Abstract
The so far unexplored H. Orientalis cv. Olympicus exhibits a unique pattern of flower senescence, involving re-greening of creamy white petaloid sepals at the later stages. The greenish sepals become photosynthetically competent immediately after pollination and persist until the seeds are set. After the seed set, the entire (green) flower abscises from the plant. Flower development of Helleborus orientalis cv. Olympicus growing in the open was divided into six stages (I–VI) from tight bud stage to the senescent stage. The average life span of an individual flower after it is fully open is about 6 days. Membrane permeability of sepal tissues estimated as electrical conductivity of leachates increased during senescence. The content of sugars and soluble proteins in the sepal tissues increased during flower opening and declined thereafter during senescence. The protease activity increased as the flower progressed towards senescence. From the present study, it becomes evident that decline in the sugar status and elevation in specific protease activity leading to degradation of proteins are the important factors regulating development and senescence in H. orientalis flowers. Although the tissue content of soluble proteins registered an overall quantitative decrease but SDS-PAGE of protein extract from sepal tissues suggested a decrease in the expression of high molecular weight proteins and an increase in low molecular weight proteins during flower development and senescence. At this stage it is not known whether the polypeptides that increased during senescence play an important role in the senescence of Helleborus orientalis flowers. The increase in these polypeptides during flower senescence is of particular interest because they may be linked to flower longevity. Understanding the nature of these proteins can provide new insights into the pathways that execute senescence and the post-transcriptional regulation of senescence in this flower system.
Keywords: α-amino acids, Flower senescence, Helleborus orientalis, Membrane permeability, Protease activity, SDS-PAGE
Introduction
Petal senescence has been shown to be genetically programmed and involves degradation of proteins, lipids, carbohydrates and nucleic acids (Rubinstein 2000; Eason et al. 2002; Wagstaff et al. 2002; van Doorn 2004; Hoeberichts et al. 2005; Zhou et al. 2005; Eason 2006; Price et al. 2008; van Doorn and Woltering 2008; Shibuya et al. 2009). Flower petals are ideal tissues for cell death studies as they are short lived, the tissue is relatively homogenous and chemical manipulation can be applied without substantial wounding. Petal senescence has been found to be accompanied by an increase in the activity of catabolic enzymes, ion leakage and nuclear fragmentation. This is all directed towards mobilization of nutrients from petals to other parts of the plant such as developing ovary (Halevy and Mayak 1979; Xu and Hanson 2000; Yamada et al. 2001; van der Kop et al. 2003; Woltering et al. 2005; Zhou et al. 2005; Rogers 2006; van Doorn and Woltering 2008). The cut stems of H. orientalis have deeply serrated long lasting leathery foliage and creamy white saucer shaped flowers (Perry and Perry 2000). The flowers of Helleborus orientalis cv. Olympicus (Lenten rose) are characterized by a showy creamy white perianth, whose usually five elements are mostly classified as sepals (Damboldt and Zimmerman 1965). The sepals are enlarged to look like petals and the whorl of petals modified into a whorl of 10–12 nectaries. A multitude of stamens with golden yellow anthers and 3 to 5 green carpels or pistils are present at the centre (Fig. 1). Their flowering season from winter through to spring brings shape and interest when gardens are otherwise bare, and their subtle colors light up gloomy corners and borders. They also make superb cut flowers, seeming subtle but on closer inspection intricately marked and remarkably beautiful. It is in this perspective; the present investigation has been undertaken on so far unexplored Helleborus orientalis cv. Olympicus to understand the changes occurring during flower development and senescence with the ultimate aim to improve the postharvest performance of this flower.
Fig. 1.
Plants of Helleborus orientalis in full bloom
Materials and methods
Plant material
Flowers of Helleborus orientalis growing in the University Botanic Garden were used. Flower development and senescence was divided into six stages on the basis of distinct morphological features (Table 1, Fig. 2a). These stages were defined as tight bud stage (I), mature bud stage (II), half open stage (III), fully open stage (IV), partially green stage (V) and green sepal stage (VI). Visible changes were recorded throughout flower development and senescence.
Table 1.
