Abstract
Fresh water resources are limited and their use in agricultural production is expected to come under increasing constraints. Eighteen Syrian lines of potato (Solanum tuberosum L.) were screened for drought tolerance by measuring aerial and root growth in vitro. Drought stress was evaluated by adding 2, 4, 6, 8 and 10 % (w:v) of sorbitol to Murashige- Skoog medium and compared to 0 % for the control. Water potential of media ranged from −0.58 MPa to −2.5 MPa. Water-stress in culture adversely affected plant growth, and genotypes differed for their responses. Plant length and stem thickness, leaf area, root number length and thickness, and plant fresh and dry weights and plant water content were measured and all decreased due to drought. Grouping lines by cluster analysis for response to drought resulted in: (1) a tolerant group of six lines, (2) a moderately tolerant group of seven lines, and (3) a susceptible group of five lines. The variation in germplasm indicated that potato varieties can be developed for production under some levels of drought.
Keywords: Solanum tuberosum, Drought, Sorbitol, Stress
Introduction
Potato (Solanum tuberosum L.) is one of the most important vegetables in the world. It provides significant amounts of starch, antioxidants, protein, vitamins, macro- and micronutrients, polyphenols, carotenoids and tocopherols (Brown 2005) to the human diet, but these values are affected by both cultivar and growing conditions. In Syria, potato production is 709,601 metric tons on 35,751 hectares in 2009 (FAOSTAT 2009). During the last years, potato consumption has increased despite the high prices. The producers in Syria tend to use imported seed tubers which increases cost of production, because of the high costs and the weak adaptation of growing varieties to local environmental conditions resulted in yield losses. In addition, the potential for importation of new diseases and pests on imported seed tubers can occur. Production of local potato varieties with high yield, improved quality, storage and processing characteristics and stress resistance is important.
In Syria, potatoes can be exposed to drought resulting from high ambient temperatures and low humidity during summer and autumn that reduces tuber yield and quality. Drought impairs mitosis, cell elongation and expansion, resulting in a reduction in plant length, leaf area and crop growth (Kaya et al. 2006; Hussain et al. 2008). Reduction of leaf size is the first morphological manifestation due to drought (Jefferies and MacKerron 1987) associated with reduced light interception and leads to a reduction in dry matter accumulation (Jefferies and MacKerron 1987; Jefferies 1993; Deblonde et al. 1999). Drought reduces numbers of leaves, leaf area, stem length, number of tubers, tuber growth and yield (Schittenhelma et al. 2006).
Effects of drought on tuber yield depend on morphological, physiological and molecular responses (Schapendonk et al. 1989). Screening for drought tolerance is complicated due to the many processes involved and their interaction with the environment. The evaluation of morphological responses to drought may contribute to an efficient selection of parents for hybridization and to development of screening tests for key factors associated with drought tolerance. The objective of this study was to screen and morphologically classify potato lines for drought tolerance.
Materials and methods
Plant material and culture conditions
The study was carried out in 2011 at the National Commission for Biotechnology (Damascus, Syria). The local potato lines SY-C.1, SY-C.2, SY-C.3, SY-C.14, SY-C.28, SY-C.29, SY-C.31, SY-C.46, SY-C.52, SY-C.53, SY-C.54, SY-C.55, SY-C.56, SY-C.57, SY-C.58, SY-C.59, SY-C.60 and SY-C.61 were used. Sprouted healthy tubers were planted in 60 × 40 × 5 cm growing tray with 84 cells of 4.8 × 4.8 × 4.8 cm containing steam disinfected compost. In order to be used as primary explants, developed stems were cut into nodal sections consisting of a single node and leaf. Nodal sections were sterilized in a solution of 0.5 % (v:v) sodium hypochlorite for 5 min. They were then rinsed with sterile distilled water three times and transferred to 15 mL of Murashige- Skoog (MS) medium (20 g.l−1 sucrose and 7 g.l−1 agar). Cultures were maintained under a 16/8 hours light/dark photoperiod with 150 μmol·m−2·s−1 light intensity at 25 ± 1 °C. In vitro grown plants were propagated by sub-culturing after 4 weeks. In order to assess drought tolerance, single nodes were transferred to MS medium containing 0, 2, 4, 6, 8 or 10 % (w:v) sorbitol with eight replicates per treatment. After 6 weeks, in vitro grown plants were harvested for measuring morphological parameters associated with water stress tolerance.
Measurements
Eight six-week old plants from each line were harvested, rinsed in sterilized water, and separated into leaves, stems and roots. Number of leaves and roots were recorded. Length and thickness of both the roots and stem were measured. Leaf areas were measured with a Li-Cor 3100 area meter (Li-Cor, Lincoln, NE). Plant fresh and dry weights (oven-dried at 70 °C for at least 72 h) were determined (Schafleitner et al. 2007). Plant water content (PWC%) was estimated according to Guo et al. (2008). Water potential of all media was measured using an isopiestic thermocouple psychrometer (Boyer and Knipling 1965).
