Abstract
Transcription factors (TFs) are an important target in understanding the regulation of plant responses to environmental stress including moisture stress. Members of the same TF family may differ in their response to moisture stress. The expression pattern could vary between shoot and root tissues depending on level of moisture stress. A set of five rarely studied TF families viz., MADS-box (MCM1, AGAMOUS, DEFICIENS and SRF), Auxin Responsive Factor (ARF), Heme Activator Protein 2 (HAP2), Multiprotein Bridging Factor (MBF) and Homeobox (HB) together having 20 members in sorghum, were expression analyzed through quantitative real-time PCR (qRT-PCR) in well watered and moisture stressed shoot and root tissues of sorghum using SYBR Green® to quantify dsDNA synthesis. Fluorescence values were used to calculate PCR efficiency by using LinRegPCR. The PTSb00029.1 and PTSb00033.1 of ARF family and PTSb00174.1 and PTSb00175.1 of HB family recorded 2 to 5, PTSb00221.1 and PTSb00208.1 of MADS family and PTSb00128.1 of HAP2 family recorded 5 to 10 fold up-regulation under moisture stress regimes. The PTSb00128.1, a HAP2 family member, recorded 15 fold up-regulation in mild moisture stressed root tissues. TF genes such as PTSb00218.1, PTSb00220.1, PTSb00031.1, PTSb00032.1, PTSb00034.1 and PTSb00223.1 were found down regulating in both tissues types under moisture stress condition. However, the PTSb00128.1, PTSb00221.1, PTSb00029.1, PTSb00033.1 and PTSb00174.1 TFs were found up-regulating to varied levels in mild and severe moisture stressed root tissues only. Verification of qRT-PCR results was done by in situ hybridization (ISH) of randomly selected two TF genes in shoot and root tissues of sorghum. Taken together, moisture stress triggered up-regulation of more genes in root tissue compared to shoot tissue in sorghum.
Keywords: Quantitative real-time PCR, Transcription factor genes, cDNA, Moisture stress, Sorghum
Introduction
Abiotic stresses represent the most limiting factors affecting crop productivity all over the world. Moisture stress affects plant growth and development and depending on the stage, the yield fall varies among crop plants. Plants have developed adaptive strategies at morphological, physiological, cellular, and metabolic levels, which allow them to survive and reproduce. These adaptive responses are governed by specific gene products that allow plants to avoid the stress or become tolerant. Considerable amount of gene variation occurs among crop plants for this trait, sorghum is one of them. Among the cereals sorghum is considered as a drought tolerant crop which is largely grown in semi-arid tropics throughout the world as staple food and cattle feed. Sorghum's ability to withstand drought stress at different developmental stages makes it an excellent candidate for drought study. Particularly, smaller genome size (730 Mb) coupled with drought tolerant genes makes it an attractive model for functional and comparative genomics of drought tolerant studies. Hence, sorghum can serve as a source of genes and regulatory elements for drought tolerance through translational genomics approach and aid in further understanding the interaction of genes and networks under adverse condition (Paterson et al. 2009).
Large number of genes and their products are involved in drought tolerance response in different pathways. Transcription factors (TF) are master regulators, generally early responding genes in drought tolerance response in plants. More than 30 families of TFs have been predicted in Arabidopsis including near about 1,922 TFs engaged in different functions. Members of DREB or CBF, MYB, bZIP, and zinc-finger families have been well characterized with roles in the regulation of plant stress responses. But very little work has been done on members of MADS box (MCM1, AGAMOUS, DEFICIENS and SRF), Auxin Responsive Factor (ARF), Heme Activator Protein 2 (HAP2), Multiprotein Bridging Factor (MBF) and Homeobox (HB) TF families in relation to moisture stress. MADS-box TFs are key regulators of several plant developmental processes. However, there are reports to indicate the role of MADS in moisture stress tolerance (Mane et al. 2007; Marino et al. 2009). The annotated functions of ARF family point to their involvement in auxin response in plants. Some of the members of ARF family are known to involve in drought tolerance besides others responses (Seo et al. 2009). HAP2 is one of the subunits of a heterotrimeric CCAAT-box-binding complex appears to control expression of genes important for mitochondrial biogenesis (Edwards et al. 1998). Plant NF-Y is one of CCAAT-box binding complex, involves in drought tolerance. NF-YB1 and its ortholog in maize ZmNF-YB2, lead to enhanced drought tolerance (Nelson et al. 2007). MBF is another TF family whose members found to work as transcriptional co-activators in plants. MBF members have been implicated in moisture stress tolerance in plants but detailed studies are still remained to be done (Kim et al. 2007). HB genes encode transcription factors that typically switch on cascades of other genes. Most of the times, homeodomain proteins act in the promoter region of their target genes together as complexes with other transcription factors in plants. Nearly 37 homeobox genes were found to be differentially expressed significantly under various abiotic stress conditions in rice (Jain et al. 2008).
Genome-wide analyses of mRNA abundance showed that expression of 5–30 % of the genes were under the control of abiotic stresses (Rabbani et al. 2003; Wang et al. 2004). In some cases, differential regulation of specific genes and pathways has been associated with improved adaptation of crop genotypes to different abiotic stress (Zhang et al. 2004). New technologies such as DNA sequencing methodologies, throughput platform DNA array, in situ hybridization (ISH), subtractive hybridization, quantitative real-time polymerase chain reaction (qRT-PCR) etc. have increased knowledge of transcriptome. But, the introduction of qRT-PCR technology has significantly changed the field of measuring gene expression in both animal and plant molecular biology research. As an assay method of gene expression qRT-PCR is the highly sensitive technique for mRNA detection and quantitation in molecular biology. This technique is sensitive enough to enable quantitation of RNA from a single cell. The development of novel chemistries and instrumentation platforms has made it a widespread tool in research field. Another powerful method for gene expression analysis is ISH. This technique can localize targeted DNA or RNA sequences in their native tissue or cell environment. ISH can also be used to quantify specific DNA or RNA sequences in a sample. The development of non-radioactive methods for nucleic acid labeling simplified the application of this technique (Karlgren et al. 2009). The present study focused on gaining insight on differential regulation of the members of the same TF family in response to moisture stress in shoot and root tissues of sorghum. We reported expressional dynamics of 20 TF genes belong to five different TF families and further localization of expression pattern of two TF genes in root and shoot tissues of sorghum experiencing moisture stress.
Materials and methods
Selection of TF genes and primer pairs
A set of five rarely studied TF families viz., MADS, ARF, HAP2, MBF and HB having 20 members in sorghum were selected for their expression analysis. Selection of TF families was done based on the available information and annotated functions of TF families in Arabidopsis and rice. To know the differential response of the members of the same family to moisture stress, most possible all the known members of selected family were selected for analysis. Function of selected TF families, number of available members within family and selected members for the experiment are presented in Table 1.
Table 1.
Details of transcription factor families and members selected for real-time quantitation in sorghum under moisture stress
| Sl. No. | TF family | Known members | Selected members | Annotated function |
|---|---|---|---|---|
| 1. | MADS | 16 | 06 | Signal-transduction pathway (drought response), floral organ identity determination |
| 2. | HB | 8 | 05 | ABA-responsive, drought responsive |
| 3. | ARF | 8 | 07 | Auxin responsive |
| 4. | MBF1 | 1 | 01 | Phytohormone response |
| 5. | CCAAT-HAP2 | 3 | 01 | Mitochondrial biogenesis |
| Total | 20 |
The nucleotide sequences of all the 20 TF genes from sorghum were carefully analyzed by comparing among the members of same family for similarity and distinction; the distinct portion of the sequence of each member to differentiate from others was identified and targeted to design the PCR primer pairs. Generally, the TF families have one or more conserved regions which are common for all the members. Non-conserved region specific to each member was scanned for primer designing. The primer pairs specific to all the 20 TF genes were designed using Primer3plus software (http://frodo.wi.mit.edu/cgi-bin/primer3plus/primer3plus_www.cgi) and the primers were synthesized at Sigma-Aldrich (Germany). To ensure maximum specificity and efficiency during PCR amplification of TF cDNA under a standard set of reaction conditions, a stringent set of criteria was used for primer design. This included predicted melting temperatures (Tm) of 60 ± 2 0C, primer lengths of 20–24 nucleotides, guanine-cytosine (GC) contents of 45–55 % and PCR amplicon length of 100–200 base pairs (bp) were adopted for designing the primer pairs. Primer sequences are presented in Table 2. Further, primer pair specificity was confirmed through BLAST analysis, PCR product size on agarose gel and sequencing of randomly selected two TF genes amplicon.
