Abstract
Hippophae salicifolia D. Don (Seabuckthorn) grows in stressful environment of high altitude under conditions of low temperature and low availability of water. We have studied gender based differences in physiochemical response to cold stress in male and female plants of Seabuckthorn. After 24 h of cold stress about 32 and 66 % higher electrolyte leakage (EL) was recorded in male and female plants respectively. Relative water content (RWC) at the end of 24 h stress was higher in male plants (~64 %) compared to female plants (~60 %). Proline content in leaf samples of cold stressed male and female plants also increased upon cold stress. After 24 h about 2.7 fold higher amount of proline was assessed in male and female in comparison to control plants. Similarly, about two fold increase in the specific activities of superoxide dismutase (SOD) and catalase was also observed upon cold stress in male and female plants. These findings have important inferences for community of molecular biologists exploring seabuckthorn genome for agronomically important genes.
Keywords: Seabuckthorn, Hippophae salicifolia, Cold stress, Dioecy, Physiochemical response
Introduction
Hippophae salicifolia D. Don [Seabuckthorn (SBT), Fam. Elaeagnaceae] is a high value medicinal dioecious shrub or small sized tree belonging to Eurasia. In India, it abundantly occurs in high altitude regions (2,000–3,600 m asl) of Himalayan range, withstanding temperature extremes (Gupta and Ahmed 2010). It has multifarious economical value in food, fodder, medicine, fuel and forestry (Gupta et al. 2011). Male and female plants are morphologically similar and cannot be distinguished prior to 3–4 years of growth, i.e., at the time of flowering.
Male and female plants in most dioecious species show variation of habits in response to the stressful environment. The differences may be occurring in terms of their occurrence, survivability, vegetative growth, etc. Interestingly, reported male and female sex ratio in SBT is 2:3 (Huxley 1992), which is against the general behaviour of occurrence of dioecious species (Lloyd and Webb 1977). Morphological and physiological differences in response to cold stress between male and female plants have been investigated in several species (Kumar et al. 2006; Li et al. 2005, 2007). Plant response to cold acclimation is complex and diverse. To count a few of the physiochemical changes in a plant cell, electrolytic leakage (EL) of the cell increases due to formation of freeze induced lesions in the plasma membrane. Measurement of relative water content (RWC) helps assessing the capability of a cell to tolerate the scarcity of water in its liquid form and has thus been tracked often as a parameter to study the capability of the cell to tolerate cold stress (Singh et al. 2011). Proline accumulates in plant cells under a broad range of stress conditions and protects folded protein structures against denaturation. Proline also functions as a hydroxyl radical scavenger and serves as an energy and nitrogen source (Claussen 2005). Elevation of activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase have also been considered often as another parameters for assessing the tolerance to cold stress by male and female plants of a species (Khare et al. 2010).
Here, we describe the results of our experiments aimed to find out the degree of the negative impact imposed by the cold stress on male plants and female plants of H. salicifolia by varying durations of cold stress.
Materials and methods
Ten root suckers were collected from mature male and the female SBT plants from Defence Institute of Bio Energy Research (DIBER) Field station at Auli (~3,000 m asl; 30.32° N, 79.36° E), India and transferred to soil:sand:vermicompost mixture (1:1:1) under artificial light at 25 °C with a 15 h light period at irradiance of 250 μM m−2 s−1 in culture room at DIBER HQ, Haldwani. Plantlets (2 month old) were given cold stress (4 °C for 6, 12 and 24 h) and leaves were harvested for different physiochemical studies.
EL was measured in excised cold stressed leaf samples (ten) of male and female SBT plants by using conductivity meter (Cond 3151/SET, WTW, Germany). Ten leaf discs (10 mm) were excised from fully expanded leaves of cold stressed male and female plants each for the determination of leaf RWC. The fresh weight of the leaf discs was recorded immediately after harvest and placed in autoclaved distilled water for 4 h at room temperature and their turgid weight was recorded. Finally, the samples were dried in oven at 80 °C overnight to obtain their dry weight. The RWC was calculated using the formula 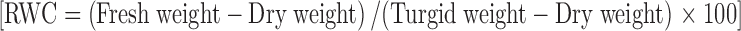 . Proline content in the leaves was estimated by the method of Bates et al. (1973) with slight modifications. Leaf tissue (500 mg) was grounded with liquid nitrogen and was mixed with 3 % sulphosalysilic acid (5 ml). Homogenate was filtered through Whatman no.1 filter paper. Glacial acetic acid (1 ml) and acid ninhydrin reagent (1 ml) were added to the 1 ml filtrate. The mixture was incubated in a boiling water bath for 30 min and cooled at room temperature. Toluene (4 ml) was added to the mixture and upper toluene level (red colour) was measured at 520 nm. The concentration of proline was estimated by referring to a standard curve made from known concentration of proline.
