Abstract
A comparative performance of two explants types (CN and Nodal) for their efficiency to induce multiple shoot regeneration in Clitoria ternatea has been carried out. Thidiazuron (TDZ) in different concentrations (0.05–2.5 μM) was used as a supplement to the Murashige and Skoog’s (MS) basal media. Explant type apart, two factors viz. concentration and exposure duration to TDZ played an important role in affecting multiple shoot regeneration. Cotyledonary node explants produced the best results at 0.1 μM TDZ, while in nodal explants the highest rate of shoot formation was achieved on MS medium supplemented with 1.0 μM TDZ. In both the explants, shoot multiplication increased when the regenerated shoots were subcultured on hormone free MS medium after 4 weeks of exposure to TDZ. Among the two, cotyledonary node explants produced considerably higher number of shoots at a comparatively lower concentration of TDZ than nodal explants. The regenerated shoots rooted best on MS medium containing 1.0 μM indole-3-butyric acid (IBA) and were successfully established in pots containing garden soil with 88 % survival rate. All the regenerated plants showed normal morphology and growth characteristics.
Keywords: Thidiazuron, Acclimatization, Butterfly pea, Cotyledonary node, Node
Introduction
Clitoria ternatea (Fabaceae) commonly known as Butterfly pea is a vigorous, strongly persistent, herbaceous perennial legume, upto 2–3 m in height and widely used in traditional Indian medicine as a brain tonic to promote memory and intelligence (Gomez and Kalamani 2003). It originated from tropical Asia but now naturalized in South and Central America, East and West Indies, China and India (Anonymous 1988).
The plant is used to cure various ailments more commonly as a rejuvenating recipe to treat neurological disorders and is considered to be wholesome for the intellect. These apart, the roots are bitter, refrigerant, laxative, diuretic, anthelmintic and are useful in dementia, hemicranias, burning sensation, leprosy, inflammation, leucoderma, bronchitis, asthma, pulmonary tuberculosis, ascites and fever while the leaves are useful in otalgia and hapatopathy (Jain et al. 2003). It is also considered to be useful for eye infections, skin diseases, urinary troubles, ulcers and has antidotal properties (Gomez and Kalamani 2003).
But due to destructive harvesting and lack of proper cultivation, the wild population of this medicinally important plant has declined rapidly and now it is listed as a rare species by the International Union for Conservation of Nature and Natural Resources (IUCN) (Panday et al. 1993). For this reason, the US Department of Agriculture is dedicated to conserving Clitoria ternatea alongwith other leguminous species with potentially useful phytochemicals (Morris 1999). Unfortunately, propagation through seeds of this species is extremely poor because of a very low percentage of germination coupled with a high rate of mortality of juvenile seedlings. Therefore, there is an urgent need to devise suitable strategies to conserve this rare medicinal flora. To balance the high market demand with supply, the only viable method seems to be appropriate cultivation and mass propagation techniques that can readily circumvent the natural population. Recent years has seen a tremendous rise in the role of Plant Tissue Culture techniques or more appropriately micropropagation for the conservation of many valuable medicinal plant species. In this sense, there is thus an urgent need to develop an appropriate in vitro micropropagation protocol for large scale multiplication and hence conservation of this rare medicinal legume.
Considering its importance and need for conservation, many reports on in vitro regeneration of Clitoria ternatea have come up lately (Lakshmanan and Dhanalakshmi 1990; Malabadi and Nataraja 2001; Rout 2005; Shahzad et al. 2007; Barik et al. 2007; Singh and Tiwari 2010, 2012; Mukhtar et al. 2010; Mohamed and Taha 2011; Ismail et al. 2011). In their studies, effect of only adenine based cytokinins viz. BA, Kin and 2 iP either singly or in combination with auxins were studied or discussed. Only few have studied the effect of TDZ, an important cytokinin analogue for affecting in vitro regeneration in Clitoria with limited success. Moreover, the existing studies lack comprehensive data on comparison between different explants types for their in vitro growth and regeneration. Taking these limitations into account, the present study was aimed at carrying out a comparative in vitro performance of two explants types viz. cotyledonary node and nodal explants of Clitoria ternatea in response to different concentrations of TDZ for enhanced plantlet production.