Experimental design and statistical analysis
The experiment was designed as completely randomized design with eight replications. Data were subjected to ANOVA analysis using the R-version 2.5.1 statistical software [The R Project for Statistical Computing, Lyon, France (www.r-project.org)] and were expressed as mean ± standard errors (mean ± SE). Using Systat software, Pearson correlation analysis was achieved to examine degrees of association between characters, and to perform cluster analysis according to the lines response to drought based on the sum of all 10 growth parameters relative values.
Results
Water potentials of the various media used are presented in Table 1. The control MS medium with 20 g · l − 1 sucrose had a water potential of −0.58 MPa. The water potential of the media decreased with the addition of sorbitol. Medium with the highest concentration (10 %) of sorbitol had a water potential of −2.5 MPa.
Table 1.
Concentrations and water potentials of media. Values are means ± standard errors (n = 10)
| Media concentration (%) | Media water potentials (MPa) |
|---|---|
| 0 | −0.82 |
| 2 | −1.09 |
| 4 | −1.44 |
| 6 | −1.79 |
| 8 | −2.14 |
| 10 | −2.5 |
Morphological parameters
In general, the lines exhibited decreases in morphological parameters as sorbitol concentration in the MS medium increased. At 6, 8 and 10 % sorbitol, plants did not produce stems and leaves. At 2 and 4 % sorbitol plants produced stems and leaves and no leaf necrosis was observed. Differences in morphological parameters occurred only at 4 % sorbitol; while at 2 % sorbitol plant responses were generally similar to the control level. Only results of 4 % sorbitol as a critical threshold for screening these lines for drought tolerance are presented.
Drought decreased stem length in all lines, ranging from 44 % in SY-C.52 to 87 % in SY-C.14 (Table 2). Decreases in stem thickness due to drought were observed in all lines except: SY-C.28, SY-C.31, SY-C.52 and SY-C.61 (Table 2).
Table 2.
Stem length and thickness of clones due to sorbitol level. Values are means ± standard errors (n = 8)
| Clone name | Stem length (cm) | Stem thickness (mm) | ||
|---|---|---|---|---|
| C | S | C | S | |
| SY-C.1 | 10 ± 0.69 | 3.5 ± 0.23 | 1.53 ± 0.05 | 1.13 ± 0.23 |
| SY-C.2 | 12.33 ± 0.44 | 3.94 ± 0.17 | 1.57 ± 0.08 | 1.32 ± 0.13 |
