Abstract
Crocus sativus is a triploid sterile plant characterized by its red stigmas, which produce significant quantities of carotenoid derivatives formed from the oxidative cleavage of β-carotene and zeaxanthin. The accumulation of three major carotenoid derivatives- crocin, picrocrocin, and safranal- is responsible for the color, bitter taste, and aroma of saffron, which is obtained from the dried stigma of Crocus. Maximum apocarotenoid accumulation occurs during fully developed scarlet stage of stigma development. Zeaxanthin is the precursor for biosynthesis of apocarotenoids. Crocus zeaxanthin 7, 8 (7, 8)-cleavage dioxygenase gene (CsZCD) encodes a chromoplast enzyme that initiates the biogenesis of these apocarotenoids by cleaving zeaxanthin. The Reverse Transcription-PCR analysis revealed that CsZCD gene expression followed different patterns during stigma development. Highest levels of CsZCD gene expression was observed in fully developed scarlet stage of stigma. Real Time PCR analysis showed that there is a sharp increase in gene expression from yellow to orange and orange to scarlet stages of stigma development. Increase in CsZCD gene expression parallels with the apocarotenoid content during the development of stigma, suggesting its regulatory role for apocarotenoid biosynthesis and stigma development in saffron.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-012-0131-9) contains supplementary material, which is available to authorized users.
Keywords: Saffron, CsZCD, Real time PCR, Gene expression, Apocarotenoid
Introduction
Crocus sativus (Saffron) belong to family Iridaceae and is most expensive spice of the world. It is characterized by its long red stigmas, which produce and store significant quantities of carotenoid derivatives, formed from the oxidative cleavage of β-carotene and zeaxanthin (Rubio et al. 2009; Bouvier et al. 2003; Moraga et al. 2004). Carotenoids and their derivatives play an important role in plants and other living organisms (Cazzonelli 2011). Biosynthesis of Crocus apocarotenoids is developmentally regulated (Grilli-Caiola and Canini 2004). During saffron development, its stigma changes in color to scarlet, passing through yellow and orange colors (Himeno and Sano 1987). This stigma growth and accumulation of apocarotenoid simultaneously occur with conversion of amyloplasts to chromoplasts (Bouvier et al. 2003). The quality of saffron is certified in the international trade market following the ISO 3632 Normative since 1993. The most important parameter is colouring strength, calculated from UV–vis measurements at 440 nm in aqueous extracts of this spice. Such measurements are related with the total crocins content. Other important components are picrocrocin and safranal, responsible for flavor and aroma respectively (Fernandez 2004). The amounts of these main compounds are used to express the quality of saffron.
Crocus zeaxanthin cleavage dioxygenase gene (CsZCD) codes for a chromoplast enzyme that initiates the biogenesis of crocetin glycosides and picrocrocin (Bouvier et al. 2003). Previous studies and literature showed the biogenesis of crocetin from β-carotene and zeaxanthin (Rubio et al. 2009; Gomez-Gomez et al. 2010). CsZCD specifically catalyzes the synthesis of crocetin dialdehyde and hydroxy-cyclocitral from zeaxanthin. Thus, CsZCD is the Crocus 7,8 (7,8)-zeaxanthin cleavage dioxygenase that initiates the synthesis of saffron pigment and aroma and is expressed specifically in the chromoplasts during the active period of zeaxanthin cleavage (Bouvier et al. 2003). The quantitative and qualitative changes in the carotenoid and the apocarotenoid profile in C. sativus stigmas have been studied previously (Castillo et al. 2005; Rubio et al. 2009; Ahrazem et al. 2010), and it has been shown that transcriptional regulation of a β-carotene hydroxylase and lycopene cyclase genes is involved in the observed changes. In this study, the relative quantification of CsZCD gene at three different stages of stigma development in C. sativus is described in order to study the involvement of this gene in the transition of immature to mature stage of stigma development in C. sativus and, therefore, in apocarotenoid accumulation. Eight stages of development have been defined for C. sativus stigmas based on the length of the tissue, pigmentation, and apocarotenoid content (Himeno and Sano 1987). Three stages of stigma development were taken in present study, stage II corresponds to a yellow undeveloped stigma, stage VIII represents scarlet fully developed stigma and stage IV represents an orange undeveloped stigma. Most likely with this paper these will be treated as yellow, orange and scarlet stages of stigma development. The apocarotenoid accumulation and expression patterns of CsZCD gene were studied during these three stages of stigma development.