Different morphological features on the basis of which the following stages were identified during flower development and senescence in Helleborus orientalis cv. Olympicus
| Stage of flower development and senescence | Features |
|---|---|
| I (Tight bud stage) | Buds completely closed; Sepals greenish in colour |
| II (mature bud stage) | Buds closed; Sepals creamy in colour |
| III (Half-open stage) | Flowers half open; Cup shaped; All stamens and nectaries clustered at center enclosing the pistils; Pistils not visible; Flowers creamy-white in colour |
| IV (fully open stage) | Flowers fully open; Saucer shaped; Outer whorls of stamens separated from the central cluster; Pistils visible; Nectaries greenish; Sepals creamy-white in colour |
| V (Partially green stage) | Flowers fully open; Saucer shaped; Outer whorls of stamens abscised while some inner whorls surround the pistils; Pistils fully visible; Nectaries dried and turned brownish. Initiation of re-greening from basal portion of sepals |
| VI (Green sepal stage) | Flowers fully open; saucer shaped; All stamens and nectaries abscised. Enlarged greenish pistils at center which develop into follicles; sepals fully green. |
Fig. 2.
a Stages of flower development and senescence in Helleborus orientalis. Note that during senescence the sepals turn green (Stages V and VI). b Increase in the pistil dimension during flower development and senescence
Floral diameter, fresh mass, dry mass and membrane permeability
Diameter, fresh and dry mass of flowers as well as individual sepals was determined at each stage. Dry mass was determined by drying the material in an oven at 70 °C for 48 h. Water content was determined as the difference between fresh and dry mass. Changes in membrane permeability were estimated by measuring the electrical conductivity (μS) of leachates of 5 sepal discs per flower (5 mm in diameter) punched from outer regions of sepals of five different flowers incubated in 15 ml glass distilled water for 15 h at 20 °C.
Estimation of sugars, amino acids and phenols
At each stage 1 g chopped material of the sepal tissue was fixed in hot 80 % ethanol. The material was macerated and centrifuged three times. The supernatants were pooled and used for the measurement of the amount of sugars, amino acids and total phenols. Reducing sugars were determined by the method of Nelson (1944) using glucose as the standard. Total soluble sugars were estimated after enzymatic conversion of non-reducing sugars into reducing sugars with invertase (BDH). Non-reducing sugars were calculated as the difference between total and reducing sugars. α-amino acids were estimated by the method of Rosen (1957) using glycine as the standard. Total phenols were estimated by the method of Swain and Hillis (1959) using gallic acid as the standard.
Estimation of protein levels and protease activity
Proteins were extracted from 1 g of sepal tissue drawn separately from different flowers. The tissue was homogenized in 5 ml of 5 % sodium sulphite (w/v) adding 0.1 g of polyvinylpyrrolidone (PVP) and centrifuged. Proteins were precipitated from a suitable volume of the cleared supernatant with equal volume of 20 % trichloroacetic acid (TCA), centrifuged at 1,000× g for 15 min and the pellet redissolved in 0.1 N NaOH. Proteins were estimated from a suitable aliquot by the method of Lowry et al. (1951) using Bovine serum albumin (BSA) as the standard.
At each stage 1 g pre-chilled sepal tissue was homogenized in 15 ml chilled 0.1 M phosphate buffer (pH 6.5) in a pre-cooled glass pestle and mortar. The contents were squeezed through four layers of muslin cloth and centrifuged for 15 min at 5,000× g in a (Remi K-24) refrigerated centrifuge at −5 °C. The supernatant was used for the assay of protease activity by the method of Tayyab and Qamar (1992), with modification. The reaction mixture comprised 1 ml of 0.1 % BSA dissolved in 0.1 M phosphate buffer (pH 6.5). The reaction was stopped by adding 2 ml of 20 % cold TCA. Blanks in which TCA was added prior to the addition of the enzyme extract were run along with each sample. The contents were centrifuged and supernatants collected. Free amino acids were estimated (as tyrosine equivalents) in a suitable aliquot of the supernatant by the method of Lowry et al. (1951) using tyrosine as the standard. The specific enzyme activity has been expressed as μg tyrosine equivalents liberated per mg protein in the tissue extract.
SDS-PAGE
At each stage 1 g sepal tissue was homogenized in 1 ml of extraction buffer (0.1 M pH 7.2) and 100 mg PVP. The mixture was centrifuged at 5,000× g at −5 °C in a refrigerated centrifuge (Remi K-24) for 15 min. The supernatant was collected and used for SDS-PAGE. The extracted protein mixture was denatured by mixing equal volumes of protein mixture and 2X sample loading buffer (0.5 M Tris pH 6.8, 10 % SDS, 10 % glycerol, 5 % β-mercaptoethanol, 0.1 % bromophenol blue). The mixture was incubated in boiling water for 5 min. The concentration of the protein was determined in both the original extracts and the TCA precipitated samples by the method of Lowry et al. (1951) using BSA as the standard.