Materials and methods
Establishment of aseptic seedlings and explants source
Seeds of C. ternatea were obtained from the botanical garden of the University and washed thoroughly under running tap water for 30 min to remove adherent particles, then treated with a liquid detergent Labolene (5 % v/v) for 20 min followed by washing in tap water, and rinsed five times with sterile distilled water. After thorough washing, the seeds were surface sterilized with freshly prepared 0.1 % (w/v) HgCl2 for 5 min followed by repeated washing with sterile double distilled water. The seeds were inoculated in Murashige and Skoog (1962) medium for germination. After 7 days, cotyledonary node explants were excised for inoculation onto shoot induction medium. For this, first primary leaves and the epicotyls were detached from the seedling using sterile surgical blades followed by excision of the seedling radicle leaving approximately 3–5 cm long hypocotyls intact. Thereafter, explants consisting of cotyledon and axillary meristem regions with hypocotyls (3–5 cm long) were used for shoot induction. Similarly, nodal segments with an axillary bud were excised from 14 days old aseptic seedlings and used as explants.
Media and culture conditions
The culture medium consisted of MS salts and vitamins with 3 % (w/v) sucrose and supplemented with varying concentrations of TDZ (0.05, 0.1, 0.5, 1.0 and 2.5 μM). The medium was gelled with 0.8 % agar, adjusted to pH 5.8 using 1 N NaOH before autoclaving at 121 °C at 1.06 kg cm−2 for 15 min. All the cultures were maintained at 24 ± 2 °C under 16 h photoperiod with a photosynthetic photon flux density (PPFD) of 50 μmol m−2 s−1 provided by cool white fluorescent lamps (Philips, India Ltd.) and with 65 % relative humidity.
Shoot induction and multiplication
For multiple shoot induction, the cotyledonary node and nodal explants were placed on MS medium supplemented with different concentrations of TDZ. MS medium without growth regulators served as control. After an induction period of 4 weeks on TDZ enriched medium, the responsive explants were transferred to control medium which served as a secondary medium. Frequency of shoot induction, number of shoots and mean shoot length were recorded after 8 weeks of culture. All cultures were subcultured to a fresh medium after every 4 weeks.
In vitro rooting and acclimatization
The elongated shoots (4–5 cm) were excised individually and transferred to 1/2 MS supplemented with IAA or IBA (0.5, 1.0, 1.5 or 2.0 μM) for rooting. Data on percentage of rooting and mean number of roots per shoot were recorded after 4 weeks of transfer. Plantlets with well developed shoots and roots were removed from the culture medium, washed gently under running tap water and transferred to plastic cups containing sterile soilrite under diffused light (16/8 h photoperiod) conditions. Potted plants were covered with polythene bags to ensure high humidity and watered every alternate day with 1/2 MS basal solution devoid of organic supplements. The plants were kept in growth room at 24 ± 2 °C for 2 weeks and thereafter bags were removed to acclimatize plants to field conditions. After 1 month, these were then transferred to earthen clay pots containing normal garden soil; maintained in a greenhouse and finally shifted to net house under normal day length conditions.
Results and discussion
Effect of thidiazuron
Thidiazuron (TDZ), a substituted phenyl urea (N-phenyl-1, 2, 3-thidiazol-5-yl urea) is a potent plant growth regulator which exhibit cytokinin like activity in various culture systems (Huetteman and Preece 1993). TDZ has been successfully used in plant regeneration systems for many plant species (Faisal et al. 2005; Ahmad et al. 2006; Ahmad and Anis 2007a) including various legumes (Malik and Saxena 1992; Kanyand et al. 1994; Sanago et al. 1996; Eapen et al. 1998; Victor et al. 1999; Ahmad and Anis 2007b; Barik et al. 2007).
In the present study, the morphogenic responses of cotyledonary node and nodal explants were explored on MS medium supplemented with different concentrations of TDZ (0.05–2.5 μM). Both cotyledonary node and nodal explants placed on control MS medium did not show any morphogenic response and failed to produce shoots/shoot buds even after 4 weeks of incubation. They remained green and fresh for about 2 weeks, eventually turned brown and finally died.
