Abstract
An experiment was conducted to find out the efficacy of putrescine and benzyladenine on photosynthesis and productivity in wheat. Seeds of wheat genotype HD 2329 (widely adapted under irrigated condition) were grown in ceramic pots under standard package and practices. Putrescine (0.1 mM) and benzyladenine (0.05 mM) were sprayed on the aerial portion of these plants at the time of anthesis. After spray, half of the plants were subjected to water stress by withholding irrigation. The non stressed plants were irrigated to keep the soil humidity at field capacity. Results showed that drought stress severly reduced the photosynthetic attributes, water status and chlorophyll content which were significantly improved by foliar application of putrescine/benzyladenine. The levels of free proline, amino acids and soluble sugars were higher under water stress conditions which were enhanced further by putrescine/benzyladenine. Memrane injury was also reduced by both the chemicals. Yield and yield attributes reduced under water stress conditions, but putrescine and benzyladenine treated plants exhibited significantly higher values over control. Most of these parameters were found significantly correlated with grain yield. It is suggested that both benyzladenine and putrescine were able to impart drought tolerance in wheat but the response of putrescine was more promising owing to better management of various physio-biochemical processes, particularly under water stress conditions.
Keywords: Benzyladenine, Photosynthesis, Putrescine, Water stress, Wheat
Drought is one of the great challenges to agricultural production worldwide. It results in altered water balance, membrane integrity and protein functioning, which in turn leads to metabolic dysfunction and yield losses (Sharma 1999). Adjustment in photosynthetic parameters, production of stress related primary and secondary metabolites, generation of reactive oxygen species, changes in gene expression are some of the strategies applied by the plant at cellular or molecular level under water stress conditions (Farooq et al. 2009). The exogenous applications of bio-regulators have been found as a good alternative to counter the toxic effects of various stresses.
Polyamines are small ubiquitous nitrogenous compounds. Increased polyamine levels in stressed plants are of adaptive significance because of their involvement in the regulation of cellular ionic environment, maintenance of membrane integrity, prevention of chlorophyll loss and stimulation of protein synthesis, nucleic acids and protective alkaloids (Bouchereau et al. 1999; Kusano et al. 2008). Stabilization of membranes and minimization of water stress of various kinds of cells are of the several known physiological effects of polyamines (eg. putrescine) in the plant system (Liu et al. 2007; Goyal and Asthir 2010).
Cytokinins are important group of plant bioregualators which play important role in greater partitioning of photosynthates towards reproductive sink thereby improving the harvest index (Sharma et al. 2008). They protect cell membranes against degradation by preventing oxidation of unsaturated fatty acids and hence increase drought tolerance in plants. Further, cytokinins inhibit formation and speed up break down of free radicals such as superoxide (O2-) and hydroxy radical (OH-) that otherwise oxidizes membrane lipids (Werner and Schmulling 2009).
The role of polyamines and cytokinins in imparting drought tolerance has been reported in wheat (Sharma 1999; Gupta et al. 2000), but their comparative tolerance to drought has not been explored. Therefore, in this experiment we have studied the effect of foliar application of putrecine and benzyladenine on various physio-biochemical and yield parameters of wheat under water stress conditions. Efforts have also been made to know that which one acts more efficiently to improve drought tolerance in wheat.
Materials and methods
Seeds of wheat genotype HD 2329 (widely adapted under irrigated condition) were sown in ceramic pots filled with sandy loam soil and farm yard manure in 6:1 ratio. The soil has bulk density of 1.50 g cm−3, pH 8.4, field capacity 11.8 % and permanent wilting point 2.8 %. It was supplemented with 60 kg each of N, P and K ha-1 at the time of sowing. Remaining 60 kg N ha−1 was given after one month of sowing. One hundred pots were used and after thinning five plants were maintained in each pot. Water stress conditions were created by withholding water supply at the time of anthesis. Non stressed plants were irrigated whenever needed to keep the soil humidity at field capacity. Fifty pots were marked for each chemical (putrescine and benzyladenine) and spray was done at the time of anthesis stage. The control plants were sprayed with distilled water.
Preparation of solution
Putrescine dihydrogen chloride (Sigma, USA Comp.) was dissolved in glass distilled water and neutralized with traces of 1 M NaOH to pH 7.0. Teepol, a wetting agent was mixed with spray solution @ 0.5 mg/l. Benzyladenine (a synthetic cytokinin) was prepared by dissolving molecular weight of the substance in glass distilled water with few traces of 0.1 M NaOH. The 0.05 M concentration was prepared by diluting the stock solution containing 0.5 mg/l Tween 20.