One dimensional vertical gel electrophoresis was carried out according to the method as described by Ausubel et al. (1989). Slab gels 0.7 mm thick containing 12 % gel {(Acrylamide + bisacrylamide), (1.5 M Tris pH 8.8), 10 % SDS, TEMED and 10 % Ammonium persulphate (APX)}. 80 μl of the SDS-denatured protein extract was loaded into each lane. Electrophoresis was carried out at room temperature with a constant voltage of 50 V during stacking and 150 V during running. GENEI molecular weight standards were used for determining approximate molecular weights (Myosin, Rabbit muscle 205,000; phosphorylase b 97,400; Bovine serum albumin 66,000; Ovalbumin 43,000; Carbonic anhydrase 29,000; Aprotinin 6,500; Insulin (α and β chains) 3,000). Following electrophoresis the gels were stained overnight in 0.25 % Coomassie brilliant blue in 45 % methanol: 10 % acetic acid. Gels were destained in 45 % methanol: 10 % acetic acid, then in 7 % methanol: 5 % acetic acid.
Statistical analysis
Each value represented in the tables corresponds to the mean ± S.E of five to ten independent replicates.
Results
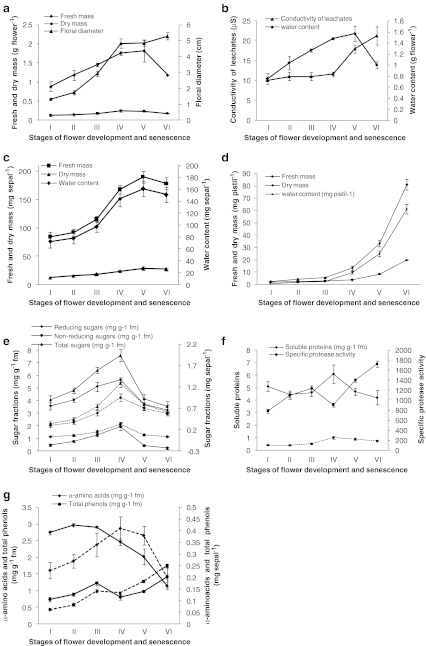
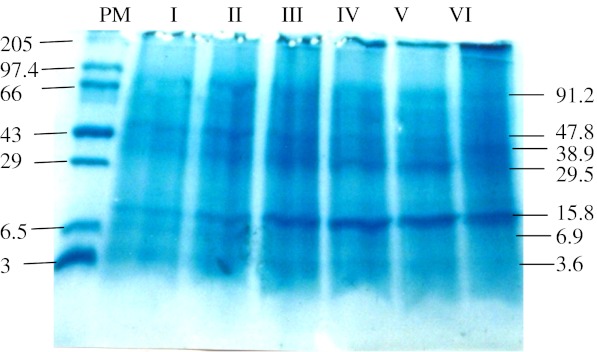
The greenish buds of Helleborus orientalis open into creamy-white flowers. Initially young pistils remain hidden and surrounded by a cluster of stamens upto stage III of flower development but as the flowers open, the pistils elongate and register an increase in their dimensions pushing away the surrounding anthers leading to their abscission. The creamy white sepals later on turn greenish during senescence (Fig. 2a). The average life span (time for a fully open flower to lose all its stamens and for the sepals to be green i.e. time from stage IV to stage VI) of an individual flower is about 6 days. Flower senescence is characterized by abscission of stamens layer by layer, followed by browning and abscission of nectaries. The sepals harden and turn greenish on senescence. The pistil dimensions increase as other floral organs undergo senescence, and develop into fruits (follicle). The greenish sepals persist as long as the seeds are set and after that they disintegrate and finally the entire flower abscises from the plant. Flower diameter increased as the flower development progressed up to stage IV and thereafter remained more or less constant, whereas fresh mass, dry mass and water content of flowers and also of individual sepals increased with flower development up to stage IV to V and declined thereafter (Fig. 3a, b and c). Membrane permeability estimated as conductivity of leachates (μS) from sepal discs generally remained constant during flower opening up to stage IV and increased thereafter as the flower progressed towards senescence (Fig. 3c).Throughout flower development and senescence the fresh and dry mass of pistil increased, however the increment of increase was pronounced during stages IV to VI (Fig. 2b) besides there was a pronounced increase in the water content of the pistil as the flower progressed from stage III to IV (Fig. 3d).The tissue content of reducing and non-reducing sugars increased through stages from I to IV and declined quickly thereafter during senescence (stages V and VI). The concentration of non-reducing sugars was much lower compared to that of reducing sugars, at each of the stages (Fig. 3e). The concentration of soluble proteins was more or less similar during stages I to III, had increased at stage IV and declined thereafter during senescence. An increase in the concentration of soluble proteins occurred during stage III to IV (Fig. 3f). The specific protease activity increased during the stages I to III, decreased at stage IV and then sharply increased as the senescence progressed through the stages V and VI (Fig. 3f). The α-amino acid content showed a slight increase during stages I to III and declined thereafter (Fig. 3g). The concentration of total phenols increased during the stages I to III, decreased during flower opening (Stage IV) and increased thereafter as senescence progressed through the stages V and VI (Fig. 3g). The SDS-PAGE of sepal proteins at various stages of flower development and senescence showed that the levels of most of the polypeptides were maintained up to full flower opening (Stages I to IV) and decreased during senescence, Stages V and VI (Fig. 3). The decrease was particularly observed in case of polypeptides with higher molecular weight (50–100 kDa). Polypeptides with a molecular weight of 29.5 and 15.8 kDa showed an increase as the flower development progressed through opening and senescence. A pronounced increase was particularly observed during opening and senescence in the polypeptide having a molecular mass of approx. 15.8 kDa (Fig. 4).