Table 2.
List of primer pairs used for the qRT-PCR amplification of 20 TF genes and two reference genes
| Sr. No. | TF genes | Primers sequence | |
|---|---|---|---|
| Database ID | Forward (5'-3') | Reverse (5'-3') | |
| 1 | PTSb00128.1 | AGCTAATCAGGCAGGCAGTC | ACAACAACCGACTGACACCA |
| 2 | PTSb00208.1 | GCAAAAGAGGAGCAGATTGG | TGGCTTTCTTGCAAGTTGTG |
| 3 | PTSb00217.1 | TAGAAGAAAGCTGGAGGAGACC | CTTCCAGGGGATGAAAGAAC |
| 4 | PTSb00218.1 | TCGGACTGGTCATCTTCTCC | AGTTCTGTGTTGGGGTTTGC |
| 5 | PTSb00219.1 | AATGTGCTGCGACTGTCTTG | TACCCAATATGCAGGGAAGG |
| 6 | PTSb00220.1 | CTGACGACTGCTCCGATACA | CGGAGAGGGCAAGTAATCAG |
| 7 | PTSb00221.1 | CAGCAGCTACACGGCAATAA | AGTTCGATCTCATGGCAACC |
| 8 | PTSb00029.1 | GGAAGCATGCCAGCTAACTC | GGACGCAGAATAGCAGAAGG |
| 9 | PTSb00030.1 | GAGGGTGACGAATCTCCTGA | GGACGCAGAATAGCAGAAGG |
| 10 | PTSb00031.1 | AGTGGAGAAGCCTGCTGGTA | CGGACAGAGAATGAGCAACA |
| 11 | PTSb00032.1 | CGAGCCCTTCAGAGTTCATC | CCTCTTCCCCTTCAAACCTC |
| 12 | PTSb00033.1 | GAAGCAGCCAGAGGAGAATG | CTGGTGTCAGAGGCTGTCAA |
| 13 | PTSb00034.1 | CCTTCGAGGTGAAGATGGAG | TGGCTTGAGCACTGGTTATG |
| 14 | PTSb00035.1 | CTCAAAATGGCGTTCCCTTA | GGTTCACAGGAGATGGGCTA |
| 15 | PTSb00172.1 | TGCATGTCATGAGTGCAGAA | AACGATGCTGCTGATCATTG |
| 16 | PTSb00173.1 | TCAACAGCTCAGGCAACAAC | TACGGGTGAAGGAAATGCTC |
| 17 | PTSb00174.1 | AACAGCGAGCTTTCCAACAG | TTGGATACGGGTGAAGGAAG |
| 18 | PTSb00175.1 | CAGCACTGGCAAAGTCTCAA | AGCTCTCGTGCCAGACTCTC |
| 19 | PTSb00176.1 | GAAAGGGAAGCTACCGAAGG | GCTGCAAGCCTCACCTTATC |
| 20 | PTSb00223.1 | AGGGAAGACAAGGCAAACTG | CAAGCAGCAAGCATTACAGG |
| 21 | Actin | TGGCATCTCTCAGCACATTC | GGGCGGAAAGAATTAGAAGC |
| 22 | 18S rRNA | GCCTTTCGAAGCACTTTCAC | AACCAAACCTCCATGCTCAC |
Moisture stress induction and tissue collection
Drought tolerant sorghum cv. E36-1 was selected for experiment. Seedlings were raised in pots containing equal proportion of coarse sand and 2–5 mm brick pieces to allow easy removal of root system at the time of tissue harvest. Slow release fertilizer, 19:19:19 (Rashtriya Chemicals and Fertilizers Ltd., India) and micronutrients were used as source of nutrients. Up to 30 days, the pots were maintained at field capacity by regular irrigation and thereafter water was withheld to impose moisture stress. Control pots were maintained at field capacity by regular watering. The relative water content (RWC) and leaf water potential (LWP) were monitored in regular intervals using Aramid-3000® (MRC Ltd., Israel) (Scholander et al. 1965). The RWC were determined as described by Netondo et al. (2004). Sorghum shoot and root tissues were harvested for mild moisture stress when the values of RWC and LWP were 75 % and −12 bars respectively. For severe moisture stress, sorghum leaf and root tissues were harvested when the values of RWC and LWP were 39.5 % and −24 bars respectively. Moisture stressed and control tissues were harvested in two sets separately. One set was subjected to paraformaldehyde fixation and another set was flash frozen in liquid nitrogen and stored at −80 °C for further RNA isolation.
RNA isolation and cDNA synthesis
Total RNA was extracted using TRIZOL reagent (Invitrogen, San Diego USA) following bench level protocol previously optimized in our laboratory. RNA was quantified using a Nanodrop Spectrophotometer® ND-100 (NanoDrop Technologies, Inc. USA). Ten μg of total RNA were digested with RNase free DNase I (Ambion, USA) according to manufacturer's protocol. RNA integrity was checked on a formaldehyde denaturing agarose gel before and after DNase I treatment. Further, resulting RNA samples were confirmed for the absence of genomic DNA contamination by PCR as suggested by Caldana et al. (2007). Desired quantity of DNA free total RNA was converted to single stranded cDNA by using High Capacity cDNA Reverse Transcription™ kit (Ambion, USA) following the instructions in the manual provided by the company. The efficiency of cDNA synthesis was assessed by normal PCR amplification of control 18S rRNA and actin genes. Only cDNA preparations that yielded sharp bands were selected for further experiment.
Internal controls
The genes such as 18S rRNA, EF-1α, β-actin, β-tubulin, and ubiquitin (UBQ) have served as good reference genes in rice and Arabidopsis (Caldana et al. 2007 and Czechowski et al. 2004). We initially selected three genes, viz, actin (AC1), β-tubulin and 18S rRNA which are common housekeeping genes in plants. The gene expression stability measure (M) was estimated in a set of 6 different cDNA samples originating from mild, severely moisture stressed and well watered root and shoot tissues using NormFinder software as described (Claus et al. 2004). The gene expression stability measure (M) was in the order of magnitude 0.301, 0.291 and 0.306 for actin (AC1), 18S rRNA and β-tubulin respectively.
Real-time PCR conditions and data analysis
PCR reactions were carried out in an Eppendorf Mastercycler® ep realplex instrument (Eppendorf, Germany) in triplicates. SYBR Green® (Ambion, USA) was used to quantify dsDNA synthesis. Reactions (10 μl total volume) were performed in an Eppenderof 96-well plate containing 5 μl 2X SYBR Green®, 2 ng cDNA and 200 nM of each gene-specific primer. The master mix of different components of real-time PCR was prepared to avoid handling and pipetting errors. All templates were amplified using the following standard PCR program: 95 °C for 10 min; 40 cycles of 95 °C for 30 s and 60 °C for 30 s, and 72 °C for 30 s. Melting curves were generated after 40 cycles by heating the sample up to 95 °C for 15 s followed by cooling down to 60 °C for 15 s and heating the samples to 95 °C for 15 s.
The Ct values and raw fluorescence data was extracted from the Eppendorf Mastercycler® ep realplex using extract PCR (Eppendorf, Germany) software. The baseline fluorescence data was collected from 3–15 cycles for background correction. The average of 3–15 cycles was subtracted from fluorescence value of all cycles. The background corrected data was used to calculate PCR efficiency by LinRegPCR (http://bioinfo.amc.uva.nl; subject: LinRegPCR). The PCR efficiency of all the TF genes was obtained from the exponential phase of each individual amplification plot using the equation (1 + E) = 10slope (Ramakers et al. 2003). Ct values of all TF genes were normalized with Ct value of 18S rRNA gene (26.53) to obtain ∆Ct value. The calculated PCR efficiency and ∆Ct values were used to derive fold expression of TF gene using the following method, 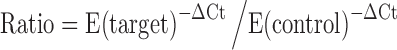 , where E (target) represents efficiency of target gene in sample, E (control) represents PCR efficiency of target gene in control and ∆Ct represents Ct of target gene—Ct of reference gene.