| SY-C.3 | 12.08 ± 1.01 | 4.14 ± 0.40 | 1.67 ± 0.13 | 1.10 ± 0.14 |
| SY-C.14 | 12.5 ± 0.41 | 1.625 ± 0.38 | 1.45 ± 0.03 | 1.21 ± 0.13 |
| SY-C.28 | 14.5 ± 0.22 | 4.411 ± 0.28 | 0.97 ± 0.10 | 1.18 ± 0.04 |
| SY-C.29 | 9.92 ± 0.55 | 3.35 ± 0.20 | 1.30 ± 0.05 | 1.13 ± 0.05 |
| SY-C.31 | 9.5 ± 0.68 | 3.75 ± 0.43 | 1.37 ± 0.16 | 1.75 ± 0.10 |
| SY-C.46 | 12.5 ± 0.86 | 2.48 ± 0.24 | 1.43 ± 0.06 | 1.20 ± 0.08 |
| SY-C.52 | 6.42 ± 0.47 | 3.6 ± 0.32 | 1.43 ± 0.05 | 1.47 ± 0.10 |
| SY-C.53 | 15.2 ± 0.37 | 3.8 ± 0.20 | 1.36 ± 0.09 | 0.87 ± 0.05 |
| SY-C.54 | 10.75 ± 0.68 | 3.21 ± 0.43 | 1.31 ± 0.01 | 0.92 ± 0.10 |
| SY-C.55 | 14.3 ± 0.95 | 3.51 ± 0.30 | 1.42 ± 0.12 | 0.88 ± 0.09 |
| SY-C.56 | 13 ± 0.89 | 3.38 ± 0.51 | 1.28 ± 0.12 | 1.1 ± 0.10 |
| SY-C.57 | 13.25 ± 0.96 | 2.15 ± 0.16 | 1.33 ± 0.03 | 1.145 ± 0.16 |
| SY-C.58 | 13.17 ± 1.55 | 2.47 ± 0.30 | 1.44 ± 0.06 | 1.15 ± 0.10 |
| SY-C.59 | 12.3 ± 0.50 | 3.13 ± 0.61 | 1.44 ± 0.07 | 0.96 ± 0.09 |
| SY-C.60 | 14.4 ± 0.45 | 2.467 ± 0.47 | 1.60 ± 0.03 | 1.21 ± 0.15 |
| SY-C.61 | 7.25 ± 0.31 | 2.78 ± 0.29 | 1.45 ± 0.06 | 1.62 ± 0.12 |
C control and S stressed plants
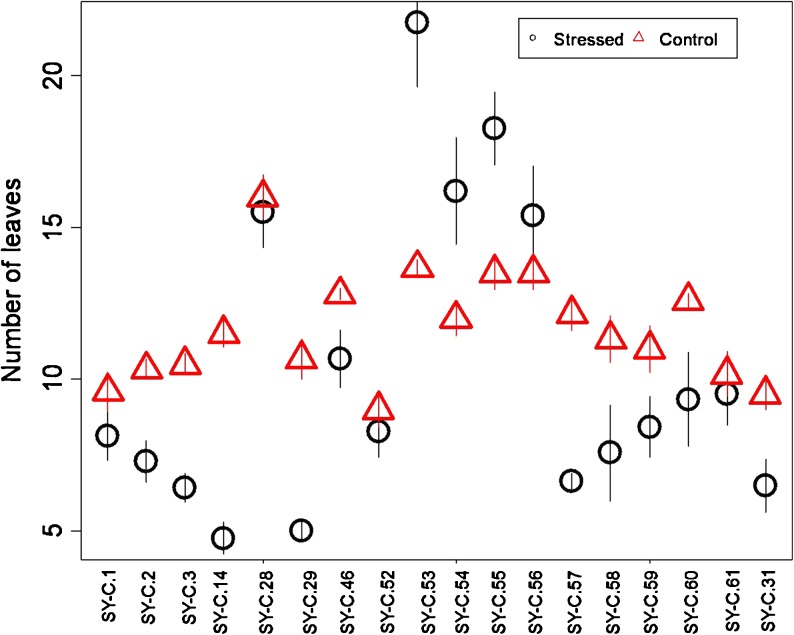
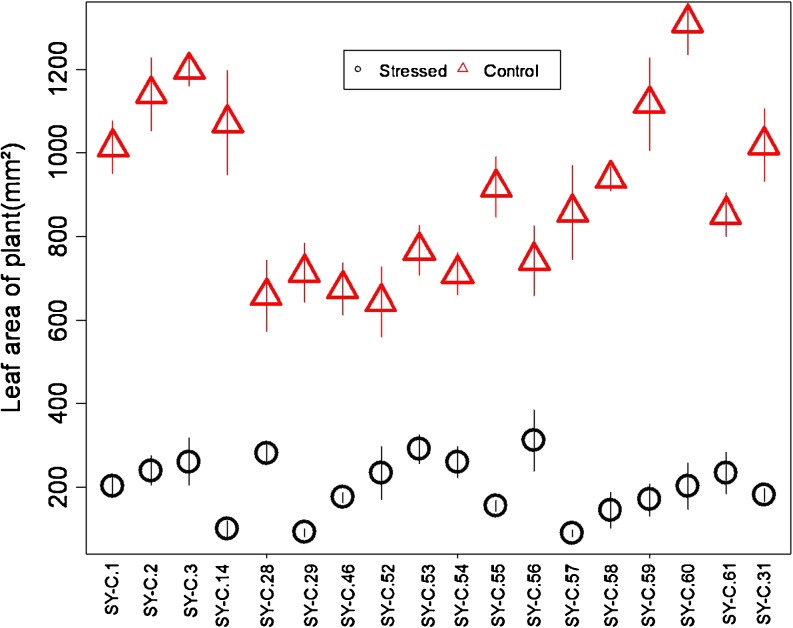
Variation in number of leaves per plant according to line and drought (Fig. 1) was observed. While number of leaves decreased due to drought in the lines SY-C.29, SY-C.31, SY-C.57, SY-C.2 and SY-C.3, it increased in lines SY-C.53, SY-C.54, SY-C.55 and SY-C.56. Total leaf area decreased in all lines due to drought (Fig. 2). Such reductions ranged from 57 % in SY-C.28 to 89 % in SY-C.58.
Fig. 1.
Number of leaves per plant according to line and drought treatment; control (— O —) and stressed (—∆—). Values are means (± S.E.) of measurements from eight plants for each treatment (n = 8)
Fig. 2.
Area of leaves per plant according to line and drought treatment; control (— O —) and stressed (—∆—). Values are means (± S.E.) of measurements from eight plants for each treatment (n = 8)
A reduction of 3 to 52 % in number of roots due to drought in most lines was observed (Table 3). Root length and thickness decreased due to drought in all lines; the reduction of root length ranged from 35 % for SY-C.28 to 67 % for SY-C.60, while the reduction in root thickness ranged from 8 % in SY-C.1 to 72 % in SY-C.54 (Table 3).
Table 3.