Fig. 3.
a Changes in fresh mass, dry mass and diameter of flowers during different stages of development and senescence in Helleborus orientalis flowers. b Changes in water content of flowers and electrical conductivity of leachates during different stages of development and senescence in Helleborus orientalis flowers. c Changes in fresh mass, dry mass and water content of sepals during different stages of development and senescence in Helleborus orientalis flowers. d Changes in fresh mass, dry mass and water content of pistils during different stages of development and senescence in Helleborus orientalis flowers. e Changes in the concentration of reducing, non-reducing and total sugars during different stages of development and senescence in Helleborus orientalis flowers. Solid lines represent values on mg per g fresh mass (mg g−1 fm) and dotted lines with the corresponding markers represent values on mg per sepal basis. f Changes in the soluble protein content and specific protease activity (μg tyrosine equivalents liberated per mg protein) during different stages of development and senescence in Helleborus orientalis flowers. Solid lines represent values on mg per g fresh mass (mg g−1 fm) and dotted lines with the corresponding markers represent values on mg per sepal basis. g Changes in the content of α-amino acids and total phenols during different stages of development and senescence in Helleborus orientalis flowers. Solid lines represent values based on mg per g fresh mass (mg g−1 fm) and dotted lines with the corresponding markers represent values on mg per sepal basis
Fig. 4.
12 % SDS-PAGE of equal amounts of extractable protein at various stages (I to VI) of flower development and senescence from sepal tissues of Helleborus orientalis. The gel was stained with coomassie blue. Numbers above the lanes correspond to developmental stages. Molecular weight standards are indicated on the left (kDa) and ca molecular weights of major polypeptides to the right of the gel (kDa)
Discussion
The results of our study suggests that flower senescence in Helleborus orientalis begins soon after anthesis and is marked by 1) the abscission of stamens and nectaries 2) change in color of sepals from creamy white to green and 3) an increase in the dimension of the pistils. The layer by layer abscission of stamens is due to the forces exerted by the developing pistils on them. During the present investigation it was observed that creamy white sepals turn greenish, the flowers become saucer shaped and the peduncle also elongates with the sharp increase in dimensions of pistils. In Helleborus niger¸ it has been shown that the removal of the pistils at the bud stage prevents (a) the development of functional chloroplasts in the perianth, (b) flower opening and (c) elongation of the peduncle (floral stem) suggesting that these morphological changes are to be triggered and/or maintained by signals originating in the pistil (Salopek-Sondi et al. 2002). The shape of flowers (Saucer shape) as well as elongation of peduncle has physiological significance for the developing pistils. The saucer shaped flowers with photosynthetically competent sepals behaves just like an antennae to trap maximum solar radiations so as to carry out photosynthesis for the developing fruits. The sepals remain photosynthetically active as long as the fruits develop and the seeds are set. It is suggestive of the fact that the sepals photosynthesize and translocate the manufactured organic materials to the developing fruits i.e. the developing pistil becomes a strong sink during senescence. It is supported by the observation that the fresh and dry mass of individual sepals decrease during senescence despite being photosynthetic while that of the pistils register a sharp increase as the development progressed through stages V and VI.