. Proline content in the leaves was estimated by the method of Bates et al. (1973) with slight modifications. Leaf tissue (500 mg) was grounded with liquid nitrogen and was mixed with 3 % sulphosalysilic acid (5 ml). Homogenate was filtered through Whatman no.1 filter paper. Glacial acetic acid (1 ml) and acid ninhydrin reagent (1 ml) were added to the 1 ml filtrate. The mixture was incubated in a boiling water bath for 30 min and cooled at room temperature. Toluene (4 ml) was added to the mixture and upper toluene level (red colour) was measured at 520 nm. The concentration of proline was estimated by referring to a standard curve made from known concentration of proline.
The activity of antioxidant enzymes like SOD (EC:1.15.1.1) and catalase (EC: 1.11.1.6) was assayed spectrophotometrically as described by Khare et al. (2010). The enzyme activity was expressed in U mg−1 of protein. Protein estimation was carried out according to the method of Bradford (1976). Each experiment was performed using biological and experimental triplicates and data obtained from different sets of experiments were analyzed using t-test.
Results and discussion
Overall differences in physiochemical parameters over 24 h in male and female plants were more or less found comparable. However, the path adopted to reach to those final values was found different in males and females. Male plants responded more quickly and adapted faster, female plants resisted the change in physiochemical values initially, and then drastically values changed to reach ultimately to the same level as in the male plants. EL at the end of 24 h was higher in female plants compared to male plants, though the difference was not statistically significant (Table 1). However, when compared to the initial values (Table 1), 65.82 % of the initial EL was observed after 24 h in female plants, compared to only 31.82 % of the initial EL in male plants. In case of male plants, maximum EL was observed within first 6 h, and thereafter it stabilized. On the other hand, female plants apparently tolerated cold stress for first 6 h and maximum damage occurred between 6 and 12 h of cold stress. The difference of EL at 6 h and 12 h time points was significant (Table 1), though in first case it was biased towards the male plants, which later turned biased to females at 12 h time point. A significant difference in EL (P < 0.001) compared to 0 h was observed at each time points in both male and female plants. Clearly, the adaptive trends were non linear with respect to prolonged low temperature exposure.
Table 1.
EL and RWC (mean ± standard error) in male and female plants of seabuckthorn
| m | EL | RWC | ||||
|---|---|---|---|---|---|---|
| Males (%) | Females (%) | P(t sex ) | Males (%) | Females (%) | P(t sex ) | |
| 0 h | 37.43 ± 2.95 | 35.64 ± 8.58 | ns | 72.73 ± 9.42 | 81.82 ± 2.36 | 0.2 < P < 0.5 |
| 6 h | 50.71 ± 5.60 | 32.80 ± 2.42 | 0.005 < P < 0.01 | 77.78 ± 3.53 | 84.61 ± 5.3 | 0.1 < P < 0.2 |
| 12 h | 45.35 ± 1.90 | 66.53 ± 8.30 | 0.002 < P < 0.005 | 63.64 ± 4.71 | 80 ± 5.89 | 0.2 < P < 0.5 |
| 24 h | 49.34 ± 3.06 | 60.44 ± 10.18 | ns | 63.64 ± 12.63 | 60 ± 11.78 | 0.02 < P < 0.05 |
| P(t time ) | P < 0.001 | P < 0.001 | ns | 0.2 < P < 0.5 | ||
ns not significant
As part of adaptability to drought conditions (Li et al. 2005), SBT has the ability to retain water even under stress to survive the dehydration. Male plants exhibited higher RWC after 24 h of stress, retaining 87.5 % of the water under non stress conditions and female plant retained 73.3 % water under similar conditions. The paths followed to arrive at those final values were again different in male and female plants, as have also been seen in the case of EL. RWC immediately reduced in male plants on the arrival of cold stress, which however, got stabilized quickly at around 12 h. From there on, it did not significantly dropped down any further. In case of female plants, a tendency to resist the water loss was observed upto 12 h. As the standard error mounted to nearly 1/5th of the average value (Table 1), i.e., 12.63 % for male plants and 11.78 %, for female plants at 24 h time point, the plant’s ability to resist dehydration cannot solely be called as a gender based parameter. The ability of different plants to survive the duration of the stress in seabuckthorn may be a result of genetic diversity, and might even be a quantitative trait. A rigorous analysis of plants spread over a wide geographical area shall be helpful in understanding this property. This would have direct inferences to the agriculture scientists and seabuckthorn breeders, who would naturally be looking for higher ability to tolerate the drought stress.