Number, root length and thickness of clones due to sorbitol level. Values means ± standard errors (n = 8)
| Clone name | Number of roots | Root length (cm) | Root thickness (mm²) | |||
|---|---|---|---|---|---|---|
| C | S | C | S | C | S | |
| SY-C.1 | 7.75 ± 0.39 | 4.75 ± 0.25 | 7 ± 0.29 | 3.71 ± 0.48 | 0.77 ± 0.3 | 0.71 ± 0.2 |
| SY-C.2 | 5.83 ± 0.48 | 3.71 ± 0.42 | 7.5 ± 0.34 | 2.76 ± 0.33 | 1.02 ± 0.08 | 0.85 ± 0.15 |
| SY-C.3 | 6.5 ± 0.43 | 4.29 ± 0.42 | 6.5 ± 0.45 | 3.5 ± 0.24 | 1.12 ± 0.13 | 0.61 ± 0.16 |
| SY-C.14 | 7.83 ± 0.65 | 4.5 ± 0.38 | 7.67 ± 0.71 | 2.94 ± 0.47 | 1.25 ± 0.26 | 0.76 ± 0.15 |
| SY-C.28 | 5.5 ± 0.43 | 4.89 ± 0.39 | 7.83 ± 0.70 | 5.12 ± 0.23 | 0.79 ± 0.04 | 0.76 ± 0.03 |
| SY-C.31 | 7.25 ± 0.48 | 6 ± 0.71 | 9.75 ± 0.25 | 4.5 ± 0.35 | 1.49 ± 0.21 | 1.01 ± 0.15 |
| SY-C.29 | 6.17 ± 0.40 | 4.5 ± 0.43 | 9.08 ± 0.55 | 4.42 ± 0.15 | 0.92 ± 0.1 | 0.44 ± 0.13 |
| SY-C.46 | 8.5 ± 0.81 | 4.67 ± 0.33 | 10.67 ± 1.09 | 6.24 ± 0.56 | 1.18 ± 0.08 | 0.61 ± 0.07 |
| SY-C.52 | 7.67 ± 0.50 | 6.86 ± 0.55 | 8.67 ± 0.50 | 4.56 ± 0.45 | 1.48 ± 0.12 | 0.95 ± 0.2 |
| SY-C.53 | 6.8 ± 1.32 | 6.6 ± 0.81 | 9 ± 0.71 | 4.24 ± 0.90 | 1.61 ± 0.19 | 0.72 ± 0.15 |
| SY-C.54 | 7.5 ± 0.76 | 5.86 ± 0.46 | 10.33 ± 1.81 | 4.11 ± 0.54 | 2.28 ± 0.4 | 0.63 ± 0.10 |
| SY-C.55 | 8.33 ± 0.95 | 5 ± 0.38 | 9.67 ± 0.50 | 3.55 ± 0.41 | 1.44 ± 0.11 | 0.57 ± 0.13 |
| SY-C.56 | 8 ± 0.77 | 7.25 ± 0.68 | 11.5 ± 0.43 | 6.63 ± 0.86 | 1.58 ± 0.3 | 0.77 ± 0.20 |
| SY-C.57 | 8.33 ± 0.56 | 4.63 ± 0.38 | 12.5 ± 0.76 | 5.15 ± 0.78 | 1.11 ± 0.17 | 0.30 ± 0.06 |
| SY-C.58 | 7.83 ± 0.75 | 4.57 ± 0.57 | 11.5 ± 0.85 | 5.73 ± 0.86 | 1.49 ± 0.13 | 0.59 ± 0.09 |
| SY-C.59 | 9.5 ± 0.43 | 5.57 ± 0.61 | 9.5 ± 0.22 | 4.07 ± 0.47 | 2.17 ± 0.2 | 0.68 ± 0.11 |
| SY-C.60 | 9 ± 0.52 | 4.5 ± 0.5 | 7.83 ± 0.40 | 2.58 ± 0.64 | 2.23 ± 0.12 | 0.89 ± 0.09 |
| SY-C.61 | 7.8 ± 0.18 | 3.75 ± 0.45 | 8.33 ± 0.61 | 3.53 ± 0.34 | 1.85 ± 0.15 | 0.62 ± 0.11 |
C control and S stressed plants
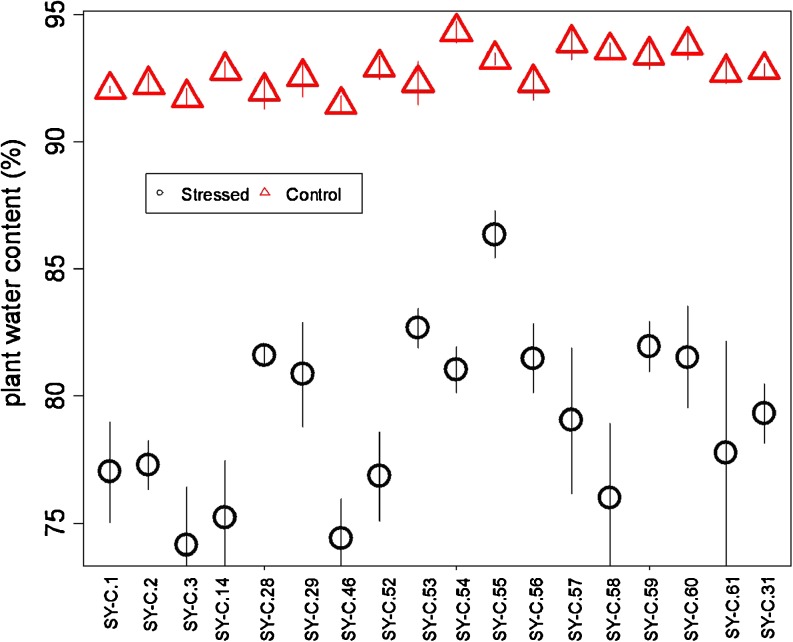
Drought caused a decrease in fresh weight of all lines, with decreases ranging from 35 % in SY-C.56 to 80 % in SY-C.14 (Table 4). The effect of drought on dry weight varied by line (Table 4); in SY-C.28, SY-C.53, SY-C.57, SY-C.3, SY-C.1 there was no reduction in dry weight, but in SY-C.31, SY-C.46, SY-C.52, SY-C.54, SY-C.2 dry weight increased, and in the remaining lines dry weight decreased under water stress. Plant water content (PWC) was reduced in all lines due to drought (Fig. 3) with decreases between 80 and 90 %.