Photosynthetic parameters
The photosynthetic rate, transpiration rate, and diffusive resistance in leaves were determined using a portable open flow, gas exchange system (CID 301, USA). The top most fully expanded leaf was enclosed in the assimilation chamber. Photosynthesis was measured while the CO2 concentration changed over a definite time interval. The system automatically calculated photosynthesis on the basis of pre loaded flow and leaf area. Leaf transpiration and diffusive resistance were also measured simultaneously on same leaf by Infra red gas analyzer (CID 301 USA).
Membrane injury
Membrane injury was estimated as per Sairam et al. (1997). 200 mg leaves from each treatment were thoroughly washed and then placed in 10 ml of double distilled water at 40 °C for 30 min. Then, their electrical conductivity was measured by conductivity meter. Subsequently, the same samples were placed on boiling water bath (100 °C) for 10 min and their electrical conductivity was recorded. The membrane injury was then calculated using the formula MI (%) = [conductivity at 40 °C/conductivity at 100 °C] × 100.
Relative water content
Fresh weight of the leaf samples was taken and then kept in distilled water for 4 h to obtain turgid weight. The turgid weight was recorded after blotting the excess water on the surfaces of the samples. Dry weight was obtained after drying the samples in oven at 60 °C till constant weight obtained. The relative water content was then calculated as RWC = 100 × (Fresh weight – Dry weight/(Turgid weight – Dry weight).
Metabolites
Chlorophyll content was estimated according to methods given by Arnon (1949). Total soluble sugars were extracted in 5 ml of 80 % ethanol from 100 mg leaf sample using anthrone reagent (Dubois et al. 1956). For proline estimation, samples were homogenized in 5 ml of 3 % aqueous sulphosalicylic acid and centrifuged at 5000 × g for 5 min (Bates et al. 1973). In the extract, an equal volume of glacial acetic acid and ninhydrin solution were added. The samples were heated to 100 °C and 5 ml of toluene was added. The absorbance of the toluene layer was read at 528 nm on a spectrophotometer. The quantity of proline was then calculated using standard curve. Free amino acid content was estimated by Yemm and Cocking (1955) and values were calculated.
Yield and yield attributes
Leaf area per plant was measured at the time of maturity using leaf area meter (LICOR- 3100, USA). The fully expanded green leaves were detached from the plants and leaf area was determined immediately to avoid wilting of leaves. Observations on plant height, number of grains per plant, 1,000- grain weight and grain yield were recorded after harvesting the crop. Harvest index was calculated from grain and biological yield. All the data were recorded in triplicates and analyzed by completely randomized design for stress, treatment and their interaction and correlation studies were carried out as described by Raghavrao (1983).
Results and discussion
Plant growth regulators act as modulators of plant responses to water stress. In recent years, polyamines are also being considered as growth regulators and secondary messengers in signaling pathways (Kusano et al. 2008). Maintenance of favourable water status is of paramount importance in plants. In present investigation, water stress conditions reduced the relative water content significantly but putrescine (put) and benzyladenine (BA) played pivotal role in maintaining higher water status (Table 1). Further, the percent increase was higher with foliar spray of putrescine (24.39 %) over benzyladenine (18.90 %) under water stress conditions. It is suggested that this increase in relative water content as a result of putrescine and benzyladenine treatment might be due to enhanced water absorption. Gupta et al. (2000) reported that exogenous cytokinin increased water absorption in wheat and thus yield was enhanced. Sharma (1999) reported that polyamines prevented the water stress induced decline in leaf relative water content in pea. A positive and significant correlation of relative water content with grain yield shows the importance of water status in wheat (Fig. 1). Loss of integrity of biological membrane is another intricate effect of drought. Our results indicated significant reduction in membrane injury (increased membrane stability) after putrescine and BA treatment, particularly under water stress conditions (Fig. 1). Further, the effect was more pronounced with putrescine than with BA and it was negatively and significantly correlated with grain yield of wheat (Table 4). Criado et al. (2009) reported that the exogenous cytokinin application increased cell membrane stability in wheat cultivars under drought stress. Farooq et al. (2009) reported that putrescine can protect the membranes and other macro molecules from oxidative damages and thus can stabilize biological membranes under stressful conditions. Delayed chlorophyll degradation on account of cytokinin treatment has been reported. (Goyal and Asthir 2010). In this investigation also, the chlorophyll content decreased significantly on account of water stress. However, the foliar spray of putrescine and benzyladenine helped in retention of chlorophyll content. Comparatively higher increment in chlorophyll content was noticed with putrescine treatment (Fig. 1). The higher chlorophyll stability index was reported with synthetic cytokinin in our previous investigation under water stress conditions (Gupta et al. 2000).