Material and methods
Plant material
Standards of crocin, safranal and picrocrocin were obtained from Sigma Aldrich. Stigmas were collected at three different stages (Yellow, orange and scarlet color). Samples were dried in saffron drier (Developed by SKUAST-K, Srinagar) at 60 °C. Dried samples were ground in pestle and mortar and were passed through a 0.5 mm mesh and stored in the dark at 4 °C until they were used for extraction and apocarotenoid estimation. For RNA isolation, stigmas were collected at Central Institute of Temperate Horticulture, Rangreth, Srinagar and quickly immersed in liquid nitrogen and then stored at −80 °C for further use.
Extraction procedure
A 100 mg mass of dried stigma was extracted with 5 ml of cold 50 % (v/v) ethanol in a pestle and mortar, it was then transferred to a screw-capped 50 ml tubes and a total volume of 20 ml 50 % (v/v) ethanol was added to it. Tubes were sonicated for 20 min on ice and then centrifuged at 4,000 rpm for 15 min and washed twice with 5 ml 50 % (v/v) ethanol. The supernatant was used for analysis by spectrophotometric procedures.
Spectrophotometery
Saffron samples were analyzed according to the ISO 3632 trade standard (ISO/TS 3632-1/2. 2003). According to ISO, picrocrocin, safranal and crocins are expressed as direct reading of the absorbance of 1 % aqueous solution of dried saffron at 257, 330 and 440 respectively. The supernatant (1 ml) was diluted to 5 ml with 50 % (v/v) ethanol for analysis using Nanodrop (Thermo scientific). A standard curve was prepared by measuring the absorption of crocin, saffranal and picrocrocin at 440 nm, 330 nm and 257 nm respectively. Sample supernatants were diluted 100 times and readings were taken at 440 nm, 330 nm and 257 nm for crocin, saffranal and picrocrocin respectively. Measurements of E 1% of an aqueous saffron extract at 440, 330, and 257 nm, respectively, were done using a 1 cm pathway quartz cell. Results are obtained by direct reading of the absorbance, D, at three wavelengths, as follows:
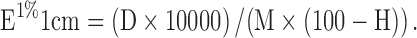 |
Where D is the specific absorbance; m is the mass of the saffron sample, in grams; H is the moisture and volatile content of the sample, expressed as a mass fraction. Moisture and volatile contents were identified by using powdered saffron stigmas. The sample were ground with a pestle and mortar and passed through a 0.5 mm mesh. After weighing, the powdered samples were kept uncovered in an oven set at 103 °C for 16 h. The moisture and volatile matter content are expressed as a percentage of the initial sample using the following relation: (initial mass - constant mass)/initial mass × 100.
RNA extraction
Frozen stigmas from three stages of stigma development (yellow, orange and scarlet) were ground in cold and sterilized mortar and pestle into fine powder and total RNA was extracted using RNA isolation kit (Roche Applied Science, Penzberg, Germany) following the manufacturer’s protocol. Quality of the extracted RNAs was checked by measuring the absorbance at 260 and 280 nm and RNAs with ratio of OD 260/280 ranging from 1.2 to 1.5 were used for cDNA synthesis.
cDNA preparation
For each sample, 5 μg of total RNA as template and 18-bp oligo dT primer and first strand cDNA synthesis kit (Roche Applied Science, Penzberg, Germany) were used for first-strand cDNA synthesis as per manufacturer’s instructions. The synthesized cDNA was stored at −20 °C for gene expression study.