Table 4.
Effect of sorbitol level on fresh and dry weights of clones. Values are means ± standard errors (n = 8)
| Clone name | Fresh weight (g) | Dry weight (g) | ||
|---|---|---|---|---|
| C | S | C | S | |
| SY-C.1 | 0.92 ± 0.17 | 0.36 ± 0.03 | 0.08 ± 0.006 | 0.08 ± 0.004 |
| SY-C.2 | 1.07 ± 0.10 | 0.43 ± 0.06 | 0.09 ± 0.007 | 0.10 ± 0.011 |
| SY-C.3 | 1.12 ± 0.08 | 0.38 ± 0.03 | 0.09 ± 0.006 | 0.09 ± 0.007 |
| SY-C.14 | 1.28 ± 0.25 | 0.24 ± 0.03 | 0.10 ± 0.009 | 0.05 ± 0.002 |
| SY-C.28 | 0.67 ± 0.14 | 0.38 ± 0.04 | 0.06 ± 0.006 | 0.06 ± 0.005 |
| SY-C.29 | 0.75 ± 0.08 | 0.23 ± 0.02 | 0.05 ± 0.006 | 0.04 ± 0.007 |
| SY-C.31 | 1.10 ± 0.12 | 0.41 ± 0.06 | 0.08 ± 0.007 | 0.09 ± 0.016 |
| SY-C.46 | 0.75 ± 0.09 | 0.35 ± 0.05 | 0.06 ± 0.007 | 0.08 ± 0.004 |
| SY-C.52 | 1.12 ± 0.06 | 0.64 ± 0.12 | 0.08 ± 0.009 | 0.10 ± 0.005 |
| SY-C.53 | 1.35 ± 0.39 | 0.57 ± 0.11 | 0.07 ± 0.005 | 0.07 ± 0.005 |
| SY-C.54 | 1.52 ± 0.21 | 0.62 ± 0.08 | 0.09 ± 0.003 | 0.11 ± 0.005 |
| SY-C.55 | 1.36 ± 0. 11 | 0.37 ± 0.03 | 0.09 ± 0.008 | 0.05 ± 0.003 |
| SY-C.56 | 1.36 ± 0.15 | 0.73 ± 0.15 | 0.09 ± 0.005 | 0.08 ± 0.004 |
| SY-C.57 | 1.28 ± 0.23 | 0.37 ± 0.04 | 0.07 ± 0.008 | 0.07 ± 0.007 |
| SY-C.58 | 1.52 ± 0.12 | 0.47 ± 0.07 | 0.11 ± 0.007 | 0.09 ± 0.009 |
| SY-C.59 | 1.76 ± 0.11 | 0.45 ± 0.07 | 0.11 ± 0.004 | 0.06 ± 0.009 |
| SY-C.60 | 1.91 ± 0.18 | 0.50 ± 0.07 | 0.11 ± 0.005 | 0.06 ± 0.002 |
| SY-C.61 | 1.38 ± 0.08 | 0.57 ± 0.08 | 0.10 ± 0.003 | 0.09 ± 0.008 |
C control and S stressed plants
Fig. 3.
Plant water content according to line and drought treatment; control (— O —) and stressed (—∆—). Values are means (± S.E.) of measurements from eight plants for each treatment (n = 8)
Correlation matrix
Correlations between morphological parameters due to drought were highly significant for most of the parameters (Table 5). For example, plant length appeared as a function of leaf area (R² = 0.88) and fresh weight (R² = 0.80), while leaf area appeared as a function of fresh weight (R² = 0.86).
Table 5.
