Abstract
Ficus religiosa (Pipal) is a long-lived valuable multipurpose forest tree. The tree is exploited because of its religious, ornamental and medicinal value and the regeneration rate in natural habitat is low. An in vitro propagation protocol has been developed from nodal segments obtained from a 45–50-year old tree. The highest bud break frequency (100 %) followed by maximum number of multiple shoots (13.9) as well as length (2.47 cm) were obtained on Woody Plant medium (WPM) supplemented with 1.0 mg/l BAP along with 0.5 mg/l IAA. Two modifications in this medium resulted in enhanced shoot regeneration-one with 200 mg/l glutamine + 150 mg/l ADS (called as MM-1) giving 32.5 shoots per nodal explant while another modification—with 200 mg/l glutamine + 150 mg/l ADS + 100 mg/l phloroglucinol (called as MM-2) giving 35.65 shoots per explant. These two media were used for sub-culturing of shoots for 4 months. The rate of shoot multiplication was same during the first three sub-cultures on MM-1 and the shoots regenerated were healthy, afterwards shoot multiplication declined. While on MM-2, shoot multiplication declined after first sub-culture and shoots underwent the problem of early leaf fall. Rooting was best induced in micro-shoots excised from proliferated shoot cultures on semi-solid as well as liquid WPM modified with 2.0 mg/l IBA and 0.5 mg/l IAA. The in vitro-raised plantlets were potted and acclimatized under culture room conditions for 25–30 days before transfer to soil conditions, where the established plants showed more than 90 % survival.
Keywords: Ficus religiosa, Pipal, Bodhi tree, Bud-break, Multiple shoots, In vitro plant regeneration, Enhanced shoot multiplication, Micropropagation
Introduction
The genus Ficus (family Moraceae) comprises of more than 1,000 species, occurring in tropical and sub tropical regions (Bailey and Bailey 1976). Ficus species are monoecious or functionally dioecious trees, shrubs, twiners and hemi-epiphytes, often with adventitious roots (Nasir and Ali 1974). Ficus religiosa L. (commonly known as Pipal, Bodhi-tree) is a long-lived, large, evergreen, ornamental and fuel wood tree, native to India and is widely found wild or cultivated throughout India, Pakistan, Bangladesh, Ceylon, China, Burma and Thailand. The tree attains large dimensions and has long life span. It is heavily branched with long petiolated, long tipped, leathery, heart-shaped leaves and is very popular as shade tree. It is commonly planted as an avenue or roadside tree and most frequently near temples.
Ficus religiosa has great mythological, religious and medicinal importance in the culture of India and Nepal since times immemorial (Prasad et al. 2006). People worship it by tying threads of white, red and yellow silk around it and the sacred value of the tree can be co-related to the great medicinal applications of it which are mentioned in Ayurveda, the indigenous Indian medicine system (Prasad et al. 2006). Various preparations from Ficus religiosa has been documented for their important medicinal applications like in the treatment of heart disease, diabetes, asthma, inflammations and glandular swelling, wound healing, stomatitis, skin diseases and women genital disorders (Shah 1982; Singh et al. 2002; Ananda and Kunjani 2000; Chandrasekar et al. 2010). Some applications have been confirmed by various scientific studies (Swami and Bisht 1996; Kirana et al. 2009; Mallurvar and Pathak 2008; Damanpreet and Rajesh 2009; Panit et al. 2010). It is also reported to exhibit acetycholinesterase inhibitory activity (Vinutha et al. 2007) which is an effective strategy for the treatment of Alzheimer’s disease. Many secondary metabolites viz. bergenin, lupin 3-one, β-sitosterol, lanosterol, n-octacosanol, stigmasterol, phytosterols, caffeic acid (hydrocarbon), amides, flavanoids, tannins, furanocoumarin derivatives namely bergapten and bergapton have been isolated from different parts of the tree (Kirana et al. 2009), while many more may still be unidentified. Various parts of the tree are used in various ayurvedic commercial preparations like Panchavalkal extract (Padmavati pharmaceuticals, http://www.padmavatiherbal.com/leucowincapsule02.htm), Lakshadi guggal (Baidyanath pharmaceuticals http://www.madanapalas.com/lakshadi-guggul-p-533.html), Leucowin capsules (Padmavati pharmaceuticals http://www.padmavatiherbal.com/leucowincapsule.htm) etc.