RT- PCR
Reverse transcription was carried for amplification of CsZCD gene and CsTUB as internal control, using AMVRT cDNA kit (Roche Applied Science, Penzberg, Germany) according to user manual. Forward and reverse primers were 5′-GTCTTCCCCGACATCCAGATC-3′& 5′-CTCTATCGGGCTCACGTTGG- 3′ for CsZCD and 5′-TGATTTCCAACTCGACCAGTGTC-3′ and 5′- ATACTCATCACCCTCGTCACCATC-3′ for CsTUB gene (Bouvier et al. 2003; Castillo et al. 2005). The lengths of the products for two genes of CsZCD and CsTUB were 241, and 225 bp, respectively. PCR was performed (Takara, Japan) with 2–5 μg of cDNA with initial denaturation at 95 °C for 5 min followed by 35 cycles of amplification according to the subsequent scheme; denaturation 1 min at 94 °C, annealing at 56.2 °C for 30 s and extension at 72 °C for 40 s and final extension at 72 °C for 7 min. The experiments were repeated twice. Subsequently 5 μl of the PCR products were used on 1.2 % (w/v) agarose (Sigma-Aldrich, St Louis, MO, USA).
Real time PCR
The real time PCR were performed in 96-well plates with a LightCycler 480 real-time PCR instrument (Roche Diagnostics) using the LightCycler 480 SYBR Green I Master kit. Reactions were performed in triplicate and contained 5 μl SYBR Green I Master, 2 μl PCR-grade water, 2 μl cDNA, and 0.5 μl of each of the 10 μM forward and reverse gene-specific primers in a final volume of 10 μl. Tubulin gene was taken as reference gene and ZCD gene expression at scarlet stage as positive control. The reactions were incubated at 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 56.2 °C for 15 s, and 72 °C for 20 s. The specificity of the PCR reaction was confirmed with a heat dissociation protocol (from 60 °C to 95 °C) following the final PCR cycle. This ensured the resulting fluorescence originated from a single PCR product, and did not represent primer dimers formed during PCR or a non-specific product. Amplification of a single product of expected size was verified by gel electrophoresis on a 2 % agarose gel (Sigma-Aldrich, St Louis, MO, USA) and ethidium bromide staining. LightCycler 480 software (version 1.5; Roche Diagnostics) was used to collect the fluorescence data. Advanced relative quantification between three stages of stigma development was done through 2−∆∆cp method (Livak and Schmittgen 2001).
Results and discussion
In C. sativus, the development of the stigmas occurs concomitantly with transition of amyloplasts to chromoplasts and parallel with biosynthesis and accumulation of apocarotenoid which relates to expression levels of CsZCD gene (Bouvier et al. 2003; Moraga et al. 2004). The pattern of accumulation of the apocarotenoid crocetin, picrocrocin and the different crocins in developing saffron stigmas was investigated by extracting stigmas corresponding to three different developmental