, where E (target) represents efficiency of target gene in sample, E (control) represents PCR efficiency of target gene in control and ∆Ct represents Ct of target gene—Ct of reference gene.
In situ hybridization
PTSb00220.1 (MADS) and PTSb00223.1 (MBF) TF genes were selected for their localization of mRNA in well watered and moisture stressed leaf and root tissues of sorghum. These two TF genes were selected on the basis of their lowest raw Ct values in both tissue types under moisture stress regimes.
The ISH was performed as described by Jackson (1991) with minor modifications. Harvested sorghum tissues were immediately placed in freshly prepared 4 % paraformaldehyde (PFA) solution and vacuum infiltrated at 450 mmHg on ice. Phosphate Buffer Saline (PBS) treatment was given twice, 30 min each, followed by an increasing series of ethanol. An increasing series of histochoice treatment was given for 30 min each and ¼ volume paraplast chips were added to the vials and kept overnight. Histochoice was replaced with freshly melted wax and left the vials open overnight at 60 °C. The wax was replaced twice everyday for 4–5 days. Tissues were placed in molds and embedded with hot wax. Tissue sections of about 7–8 μm thick were taken using microtome (Leica, Germany). The sections were pre-treated to optimize the specificity and signal intensity of hybridization. The tissue sections were first deparaffinised by histochoice treatment and rehydrated through a decreasing ethanol series. The Sodium Citrate Saline (SSC) treatment was given for 15–20 min followed by Tris-EDTA (TE) with proteinase K (1 μg/ml) treatment for 30 min at 37 °C followed by 0.2 % glycine in PBS for 2 min. The slides were treated for 10 min with PFA. Again, PBS wash was given for 5 min twice, followed by acetic anhydride treatment for 10 min. The slides were finally washed in PBS for 5 min twice, followed by an increasing ethanol series treatment for dehydration of tissues on the slides. Slides were hybridized with DIG-labeled riboprobes and kept at 50 °C for 8 h. The slides were washed to remove the hybridization solution and subsequently treated with RNase A (Roche Diagnostics, Germany) to remove single stranded RNA. SSC and NTE solution treatment was given. The anti-DIG antibody was diluted (1:1250) in 1 % Bovine Serum Albumin (BSA), 0.3 % Triton X-100 in PBS solution and used for treatment. Slides were sandwiched together and dipped in the substrate solution and kept at dark for 1–5 days. Every day slides were checked for colour development, the and solution was changed. After the colour development, the slides were washed with TE solution to stop the alkaline phosphates activity. The sections were dehydrated through an increasing ethanol series followed by histochoice treatment. Finally the slides were dried in a fume hood and mounted with drops of cytoseal. The slides were left overnight to dry in laminar air flow.
Results and discussion
The RWC and LWP were regularly monitored to harvest the tissues. In well watered leaves of sorghum, the values of RWC and LWP at full turgor were 96–97 % and −5 to −6 bars, respectively. Sorghum shoot and root tissues were harvested for mild moisture stress when the values of RWC and LWP were 75 % and −12 bars respectively. During this period the leaves tended to roll slightly. For severe moisture stressed sorghum leaf and root tissues, tissue harvesting was done when the values of RWC and LWP were 39.5 % and −24 bars respectively. At this stage the leaves were found to be severely desiccated and drooping.
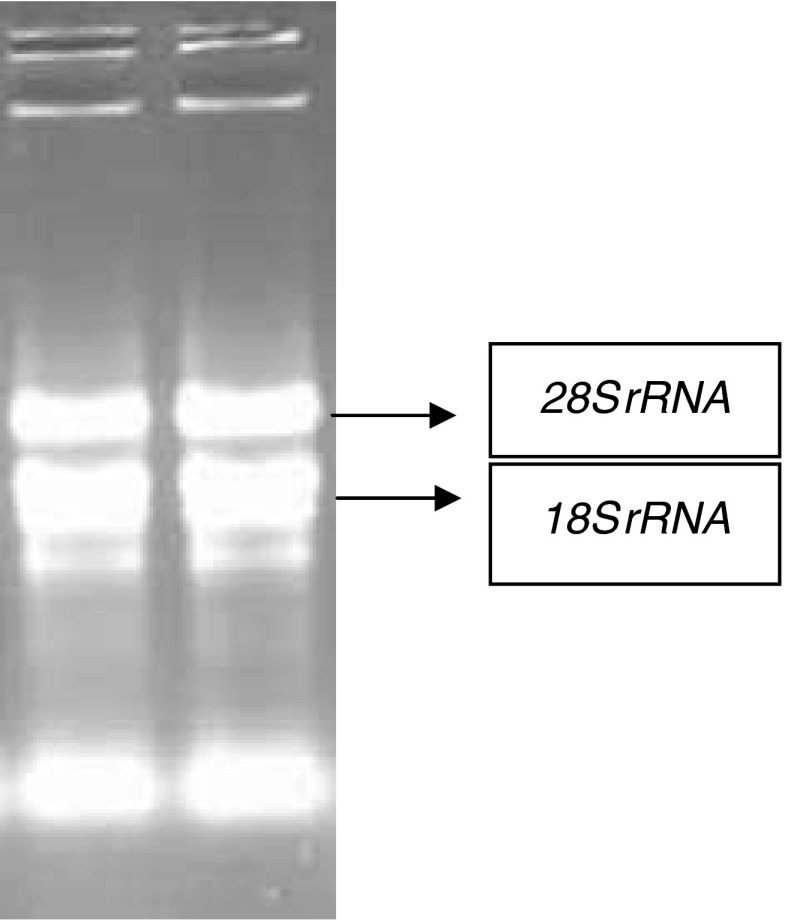
The total RNA was isolated from moisture stressed and well watered shoot and root tissues using TRIZOL reagent. The integrity of total RNA was examined by electrophoresing the individual RNA samples on denaturing, formaldehyde 1 % agarose gel stained with ethidium bromide which clearly showed the presence of two bright bands corresponding to ribosomal 28S rRNA and 18S rRNA with a ratio of intensities of ~ 2:1 (Fig. 1). In order to eliminate genomic DNA contamination, about 10 μg of total RNA from each treatment was treated with DNase I enzyme. Resulting total RNA as template and sorghum gene specific primers were used in the PCR reaction and confirmed absence of genomic DNA traces in RNA samples as there was not amplification detected.
Fig. 1.
Integrity of total RNA isolated from sorghum: two bright bands corresponding to ribosomal 28S rRNA and 18S rRNA with a ratio of intensities of ~ 2:1 are visible in gel photograph
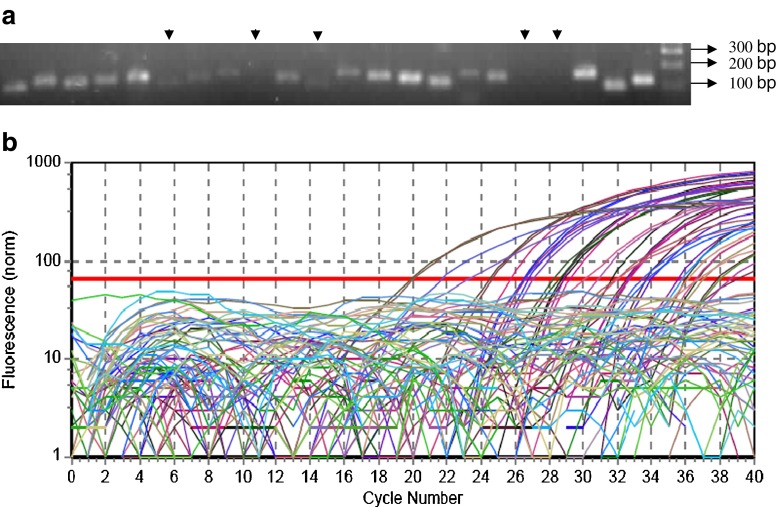
Specificity of PCR primers was assessed in three ways: by melting curve analysis of PCR reaction products to confirm the occurrence of specific amplification peaks and an absence of primer-dimer formation. Fractionation of PCR products on 3 % agarose gel and by sequencing the products of two randomly selected TF genes conformed their specificity to the target members of the chosen TF families (Table 3; Fig. 2a). Despite high degree of sequence similarity among the members of TF families, it was possible to identify the distinct region that distinguished from one another in a PCR reaction. The distinct regions of homologous genes indeed pave way for their expression detection through strategically pointed primer pairs to such regions (Sadelin and wasserman 2004; Shaw et al. 2009). Out of 20 TF genes, 15 of them yielded expected amplicon and five primer pairs of the reactions yielded no detectable PCR product from both shoot or root cDNA at the end of 40 cycles indicating that the target genes were probably not expressed or expressed at very low level in these tissues and growth conditions. A typical qRT-PCR amplification plot is presented in Fig. 2b. Sequence of PCR product of PTSb00220.1 and PTSb00223.1 matched that of the intended target cDNA. Sequence analysis revealed that designed primers are highly specific (Table 3). The average PCR efficiency was computed for each individual primer pairs across all analyzed samples. Out of 20 TF genes tested, 2 genes recorded PCR efficiency greater than 1.91, and 10 recorded a PCR efficiency between 1.71 and 1.90. Three primer pairs had PCR efficiency between 1.60 and 1.70, and remaining 5 showed lower than 1.4.