Conventionally, the tree is propagated by seeds, which remain viable for a few months. Also the vegetative propagation by cutting is not efficient under varied climatic conditions. Because of its medicinal importance and potential, there is a need to carry out rapid, mass propagation of the species. The in vitro culture techniques can be the alternative for the continuous provisions of the woody plantlet stocks for large scale field cultivation (Ahmad and Anis 2007; Beruto et al. 2004; Husain et al. 2008).
So far, only three studies on in vitro propagation of Ficus religiosa L. are available (Jaiswal and Narayan 1985; Deshpande et al. 1998 and Hassan et al. 2009). Jaiswal and Narayan (1985) have reported shoot regeneration via indirect organogenesis through callus phase, maximum number of 6–10 shoots being obtained from a callus piece. Micropropagation via callus phase has the chances of somaclonal variations and so cannot be said as clonal propagation. Direct shoot regeneration in Ficus religiosa L. has been reported by Deshpande et al. (1998) and in their study, maximum of 5–6 shoots were obtained from a single explant which may be considered as low regeneration rate for a commercially efficient and economically viable micropropagation system. Further no observation has been made for multiplying the shoots after repeated sub-culturing, no mention of any observation/problem common to tissue culture of higher age woody perennials, has been made. Hassan et al. (2009) have reported a comparative high rate of multiplication but still the study lacks the observations regarding the shoot multiplication as well as health of the shoots during continuous sub-culturing process. So there is a need to further modify the micropropagation protocol for Ficus religiosa which can fulfil the large scale commercial demand of planting material of this tree.
Several factors influence the efficiency of in vitro regeneration, such as basal medium, growth regulators, type of additives, age of explants, age of culture, photoperiod, to quote a few. Woody plant medium (Lloyd and McCown 1980) has been reported as more suitable media for in vitro regeneration study of woody tree species (Feng et al. 2010). Thidiazuron (TDZ), a synthetic phenylurea derivative (N-phenyl-1,2,3-thidiazol-5-yl urea), is among the most active cytokinin like substance for woody plant tissue culture (Huetteman and Preece 1993). It is more effective than adenine based compounds for inducing axillary shoot formation in many woody species (Thimmappaiah and Sadhana 2002; Ahmad and Anis 2007). The nature of nitrogen source significantly affects the in vitro response of the explants (Green et al. 1990). Many reports specify that organic nitrogen source like amino acids instead of inorganic source like nitrates or ammonium sulphates; can improve the cell proliferation as well as regeneration in specific genotypes (Vasudevan et al. 2004). Phloroglucinol (1,3,5-trihydroxybenzene; PG) is a phenolic compound and is reported to promote the shoot proliferation in many plant species like Vitex negundo L. (Steephen et al. 2010), Minuartia valentine (Ibanez and Amo-marco 1998). Adenine, in various forms enhances the growth of isolated meristem tips, induces the proliferation of axillary shoots in shoot cultures and promotes the adventitious shoot formation indirectly from calli or directly from the explants (Van Stedan et al. 2008). In the present paper, we report the accomplishment of a highly efficient micropropagation system for Ficus religiosa L. with very high shoot regeneration rate by using various concentrations and combinations of ADS (50–200 mg/l), glutamine (50–400 mg/l) and phloroglucinol in woody plant medium supplemented with various concentration and combinations of different PGRs.
Materials and methods
Plant materials
Nodal region, bearing single axillary buds were excised from a 45–50 year old mature Ficus religiosa tree, growing near the campus area of Chaudhary Devi Lal University, Sirsa, Haryana, India, during May to September, 2008. The healthy and juvenile multi nodal shoots were defoliated after bringing to the laboratory and were kept for 30 min under running tap water in order to remove dust and dirt. These were cut down into single node state (3–4 cm), and were swabbed with an alcohol (50 %, v/v) absorbed muslin cloth followed by washing with 2–3 drops of tween-20 (1 %, v/v) for 10 min. Then rinsing of the explants was carried out three times with sterile distilled water. Thereafter, in aseptic conditions, disinfection was done with bavistin (0.1 %, w/v) for 7 min followed by with mercuric chloride (0.1 %, w/v) solution for 8 min. After each surface sterilization treatment, explants were thoroughly rinsed 5 times with sterilized distilled water. The explants were dried by blotting on sterile filter paper before trimming to a final size of 1–2 cm.