Table 1.
Efficacy of putrescine and benzyladenine on relative water content, and osmotically active metabolites in wheat (data in parenthesis indicate ± over control)
| Treatment | Relative water content (%) | Proline content (mg.g.−1 f.w) | Amino acids (mg.g.−1 f.w) | Soluble sugars (mg.g.−1 f.w) |
|---|---|---|---|---|
| Non Stress | ||||
| Control | 69.88 | 0.23 | 3.02 | 7.49 |
| Put (0.1 mM) | 72.32 (3.49) | 0.37 (60.87) | 3.34 (10.60) | 7.92 (5.74) |
| BA (0.05 mM) | 73.89 (5.74) | 0.31 (34.78) | 3.30 (09.27) | 7.74 (3.34) |
| Water stress | ||||
| Control | 53.76 | 0.42 | 3.19 | 8.13 |
| Put (0.1 mM) | 66.87 (24.39) | 0.66 (57.14) | 3.63 (13.79) | 8.71 (7.13) |
| BA (0.05 mM) | 63.92 (18.90) | 0.61 (45.24) | 3.53 (10.66) | 8.54 (5.04) |
| CD 0.05P | ||||
| Treatment (T) | 1.31 | 0.07 | 0.17 | 0.12 |
| Stress (S) | 1.08 | 0.05 | 0.11 | 0.09 |
| T x S | 1.86 | 0.04 | 0.09 | 0.14 |
Fig. 1.
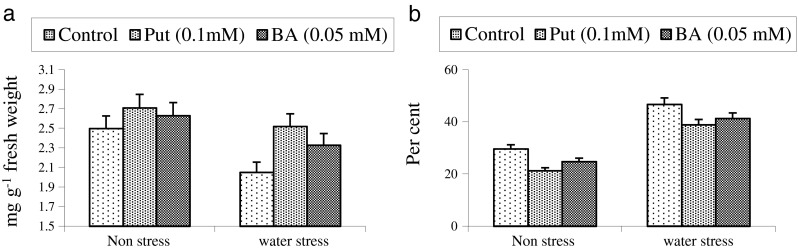
Comparative effect of putrescine (Put) and benzyladenine (BA) on chlorophyll content (a) and membrane injury (b) in wheat
Table 4.
Simple correlation coefficient of various morpho-physiological and yield parameters with grain yield of wheat treated with putrescine and benzyladenine under water stress conditions
| Parameter | Grain yield |
|---|---|
| Relative water content | +0.84** |
| Membrane injury | −0.89** |
| Chlorophyll content | +71* |
| Amino acids | +69* |
| Proline | +63* |
| Soluble sugars | +68* |
| Leaf photosynthesis rate | +85** |
| Leaf transpiration rate | +65* |
| Leaf diffusive resistance | −62* |
| Plant height | +65* |
| Leaf area | +89** |
| Number of grains | +97** |
| Harvest index | +76** |
* significant at 5 %; ** significant at 1 %
Osmotically active metabolites are directly linked with stress tolerance. The minimum concentrations of free prolines, amino acids and soluble sugars were measured in wheat leaves raised under well-watered conditions. The levels of these metabolites were increased upon exposure to drought. The effect of putrescine was more perceptible than benzyladenine (Table 1). Correlations of these metabolites with grain yield of wheat were positive and significant (Table 4). Contribution of osmolytes in osmotic adjustment and maintenance of cell turgor has been reported in maize (Mohammadkhani and Heidari 2008). Yadav et al. (1994) also observed that BA stimulated the accumulation of proline, amino acids and soluble sugars in chick pea under water stress.
The water stress conditions seriously hampered the rate of photosynthesis and transpiration in wheat leaves. Foliar spray of putrescine and benzyladenine increased the photosynthetic rates by 40.41 and 30.10 %, respectively under water stress conditions (Table 2). Similarly, the transpiration rate was also increased by 22.76 % (putrescine) and 17.89 % (benzyladenine) under water stress conditions. It has been reported that the application of synthetic cytokinin helps not only in mitigating the effect of stress through maintaining a high CK/ABA ratio but also increased crop productivity per se via enhanced uptake of CO2 in soybean (Sharma 1999). The higher internal CO2 concentration in water stress conditions reveals its inefficient utilization in photosynthesis. Further, the reduction in internal CO2 concentration might be due to the increased rubisco activity and enhanced CO2 fixation on account of Put/BA treatment. The BA induced increase in photosynthesis and rubisco activity has been reported in wheat (Xie et al. 2004). The increase in transpiration rate was elicited through enhanced effects of putrescine and BA on stomatal opening. Yadav et al. (1994) observed an increase in transpiration rate in wheat genotypes contrasting in drought tolerance in response to BA treatment. In this study, the leaf diffusive resistance decreased in response to putrescine (19.88 %) and benzyladenine (29.58 %). The decrease in diffusive resistance and increase in transpiration rate under water stress indicate the possible role played by these chemicals in stomatal regulation. It is suggested that ABA acts as a ‘distress’ signal which is passed to guard cells. This ABA induces stomatal closure by inhibiting K+ uptake by guard cells, starch hydrolysis and proton expulsion from surrounding cells (Gupta et al. 2003). Our result further indicate positive correlation of photosynthesis and transpiration rate and negative correlation of leaf diffusive resistance with grain yield of wheat (Table 4). Farooq et al. (2009) also found positive and significant correlation between stomatal conductance and grain yield.