stages as previously described (Castillo et al. 2005). In the present study apocarotenoid accumulation was correlated with the expression of CsZCD gene during three developmental stages of saffron stigma. It was observed that apocarotenoid synthesis at three different stages of stigma development varied significantly. An apocarotenoid accumulation start from yellow stage but maximum accumulation was observed in scarlet stage of stigma development (Table 1). Carotenoid accumulation and composition during stigma development of C. sativus is highly regulated by the coordinated transcriptional activation of carotenoid biosynthetic genes (Castillo et al. 2005). The expression levels of CsZCD gene parallels the accumulation of the apocarotenoid compounds, suggesting that the formation of these compounds is controlled at a different level, by CsZCD gene expression (Rubio et al. 2008). Biogenesis of crocetin glucosides and picrocrocin are initiated by zeaxanthin cleavage by dioxygenase, which is coded by CsZCD has been reported (Bouvier et al. 2003). The gene CsZCD, which codes for a chromoplast enzyme that initiates the biogenesis of crocetin glycosides and picrocrocin, was highly expressed in the scarlet stigma when the highest levels of zeaxanthin and apocarotenoids have been detected (Castillo et al. 2005). During the development of saffron, the stigma changes in color from white to scarlet, passing through yellow and orange stages, parallel stigma growth, and apocarotenoid accumulation. Apocarotenoids increased 7 % from the yellow to the orange stage and up to 88 % in the scarlet stage (Himeno and Sano 1987). In the present study picocrocin increased 2.5 folds from the yellow to the orange stage and further 4 folds from orange to scarlet stage. Crocin content showed 10 and 3 folds increase from yellow to orange and orange to scarlet stages respectively and safranal showed 8 and 4 folds increase from yellow to orange and orange to scarlet stages respectively. Accumulation of carotenoids during this process, which reach their maximum levels at the time of anthesis. β-Carotene and zeaxanthin increased by 60.5 % and 85 %, respectively, from the orange to the scarlet stage (Castillo et al. 2005).
Table 1.
Apocarotenoid contents in saffron stigma during different stages of stigma development
| Apocarotenoid | Stage of flower development | ISO/TS 3632 value |
|---|---|---|
| Picrocrocin | Scarlet | 79 ± 0.67 |
| Orange | 20.0 ± 1.11 | |
| Yellow | 8 ± 0.30 | |
| Crocin | Scarlet | 188 ± 1.16 |
| Orange | 60 ± 1.12 | |
| Yellow | 6 ± 0.10 | |
| Safranal | Scarlet | 23.2 ± 1.16 |
| Orange | 8 ± 0.04 | |
| Yellow | 1.02 ± 0.02 |
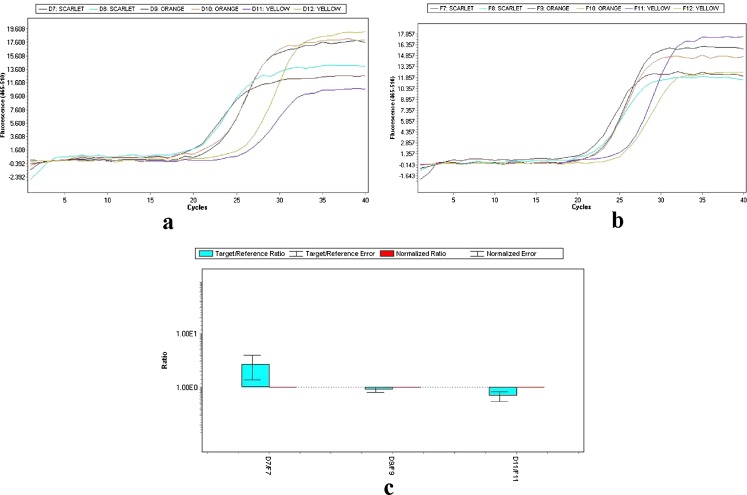