Table 3.
Nucleotide sequences of PTSb00220.1and PTSb00223.1TF genes obtained after sequencing
| Sl. No. | TF gene ID | Nucleotide sequences (5' - 3') |
|---|---|---|
| 1 | PTSb00220.1 | GGCGTCGATTCAGAAATTGTGTATGAGGAAGGGCAGTCATCTGAATCTGTGACAAACACATCCT ATCCGCGCCCATCAACAGACACTGACGACTGCTCCGATACA |
| 2 | PTSb00223.1 | AGGGAAGACAAGGCAAACTGTCTGCTTATTATCATCTATGTTTGGTATCTCAAGTCATGCGGACT GTTTCTGTCGGTACAAGAGTGAATAACTGTGTTCTCAGACC |
Fig. 2.
Specificity of qRT-PCR reaction. a Separation of qRT-PCR products of 22 genes on 3 % agarose gel gives single product of the expected size for most of the reactions with few reactions yielding no product (arrow). b Typical real-time PCR amplification plot of 22 genes for two types of cDNA samples of shoot and root tissues of sorghum. As number of PCR cycle increases there is increase in fluorescence. Some curves do not cross threshold line in such reactions there is no detectable PCR product when loaded on 3 % agrose gel
Data normalization for relative quantitation of expression of genes was approached by identifying the most suitable reference gene among tested actin (AC1), β-tubulin and 18S rRNA in sorghum. The M value was estimated through qRT-PCR in a set of 6 different cDNA samples corresponding to mild shoot, mild root, severe shoot, severe root, control shoot, and control root. On a model-based variance estimation approach, the M value was in the order of magnitude 0.301, 0.291 and 0.306 for actin (AC1), 18S rRNA and β-tubulin respectively; hence, 18S rRNA and actin genes were selected as reference genes. This combination of reference genes recorded a M value of 0.222, indicating this as a most stable combination and absence of significant differences in the expression levels in varied experimental conditions. In several instances of plant gene expression analysis by qRT-PCR these genes with similar combination have been adopted (Marino et al. 2003; Ruth et al. 2008).
The technical precision or reproducibility of qRT-PCR was assessed by performing replicated measurements in separate PCR runs. The same pool of cDNA to account the precision in technique employed and two different pools of cDNA obtained independently from two different batches of total RNA under the same condition to test precision of biological responses of plant to moisture stress were used. Precision, as reflected by the correlation coefficient, was r > 0.97 for technical replicate and r > 0.96 for biological replicates for both shoot and root tissues. The scattered plot depicting correlation coefficient between duplicate measurements of cDNA levels from the same and different pool of total RNA harvested from same treatment is presented in Fig. 3. The correlation coefficient, was high in both the cases; technical and biological replicates recorded correlation coefficient values greater than 0.97 and 0.96 respectively in shoot and root tissues indicating high precision of technical and biological treatments and response of sorghum seedlings. A similar strategy to monitor the technical and biological precision of experiment was adopted by Czechowski and coworkers (2004) in Arabidopsis and by Kakar et al. (2008) in Medicago for TF genes to assign putative TF gene status. Besides separating the PCR products on an agarose gel, melt curve analyses was also performed for all PCR products to confirm the occurrence of specific amplification peaks and the absence of primer-dimer formation. Similar technical and biological precision in moisture stress experiments leading to qRT-PCR testing of TF gene was reported in Arabidopsis (Huang et al. 2008; Seo et al. 2009), soybean (Moreira et al. 2010) and maize (Li et al. 2007; Xiao-feng et al. 2009).
Fig. 3.
Technical precision of qRT-PCR reaction. a Technical precision of real-time PCR reflected as correlation coefficient between the duplicate measurements of cDNA levels of TF genes from the separate reverse transcription reaction (biological replicates). The correlation coefficient values for biological replicate is 0.97. b Technical precision of real-time PCR reflected as correlation coefficient between the duplicate measurements of cDNA levels of TF genes from the same reverse transcription reaction (technical replicates). The correlation coefficient values for technical replicate is 0.96
Different methods are available for estimating PCR efficiency. Classical methods use Ct values obtained from experiment while, alternative methods utilize fluorescence data captured during the exponential phase of amplification. We followed the later approach to calculate PCR efficiency by using LinRegPCR software to determine the PCR efficiency of each primer pair of the TF. In this method E value is derived from the log slope of the fluorescence versus cycle number curve for a particular primer pair, using the equation (1 + E) = 10slope (Ramakers et al. 2003).
Expression status of TF genes
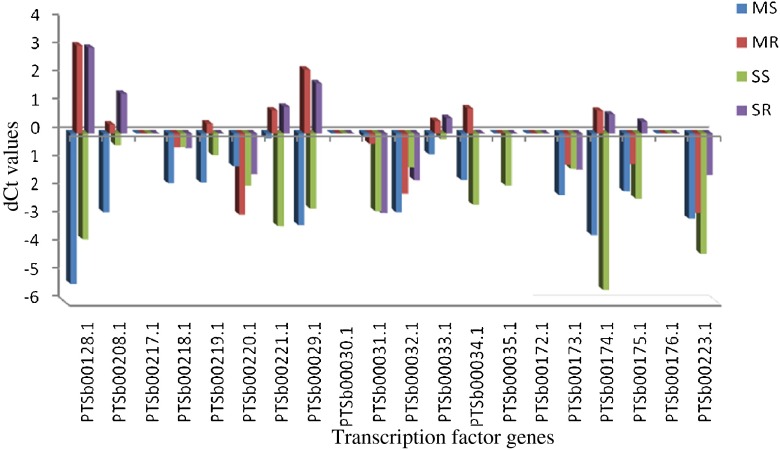
The expression levels selected TF genes were analyzed in mild moisture stressed shoot and root tissues in comparison to respective well watered controls (Table 4 and Fig. 4). In general, many genes were down-regulated in moisture stressed shoot tissues as compared to moisture stressed root tissues. In mild moisture stressed shoot tissues none of the tested genes up-regulated, while 11 genes viz. PTSb00128.1, PTSb00218.1, PTSb00220.1, PTSb00029.1, PTSb00031.1, PTSb00032.1, PTSb00033.1, PTSb00034.1, PTSb00174.1, PTSb00175.1 and PTSb00223.1 were found down-regulating. In mild moisture stressed root tissues PTSb00128.1, PTSb00221.1, PTSb00029.1, PTSb00033.1 and PTSb00174.1 were up-regulated and 8 genes viz. PTSb00218.1, PTSb00220.1, PTSb00031.1, PTSb00032.1, PTSb00034.1, PTSb00173.1, PTSb00175.1 and PTSb00223.1 genes were down regulated. Seven genes were commonly down-regulated in mild moisture stressed shoot and root tissues. In case of root tissues, PTSb00128.1, PTSb00221.1, PTSb00029.1, PTSb00033.1 and PTSb00174.1 were up-regulated (Table 5). Fold change analysis indicated that PTSb00029.1, PTSb00033.1 and PTSb00174.1 recorded a fold change range of 2 to 5; PTSb00221.1 TF recorded a fold change range of 5 to 10 while PTSb00128.1 recorded a fold change range of 10 to 20.
Table 4.