Pearson correlation of the morphological parameters measured per plant of combined data on 36 clones (stressed and control)
| PL | NL | LA | STh | NR | RL | RTh | FW | DW | |
|---|---|---|---|---|---|---|---|---|---|
| NL | 0.43** | ||||||||
| LA | 0.88** | 0.36** | |||||||
| STh | 0.30** | −0.07ns | 0.35** | ||||||
| NR | 0.67** | 0.44** | 0.63** | 0.22** | |||||
| RL | 0.78** | 0.42** | 0.68** | 0.20** | 0.66** | ||||
| RTh | 0.65** | 0.24 ns | 0.67** | 0.36** | 0.67** | 0.54** | |||
| FW | 0.80** | 0.34** | 0.86** | 0.32** | 0.73** | 0.70** | 0.79** | ||
| DW | 0.26** | 0.12 ns | 0.41** | 0.23** | 0.38** | 0.18 ns | 0.42** | 0.55** | |
| PWC | 0.81** | 0.44** | 0.78** | 0.29** | 0.68** | 0.75** | 0.65** | 0.77** | 0.09 ns |
ns, *,** = non-significant or significant at P = 0.05 and 0.01 probability levels, respectively.
PL plant length (cm), NL number of leaves, LA leaf area (mm²), STh stem thickness (mm), NR number of roots, RL root length (cm), RTh root thickness (mm), FW fresh weight, DW dry weight (g), PWC plant water content (%)
Cluster analyses
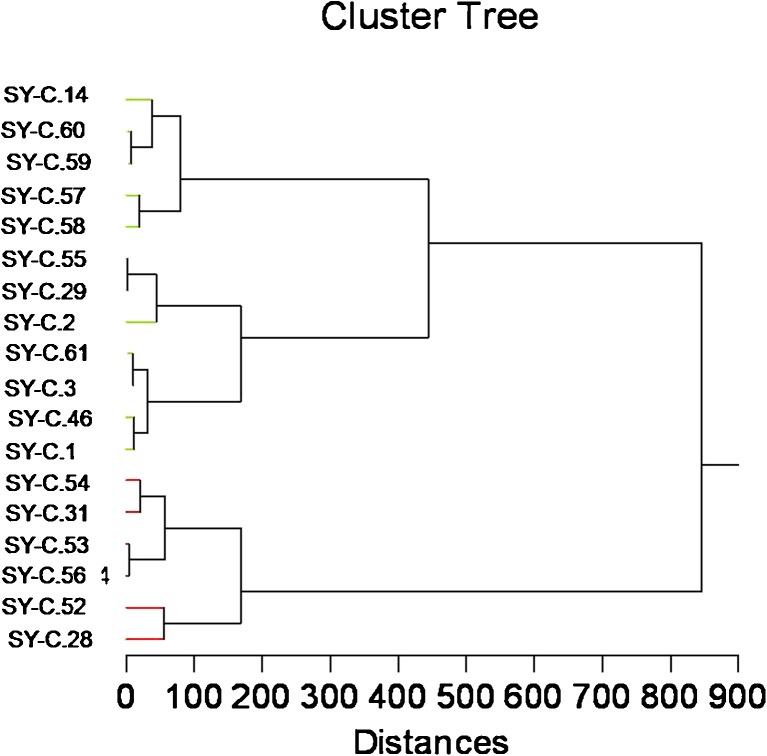
Clustering for response to drought resulted in three distinct groups: (1) drought tolerant group consisting of lines SY-C.28, SY-C.52, SY-C.56, SY-C.53, SY-C.31, and SY-C.54; (2) a moderately tolerant group consisting of lines SY-C.1, SY-C.46, SY-C.3, SY-C.61, SY-C.2, SY-C.29, and SY-C.55, and (3) a susceptible group consisting of lines SY-C.58, SY-C.57, SY-C.59, SY-C.60, and SY-C.14 (Fig. 4).
Fig. 4.
Dendrogram based on relative values of ten morphological and physiological parameters of growth of potato lines under drought
Discussion
Screening a large number of genotypes for drought tolerance in the field is difficult, due to spatial heterogeneity of soil chemical and physical properties and seasonal fluctuations. In vitro screening of potato genotypes for water stress tolerance has been proposed as an alternative approach to costly, labor-intensive and sometimes problematic field-based screening (Rahman et al. 2008). The effect of water or salinity stress on in vitro potato growth has been reported to be similar to that observed under field conditions (Zhang and Donnelly 1997; Gopal and Iwama 2007; Aghaei et al. 2008). Limited information is available about effects of sorbitol on in vitro potato growth. However, Gopal and Iwama (2007) reported that addition of sorbitol to MS medium decreased water potential, inducing drought stress affecting shoot and root growth. Moreover, the water potential values of MS media in the present study were consistent with that reported by Gopal and Iwama (2007) who concluded the possibility to use the in vitro system as an alternative to field evaluations for studying the general effect of water-stress on plant growth and development.
Drought is known to adversely affect plant height and weight (Deblonde and Ledent 2001; Tourneux et al. 2003; Lahlou and Ledent 2005). Studies have shown that the first morphological effect of drought is leaf size reduction (Jefferies and MacKerron 1987), which results in lessened photosynthesis and reduction in dry matter accumulation in tubers (Jefferies 1993; Deblonde et al. 1999). Drought leads to decreased tissue water content resulting in reduced leaf turgor, and consequently inhibited cell elongation (Taiz and Zeiger 2006). These results indicated decrease in stem length and thickness, number of leaves, total area of leaves, number of roots, length and thickness of roots, fresh and dry weight, and water content due to drought with variation among lines for these traits. These results agree with other studies reporting decreased leaf number and plant water potentials (Frensh 1997), leaf area, stem height, growth and yield, canopy radiation interception and tuber dry matter concentration under drought (Schittenhelma et al. 2006; Sanchez-Rodriguez et al. 2010).