Reverse transcription and real time PCR was performed with RNA purified from stigmas in stages. The Reverse Transcription-PCR analysis revealed that accumulation of the mRNAs corresponding to the CsZCD gene studied during stigma development followed different patterns during stigma development (Fig. 1). Highest levels of CsZCD gene expression was observed in the scarlet stage of stigma development. These results are in accordance with the results observed by Castillo et al. 2005. In addition, the expression levels of CsZCD were analyzed in each step (Fig. 1). The expression levels were correlated with the accumulation of apocarotenoids during these stages of stigma development. Therefore, both CsZCD gene expression and accumulation of apocarotenoids occur in parallel (Bustin 2000). Real Time PCR amplification of CsZCD and tubulin genes at yellow, orange and scarlet stages of stigma development is shown in Fig. 2. There was 2.69 folds relative increase in CsZCD gene expression over tubulin gene expression in scarlet stage and 0.90 and 0.69 folds decrease in CsZCD gene expression over tubulin gene expression in orange and yellow stages respectively (Fig. 2c). Real Time PCR analysis revealed that CsZCD gene expression gets up-regulated by 8 % from yellow to orange and by 33 % from orange to scarlet stage. Yellow stage of stigma development showed 25 % of gene expression with respect to scarlet stage of stigma development. So there is an abrupt increase in gene expression of CsZCD gene from orange to scarlet stage of stigma development. Scarlet stage which is fully developed stigma was treated as positive calibrator. Similar results using reverse transcription PCR have been observed in saffron (Rubio et al. 2004; Castillo et al. 2005). This is the first report which gives fold changes of CsZCD gene expression with respect to internal control during stigma development and correlates the CsZCD gene expression with apocarotenoid accumulation. To the best of our knowledge, no other study has attempted to analyze relative gene expression of CsZCD using real time in saffron. To successfully commercialize saffron spice specific for apocarotenoid values, an understanding of gene expression of key genes responsible for apocarotenoid biosynthesis during stigma development is necessary before we can harness the power of biotechnology for further utilization of these genes and mechanism for its improvement. The prelude to this would be to validate a set of reference genes along with other important genes like lycopene cyclase, β -carotene hydroxylase in order to harmonise the data from various experiments that are expected to follow suit.
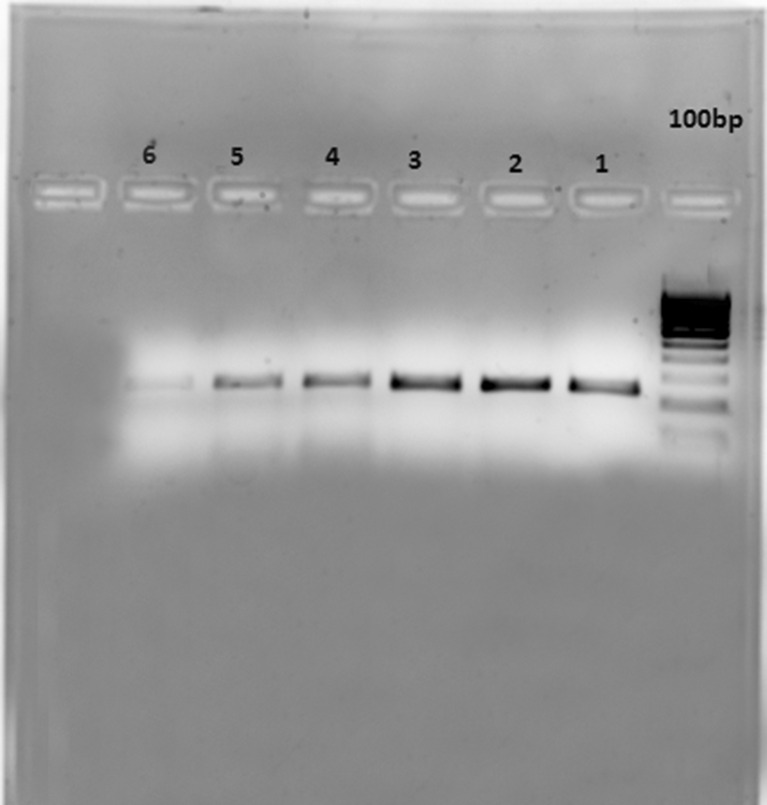
Fig. 1.
CsZCD (Lane 4–6) and reference Tubulin (Lane 1–3) gene expression at three developmental stages of saffron stigma (Lane 1 &, 4 for Scarlet, 2 & 5 for orange and 3 & 6 for yellow)
Fig. 2.