Fold change of 20 TF genes under moisture stress in sorghum
| Sl. No. | Database gene ID | TF family | Fold expression for mild moisture stress | Fold expression for severe moisture stress | ||
|---|---|---|---|---|---|---|
| shoot | root | shoot | root | |||
| 1 | PTSb00128.1 | HAP2 | 0.0520 | 15.8390 | 0.1092 | 5.1539 |
| 2 | PTSb00208.1 | MADS | 1.3545 | 1.1687 | 6.7597 | 7.1872 |
| 3 | PTSb00217.1 | MADS | 0.3557 | 0.0334 | 2.3357 | 4.7113 |
| 4 | PTSb00218.1 | MADS | 0.5310 | 0.8566 | 0.7173 | 0.8045 |
| 5 | PTSb00219.1 | MADS | 0.2671 | 3.6418 | 1.7407 | 2.4019 |
| 6 | PTSb00220.1 | MADS | 0.5117 | 0.8495 | 0.4109 | 0.2762 |
| 7 | PTSb00221.1 | MADS | 0.9941 | 5.1515 | 3.7656 | 7.2070 |
| 8 | PTSb00029.1 | ARF | 0.1696 | 2.1799 | 0.2731 | 4.5698 |
| 9 | PTSb00030.1 | ARF | 0.4808 | 1.1755 | 1.7149 | 4.5178 |
| 10 | PTSb00031.1 | ARF | 0.7872 | 0.6601 | 0.2924 | 0.5723 |
| 11 | PTSb00032.1 | ARF | 0.2053 | 0.5956 | 0.5211 | 0.7693 |
| 12 | PTSb00033.1 | ARF | 0.7018 | 1.5782 | 0.6298 | 1.4689 |
| 13 | PTSb00034.1 | ARF | 0.9677 | 0.5148 | 0.2099 | 0.7671 |
| 14 | PTSb00035.1 | ARF | 0.9807 | 0.3235 | 0.2722 | 0.4511 |
| 15 | PTSb00172.1 | HB | 0.3471 | 14.1505 | 1.0057 | 0.7871 |
| 16 | PTSb00173.1 | HB | 1.0892 | 0.7421 | 0.4372 | 1.0225 |
| 17 | PTSb00174.1 | HB | 0.0708 | 3.2442 | 0.0876 | 1.9090 |
| 18 | PTSb00175.1 | HB | 0.3371 | 0.1149 | 0.3260 | 1.8753 |
| 19 | PTSb00176.1 | HB | 0.4060 | 1.2728 | 0.0299 | 0.5774 |
| 20 | PTSb00223.1 | MBF | 0.1698 | 0.0213 | 0.0839 | 0.2558 |
Relative change in up and down-regulation of TF genes in mild and severe moisture stressed shoot and root tissues compared to respective well watered controls of sorghum seedlings and final fold change is calculated in moisture stressed root and shoot tissues. As compare to moisture stressed shoot tissues more number of genes are up-regulated in moisture stressed root tissues of sorghum seedlings
Fig. 4.
Trends of regulated expression of TFs during moisture stress in sorghum shoot and root tissues. Relative change in up and down-regulation of transcription factor genes in mild and severe moisture stressed shoot and root tissues compared to respective well watered control. The x-axis showes the TF gene ID, and the y-axis shows the diffrence between Ct values of moisture stressed samples and respective well watered control. MS = Mild Root; MR = Mild Root; SS = Severe stressed Shoot; SR = Severe stressed Root
Table 5.
Down-regulated and up-regulated TF genes under drought regimes in sorghum
| Mild moisture stressed shoot | Mild moisture stressed root | Severe moisture stressed shoot | Severe moisture stressed root | Commonly down-regulated TF |
|---|---|---|---|---|
| a) | ||||
| PTSb00128.1 | PTSb00218.1 | PTSb00128.1 | PTSb00218.1 | PTSb00218.1 |
| PTSb00218.1 | PTSb00220.1 | PTSb00218.1 | PTSb00220.1 | PTSb00220.1 |
| PTSb00220.1 | PTSb00031.1 | PTSb00220.1 | PTSb00031.1 | PTSb00031.1 |
| PTSb00029.1 | PTSb00032.1 | PTSb00029.1 | PTSb00032.1 | PTSb00032.1 |
| PTSb00031.1 | PTSb00034.1 | PTSb00031.1 | PTSb00034.1 | PTSb00034.1 |
| PTSb00032.1 | PTSb00173.1 | PTSb00032.1 | PTSb00223.1 | PTSb00175.1 |
| PTSb00033.1 | PTSb00175.1 | PTSb00033.1 | PTSb00223.1 | |
| PTSb00034.1 | PTSb00223.1 | PTSb00034.1 | ||
| PTSb00174.1 | PTSb00173.1 | |||
| PTSb00175.1 | PTSb00174.1 | |||
| PTSb00223.1 | PTSb00175.1 | |||
| PTSb00223.1 | ||||
| b) | ||||
| Nil | PTSb00128.1 | PTSb00208.1 | PTSb00128.1 | Nil |
| PTSb00221.1 | PTSb00208.1 | |||
| PTSb00029.1 | PTSb00221.1 | |||
| PTSb00033.1 | PTSb00029.1 | |||
| PTSb00174.1 | PTSb00033.1 | |||
| PTSb00174.1 | ||||
| PTSb00175.1 | ||||
List of TF genes which recorded up- and down-regulation in shoot and root tissues of sorghum in moisture stress. Based on obtained fold change in qRT-PCR experiment up-regulated and down-regulated TF were identified as per stress and tissue type. More number of genes were down-regulated in moisture stressed shoot tissues whereas, more number of genes were up-regulated in moisture stressed root tissues
The TFs are known to influence many biological processes, including cell cycle progression, metabolism, growth and development and responses to external environments. The transcription levels of TFs are altered by moisture stress. In this experiment we found differential up- and down-regulation of TF genes in response to moisture stress. The expression profiling revealed that more genes from among the tested ones up-regulated in root tissues as compared to shoot tissues during mild and severe moisture stressed conditions. The root system is critical to plant adaption and crop productivity in drought-prone environments. Roots are known to have the ability to maintain elongation under severe moisture deficit levels which completely inhibit shoot growth. It is therefore the root growth is tightly regulated both by intrinsic developmental cues, such as abscisic acid (ABA) and auxin, and by the external growth conditions such as scanty moisture and high salinity in the soil. Molina et al. (2008) and Seo and Park (2009) recorded up-regulation of several drought responsive genes including transcription factors in chickpea and Arabidopsis roots respectively. Earlier Jiang and Deyholos (2006) found similar type of results, who examined various members of ARF, MADS, HB and some other TF families in moisture stressed root tissues of Arabidopsis.
Expression levels of selected genes were compared in severe moisture stressed shoot and root tissues with respective well watered controls. In severe moisture stressed shoot tissues, PTSb00208.1 was up-regulated, while other 12 genes, PTSb00128.1, PTSb00218.1, PTSb00220.1, PTSb00029.1, PTSb00031.1, PTSb00032.1, PTSb00033.1, PTSb00034.1, PTSb00173.1, PTSb00174.1, PTSb00175.1 and PTSb00223.1 were down regulated. In severe moisture stressed root tissues 7 TF genes viz. PTSb00128.1, PTSb00208.1, PTSb00221.1, PTSb00029.1, PTSb00033.1, PTSb00174.1 and PTSb00175.1 were up-regulated and 6 were down-regulated (PTSb00218.1, PTSb00220.1, PTSb00031.1, PTSb00032.1, PTSb00034.1 and PTSb00223.1). Six TF genes were found to be commonly down regulated in severe moisture stressed shoot and root tissues. Whereas, PTSb00208.1 was found to be commonly up-regulated in severe moisture stressed shoot and root tissues (Table 5). In case of root tissues, 7 TF genes up-regulated; PTSb00029.1, PTSb00033.1, PTSb00174.1 and PTSb00175.1 recorded a fold change of 2–5; PTSb00128.1, PTSb00208.1 and PTSb00221.1 recorded a fold change of 5–10. In case of shoot tissue PTSb00208.1 recorded a fold change of 5–10. A web-based annotation of the genes used in the current study is presented in the Table 6.
Table 6.