Effect of different PGRs on shoot organogenesis and proliferation
The nodal segments (1–2 cm) were inoculated on basal medium (WPM) supplemented with 1.0 mg/l of each viz. BAP, 2-ip, TDZ, Zeatin, IAA and NAA, individually, for observing the type of morphogenic response (Table 1). The response was observed after 3 weeks of culture. Based on the observations, growth regulators inducing the efficient shoot bud formation were selected and used, in different concentrations as well as in combinations with IAA, for the shoot organogenesis from nodal segments (Table 2). The number of explants initiating shoot buds (per cent bud break) and the average number of shoot buds per explant was recorded after 21 days of culture. The explants with sprouted shoot buds/shoots were shifted to the same medium as such within an interval of 30 days so as to score the number of shoots and the length of shoots after 60 days of culture (Table 2).
Table 1.
Effect of various plant growth regulators supplemented to woody plant medium (WPM) on shoot organogenesis from nodal segments, after 3 weeks of culture
| PGR (mg/l) | Nature of response |
|---|---|
| 0 | No response, explants died |
| 1.0 BAP | Shoot bud induction, No callus |
| 1.0 TDZ | Shoot bud induction along with callus formation at the base of the explant |
| 1.0 2-ip | Bud break in some explants, yellowing of explant, necrosis |
| 1.0 ZEA | Induction of shoot buds in some explants, wrinkled appearance |
| 1.0 IAA | Callus formation in some explants at the base, shoot bud like protuberances at the surface of some explants |
| 1.0 NAA | Initiation of callus formation, unhealthy appearance |
Table 2.
Effect of different concentrations of cytokinins, benzylaminopurine (BAP) and thidiazuron (TDZ), supplemented individually and in combination with indole acetic acid (IAA) to woody plant basal medium, on per cent response and shoot organogenesis
| PGR | Per cent bud break (after 21 days) | Number of shoot buds per explants (after 21 days) | Number of shoots per explant (after 60 days) | Length of the shoots (after 60 days) | Comments | ||
|---|---|---|---|---|---|---|---|
| BAP | TDZ | IAA | |||||
| 0.5 | – | – | 90 ± 6.88ab | 2.15 ± 0.109de | 2.9 ± 0.32d | 2.38 ± 0.12a | y |
| 1.0 | – | – | 100 ± 0a | 3.25 ± 0.239d | 4.85 ± 0.49c | 2.42 ± 0.13a | y |
| 1.5 | – | – | 95 ± 5ab | 1.3 ± 0.105e | 3.65 ± 0.26cd | 1.98 ± 0.126b | y |
| – | 0.5 | – | 90 ± 6.88ab | 11.85 ± 0.36a | 4.15 ± 0.39cd | 1.53 ± 0.121c | x |
| – | 1.0 | – | 80 ± 9.17ab | 11.2 ± 0.66ab | 1.2 ± 0.09ef | 1.043 ± 0.059d | x |
| – | 1.5 | – | 85 ± 8.19ab | 9.9 ± 0.42b | 0.35 ± 0.109f | 0.72 ± 0.066e | x |
| 1.0 | – | 0.5 | 100 ± 0a | 6.4 ± 0.49c | 13.9 ± 0.68a | 2.47 ± 0.072a | y |
| 1.0 | – | 1.0 | 95 ± 5ab | 5.35 ± 0.109c | 10.35 ± 0.74b | 2.306 ± 0.191a | y |
| – | 0.5 | 0.5 | 90 ± 6.88ab | 11.65 ± 0.949a | 3.2 ± 0.46d | 1.245 ± 0.102cd | x |
| – | 0.5 | 1.0 | 75 ± 9.93b | 10.4 ± 0.81ab | 1.65 ± 0.209e | 0.576 ± 0.04e | x |
Data are means from 20 replicates ± SE and those representing similar letter in the appropriate column are not significantly different (ANOVA, P ≤ 0.05)
xThick, hyperhydric and stunted shoots
ySlender shoots with small diameter and exhibited pre-mature leaf fall
For each of the above treatment 20 explants were used and the experiment was repeated thrice.