Amplification curves of CsZCD (a) and Tubulin (b) genes at scarlet, orange and yellow stages of stigma development. Bar graph (c) representing the relative expression (2−ΔΔCt) of CsZCD over tubulin gene, bars represent means for three replications and error bars represent standard error of the mean
Conclusion
During the development of the stigma tissue clear changes in levels apocarotenoid and CsZCD gene were observed. A dramatic increase of the apocarotenoid accumulation and CsZCD gene expression was observed in the fully developed stigma. The magnitude of expression of CsZCD gene need further to be correlated with other genes involved in apocarotenoid biosynthesis, so that complete information about gene regulation in apocarotenoid biosynthetic pathway can be deciphered which can be further utilized in saffron improvement programme.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PNG 333 kb)
(PDF 402 kb)
References
- Ahrazem O, Moraga AR, Castillo LR, Gomez Gomez L. The expression of a chromoplast-specific lycopene beta-cyclase gene is involved in the high production of saffron’s apocarotenoid precursors. J Exp Bot. 2010;61(1):105–119. doi: 10.1093/jxb/erp283. [DOI] [PubMed] [Google Scholar]
- Bouvier F, Suire C, Mutterer J, Camara B. Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell. 2003;15:47–62. doi: 10.1105/tpc.006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Castillo R, Fernandez JA, Gomez-Gomez L. Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crocus sativus and its closer relatives. Plant Physiol. 2005;139:674–689. doi: 10.1104/pp.105.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli CI. Carotenoids in nature: insights from plants and beyond. Func Plant Biol. 2011;38:833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- Fernandez JA. Biology, biotechnology and biomedicine of saffron. Recent Res Devel Plant Sci. 2004;2:127–159. [Google Scholar]
- Gomez-Gomez L, Moraga AR, Ahrazen O. Understanding carotenoid metabolism in saffron stigmas: unraveling aroma and color formation. Func Plant Sci Biotech. 2010;4:56–63. [Google Scholar]
- Grilli-Caiola M, Canini A. Ultrastructure of chromoplasts and other plastids in Crocus sativus L. (Iridiaceae) Plant Biosytematics. 2004;138:43–52. doi: 10.1080/11263500410001684116. [DOI] [Google Scholar]
- Himeno H, Sano K. Synthesis of crocin, picrocrocin and safranal by saffron stigma-like structures proliferated in vitro. Agric Biol Chem. 1987;51:2395–2400. doi: 10.1271/bbb1961.51.2395. [DOI] [Google Scholar]
- ISO/TS 3632-1/2 . In: Technical specification. Crocus sativus. Saffron L, editor. Geneva: ISO; 2003. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real- time quantitative PCR and the 2−∆∆cp method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Moraga AR, Nohales PF, Perez JA, Gômez Gômez L. “Glucosylation of the saffron apocarotenoid crocetin by a glucosyltransferase isolated from Crocus sativus stigmas.”. Planta. 2004;219(6):955–966. doi: 10.1007/s00425-004-1299-1. [DOI] [PubMed] [Google Scholar]
- Rubio A, Fernández NP, Fernández JA, Gomez-Gomez L. Glucosylation of the saffron apocarotenoid crocetin by a glucosyltransferase isolated from Crocus sativus stigmas. Planta. 2004;219:955–966. doi: 10.1007/s00425-004-1299-1. [DOI] [PubMed] [Google Scholar]
- Rubio A, Rambla JL, Santoella M, Gomez MD, Orzaez D, Granell A, Gomez-Gomez L. Cytosolic and plastoglobule targeted caroteniod dioxygenase from Crocus sativus are both involved in β-ionone release. J Biol Chem. 2008;283:24816–24825. doi: 10.1074/jbc.M804000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A, Rambla JL, Ahrazem O, Granell A, Gomez-Gomez L. Metabolite and target transcript analyses during Crocus sativus stigma development. Photochemistry. 2009;70:1009–1016. doi: 10.1016/j.phytochem.2009.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 333 kb)
(PDF 402 kb)