Compared to other species, potato is sensitive to drought (Harris 1978; Van Loon 1981; Frusciante et al. 1999; Hassanpanah et al. 2008) because of its shallow root system (Iwama and Yamaguchi 2006). There are differences in potato cultivar susceptibility to drought (Steckel and Gray 1979; Levy 1983, 1986; Susnoschi and Shimshi 1985). Screening for drought susceptibility is complicated. Frusciante et al. (1999) used parameters correlated with drought tolerance, i.e., canopy expansion, canopy temperature, chlorophyll fluorescence, and leaf relative water content, appear very promising, especially if used in the first generations of clonal selection. Based on the relative values of shoot length, fresh and dry weight as well as root length, fresh and dry weight, Zhang and Donnelly (1997) screened in vitro genotypes of potato for salinity tolerance. Physiological parameters were used by Aghaei et al. (2008), with random amplification of polymorphic DNA screening to confirm the reliability of screening using physiological parameters. A single parameter has been used for screening. For example, Ranalli et al. (1996) showed that differences in canopy temperature between irrigated and stressed treatments can be used for screening for drought tolerance among potato genotypes. Moreover, leaf relative water content was used as one of the most reliable indicators for defining plant sensitivity to drought (Rampino et al. 2006).
In conclusion, water stress tolerance of potato genotypes can be evaluated in vitro, and screening growth parameters can be done at 4 % sorbitol for in vitro potato genotypes. Many potato genotypes could be easily evaluated by this method for the identification of suitable parental lines with improved stress tolerance. Although, the results of the previous study clearly showed that screening of potato genotypes under field conditions provide a high efficacy in vitro screening method for drought tolerance, the effectiveness of this approach, should be further tested on theses potato genotypes with known performance for root and shoot growth characteristics related to drought-tolerance under field conditions.
Acknowledgements
The authors thank Miss Zenab Najla for revising the manuscript and improving the English. This research took place at NCBT (National Commission for Biotechnology, Damascus, Syria) and was supported by the Higher Commission for Scientific Research in Syrian Arab Republic.
References
- Aghaei K, Ehsanpour AA, Balali G, Mostajeran A. In vitro screening of potato (Solanum tuberosum L.) cultivars for salt tolerance using physiological parameters and RAPD analysis. Am-Eurasian J Agric Environ Sci. 2008;3(2):159–164. [Google Scholar]
- Boyer JS, Knipling EB. Isopiestic technique for measuring leaf water potential with a thermocouple psychrometer. Proc Natl Acad Sci USA. 1965;54:1044–1051. doi: 10.1073/pnas.54.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR. Antioxidants in potato. Amer J Potato Rsch. 2005;82:163–172. doi: 10.1007/BF02853654. [DOI] [Google Scholar]
- Deblonde PMK, Ledent JF. Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur J Agron. 2001;14:31–41. doi: 10.1016/S1161-0301(00)00081-2. [DOI] [Google Scholar]
- Deblonde PMK, Haverkort AJ, Ledent JF. Responses of early and late potato cultivars to moderate drought conditions. Agronomic parameters and carbon isotope discrimination. Eur J Agron. 1999;11(2):91–105. doi: 10.1016/S1161-0301(99)00019-2. [DOI] [Google Scholar]
- Food and agriculture organization of the united nations (2009) FAOSTAT. http://faostat.fao.org
- Frensh J. Primary response of root and leaf elongation to water deficits in the atmosphere and soil solution. J Exp Bot. 1997;48:985–999. [Google Scholar]
- Frusciante L, Amalia B, Carputo D, Ranalli P. Breeding and physiological aspects of potato cultivation in the Mediterranean region. Potato Rsch. 1999;42:265–277. doi: 10.1007/BF02357857. [DOI] [Google Scholar]
- Gopal J, Iwama K. In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol. Plant Cell Rep. 2007;26:693–700. doi: 10.1007/s00299-006-0275-6. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhang J, Gao Q, Xing S, Li F, Wang W. Drought tolerance through over expression of monoubiquitin in transgenic tobacco. J Plant Physiol. 2008;165:1745–1755. doi: 10.1016/j.jplph.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Harris PM. The Potato crop: The scientific basis for improvement. London: Chapman and Hall; 1978. [Google Scholar]
- Hassanpanah D, Gurbanov E, Gadimov A, Shahriari R. Determination of yield stability in advanced potato cultivars as affected by water deficit and potassium humate in Ardabil region. Iran Pak J Biol Sci. 2008;15:1330–1335. doi: 10.3923/pjbs.2008.1354.1359. [DOI] [PubMed] [Google Scholar]