The fresh and dry mass, water content and soluble carbohydrates of flowers as well as individual sepals showed an increase in the sepal tissues during the process of floral development from bud to fully open bloom after which a declining trend was found during senescence. The decrease in fresh and dry mass of flowers was found to be partly due to abscission of stamens and nectaries and in part due to decrease in fresh and dry mass of individual sepals. It has been shown that flowers (sepal/petal tissues) switch from being a sink to a source during senescence and the changes such as decline of fresh mass, dry mass and soluble carbohydrates are often linked to PCD (Zhou et al. 2005). Flower maturation and senescence has been found to be accompanied by a decline in total soluble carbohydrate content in various flowers such as carnations, Hemerocallis, Iris and rose (Nichols 1973; Paulin and Jamain 1982; Lukaszewski and Reid 1989; Lay-yee et al. 1992; Beileski 1993; Mwangi et al. 2003; Gulzar et al. 2005; Reid 2005). It has been suggested that sugar metabolism is active in senescent cells as many carbon skeletons that are remobilized from macromolecules are transported out of the petal, mainly as sucrose (van Doorn and Woltering 2008).
The sepal senescence of Helleborus orientalis exhibited two stages of ion leakage. A dramatic increase in ion leakage was observed as the senescence progressed to stages V and VI. A consistent feature of senescence is the loss of differential permeability of cell membranes leading to the loss of ionic gradients and pumps. Membrane permeability has been shown to increase with age in various flowers such as rose, Hemerocallis, carnations, Iris and Petunia. (Borochov and Woodson 1989; Stead and van Doorn 1994; Celikel and van Doorn 1995; van Doorn 2004; Gulzar et al. 2005). It has been suggested that leakage may rather be an indicator of cell death and its increase is a measure of dead cells (van Doorn and Woltering 2008). We observed that there was a loss of proteins when the flower senesced. The amino acid content also decreased during the final stage of senescence. It has been shown in some flowers e.g. Hemerocallis that after the flower opens it abruptly changes from sink to source for transporting breakdown products of proteins (Beileski 1995). An overall decrease in cell protein levels has been reported in various ethylene sensitive and insensitive flower senescence (van Doorn and Stead 1994; van Doorn and Woltering 2008). An apparent decrease in the amino acid content during final stages of senescence as observed by us may be due to rapid export of amino acids to the developing pistil.
During the current study it was observed that there was a pronounced increase in the protease activity as the flowers progressed towards senescence. The activity was commensurate with a drastic decrease in soluble proteins. The expression of transcripts encoding proteases is one of the earliest senescence-related gene changes (Eason et al. 2002). A marked increase in the protease activity during petal senescence has also been reported in flowers such as Hemerocallis, Iris and Petunia (Stephenson and Rubinstein 1998; Pak and van Doorn 2005; Jones et al. 2005). The present study suggests that although total protein levels may decline quickly after flower opening, SDS-PAGE revealed a decrease in higher molecular weight proteins while the content of low molecular weight proteins increased. This corroborates the similar finding on flowers such as Hibiscus and Hemerocallis (Woodson and Handa 1987; Courtney et al. 1994). At this stage we do not know whether the polypeptides that increased during senescence having a molecular weight of approx. 15.8 and 29.5 kDa play an important role in the senescence of Helleborus orientalis flowers.