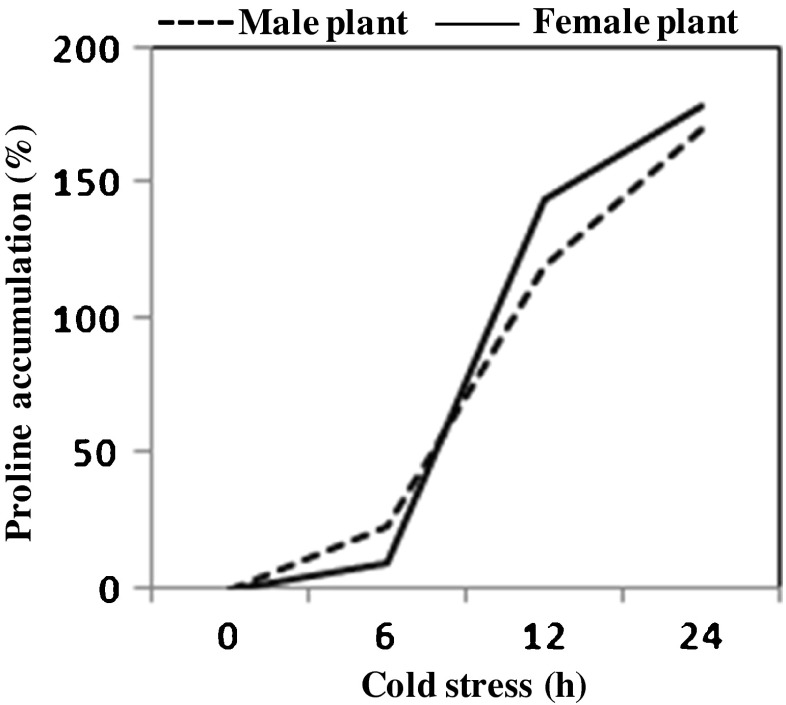
After 24 of cold stress proline content increased from 5.96 to 16.08 (2.7 folds increase) and 6.97–19.44 (2.8 folds increase) μM gFW−1 in male and female plants, respectively. However, in the first 6 h, more proline was accumulated in male plants compared to female plants, as we observed that the change in total proline in cells in male plants increased by 23 % compared to only 10 % in female plants in the same duration (Fig. 1). Clearly, proline accumulation was twice more rapid in male plants in initial hours, though at the end of 24 h, more proline was found accumulated in female plants. Nevertheless, overall difference in proline accumulation in male and female plants at 24 h time point has not been found significant, but the amount of proline accumulated at each of the time points studied (Fig. 1), was significantly higher than the proline accumulated at previous time points.
Fig. 1.
Overall trends of proline accumulation in male and female plants of seabuckthorn, in terms of percentage increase from 0 h time point of cold stress
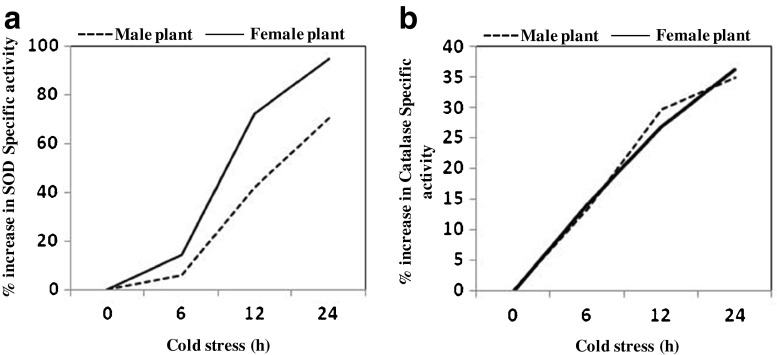
The increase in specific activity of SOD over 24 h of cold exposure was found non linear in both male and female plants, as in the case of biochemical parameters described above. However, in case of female plants, the increase in activity was sharper, between any two times points, as compared to male plants, thereby creating a significant difference between the SOD activities of male and female plants at 12 h and 24 h (Fig. 2a). The difference of the factor by which enzyme activity increased in male and female plants was sharpest in first 6 h, when the rate of increase of activity in female plants was 2.5 times higher than that in male plants.
Fig. 2.
Percentage increase (cumulative) in SOD (a) and Catalase (b) activity in response to cold stress at different time intervals with respect to 0 h time point of cold stress in male and female plants of seabuckthorn
Specific activity of catalase increases upon cold stress from 0.26 to 0.43 (1.6 fold increase) and 0.3 to 0.51 (1.7 fold increase) U mg−1 protein in male and female plants, respectively. Interestingly, unlike other parameters studied, increase in the activity of catalase was not only uniform in both male and female plants; it also increased more or less in a linear fashion in both the sexes (Fig. 2b). The final values attained at the end of 24 h was significantly higher compared to the initial values of catalase activity (P = 0.3). However, as the difference between the catalase activity at the end of 12 h and 24 h was not significant, it can be assumed that the maximum elevation in the activity of catalase enzyme is witnessed in first 12 h of cold exposure. Conversely, scavenging of free radicals/H2O2 by the elevated level of antioxidant enzymes like SOD and catalase in response to cold stress occur maximum in first 12 h of cold exposure.
