environ. 2002;25:461–468. doi: 10.1046/j.0016-8025.2001.00819.x. [DOI] [Google Scholar]
- De Agazio M, Zacchini M, Federico R, Grego S. Putrescine accumulation in maize roots treated with spermidine: evidence for spermidine to putrescine conversion. Plant Sci. 1995;111:181–185. doi: 10.1016/0168-9452(95)04245-P. [DOI] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34:35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- Ha CH, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA. The natural polyamine spermine functions directly as a natural radical scavenger. Proc Natl Acad Sci USA. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Ban Y, Inoue H, Matsuda N, Liu J, Moriguchi T. Enhancement of spermidine content and antioxidant capacity in transgenic pear shoots over-expressing apple spermidine synthase in response to salinity and hyperosmosis. Phytochemistry. 2008;69:2133–2141. doi: 10.1016/j.phytochem.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Islam A. Fungus resistant transgenic plants: strategies, progress and lessons learnt. Plant Tissue Cult Biotechnol. 2006;16:117–138. [Google Scholar]
- Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S. Over-expression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004;45:712–722. doi: 10.1093/pcp/pch083. [DOI] [PubMed] [Google Scholar]
- Kasukabe Y, He L, Watakabe Y, Otani M, Shimada T, Tachibana S. mprovement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol. 2006;23:75–83. doi: 10.5511/plantbiotechnology.23.75. [DOI] [Google Scholar]
- Kooter JM, Matzke MA, Meyer P. Listening to the silent genes: transgene silencing, gene regulation and pathogen control. Trends Plant Sci. 1999;4:340–347. doi: 10.1016/S1360-1385(99)01467-3. [DOI] [PubMed] [Google Scholar]
- Kubis J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and a superoxide radical level in water-stressed cucumber leaves. J Plant Physiol. 2008;165:397–406. doi: 10.1016/j.jplph.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kumar SV, Sharma ML, Rajam MV. Polyamine Biosynthetic Pathway as a novel target for potential applications in plant biotechnology. Physiol Mol Biol Plants. 2006;12:13–28. [Google Scholar]
- Kumria R, Rajam MV. Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro morphogenesis and response to salt stress. J Plant Physiol. 2002;159:983–990. doi: 10.1078/0176-1617-00822. [DOI] [Google Scholar]
- Kusano T, Yamaguchi K, Berberich T, Takahashi Y. Advances in polyamine research in 2007. J Plant Res. 2007;120:345–350. doi: 10.1007/s10265-007-0074-3. [DOI] [PubMed] [Google Scholar]
- Lin B, Xiao Y. Sources of resistance to Verticillium wilt in Solanum melongena and its affinities identified by improved root dip method. Capsicum Eggplant Newslett. 1995;14:81–84. [Google Scholar]
- Madhulatha P, Pandey R, Hazarika P, Rajam MV. Polyamines and maltose significantly enhances shoot regeneration in tomato. Physiol Mol Biol Plants. 2006;12:295–301. [Google Scholar]
- Madhulatha P, Pandey R, Hazarika P, Rajam MV. High Transformation frequency in Agrobacterium-mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiol Mol Biol Plants. 2007;13:191–198. [Google Scholar]
- Martin-Tanguy J. The occurrence and possible function of hydroxycinnamoyl acid amides in plants. Plant Growth Regul. 1985;3:381–399. doi: 10.1007/BF00117595. [DOI] [Google Scholar]
- Martin-Tanguy J. Metabolism and function of polyamines in plants: recent development (new approaches) Plant Growth Regul. 2001;34:135–148. doi: 10.1023/A:1013343106574. [DOI] [Google Scholar]
- Oerke EC, Dehne HW, Schoebeck F, Weber A. Crop production and crop protection. Amsterdam: Elsevier; 1994. [Google Scholar]
- Prabhavathi VR, Rajam MV. Polyamine accumulation in transgenic eggplant enhances tolerance to multiple abiotic stresses and fungal resistance. Plant Biotechnol. 2007;24:273–282. doi: 10.5511/plantbiotechnology.24.273. [DOI] [Google Scholar]
- Punja ZK. Genetic engineering of plants to enhance resistance to fungal pathogens- a review of progress and future prospects. Can J Plant Pathol. 2001;23:216–235. doi: 10.1080/07060660109506935. [DOI] [Google Scholar]
- Rajam MV. Polyamines. In: Prasad MNV, editor. Plant ecophysiology. New York: Wiley; 1997. pp. 343–374. [Google Scholar]
- Rajam MV, Chandola N, Saiprasad PG, Singh D, Kashyap V, Choudhary ML, Sihachakr D. Thaumatin gene confers resistance to fungal pathogen as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol Plant. 2007;1:35–141. [Google Scholar]
- Rao G, Kaur M, Verma A, Sihachakr D, Rajam MV. Genetic engineering of crop plants for resistance to fungal pathogens. J Plant Biol. 1999;26:31–42. [Google Scholar]
- Roy M, Wu R. Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 2001;160:869–875. doi: 10.1016/S0168-9452(01)00337-5. [DOI] [PubMed] [Google Scholar]
- Roy M, Wu R. Over-expression of S-adenosyl methionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 2002;163:987–992. doi: 10.1016/S0168-9452(02)00272-8. [DOI] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Scott JW, Gardner RG. Breeding for resistance to fungal pathogens. In: Razdan MK, Mattoo AK, editors. Tomato genetic improvement of solanaceae crops, vol. 2. Enfield: Science publishers; 2007. pp. 421–456. [Google Scholar]
- Shevyakova NI, Rakitin VY, Stetsenko LA, Aronova EE, Kuznetsov VV. Oxidative stress and fluctuations of free and conjugated polyamines in the halophyte Mesembryanthemum crystallinum L. under NaCl salinity. Plant Growth Regul. 2006;50:69–78. doi: 10.1007/s10725-006-9127-1. [DOI] [Google Scholar]
- Tassoni A, Franceschetti M, Bagni N. Polyamines and salt stress response and tolerance in Arabidopsis thaliana flowers. Plant Physiol Biochem. 2008;46:607–613. doi: 10.1016/j.plaphy.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Waie B, Rajam MV. Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci. 2003;164:727–734. doi: 10.1016/S0168-9452(03)00030-X. [DOI] [Google Scholar]
- Walters D. Resistance to plant pathogens: possible role for free polyamines and polyamine metabolism. New Phytol. 2003;159:109–115. doi: 10.1046/j.1469-8137.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- Walters DR. Polyamines and plant disease. Phytochemistry. 2003;64:97–107. doi: 10.1016/S0031-9422(03)00329-7. [DOI] [PubMed] [Google Scholar]
- Wen XP, Pang XM, Matsuda N, Kita M, Inoue H, Hao YJ, Honda C, Moriguchi T. Over-expression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res. 2008;17:251–263. doi: 10.1007/s11248-007-9098-7. [DOI] [PubMed] [Google Scholar]
- Wi SJ, Kim WT, Park KY. Over-expression of carnation S-denosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006;25:1111–1121. doi: 10.1007/s00299-006-0160-3. [DOI] [PubMed] [Google Scholar]
- Witzell J, Kuusela T, Sarjala T. Polyamine profiles of healthy and parasite-infected Vaccinium myrtillus plants under nitrogen enrichment. J Chem Ecol. 2005;31:561–574. doi: 10.1007/s10886-005-2041-6. [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Kamada H, Satoh M, Ohashi Y. Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol. 1998;118:1213–1222. doi: 10.1104/pp.118.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]