Influence of different additives on shoot multiplication
The nodal segments with induced shoot buds on WPM supplemented with 1.0 mg/l BAP and 0.5 mg/l IAA (the medium yielding maximum number of healthy looking shoot buds), were taken out from this medium after 21 days. These were then used as the explants to study the influence of different additives (glutamine, ADS and phloroglucinol) on shoot multiplication. The woody plant medium supplemented with 1.0 mg/l BAP along with 0.5 mg/l IAA was further modified with different concentrations and combinations of ADS (50–200 mg/l), glutamine (50–400 mg/l) and phloroglucinol (50–200 mg/l) for evaluating the effect of these additives on shoot proliferation and multiplication (Table 3). The number of shoots per explant and the length of the shoots were recorded after 45 days of culturing. Every treatment contained 20 replicates and the experiment was repeated thrice
Table 3.
Effect of glutamine, ADS and phlouroglucinol, supplemented in different concentrations and combinations to WPM having 1.0 BAP along with 0.5 mg/l IAA on shoot multiplication, as observed after 45 days of culture
| Glutamine | ADS | PG | Number of shoots | Length of shoots (cm) | Comments |
|---|---|---|---|---|---|
| – | – | – | 13.75 ± 0.421h | 2.46 ± 0.07def | −a |
| 50 | – | – | 12.89 ± 0.272hi | 2.365 ± 0.11f | − |
| 100 | – | – | 13.58 ± 0.27h | 2.385 ± 0.13ef | + |
| 200 | – | – | 12.5 ± 0.29i | 2.63 ± 0.067cdef | +++ |
| 400 | – | – | 13.39 ± 0.31hi | 2.607 ± 0.13cdef | − |
| 200 | 50 | – | 17.7 ± 0.48e | 2.58 ± 0.07cdef | ++ |
| 200 | 100 | – | 21.72 ± 0.37c | 2.8 ± 0.129cd | ++ |
| 200 | 150 | – | 32.5 ± 0.35b | 3.3 ± 0.11b | +++ |
| 200 | 200 | – | 13.33 ± 0.20hi | 2.67 ± 0.108cdef | ++ |
| 200 | – | 50 | 16.67 ± 0.24f | 2.7 ± 0.10cde | ++ |
| 200 | – | 100 | 19.29 ± 0.16d | 3.3 ± 0.14b | ++ |
| 200 | – | 200 | 15.05 ± 0.30g | 2.87 ± 0.107c | ++ |
| 200 | 150 | 50 | 21.6 ± 0.365c | 2.71 ± 0.07cde | +++ |
| 200 | 150 | 100 | 35.65 ± 0.27a | 3.74 ± 0.04a | ++++ |
Values represent means ± SE of 20 replicates, those representing similar letter in the appropriate column are not significantly different (ANOVA, P < 0.05)
aControl, from Table 2
− Leaf fall
+ Leaf fall at low frequency
++ No leaf fall
+++ No leaf fall, healthy shoots with increased girth
++++ No leaf fall, increased vigour of shoots
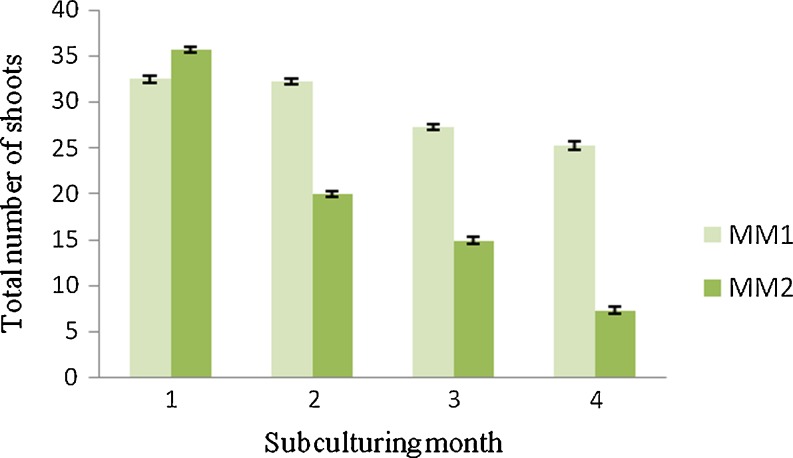
The shoots so multiplied were separated into clumps of 3–4 shoots after 45 days and sub-cultured on two medium (giving maximum number of shoots, as observed from Table 3). This process was repeated for another 4 subcultures to study the effect of subculture and media components on shoot multiplication (Fig. 5). Every treatment contained 20 replicates and the experiment was repeated thrice.