BLASTX based annotated function of 20 TF genes used in the present study
| Sl. No. | Database gene ID | TF family | Annotated function |
|---|---|---|---|
| 1 | PTSb00128.1 | HAP2 | CCAAT-box TF complex |
| 2 | PTSb00208.1 | MADS | MADS-box TF (rice and wheat) |
| 3 | PTSb00217.1 | MADS | Putative MADS TF (maize) |
| 4 | PTSb00218.1 | MADS | Hypothetical protein (rice), MADS TF (wheat) |
| 5 | PTSb00219.1 | MADS | MADS-box TF (maize and wheat) |
| 6 | PTSb00220.1 | MADS | MADS-box TF (maize), hypothetical protein (rice) |
| 7 | PTSb00221.1 | MADS | MADS-box TF (maize, wheat and Oat) |
| 8 | PTSb00029.1 | ARF | Auxin Responsive Factor (maize), hypothetical protein (rice) |
| 9 | PTSb00030.1 | ARF | Auxin Responsive Factor (maize), hypothetical protein (rice) |
| 10 | PTSb00031.1 | ARF | Auxin Responsive Factor (maize), hypothetical protein (rice) |
| 11 | PTSb00032.1 | ARF | Auxin Responsive Factor (maize), hypothetical protein (rice) |
| 12 | PTSb00033.1 | ARF | Auxin Responsive Factor (maize and rice) |
| 13 | PTSb00034.1 | ARF | Hypothetical protein (maize and rice) |
| 14 | PTSb00035.1 | ARF | Auxin Responsive Factor (maize and rice) |
| 15 | PTSb00172.1 | HB | Unknown (rice and grape) |
| 16 | PTSb00173.1 | HB | Hypothetical protein (maize and rice) |
| 17 | PTSb00174.1 | HB | BEL1-related protein (maize), Benzothiadiazole-induced homeodomain protein (rice) |
| 18 | PTSb00175.1 | HB | Homobox3 (maize),PHD-finger family homeodomain protein (rice) |
| 19 | PTSb00176.1 | HB | Homeobox protein OSH1 (maize and rice) |
| 20 | PTSb00223.1 | MBF | Putative ethylene responsive TF, endothelial differentiation related factor-1 (rice) |
Annotation of 20 TF genes was done with BLASTX analysis by comparing TF genes with all the proteins in GenBank. Gene products of most of the genes are hypothetical DNA-binding proteins
Members of ARF family, PTSb00031.1, PTSb00032.1 and PTSb00034.1 showed down-regulation in mild and severe moisture stressed shoot and root tissues of sorghum. Whereas, PTSb00033.1 showed up-regulation in mild and severe moisture stressed root tissues. Some of the members of ARF family are known to respond differentially in a moisture stressed manner in Arabidopsis. Seo et al. (2009) recorded up-regulation of ARF17 in Arabidopsis mutant which was drought tolerant. However, in the same mutant no significance difference was noticed in expression levels of ARF1, ARF5 and ARF7 as compared with normal Arabidopsis plant, indicating differential response of ARF members in moisture stress. PTSb00128.1 TF is the member of HAP2 family and it showed up-regulation in mild and severe moisture stressed root tissues. On the other hand, it showed down-regulation in mild and severe moisture stressed shoot tissues. Cohen et al. (2010) also reported up-regulation of putative NF-YA and NF-YB in drought induced populos roots and they also carried out meta analysis of genome wide expression profiling in mature leaves and root apices. Further, the TF gene PTSb00128.1 revealed homology with OsHAP2B and AtNF-YA subunit 7. Up-regulation of HAP2 TF family member in drought tolerant landraces of maize has been reported previously (Kanashiro et al. 2009).
Verification of qRT-PCR results by in situ hybridization
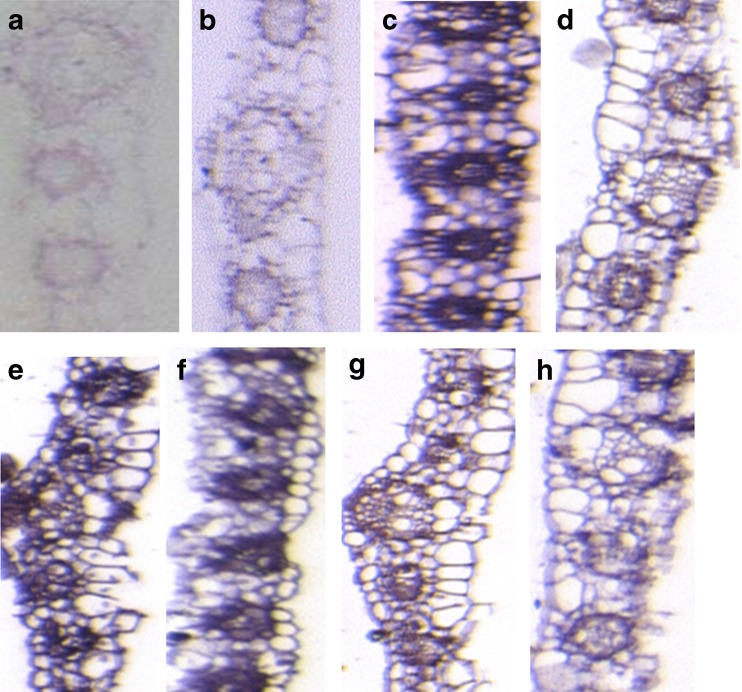
Confirmation of the qRT-PCR result was approached through ISH analyses of randomly selected PTSb00220.1 and PTSb00223.1 TF genes. The signal was detected in all hybridized samples except the ones without probe controls (Figs. 5 and 6a and b). The expression of PTSb00220.1 was detected throughout the leaf. Accumulation of transcript was found more in lower and upper epidermis in well watered leaf sections as compared to moisture stressed leaf sections. Intensity of hybridization signal was slightly less in mild moisture stressed and severe moisture stressed leaf tissue as compared to well watered leaf tissues. In moisture stressed leaf tissues, the expression of PTSb00220.1 was conspicuous in vascular bundles and it was very less in epidermis cells. In severe moisture stressed leaf tissue sections, in lower epidermis and spongy tissues the expression of PTSb00220.1 was more as compared to other parts of tissues (Fig. 5c to e). The pattern was similar in root tissues as well. Signal intensity was more in epidermis and phloem cells of vascular bundles whereas in endodermis and xylem cells of well watered root tissues, the signal was very low or negligible: the expression pattern was comparable in moisture stressed root tissues (Fig. 6c to e). The expression pattern of PTSb00223.1, a MBF family member, was similar in both root and leaf tissues, where expression was strong in well watered situation compared to both mild and severe moisture stress conditions. The expression of gene was observed in all part of leaf such as upper and lower epidermis, cuticle, palisade and spongy cells but it was more in vascular bundle in well watered and moisture stressed leaf tissues (Fig. 5f to h). In case of root tissues signal intensity was more in epidermis and phloem cells of vascular bundle. The signal was very low or negligible in endodermis and xylem cells of well watered root tissues (Fig. 6f to h). Comparison of the results from qRT-PCR with those from ISH analyses revealed similar patterns or tendencies of expression of TFs as response to moisture stress.
Fig. 5.
Sorghum leaf sections hybridized with DIG-labeled RNA probe: presence of targeted mRNA is indicated by pinkish purple colour. a, b leaf sections; negative (without probe) control c Well watered leaf section hybridized with PSTb00220.1 DIG-labeled RNA probe d Mild moisture stressed leaf section hybridized with PSTb00220.1 DIG-labeled RNA probe e Severe moisture stressed leaf section hybridized with PSTb00220.1 DIG-labeled RNA probe. f Well watered leaf section hybridized with PSTb00223.1 DIG-labeled RNA probe g Mild moisture stressed leaf section hybridized with PSTb00223.1 DIG-labeled RNA probe h Severe moisture stressed leaf section hybridized with PSTb00223.1 DIG-labeled RNA probe
Fig. 6.