Table 2.
Efficacy of putrescine and benzyladenine on photosynthetic parameters in wheat (data in parenthesis indicate ± over control)
| Treatment | Photosynthesis | Transpiration | Diffusive resistance | Internal CO2 concentration |
|---|---|---|---|---|
| (μmol CO2 m−2 s−1) | (μmol H2O m−2 s−1) | (mmol m−2 s−1) | (ppm) | |
| Non Stress | ||||
| Control | 20.45 | 1.96 | 5.92 | 159.7 |
| Put (0.1 mM) | 28.76 (40.64) | 2.16 (10.20) | 4.31 (−27.20) | 154.3 (−3.38) |
| BA (0.05 mM) | 26.15 (27.87) | 2.14 (09.18) | 4.13 (−30.24) | 159.1 (−0.38) |
| Water stress | ||||
| Control | 16.21 | 1.23 | 10.21 | 342.2 |
| Put (0.1 mM) | 22.76 (40.41) | 1.51 (22.76) | 8.18 (−19.88) | 321.8 (−5.96) |
| BA (0.05 mM) | 21.09 (30.10) | 1.45 (17.89) | 7.19 (−29.58) | 319.6 (−6.60) |
| CD 0.05P | ||||
| Treatment (T) | 1.83 | 0.73 | 1.07 | 19.4 |
| Stress (S) | 1.64 | 0.67 | 0.87 | 15.7 |
| T x S | 2.29 | 1.0 | 1.52 | 22.7 |
Yield and yield contributing parameters reduced significantly under water stress conditions (Table 3, Fig. 2). This reduction was found to alleviated through exogenous applications of putrescine and benzyladenine. Leaf area increased by 23.25 % and 16.37 % with foliar sprays of putrescine and benzyladenine under water stress conditions. Number of grains per plant were also increased by these chemicals under both the conditions. Grain yield and harvest index also increased, however, the response of putrescine was better over benyladenine (Fig. 2). Analysis of data showed the positive correlation of these parameters with grain yield (Table 4). Improvement in grain yield of wheat by exogenous application of cytokinin and polyamines have been reported individually but comparative efficacy has not been evaluated (Gupta et al. 2003; Mostafa et al. 2010). We conclude that both benyzladenine and putrescine were able to impart drought tolerance in wheat but the response of putrescine was more promising. It is suggested that the establishment of such roles of putrescine are likely to play a greater role towards drought tolerance in wheat and other water consuming crops.
Table 3.
Efficacy of putrescine and benzyladenine on yield and yield contributing parameters at harvest in wheat under water stress conditions (data in parenthesis indicate ± over control)
| Treatment | Leaf area (cm2 plant−1) | Plant height (cm plant−1) | No of grains (plant−1) |
|---|---|---|---|
| Non Stress | |||
| Control | 157.81 | 67.82 | 86.70 |
| Put (0.1 mM) | 167.51 (6.15) | 72.32 (6.64) | 93.00 (7.27) |
| BA (0.05 mM) | 164.92 (4.51) | 70.78 (4.36) | 89.33 (3.03) |
| Water stress | |||
| Control | 105.12 | 61.34 | 56.43 |
| Put (0.1 mM) | 129.56 (23.25) | 64.31 (4.84) | 66.94 (18.62) |
| BA (0.05 mM) | 122.33 (16.37) | 63.20 (3.03) | 63.23 (12.05) |
| CD 0.05P | |||
| Treatment (T) | 0.58 | 1.36 | 2.12 |
| Stress (S) | 0.44 | 1.02 | 1.72 |
| T x S | 0.89 | 2.51 | 2.69 |
Fig. 2.