The frequency and response of in vitro shoot regeneration differed depending on the type of explants and both the type and concentration of growth regulators added to the regeneration medium (Gubiš et al. 2003; Ishag et al. 2009). Likewise, shoot bud induction from both CN and nodal explants was observed in all treatments containing TDZ though with varied frequencies. Of various levels of TDZ tested, 0.1 μM proved to most effective and optimum for inducing maximum percent regeneration (49.6 ± 3.69 %), maximum number of shoots (5.2 ± 0.37) and shoot length (5.8 ± 0.23 cm) (Table 1; Fig. 1b) in CN explants. This concentration however, was less effective in nodal explants where only 31.6 ± 2.54 % of the explants responded with 2.6 ± 0.50 shoots and 3.2 ± 0.25 cm shoot length in 4 weeks. In nodal explants, the most effective concentration for inducing maximum shoot regeneration in vitro was 1.0 μM where the highest percentage (45.8 ± 4.14 %) with maximum number of shoots (3.6 ± 0.24) and shoot length (5.0 ± 0.10) was obtained on completion of 4 weeks (Table 2; Fig. 1a). The better response of CN explants towards TDZ treatment may be due to the presence of expanded cotyledons since cell wall polysaccharides stored in them might be degraded and mobilized to the developing shoots and thereby enhancing the growth of developing shoots (Rajeswari and Paliwal 2008). While on the other hand, due to the absence of such features nodal segments needed a higher dose of the growth regulator to elicit response.
Table 1.
Effect of TDZ on multiple shoot induction from cotyledonary node explants of Clitoria ternatea in MS medium after 4 weeks of culture
| TDZ (μM) | % Regeneration | Mean no. of shoots | Mean shoot length (cm) |
|---|---|---|---|
| 0.05 | 37.2 ± 3.03bc | 2.6 ± 0.24bc | 3.4 ± 0.08bc |
| 0.1 | 49.6 ± 3.69a | 5.2 ± 0.37a | 5.8 ± 0.23a |
| 0.5 | 42.4 ± 5.39ab | 3.2 ± 0.58b | 3.7 ± 0.21b |
| 1.0 | 30.8 ± 0.24cd | 1.8 ± 0.37cd | 2.9 ± 0.36c |
| 2.5 | 24.0 ± 1.22d | 1.4 ± 0.24d | 1.7 ± 0.15d |
Values represent means ± SE. Means followed by the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test
Fig. 1.
a Distorted shoots of C. ternatea derived from nodal segment on continuous exposure with TDZ (1.0 μM). b Multiple shoot induction from CN explant of C. ternatea on MS + TDZ (0.1 μM) after 4 weeks of culture. c Multiplication and elongation of TDZ exposed shoots on control MS medium after 8 weeks of culture. d Properly rooted shootlets of C. ternatea (using 1.0 μM IBA) ready for transplant. e 6 weeks old acclimatized plants in pots
Table 2.
Effect of TDZ on multiple shoot induction from nodal explants of Clitoria ternatea on MS medium after 4 weeks of culture
| TDZ (μM) | % Regeneration | Mean no. of shoots | Mean shoot length (cm) |
|---|---|---|---|
| 0.05 | 26.8 ± 1.98bc | 1.8 ± 0.37bc | 2.2 ± 0.38d |
| 0.1 | 31.6 ± 2.54bc | 2.6 ± 0.50ab | 3.2 ± 0.25c |
| 0.5 | 33.0 ± 3.30b | 2.8 ± 0.58ab | 4.0 ± 0.16b |
| 1.0 | 45.8 ± 4.14a | 3.6 ± 0.24a | 5.0 ± 0.10a |
| 2.5 | 23.2 ± 1.06c | 1.2 ± 0.37bc | 1.9 ± 0.41d |
Values represent means ± SE. Means followed by the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test
The stimulatory effect of TDZ on multiple shoot formation has been reported earlier by Huetteman and Preece (1993) in woody trees, Kanyand et al. (1994) in Arachis hypogea, Ahmad and Anis (2007) in Cyamopsis tetragonoloba and Siddique and Anis (2007) in Cassia angustifolia. Similarly, Ismail et al. (2011) successfully regenerated multiple shoots in nodal segments of C. ternatea using TDZ. They however reported 2.5 μM as the optimum concentration which is contradictory to our findings. The mode of action of TDZ for affecting in vitro shoot regeneration may be attributed to its low ability to induce cytokinin accumulation (Victor et al. 1999) and or may be due to enhanced accumulation and translocation of auxin (Murch and Saxena 2001).