- Hussain M, Malik MA, Farooq M, Ashraf MY, Cheema MA. Improving Drought tolerance by exogenous application of glycine-betaine and salicylic acid in sunflower. J Agron Crop Sci. 2008;194:193–199. doi: 10.1111/j.1439-037X.2008.00305.x. [DOI] [Google Scholar]
- Iwama K, Yamaguchi J. Abiotic stresses. In: Gopal J, Khurana SM, editors. Handbook of potato production, improvement and postharvest management. New York: Food Product Press; 2006. pp. 231–278. [Google Scholar]
- Jefferies RA. Responses of potato genotypes to drought. I. Expansion of individual leaves and osmotic adjustment. Ann Rev Appl Biol. 1993;122:93–104. doi: 10.1111/j.1744-7348.1993.tb04017.x. [DOI] [Google Scholar]
- Jefferies RA, MacKerron DKL. Aspects of the physiological basis of cultivar differences in yield of potato under droughted and irrigated conditions. Potato Rsch. 1987;30:201–217. doi: 10.1007/BF02357663. [DOI] [Google Scholar]
- Kaya MD, Okçub G, Ataka M, Çıkılıc Y, Kolsarıcıa O. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.) Eur J Agron. 2006;24:291–295. doi: 10.1016/j.eja.2005.08.001. [DOI] [Google Scholar]
- Lahlou O, Ledent JF. Root mass and depth, stolons and roots formed on stolons in four cultivars of potato under water stress. Eur J Agron. 2005;22:159–173. doi: 10.1016/j.eja.2004.02.004. [DOI] [Google Scholar]
- Levy D. Varietal differences in the response of potatoes to repeated short periods of water stress in hot climates. 2. Tuber yield and dry matter accumulation and other tuber properties. Potato Rsch. 1983;26:315–321. doi: 10.1007/BF02356153. [DOI] [Google Scholar]
- Levy D. Genotypic variation in the response of potatoes (Solanum tuberosum L.) to high ambient temperatures and water deficit. Field Crops Res. 1986;15:85–96. doi: 10.1016/0378-4290(86)90103-6. [DOI] [Google Scholar]
- Rahman MH, Islam R, Hossain M, Haider SA. Differential response of potato under sodium chloride stress conditions in vitro. J Bio-Sci. 2008;16:79–83. [Google Scholar]
- Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C. Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006;29:2143–2152. doi: 10.1111/j.1365-3040.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- Ranalli P, Di Candilo M, Ruaro G, Marino A (1996) Drought effects on chlorophyll fluorescence and canopy temperature. 14th Triennial Conference of the European Association for Potato Research, 2–7 May 1996, Sorrento, Italy
- Sanchez-Rodriguez E, Rubio-Wilhelmi MM, Cervilla LM, Blasco B, Rios JJ, Rosales MA, Romero L, Ruiz JM. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010;178:30–40. doi: 10.1016/j.plantsci.2009.10.001. [DOI] [Google Scholar]
- Schafleitner R, Rosales ROG, Gaudin A, Aliaga CAA, Martinez GN, Marca LRT, Bolivar LA, Delgado FM, Simon R, Bonierbale M. Capturing candidate drought tolerance traits in two native Andean potato lines by transcription profiling of field grown plants under water stress. Plant Physiol Biochem. 2007;45:673–690. doi: 10.1016/j.plaphy.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Schapendonk AHCM, Spitters CJT, Groot PJ. Effects of water stress on photosynthesis and chlorophyll fluorescence of five potato cultivars. Potato Rsch. 1989;32:7–32. [Google Scholar]
- Schittenhelma S, Sourell H, Lopmeierc F. Drought resistance of potato cultivars with contrasting canopy architecture. Eur J Agron. 2006;24:193–202. doi: 10.1016/j.eja.2005.05.004. [DOI] [Google Scholar]
- Steckel JR, Gray D. Drought tolerance of potatoes. J Agric Sci Cambridge. 1979;47:770–775. [Google Scholar]
- Susnoschi M, Shimshi D. Growth and yield studies of potato development in a semi-arid region. 2. Effect of water stress and amounts of nitrogen top dressing on growth of several cultivars. Potato Res. 1985;28:161–176. doi: 10.1007/BF02357442. [DOI] [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. 4. Sunderland, Massachusetts: Sinauer Associates Inc; 2006. [Google Scholar]
- Tourneux C, Devaux A, Camacho MR, Mamani P, Ledent JF. Effect of water shortage on six potato genotypes in the highlands of Bolivia (II): water relations, physiological parameters. Agronomie. 2003;23:181–190. doi: 10.1051/agro:2002080. [DOI] [Google Scholar]
- Van Loon CD. The effect of water stress on potato growth development and yield. Am Potato J. 1981;58:51–69. doi: 10.1007/BF02855380. [DOI] [Google Scholar]
- Zhang Y, Donnelly DJ. In vitro bioassays for salinity tolerance screening of potato. Potato Rsch. 1997;40:285–295. doi: 10.1007/BF02358010. [DOI] [Google Scholar]