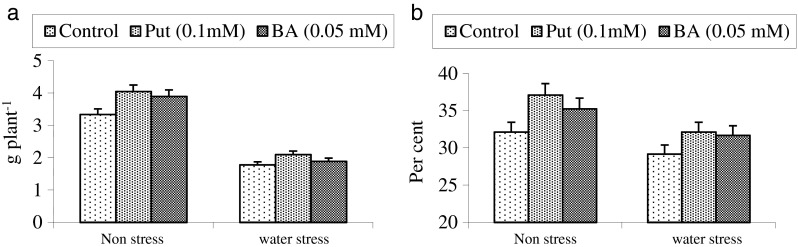
Comparative effect of putrescine (Put) and benzyladenine (BA) on grain yield (a) and harvest index (b) in wheat
Contributor Information
Sunita Gupta, Email: sunitagupta69@rediffmail.com.
Vishnu Prakash Agarwal, Email: vishnuprakash.agarwal@rediffmail.com.
N. K. Gupta, Email: nkgupta69@yahoo.co.in
References
- Arnon DI. Copper enzymes in isolated chloroplast: I. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldrenand RP, Teare IP. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140:103–125. doi: 10.1016/S0168-9452(98)00218-0. [DOI] [Google Scholar]
- Criado MV, Caputo C, Roberts IN, Castro MA, Barneix A. Cytokinin induced changes of nitrogen mobilization and chloroplast ultrastructure in wheat. J Plant Physiol. 2009;16:1775–85. doi: 10.1016/j.jplph.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hemilton JK, Robbersand PA, Smith F. Colorimetric method for determination of sugars and related substances. Analyt Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Farooq M, Wahid A, Lee DJ. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009;31:937–945. doi: 10.1007/s11738-009-0307-2. [DOI] [Google Scholar]
- Goyal M, Asthir B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 2010;60:13–25. doi: 10.1007/s10725-009-9414-8. [DOI] [Google Scholar]
- Gupta NK, Gupta S, Kumar A. Exogenous cytokinin application increases chlorophyll and cell membrane stability index in wheat (Triticum aestivum L.) Cereal Res Comm. 2000;28:287–291. [Google Scholar]
- Gupta NK, Gupta S, Shukla DS, Deshmukh PS. Differential response of BA injection on yield and specific grain weight in wheat genotypes recommenced for normal and late sown conditions. Plant Growth Regul. 2003;40:201–205. doi: 10.1023/A:1025023822806. [DOI] [Google Scholar]
- Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotech. 2007;24:117–126. doi: 10.5511/plantbiotechnology.24.117. [DOI] [Google Scholar]
- Mohammadkhani N, Heidari R. Drought induced accumulation of soluble sugars and proline in two maize varieties. West Indies Applied Sci J. 2008;3:448–453. [Google Scholar]
- Mostafa HAM, Hassanein RA, Khalil SI, El-Khawas SA, El-Bassiouny HMS, El-Momenad A. Effect of putrescine on growth, yield and yield components of late sowing wheat. J Applied Sci Res. 2010;6:177–183. [Google Scholar]
- Raghavrao D. Design of experiment. statistical techniques in agriculture and biological research. New Delhi: Oxford& IBH Publishing Comp; 1983. [Google Scholar]
- Sairam RK, Deshmukh PS, Shukla DS. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178:171–177. doi: 10.1111/j.1439-037X.1997.tb00486.x. [DOI] [Google Scholar]
- Sharma ML. Polyamine metabolism under abiotic stress in higher plants: salinity, drought and high temperature. Physiol Mol Biol Plants. 1999;5:103–113. [Google Scholar]
- Sharma KM, Sharma DD, Shukla KB, Upashyay B. Growth, partitioning and productivity of wheat as influenced by fertilization and foliar application of bioregulators. Indian J Plant Physiol. 2008;13:387–393. [Google Scholar]
- Werner T, Schmulling T. Cytokinin action in plant development. Curr Opinion Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Xie Z, Jiang D, Dai T, Jing Q, Lao W. Effects of exogenous ABA and cytokinin on leaf photosynthesis and grain protein accumulation in wheat ears culture in vitro. Plant Growth Regul. 2004;44:25–32. doi: 10.1007/s10725-004-1880-4. [DOI] [Google Scholar]
- Yadav N, Yadav VK, Kumar A. Effect of benzyladenine on transpiration, water potential and its components in genotypes of wheat contrasting in drought tolerance. J Agron Crop Sci. 1994;173:61–68. doi: 10.1111/j.1439-037X.1994.tb00574.x. [DOI] [Google Scholar]
- Yemm EW, Cocking FC. The determination of amino acids with ninhydrin. Analyst. 1955;80:208–213. doi: 10.1039/an9558000209. [DOI] [Google Scholar]