Increase in concentration of TDZ beyond the optimal level resulted in low regeneration frequencies, with low shoot number and shoot length irrespective of the explant type used. In the present study, higher concentrations drastically reduced the in vitro response and 2.5 μM TDZ was found to be least productive (1.4 ± 0.24 shoots/explant). Contrary to an earlier report (Ismail et al. 2011), the lowest regeneration frequency with the lowest number of shoots per explant (1.2 ± 0.37) was obtained in MS medium supplemented with 2.5 μM TDZ using nodal segments in the present study (Table 2). Reduction in the number of shoots generated from each explant at TDZ concentration higher than optimal level has also been reported for several plant species like Adhatoda beddomei (Sudha and Seeni 1994); Ocimum americanum (Pattnaik and Chand 1996) and Cassia angustifolia (Siddique and Anis 2007).
TDZ being a cytokinin analogue may negatively affect the shoot regeneration and shoot quality on prolonged culture. Therefore, after an initial induction period of 4 weeks, the TDZ exposed shoots/explants were transferred to a secondary medium lacking TDZ for shoot multiplication and elongation. Shoot multiplication increased considerably on transfer to control MS medium resulting in 7.4 ± 0.60 and 6.8 ± 0.58 shoots per CN and Nodal explants respectively at the 4th subculture (Fig. 1c). Such type of culture strategy employing different media for initiation and multiplication have been worked out successfully in many species (Faisal et al. 2005; Ahmad and Anis 2007; Siddique and Anis 2007). Reportedly, TDZ exposed shoots/explants carry a high potential of multiplication on a basal media devoid of TDZ (Mok et al. 1982; Capelle et al. 1983). In contrast, the cultures grown continuously on TDZ containing media have fasciated and distorted shoots (Fig. 1a). This formation of stunted or fasciation of the shoots on TDZ-supplemented media has been reported in several plant species such as Rauvolfia tetraphylla (Faisal et al. 2005) and Capsicum annuum (Ahmad et al. 2006). The inhibition of shoot elongation may be due to the high cytokinin activity of TDZ and the presence of a phenyl group in TDZ may be the possible cause of shoot bud fasciation (Huetteman and Preece 1993).
Effect of subculture passages
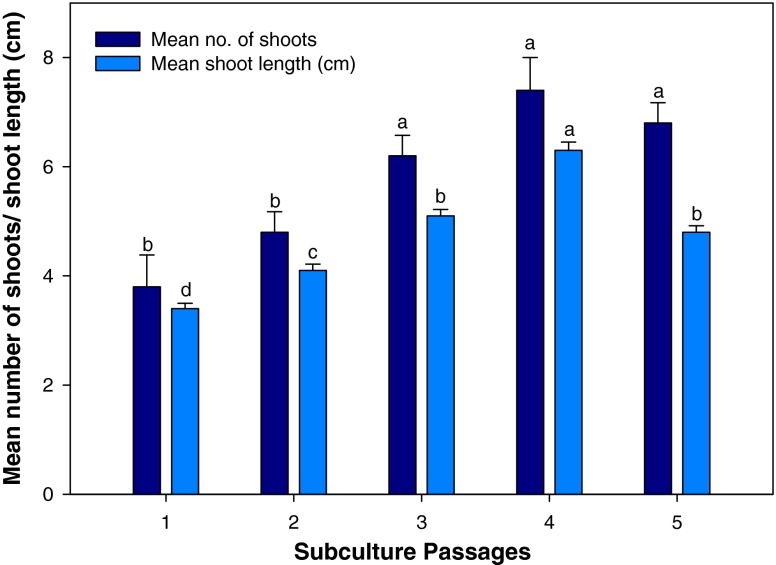