Sorghum leaf sections hybridized with DIG-labeled RNA probe: The presence of targeted mRNA is indicated by pinkish purple colour. a, b root sections; negative (without probe) control c Well watered root section hybridized with PSTb00220.1 DIG-labeled RNA probe d Mild moisture stressed root section hybridized with PSTb00220.1 DIG-labeled RNA probe e Severe moisture stressed root section hybridized with PSTb00220.1 DIG-labeled RNA probe. f Well watered root section hybridized with PSTb00223.1 DIG-labeled RNA probe g Mild moisture stressed root section hybridized with PSTb00223.1 DIG-labeled RNA probe h Severe moisture stressed root section hybridized with PSTb00223.1 DIG-labeled RNA probe
The MADS family members, PTSb00218.1 and PTSb00220.1 recorded down-regulation in mild and severe moisture stressed shoot and root tissues of sorghum. Both TF genes recorded two folds down-regulations in mild moisture stressed shoot tissues and it was 1.5-2 folds down regulation for other tissues. BLASTN analysis of the PTSb00218.1 and PTSb00220.1 showed significant sequence similarity with SVP and AGL29 of Arabidopsis thaliana: the SVP gene is known to involve in late flowering in Arabidopsis (Masiero et al. 2004). SVP represses FLOWERING LOCUS T (FT) expression by directly binding to a CArG box motif in the FT promoter and block the expression of floral stimulus in the leaf (Lee et al. 2007). These two MADS family member might have a role in flower induction pathway in sorghum by acting as a repressor of flowering. Drought seems to down-regulate expression of these two genes in order to achieve early flowering in sorghum so that plants could complete their life cycle early: one of the mechanisms of drought escape. Arora et al. (2007) also recorded down-regulation of MADS family members in response to drought stress and in rice they showed that three genes, OsMADS2, OsMADS30 and OsMADS55 showing more than 2-fold down-regulation in response to dehydration and salt stress. In the present study, another members of MADS family, PTSb00221.1 up-regulated in mild and severe moisture stressed root tissues whereas, PTSb00208.1 found up-regulating in mild moisture stressed shoot tissues. Though, MADS members have been well characterized for their role in floral identity in Arabidopsis (Pelaz et al. 2000), recently some MADS TFs are found to be involved in moisture stress response of Arabidopsis (Mane et al. 2007). Previous reports of Fujita et al. (2004) and Tran et al. (2004) also point at an involvement of MADS in abiotic stresses response in plants. All these reports including the results of this study revealed role of MADS family genes in overcoming stress responses in plants but detailed studies are still remains to be done.
MBF family member, PTSb00223.1 showed down-regulation in mild and severe moisture stressed shoot and root tissues. The members of MBF family are known to work as a transcriptional co-activator that mediates transcriptional activation by bridging between an activator and a TATA-box binding protein. Only one gene for MBF1 has been reported in all eukaryotes but Arabidopsis consist three different genes encoding MBF1. MBF1a (At2g42680) and MBF1b (At3g58680) are developmentally regulated (Tsuda et al. 2004). In contrast, the steady-state level of transcripts encoding MBF1c (At3g24500) is specifically elevated in Arabidopsis in response to pathogen infection, salinity, drought, heat, hydrogen peroxide, and application of the plant hormones abscisic acid or salicylic acid (Rizhsky et al. 2004; Tsuda et al. 2004). In present study BLASTN revealed 85 % sequence similarity between PTSb00223.1 and MBF1a. There was no significant sequence similarity with MBF1b and MBF1c. As PTSb00223.1 shares 85 % sequence similarity with MBF1a it may be developmentally regulated and it has no direct role in drought tolerance in sorghum.
The PTSb00175.1, a HB family down-regulated in mild and severe moisture stressed shoot and root tissues and PTSb00173.1 recorded a down-regulation in mild moisture stressed root tissues and severe moisture stressed shoot tissues. Whereas, PTSb00174.1 TF gene, close to homeobox ATHB7 and ATHB12, shows up-regulation in mild and severe moisture stressed root tissues. Role of ATHB12 and ATHB7 is well-known in Arabidopsis under water stress conditions (Olsson et al. 2004). Cohen et al. (2010) also reported up-regulation of putative TF close to ATHB7 and ATHB12 in populous. Recently, Ariel et al. (2010) recorded role of one of the HB family member HB1 in lateral root emergence in Medicago. A differential expression of HB TF family members in leaf tissues of drought tolerant cultivar of Mexican maize as compared to well watered control has been reported by Kanashiro et al. (2009). Nonetheless, the HB family is known to be a moisture stress up-regulator in plants (Rocha et al. 2005; Zhou et al. 2007) and its members are involved in ABA dependent drought response. Hence, PTSb00174.1 seems to have a functional role in making sorghum a drought tolerant plant: a detailed characterization of this gene for its involvement in moisture stress response in sorghum will serve as a reference for comparative studies in closely related crop plants.
Apart from role in moisture stress some of the members of the selected families are involved in more than one type of stresses such as biotic and other abiotic stresses. The MBF1c (At3g24500) is specifically elevated in Arabidopsis in response to pathogen infection, salinity, drought, heat, hydrogen peroxide, and application of the plant hormones abscisic acid or salicylic acid. The MBF members are also known to participate in ethylene signaling (Kim et al. 2007). Recently, Yang et al. (2010) recorded that the involvement of MBF (GenBank accession numbers HO762735) in salt stress. OsBIHD1, a HB member is involved in different signal transduction pathways to regulate developmental processes and responses to biotic and abiotic stress. This family is also known to positively regulate disease-resistance responses and act as a negative regulator of abiotic stress tolerance (Luo et al. 2005). Further, auxin acts as an important component involved in defense responses via regulating the expression of a large number of genes and mediates crosstalk between abiotic and biotic stress responses (Ghanashyam and Jain 2009). Ghanashyam and Jain (2009) analyzed the expression profiles of auxin related genes in which some of the members of the ARF family were differentially regulated under different abiotic stresses. Several HAP2 transcription factor transcripts including At3g05690/NF-YA2, At1g54160/NF-YA5, At3g14020/NF-YA6, At1g72830/NF-YA8, and At5g06510/NF-YA10, increase during N and P limitation (Scheible et al. 2004; Pant et al. 2009).
Moisture stress response of crop plants is a complex phenomena involving a large number of genes and understanding the specific role of these gene in crop like sorghum, known to endure the moisture stress relatively better compared to other cereals, will help deeper understanding of possible mechanisms of drought tolerance and its possible cross comparison with other crop plants. Taken together, the results of present study provide insight into role of selected TF families and their members in moisture stress response of sorghum. Differential regulation of these genes under moisture stress also pointed at their role in drought tolerance in plants. This information will be a valuable starting point for further research on these genes.
Acknowledgements
We thank Dr. N Seetharama for encouragement. The research in the laboratory of BF is funded by Department of Biotechnology, Government of India, New Delhi, Indo-US (ICAR-AKI PGI project) and UAS, Dharwad. SBA was a recipient of JNU fellowship. We would like to thank the anonymous reviewers for their valuable suggestions to increase the scientific merit of this paper and encouragement and constant perusal of Dr. N. Raghuram.