Acknowledgement
The authors thank the Head, Department of Botany for providing facilities and Dr. A.Q. John (Professor Emeritus, SKUAST, Kashmir) for cultivar identification. Waseem Shahri thanks University Grants Commission (India) for providing a Junior Research Fellowship. The authors are extremely indebted to Dr. W.G. van Doorn (Mann Laboratory, University of California, Davis) for critically going through the manuscript and giving valuable suggestions.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JC, Struhl K. Current protocols in molecular biology. New York: John Wiley and Sons; 1989. [Google Scholar]
- Beileski RL. Fructan hydrolysis drives petal expansion in the ephemeral daylily flower. Plant Physiol. 1993;103:213–219. doi: 10.1104/pp.103.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beileski RL. Onset of phloem export from senescent petals of daylily. Plant Physiol. 1995;109:557–565. doi: 10.1104/pp.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A, Woodson WR. Physiology and biochemistry of flower petal senescence. Hort Rev. 1989;11:15–43. [Google Scholar]
- Celikel FG, van Doorn WG. Solute leakage, lipid peroxidation and protein degradation during senescence of Iris tepals. Physiol Plant. 1995;94:515–521. doi: 10.1111/j.1399-3054.1995.tb00962.x. [DOI] [Google Scholar]
- Courtney SE, Rider CC, Stead AD. Changes in protein ubiquitination and the expression of ubiquitin-encoding transcripts in daylily petal during floral development and senescence. Physiol Plant. 1994;9:196–204. doi: 10.1111/j.1399-3054.1994.tb00419.x. [DOI] [Google Scholar]
- Damboldt J, Zimmerman W. Helleborus. In: Hegi G, editor. Illustrierte Flora von Mittel-Europa. Munchen: Carl Hanser-Verlag; 1965. pp. 91–107. [Google Scholar]
- Eason JR, Ryan DJ, Pinkney TT, O’Donoghue EM. Programmed cell death during flower senescence: Isolation and characterization of cysteine proteinases from Sandersonia aurantiaca. Funct Plant Biol. 2002;29:1055–1064. doi: 10.1071/PP01174. [DOI] [PubMed] [Google Scholar]
- Eason JR. Molecular and genetic aspects of flower senescence. Steward Postharvest Rev. 2006;2:1–7. [Google Scholar]
- Gulzar S, Amin I, Tahir I, Farooq S, Sultan SM. Effect of cytokinins on the senescence and longevity of isolated flowers of daylily (Hemerocallis fulva) cv. Royal crown sprayed with cycloheximide. Acta Hort. 2005;669:395–403. [Google Scholar]
- Halevy AH, Mayak S. Senescence and postharvest physiology of cut flowers, Part 1. Hort Rev. 1979;1:204–236. [Google Scholar]
- Hoeberichts FA, de Jong AJ, Woltering EJ. Apoptotic like cell death marks the early stages of gypsophila (Gypsophila paniculata) petal senescence. Postharvest Biol Technol. 2005;35:229–236. doi: 10.1016/j.postharvbio.2004.10.005. [DOI] [Google Scholar]
- Jones ML, Chaffin GS, Eason JR, Clark DG. Ethylene sensitivity regulates Proteolytic activity and cysteine protease gene expression in petunia corollas. J Exp Bot. 2005;56:2733–2744. doi: 10.1093/jxb/eri266. [DOI] [PubMed] [Google Scholar]
- Lay-Yee M, Stead AD, Reid MS. Flower senescence in daylily (Hemerocallis) Physiol Plant. 1992;86:308–314. doi: 10.1034/j.1399-3054.1992.860218.x. [DOI] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lukaszewski TA, Reid MS. Bulb type flower senescence. Acta Hort. 1989;261:59–62. [Google Scholar]
- Mwangi M, Chatterjee SR, Bhattacharjee SK. Changes in the biochemical constituents of “Golden gate” cut rose petals as affected by precooling with ice cold water spray, pulsing and packaging. J Plant Biol. 2003;30:95–97. [Google Scholar]
- Nelson N. Photometric adaptation of Smogy’s method for determination of glucose. J Biol Chem. 1944;153:375. [Google Scholar]
- Nichols R. Senescence of cut carnation flower: respiration and sugar status. J Hort Sci. 1973;48:111–121. [Google Scholar]
- Pak C, van Doorn WG. Delay of Iris flower senescence by protease inhibitors. New Phytol. 2005;165:473–480. doi: 10.1111/j.1469-8137.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- Paulin A, Jamain C. Development of flowers and changes in various sugars during opening of cut carnations. J Amer Soc Hort Sci. 1982;107:258–261. [Google Scholar]
- Perry M, Perry C (2000) Helleborus. In: Kyle C (ed) Fantastic flowers. London, pp 88–91