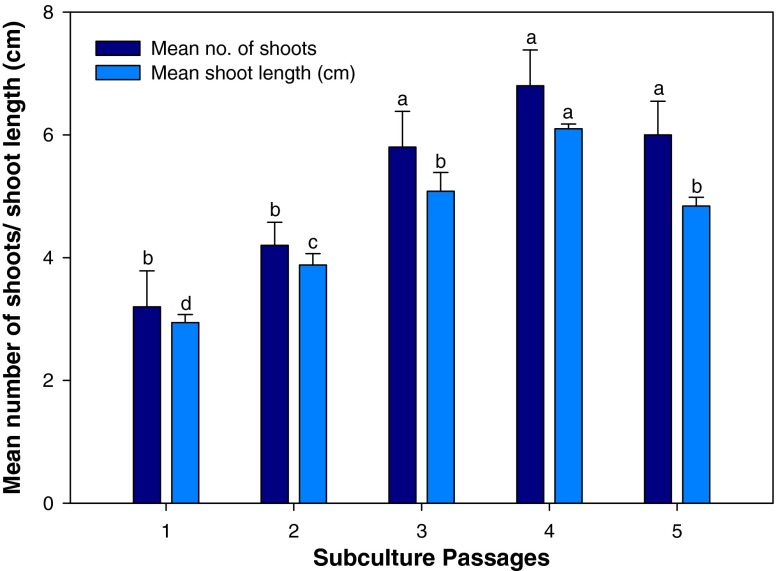
The effect of subculture passages on multiple shoot production of TDZ exposed shoots was evaluated after transferring them to control MS medium. In cotyledonary node explants, 0.1 μM TDZ exposed shoots were used for the purpose whereas shoots obtained on 1.0 μM TDZ was used for the same in the case of nodal explants. Shoot proliferation increased on every subculture and transfer to fresh media. Using cotyledonary node explants, the highest number of shoots (7.4 ± 0.60) and shoot length (6.3 ± 0.15 cm) per explant was obtained at the fourth subculture passage (Fig. 2) which gradually declined thereafter. Similarly in nodal explants, the number of shoots and shoot length increased after every subculturing (Fig. 3) and declined after fourth passage. The highest shoot multiplication (6.8 ± 0.58) and shoot length (6.1 ± 0.07 cm) were recorded at fourth passage. The promotive effects of subculturing on shoot multiplication is a common phenomenon and reported in a number of cases (Tiwari et al. 2001; Siddique and Anis 2007).
Fig. 2.
Effect of subculture passages on in vitro multiple shoot induction in cotyledonary node explant of Clitoria ternatea on MS medium supplemented with TDZ (0.1 μM). The bars represent mean ± SE. Bars denoted by the same letter within response variables are not significantly different (P = 0.05) using DMRT
Fig. 3.
Effect of subculture passages on in vitro multiple shoot induction in nodal explant of Clitoria ternatea on MS medium supplemented with TDZ (1.0 μM). The bars represent mean ± SE. Bars denoted by the same letter within response variables are not significantly different (P = 0.05) using DMRT
In vitro rooting
For rooting, individual microshoots were transferred to 1/2 MS basal medium supplemented with different concentrations of IAA and IBA (Table 3). The best rooting percentage (88.4 ± 1.20 %) and the highest mean number of roots (5.2 ± 0.37) per shoot was obtained on 1/2 MS supplemented with 1.0 μM IBA (Fig. 1d). The superiority of IBA over other auxins for in vitro rooting has been reported in Clitoria ternatea (Barik et al. 2007; Mukhtar et al. 2010; Singh and Tiwari 2012) and several other medicinal plants like Cunila galiodes (Fracaro and Echiverrigaray 2001), Mucuna pruriens (Faisal et al. 2006), Psoralea corylifolia (Faisal and Anis 2006).
Table 3.