References
- Ariel F, Diet D, Verdenaud M, Gruber V, Frugier F, Chan R, Crespi M. Environmental regulation of lateral root emergence in Medicago truncatula requires the HD-Zip I transcription factor HB1. Plant Cell. 2010;22:2171–2183. doi: 10.1105/tpc.110.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Agarwal P, Ray S, Singh AK, Singh VP, Tyagi AK, Kapoor S. MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics. 2007;8:242–255. doi: 10.1186/1471-2164-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana C, Scheible WR, Mueller-Roeber B, Ruzicic S. A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Meth. 2007;3:1746–1748. doi: 10.1186/1746-4811-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus AL, Jens JL, Torben OF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Cohen D, Bogeat-Triboulotl M, Tisserant E, Balzergue S, Martin-Magniette M, Lelandais G, Ningre N, Renou J, Tamby J, Thiec DL, Hummel I. Comparative transcriptomics of drought responses in Populous: a meta-analysis of genome-wide expression profiling in mature leaves and root apices across two genotypes. BMC Genomics. 2010;11:630–642. doi: 10.1186/1471-2164-11-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Bari RP, Stitt M, Scheible WR, Udvardi MK. Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J. 2004;38:366–379. doi: 10.1111/j.1365-313X.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- Edwards D, Murray JA, Smith AG. Multiple genes encoding the conserved CCAAT-box transcription factor complex are expressed in Arabidopsis. Plant Physiol. 1998;117(3):1015–1022. doi: 10.1104/pp.117.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran LSP, Yamaguchi-Shinozaki K, Shinozaki K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004;39:863–876. doi: 10.1111/j.1365-313X.2004.02171.x. [DOI] [PubMed] [Google Scholar]
- Ghanashyam C, Jain M. Role of auxin-responsive genes in biotic stress responses. Plant Signa Behav. 2009;4(9):846–848. doi: 10.4161/psb.4.9.9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wu W, Abrams SR, Cutler AJ. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot. 2008;59(11):2991–3007. doi: 10.1093/jxb/ern155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D (1991). In-situ hybridisation in plants. Molecular plant pathology: a practical approach. In: Bowles DJ, Gurr SJ, McPherson M (eds.). Oxford University Press
- Jain M, Tyagi AK, Khurana J. Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS J. 2008;275(11):2845–2861. doi: 10.1111/j.1742-4658.2008.06424.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Deyholos MK. Compressive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006;47:6–25. doi: 10.1186/1471-2229-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar K, Wandrey M, Czechowski T, Gaertner T, Scheible WR, Stitt S, Torres-Jerez I, Xiao X, Redman JC, Wu HC, Cheung F, Town CD, Udvardi MK. A community resource for high-throughput quantitative RT-PCR analysis of transcription factor gene expression in Medicago truncatula. Plant Meth. 2008;4:18–23. doi: 10.1186/1746-4811-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanashiro C, Vazquez CC, Ibarra-Laclette E, Herrera-Estrella L and Simpson J (2009). Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS One 4(10):e7531 [DOI] [PMC free article] [PubMed]
- Karlgren A, Carlsson J, Gyllenstrand N, Lagercrantz U, Sundstrom JF. Non-radioactive in situ hybridization protocol applicable for Norway spruce and a range of plant species. J Vis Exp. 2009;23:43–48. doi: 10.3791/1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lim GH, Kim ES, Ko CB, Yang KY, Jeong JA, Lee MC, Kim CS. Abiotic and biotic stress tolerance in Arabidopsis overexpressing the multiprotein bridging factor 1a (MBF1a) transcriptional coactivator gene. Biochem Biophys Res Commun. 2007;354:440–446. doi: 10.1016/j.bbrc.2006.12.212. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007;21:397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FH, Fu FL, Sha LN, Li WC. Identification of drought-responsive genes from maize inbred lines. J Plant Physiol Mol Biol. 2007;33(6):607–611. [PubMed] [Google Scholar]
- Luo H, Song F, Zheng Z. Overexpression in transgenic tobacco reveals different roles for the rice homeodomain gene OsBIHD1 in biotic and abiotic stress responses. J Exp Bot. 2005;56(420):2673–2682. doi: 10.1093/jxb/eri260. [DOI] [PubMed] [Google Scholar]
- Mane SP, Vasquez-Robinet C, Sioson AA, Heath LS, Grene R. Early PLDα-mediated events in response to progressive drought stress in Arabidopsis: a transcriptome analysis. J Exp Bot. 2007;58(2):241–252. doi: 10.1093/jxb/erl262. [DOI] [PubMed] [Google Scholar]
- Marino HJ, Cook P, Miller KS. Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and Real-Time RT-PCR. J Immunol Meth. 2003;283:291–306. doi: 10.1016/S0022-1759(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Marino R, Ponnaiah M, Krajewski P, Frova C, Gianfranceschi L, Enrico M, Sari-Gorla P. Addressing drought tolerance in maize by transcriptional profiling and mapping. Mol Genet Genom. 2009;281:163–179. doi: 10.1007/s00438-008-0401-y. [DOI] [PubMed] [Google Scholar]
- Masiero S, Li MA, Will I, Hartmann U, Saedler H, Huijser P, Schwarz-Sommer Z, Sommer H. INCOMPOSITA: a MADS-box gene controlling prophyll development and floral meristem identity in Antirrhinum. Development. 2004;131:5981–5990. doi: 10.1242/dev.01517. [DOI] [PubMed] [Google Scholar]
- Molina C, Rotter B, Horres R, Udupa SM, Besser B, Bellarmino L, Baum M, Matsumura H, Terauchi R, Kahl G, Winter P. SuperSAGE: the drought stress-responsive transcriptome of chickpea roots. BMC Genomics. 2008;9:553–559. doi: 10.1186/1471-2164-9-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira RS, Medri ME, Neumaier N, Lemos NG, Brogin RL, Marcelino FC, de Oliveira MCN, Farias JRB, Abdelnoor RV, Nepomuceno AL. Cloning and quantitative expression analysis of drought-induced genes in soybean. Genet Mol Res. 2010;9(2):858–867. doi: 10.4238/vol9-2gmr701. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA. 2007;104:16450–16455. doi: 10.1073/pnas.0707193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netondo GW, Onyango JC, Beck E. Sorghum and salinity: I. response of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci. 2004;44:797–805. doi: 10.2135/cropsci2004.0797. [DOI] [Google Scholar]
- Olsson ASB, Engstrom P, Soderman E. The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol. 2004;55:663–677. doi: 10.1007/s11103-004-1581-4. [DOI] [PubMed] [Google Scholar]
- Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR. Identification of nutrient-responsive Arabidopsis and rapeseed MicroRNAs by comprehensive Real-Time Polymerase Chain Reaction profiling and small RNA sequencing. Plant Physiol. 2009;150(3):1541–1555. doi: 10.1104/pp.109.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman JF, Feltus A, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Rahman M, Ware D, Westhoff P, Messing J, Rokhsar DS. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003;133:1755–1767. doi: 10.1104/pp.103.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruitjer JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;13:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683–1696. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha GCG, Correa RL, Borges ACN, Sa CBP, Ferreira MA. Identification and characterization of homeobox genes in Eucalyptus. Genet Mol Biol. 2005;28(3):165–173. doi: 10.1590/S1415-47572005000400005. [DOI] [Google Scholar]
- Ruth C, Martin VG, Hollenbeck T, James E, Dombrowski G. Evaluation of reference genes for quantitative RT-PCR in Lolium perenne. Crop Sci. 2008;48:1881–1887. doi: 10.2135/cropsci2007.10.0597. [DOI] [Google Scholar]
- Sadelin A, Wasserman W. Constrained binding site diversity within families of transcription factors enhances pattern discovery bioinformatics. J Mol Biol. 2004;338(2):207–215. doi: 10.1016/j.jmb.2004.02.048. [DOI] [PubMed] [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004;136:2483–2499. doi: 10.1104/pp.104.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander PF, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Sci. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Seo PJ, Park CM. Auxin homeostasis during lateral root development under drought condition. Plant Signal Behav. 2009;4(10):1002–1004. doi: 10.4161/psb.4.10.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009;151:275–289. doi: 10.1104/pp.109.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, McIntyre CL, Gresshoff PM, Xue GP. Members of the Dof transcription factor family in Triticum aestivum are associated with light-mediated gene regulation. Funct Integr Genomics. 2009;9:485–498. doi: 10.1007/s10142-009-0130-2. [DOI] [PubMed] [Google Scholar]
- Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004;16:2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Tsuji T, Hirose S, Yamazaki K. Three Arabidopsis MBF1 homologs with distinct expression profiles play roles as transcriptional co-activators. Plant Cell Physiol. 2004;45(2):225–231. doi: 10.1093/pcp/pch017. [DOI] [PubMed] [Google Scholar]
- Wang R, Tischner R, Gutierrez RA, Hoffman M, Xing X, Chen M, Coruzzi G, Crawford NM. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136:2512–2522. doi: 10.1104/pp.104.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao-feng D, Feng-ling FU, Na NI, Wan-chen LI. Differential gene expression in response to drought stress in maize seedling. Agril Sci China. 2009;8(7):767–776. doi: 10.1016/S1671-2927(08)60277-1. [DOI] [Google Scholar]
- Yang SS, Valdés-López O, Xu WW, Bucciarellil B, Gronwald JW, Hernández G, Vancel CP. Transcript profiling of common bean (Phaseolus vulgaris L.) using the genechip soybean genome array: Optimizing analysis by masking biased probes. BMC Plant Biol. 2010;10:1–58. doi: 10.1186/1471-2229-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Liu C, Shi YS, Song YC, Bai BZ, Li Y, Wang TY. QTL analysis of parameters related to flowering in maize under drought stress and normal irrigation condition. J Plant Gen Res. 2004;5:161–165. [Google Scholar]
- Zhou S, Bechner MC, Place M, Churas CP, Pape L, Leong SA, Runnheim R, Forrest DK, Goldstein S, Livny M, Schwartz DC. Validation of rice genome sequence by optical mapping. BMC Genomics. 2007;8:270–278. doi: 10.1186/1471-2164-8-270. [DOI] [PMC free article] [PubMed] [Google Scholar]