- Price AM, DF Aros orellana, Salleh FM, Stevens R, Acock R, Buchanan-Wollaston V, Stead AD, Rogers HJ. A comparison of leaf and petal senescence in wall flower reveals common and distinct patterns of gene expression and physiology. Plant Physiol. 2008;147:1898–1912. doi: 10.1104/pp.108.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS. Flower development: from bud to bloom. Acta Hort. 2005;669:105–107. [Google Scholar]
- Rogers HJ. Programmed cell death in floral organs: how and why do flowers die? Ann Bot. 2006;97:309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. A modified ninhydrin colorimetric method for amino acids. Arch Biochem Biophys. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Rubinstein B. Regulation of cell death in flower petals. Plant Mol Biol. 2000;44:303–318. doi: 10.1023/A:1026540524990. [DOI] [PubMed] [Google Scholar]
- Salopek-sondi B, Kova M, Prebeg T, Magnus V. Developing fruit direct post-floral morphogenesis in Helleborus niger L. J Exp Bot. 2002;53:1949–1957. doi: 10.1093/jxb/erf047. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Yamada T, Suzuki T, Shimizu K, Ichimura K. InPSR26, a putative membrane protein, regulates programmed cell death during petal senescence in Japanese morning glory. Plant Physiol. 2009;149:816–824. doi: 10.1104/pp.108.127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead AD, van Doorn WG. Strategies of flower senescence—a review. In: Scott RJ, Stead AD, editors. Molecular and cellular aspects of plant reproduction. Cambridge: Cambridge University Press; 1994. pp. 215–238. [Google Scholar]
- Stephenson P, Rubinstein B. Characterization of proteolytic activity during senescence in daylilies. Physiol Plant. 1998;104:463–473. doi: 10.1034/j.1399-3054.1998.1040323.x. [DOI] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica L. The quantitative analysis of phenolic constituents. J Fd Sci Agri. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tayyab J, Qamar S. A look into enzyme kinetics: some introductory experiments. Biochem Edu. 1992;20:116–118. doi: 10.1016/0307-4412(92)90121-2. [DOI] [Google Scholar]
- van der Kop DAM, Ruys G, Dees D, van der Schoot C, de Boer AD, van Doorn WG. Expression of defender against apoptotic death (DAD-1) in Iris and Dianthus petals. Physiol Plant. 2003;117:256–263. doi: 10.1034/j.1399-3054.2003.1170213.x. [DOI] [Google Scholar]
- van Doorn WG. Is petal senescence due to sugar starvation? Plant Physiol. 2004;134:35–42. doi: 10.1104/pp.103.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Stead AD. The physiology of petal senescence which is not initiated by ethylene. In: Scott RJ, Stead AD, editors. Molecular and cellular aspects of plant reproduction. Cambridge: Cambridge University Press; 1994. pp. 215–238. [Google Scholar]
- van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence. J Exp Bot. 2008;59:453–480. doi: 10.1093/jxb/erm356. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Leverentz MK, Griffiths G, Thomas B, Chanasut U, Stead AD, Rogers HJ. Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J Exp Bot. 2002;53:233–240. doi: 10.1093/jexbot/53.367.233. [DOI] [PubMed] [Google Scholar]
- Woltering EJ, de Jong A, Hoeberichts FA, Iakimova E, Kapchina V. Plant programmed cell death, ethylene and flower senescence. Acta Hort. 2005;669:159–169. [Google Scholar]
- Woodson WR, Handa AK. Changes in protein patterns and in vivo protein synthesis during presenescence and senescence of Hibiscus petals. J Plant Physiol. 1987;128:67–75. [Google Scholar]
- Xu Y, Hanson MR. Programmed cell death during pollination-induced petal senescence in Petunia. Plant Physiol. 2000;122:1323–1333. doi: 10.1104/pp.122.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Takatsu Y, Kasumi M, Manabe T, Hyashi M, Marubashi W, Niwa M. Novel evaluation method of flower senescence in Freesia based on apoptosis as an indicator. Plant Biotechnol. 2001;18:215–218. doi: 10.5511/plantbiotechnology.18.215. [DOI] [Google Scholar]
- Zhou Y, Wang C, Hong GE, Hoeberichts FA, Visser PB. Programmed cell death in relation to petal senescence in ornamental plants. J Integ Plant Biol. 2005;47:641–650. doi: 10.1111/j.1744-7909.2005.00112.x. [DOI] [Google Scholar]