Effect of MS strength and auxin concentrations on root induction from in vitro raised microshoots of Clitoria ternatea after 4 weeks of culture
| Treatments (μM) | % Rooting | Mean no. of roots | Mean shoot length (cm) |
|---|---|---|---|
| 1/2 MS + IBA (0.5) | 60.2 ± 3.99e | 2.2 ± 0.37de | 4.6 ± 0.87b |
| 1/2 MS + IBA (1.0) | 88.4 ± 1.20a | 5.2 ± 0.37a | 7.9 ± 0.59a |
| 1/2 MS + IBA (1.5) | 81.2 ± 1.88abc | 4.2 ± 0.37ab | 7.0 ± 0.42a |
| 1/2 MS + IBA (2.0) | 70.6 ± 3.69d | 2.0 ± 0.44de | 3.0 ± 0.92bc |
| 1/2 MS + IAA (0.5) | 58.6 ± 4.92e | 1.8 ± 0.37e | 2.6 ± 0.52bc |
| 1/2 MS + IAA (1.0) | 84.6 ± 1.80a | 4.8 ± 0.37ab | 7.8 ± 0.34a |
| 1/2 MS + IAA (1.5) | 73.6 ± 2.61cd | 3.8 ± 0.37bc | 7.1 ± 0.28a |
| 1/2 MS + IAA (2.0) | 70.8 ± 3.21d | 1.6 ± 0.40e | 1.8 ± 0.85c |
Values represent means ± SE. Means followed by the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test
Hardening and Acclimatization
Rooted plantlets with 4–5 fully expanded leaves and well developed roots were transferred to pots containing soilrite and hardened off in growth room for 4 weeks (Fig. 1e) as described in materials and methods. After 1 month, the micropropagated plants were planted in earthen pots containing garden soil and shifted to greenhouse conditions and finally to field under full sun. About 88 % plants survived the hardening procedure without any detectable morphological variations. Such process of slow hardening and acclimatization of tissue culture raised plantlets have been found suitable with many micropropagation protocols (Ahmad and Anis 2007; Khan et al. 2011).
Conclusion
Micropropagation plays a key role in the successful multiplication and conservation of many valuable medicinal plants. The present study demonstrates the successful use of tissue culture techniques in both CN and nodal explants of C. ternatea for multiple shoot induction and proliferation which may in turn facilitate the conservation of this important medicinal legume. The micropropagation technique described here is convenient and affordable. The comparative study using two explants types could provide a deeper understanding of the possible mechanisms and requirements of each tissue type during various micropropagation stages. Although both explants types were found to be highly competent for in vitro shoot production, CN explants seems to be more responsive in comparision to nodal explants.
Acknowledgements
Authors appreciate the financial assistance provided by the University Grants Commission, Govt. of India, under UGC-SAP (DRS-I) program (2009) and Department of Science and Technology (DST) Govt. of India, in the form of DST-FIST program (2011) to the Department of Botany, Aligarh Muslim University.
Footnotes
An erratum to this article can be found online at 10.1007/s12298-016-0352-4.
The corresponding author retracts this article due to the mistaken inclusion of Table 3 and Fig. 1C from the previously published article by Mukhtar, S., Anis, M., & Ahmad, N. (2010) titled "In vitro optimization of phytohormones on micropropagation in Butterfly pea (Clitoria ternatea L.)" in the Journal of herbs, spices & medicinal plants 16:2, 98-105. The authors regret the error due to oversight and thank the anonymous complainant and the editor of PMBP for bringing it to our notice.
References
- Ahmad N, Anis M. Rapid plant regeneration protocol for cluster bean (Cyamopsis tetragonoloba L. Taub.) J Hort Sci Biotechnol. 2007;82:585–589. [Google Scholar]
- Ahmad N, Siddique I, Anis M. Improved plant regeneration in Capsicum annuum from nodal segment. Biol Plant. 2006;50:701–704. doi: 10.1007/s10535-006-0110-5. [DOI] [Google Scholar]
- Anonymous (1988) The wealth of India: a dictionary of Indian raw materials and industrial products. Vol. II. New Delhi: Publication and Information Directorate. CSIR