Differences in physiochemical and growth characteristics in response to abiotic stress in different dioecious plants have been recorded earlier as well (Kumar et al. 2006; Li et al. 2005, 2007; Wang and Griffin 2003), and in most of these studies female plants have been found more susceptible. In fact, females are more scarcely found in stressful environments compared to male members of the same plant species (Dawson and Ehleringer 1993). This may be attributed to the fact that nutrient requirements for reproduction in females are greater than that to males. Seed production is assumed to reduce the vegetative growth and survivability of the females (Dawson and Ehleringer 1993). In the present context, at the end of 24 h of cold stress, higher electrolyte leakage and lower relative water content were observed in female plants compared to male plants, implying higher damage. On the other hand, higher proline accumulation as well as SOD activity too has been found in the female plants only, suggesting higher adaptability as well. Under the conditions of abiotic stress, a balance is required between the reproductive ability and the growth cum survival, and either of these has to be compromised. Probably, the different paths leading to adaptability of male and female plants observed in this study are outcome of this biological dilemma of the female plants. In general, females are more negatively affected by stress conditions (Li et al. 2007). Nevertheless, the response of female plants of seabuckthorn to cold stress is more prominent but complex, to which a more rigorous attention is required to be paid, as these are the fruit bearing trees and thus economically more important to mankind.
Acknowledgements
Financial and logistic help received from Defence Research and Development Organisation (DRDO), HQ, New Delhi is duly acknowledged.
References
- Bates LS, Waldran LP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Claussen W. Proline as a measure of stress in tomato plants. Plant Sci. 2005;168:241–248. doi: 10.1016/j.plantsci.2004.07.039. [DOI] [Google Scholar]
- Dawson TE, Ehleringer JR. Gender-specific physiology, carbon isotope discrimination and habitat distribution in box elder Acer negundo. Ecology. 1993;74:798–815. doi: 10.2307/1940807. [DOI] [Google Scholar]
- Gupta SM, Ahmed Z. Seabuckthorn (Hippophae salicifolia L.) plant: as source donor of cold tolerant genes for improving high altitude agriculture during cold stress. Res Environ Life Sci. 2010;3:105–112. [Google Scholar]
- Gupta SM, Gupta AK, Ahmed Z, Kumar A (2011) Antibacterial and antifungal activity in leaf, seed extract and seed oil of Seabuckthorn (Hippophae salicifolia D. Don) Plant. J Plant Pathol Microbiol 2:105. doi:10.4172/2157-7471.1000105
- Huxley A. The new RHS dictionary of gardening. Portland: MacMillan Press; 1992. [Google Scholar]
- Khare N, Goyary D, Singh NK, Shah P, Rathore M, Anandhan S, Sharma D, Arif M, Ahmed Z. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tiss Organ Cult. 2010;103:267–277. doi: 10.1007/s11240-010-9776-7. [DOI] [Google Scholar]
- Kumar N, Gupta S, Tripathi AN. Gender-specific responses of Piper betle L. to low temperature stress: changes in chlorophyllase activity. Biol Plant. 2006;50:705–708. doi: 10.1007/s10535-006-0111-4. [DOI] [Google Scholar]
- Li C, Yang Y, Junttila O, Palva ET. Sexual differences in cold acclimation and freezing tolerance development in Seabuckthorn (Hippophae rhamnoides L.) ecotypes. Plant Sci. 2005;168:1365–1370. doi: 10.1016/j.plantsci.2005.02.001. [DOI] [Google Scholar]
- Li C, Xu G, Zang R, Korpelainen H, Berninger F. Sex-related differences in leaf morphological and physiological responses in Hippophae rhamnoides along an altitude gradient. Tree Physiol. 2007;27:399–406. doi: 10.1093/treephys/27.3.399. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Webb CJ. Secondary sex characters in plants. Bot Rev. 1977;43:177–216. doi: 10.1007/BF02860717. [DOI] [Google Scholar]
- Singh S, Rathore M, Singh RK, Sharma DK, Goyary D, Anandhan S, Ahmed Z. Induced ectopic expression of At-CBF1 in marker-free transgenic tomatoes confers enhanced chilling tolerance. Plant Cell Rep. 2011;30:1019–1028. doi: 10.1007/s00299-011-1007-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Griffin KL. Sex-specific physiological and growth responses to elevated atmospheric CO2 in Silene latifolia Poiret. Glob Change Biol. 2003;9:612–618. doi: 10.1046/j.1365-2486.2003.00615.x. [DOI] [Google Scholar]