- Barik DP, Naik SK, Mudgal A, Chand PK. Rapid plant regeneration through in vitro axillary shoot proliferation of butterfly pea (Clitoria ternatea L.)—a twinning legume. In Vitro Cell Dev Biol Plant. 2007;43:144–148. doi: 10.1007/s11627-007-9040-y. [DOI] [Google Scholar]
- Capelle SC, Mok DWS, Kirchner SC, Mok MC. Effects of thidiazuron on cytokinin autonomy and the metabolism of N6 (2-isopentenyl)[8-'4C]adenosine in callus tissue of Phaseolus lunatus L. Plant Physiol. 1983;73:796–802. doi: 10.1104/pp.73.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen S, Tivarekar S, George L. Thidiazuron induced shoot regeneration in pigeon pea (Cajanus cajan L.) Plant Cell Tissue Organ Cult. 1998;53:217–220. doi: 10.1023/A:1006060318752. [DOI] [Google Scholar]
- Faisal M, Anis M. Thidiazuron induced high frequency axillary shoot multiplication in Psoralea corylifolia. Biol Plant. 2006;50:437–440. doi: 10.1007/s10535-006-0064-7. [DOI] [Google Scholar]
- Faisal M, Ahmad N, Anis M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005;80:187–190. doi: 10.1007/s11240-004-0567-x. [DOI] [Google Scholar]
- Faisal M, Siddique I, Anis M. In vitro rapid regeneration of plantlets from nodal explants of Mucuna pruriens—a valuable medicinal plant. Ann App Biol. 2006;148:1–6. doi: 10.1111/j.1744-7348.2005.00034.x. [DOI] [Google Scholar]
- Fracaro F, Echiverrigaray S. Micropropagation of Cunila galioides, a popular medicinal plant of South Brazil. Plant Cell Tissue Organ Cult. 2001;64:1–4. doi: 10.1023/A:1010626200045. [DOI] [Google Scholar]
- Gomez SM, Kalamani A. Butterfly pea (Clitoria ternatea): a nutritive multipurpose forage legume for the tropics—an overview. Pak J Nutr. 2003;2:374–379. doi: 10.3923/pjn.2003.374.379. [DOI] [Google Scholar]
- Gubiš J, Lajchová Z, Faragó J, Jureková Z. Effect of genotype and explant type on shoot regeneration in tomato (Lycopersicon esculentum Mill.) in vitro. Czech J Genet Plant Breed. 2003;39:9–14. [Google Scholar]
- Huetteman CA, Preece JE. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993;33:105–119. doi: 10.1007/BF01983223. [DOI] [Google Scholar]
- Ishag S, Osman MG, Khalafalla MM. Effects of growth regulators, explant and genotype on shoot regeneration in tomato (Lycopersicon esculentum c.v. Omdurman) Int J Sustain Crop Prod. 2009;4:7–13. [Google Scholar]
- Ismail N, Rani U, Batra A. Crucial role of TDZ in the quick regeneration of multiple shoots of Clitoria ternatea L. Int J Pharma Sci Rev Res. 2011;6:23–26. [Google Scholar]
- Jain MN, Dhal CC, Shroff RH, Bhutada RH, Somani RS, Kasture VS, Kasture SB. Clitoria ternatea and the CNS. Pharmacol Biochem Behav. 2003;75:529–536. doi: 10.1016/S0091-3057(03)00130-8. [DOI] [PubMed] [Google Scholar]
- Kanyand N, Dessai AP, Prakash SC. Thidiazuron promotes high frequency regeneration of peanut (Arachis hypogea) plants in vitro. Plant Cell Rep. 1994;14:1–5. doi: 10.1007/BF00233288. [DOI] [PubMed] [Google Scholar]
- Khan MI, Ahmad N, Anis M. The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb.: a tree of ecological importance. Trees. 2011;25:577–584. doi: 10.1007/s00468-010-0534-6. [DOI] [Google Scholar]
- Lakshmanan KK, Dhanalakshmi S. Callus, organogenesis and plantlet formation in tissue cultures of Clitoria ternatea. Ann Bot. 1990;66:451–455. [Google Scholar]
- Malabadi RB, Nataraja K. Shoot regeneration in leaf explants of Clitoria ternatea L. cultured in vitro. Phytomorphology. 2001;51:169–171. [Google Scholar]
- Malik KA, Saxena PK. Regeneration in Phaseolus vulgaris L. high frequency induction of direct shoot formation in intact seedlings by N6-benzylamino purine and thidiazuron. Planta. 1992;186:384–389. doi: 10.1007/BF00195319. [DOI] [PubMed] [Google Scholar]
- Mohamed N, Taha RM. Plant regeneration of Clitoria ternatea from leaf explants cultured in vitro. J Food Agric Environ. 2011;9:268–270. [Google Scholar]
- Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, Okamoto T. Cytokinin activity of N-phenyl-N’-1,2,3-thidiazol-5-ylurea (thidiazuron) Phytochemistry. 1982;21:1509–1511. doi: 10.1016/S0031-9422(82)85007-3. [DOI] [Google Scholar]
- Morris JB. Legume genetic resources with novel ‘value added’ industrial and pharmaceutical use. In: Janick J, editor. Perspectives on new crops and new uses. Alexandria: ASHS; 1999. pp. 196–201. [Google Scholar]
- Mukhtar S, Anis M, Ahmad N. In vitro optimization of phytohormones on micropropagation in Butterfly pea (Clitoria ternatea L.) J Herbs Spices Med Plants. 2010;16:98–105. doi: 10.1080/10496475.2010.499310. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tiossue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murch SJ, Saxena PK. Molecular fate of thidiazuron and its effects on auxin transport in hypocotyls tissues of Pelargonium x hortorum Bailey. Plant Growth Regul. 2001;35:269–275. doi: 10.1023/A:1014468905953. [DOI] [Google Scholar]
- Panday NK, Tewari KC, Tewari RN, Joshi GC, Pande VN, Pandey G. Medicinal plants of Kumaon Himalaya: strategies for conservation. In: Dhar U, editor. Himalayan biodiversity conservation strategies, no. 3. Nanital: Himavikas; 1993. pp. 293–302. [Google Scholar]
- Pattnaik S, Chand PK. In vitro propagation of the medicinal herbs Ocimum americanum L. Syn. O. canum Sims. (Hoary bsil) and Ocimum sanctum L. (Holy basil) Plant Cell Rep. 1996;15:846–850. doi: 10.1007/BF00233154. [DOI] [PubMed] [Google Scholar]
- Rajeswari V, Paliwal K. In vitro plant regeneration of red sanders (Pterocarpus santalinus L.f.) from cotyledonary nodes. Ind J Biotechnol. 2008;7:541–546. [Google Scholar]
- Rout GR. Micropropagation of Clitoria ternatea Linn. (Fabaceae): an important medicinal plant. In vitro Cell Dev Biol-Plant. 2005;41:516–519. doi: 10.1079/IVP2005675. [DOI] [Google Scholar]
- Sanago MHM, Shataluk VI, Stromme J. Rapid plant regeneration of pea using thidiazuron. Plant Cell Tissue Org Cult. 1996;45:165–168. doi: 10.1007/BF00048761. [DOI] [Google Scholar]
- Shahzad A, Faisal M, Anis M. Micropropagation through excised root culture of Clitoria ternatea L. and comparison between in vitro regenerated plants and seedling. Ann Appl Biol. 2007;150:341–349. doi: 10.1111/j.1744-7348.2007.00132.x. [DOI] [Google Scholar]
- Siddique I, Anis M. In vitro shoot multiplication and plantlet regeneration from nodal explants of Cassia angustifolia (Vahl.)—a medicinal plant. Acta Physiol Plant. 2007;29:333–338. doi: 10.1007/s11738-007-0029-2. [DOI] [Google Scholar]
- Singh J, Tiwari KN. High-frequency in vitro multiplication system for commercial propagation of pharmaceutically important Clitoria ternatea L.—a valuable medicinal plant. Ind Crop Prod. 2010;32:534–538. doi: 10.1016/j.indcrop.2010.07.001. [DOI] [Google Scholar]
- Singh J, Tiwari KN. In vitro plant regeneration from decapitated embryonic axes of Clitoria ternatea L.—an important medicinal plant. Ind Crop Prod. 2012;35:224–229. doi: 10.1016/j.indcrop.2011.07.008. [DOI] [Google Scholar]
- Sudha CG, Seeni S. In vitro multiplication and field establishment Adhotoda beddomei GB, Clarke, a rare medicinal plant. Plant Cell Rep. 1994;13:203–207. doi: 10.1007/BF00239893. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Tiwari KN, Singh BD. Comparitive studies of cytokinins on in vitro propagation of Bacopa monniera. Plant Cell Tissue Org Cult. 2001;66:9–16. doi: 10.1023/A:1010652006417. [DOI] [Google Scholar]
- Victor JMR, Murthy BNS, Murch SJ, Krihnaraj S, Saxena PK. Role of endogenous purine metabolism in thidiazuron-induced somatic embryogenesis of peanut (Arachis hypogea) Plant Growth Regul. 1999;28:41–47. doi: 10.1023/A:1006251531319. [DOI] [Google Scholar]