Fig. 5.
Effect of different media and sub-culture on shoot multiplication
Shoot elongation and rooting
Individual shoots, grown to 2–3 cm long, were separated from the multiple shoots clump and transferred to elongation medium (WPM supplemented with 0.5 mg/l BAP, 0.5 mg/l IAA and 0.5 mg/l GA3) with (200 mg/l) and without glutamine. After 4 weeks, elongated shoots were transferred to liquid as well as semi-solid rooting medium, supplemented with different concentrations and combinations of IAA, NAA and IBA (Table 4). After 5 weeks percentage of root induction, number of roots per shoot and the roots lengths were recorded. In each treatment, 20 shoots were used and the experiment was repeated thrice.
Table 4.
Effect of different concentrations and combinations of NAA, IBA and IAA on rooting frequency, mean root number and length (cm) of roots, after 4 weeks of culture
| NAA | IBA | IAA | Rooting (%) | Mean number of roots per shoot | Longest root length (cm) |
|---|---|---|---|---|---|
| Control | - | – | 00.00 ± 00.00d | 0 ± 0f | 0 ± 0f |
| 0.1 | 1.0 | – | 60.00 ± 11.23c | 1.4 ± 0.169e | 3.51 ± 0.11d |
| 0.5 | 1.0 | – | 65.00 ± 10.94bc | 3.25 ± 0.09d | 2.88 ± 0.13e |
| – | 1.0 | 0.1 | 80.00 ± 09.17abc | 2.8 ± 0.329d | 4.05 ± 0.17c |
| – | 1.0 | 0.5 | 75.00 ± 09.93abc | 4.25 ± 0.123c | 4.08 ± 0.07bc |
| 0.1 | 2.0 | – | 90.00 ± 06.88ab | 3.3 ± 0.105d | 3.67 ± 0.17d |
| 0.5 | 2.0 | – | 85.00 ± 08.19abc | 5.35 ± 0.109b | 4.67 ± 0.14a |
| – | 2.0 | 0.1 | 90.00 ± 06.88ab | 4.65 ± 0.27c | 4.235 ± 0.073bc |
| – | 2.0 | 0.5 | 95.00 ± 05.00a | 6.4 ± 0.11a | 4.405 ± 0.057ab |
Values represent means ± SE of 20 replicates, those representing similar letter in the appropriate column are not significantly different (ANOVA, P < 0.05)
Culture medium and culture conditions
The woody plant medium (WPM) (Lloyd and McCown 1980), supplemented with 3 % (w/v) sucrose and solidified with 0.8 % (w/v) agar, was used as basal medium throughout the study. The basal medium was modified with different concentrations and combinations of various growth regulators and additives, as per different requirements as reported above, prior to pH adjustment to 5.8 using 0.1 N NaOH or 0.1 N HCl. The culture vessels containing the media were autoclaved at 15 lb and 121 °C for 20 min. Cultures were maintained at 25 °C ± 2 °C, at a photoperiod of 16 h (80 μmol m−2 s−1) for 4 weeks.
Acclimatization and transplantation
Plantlets with well developed roots were removed from culture, washed thoroughly with sterile water and transferred to potting mixture comprising autoclaved garden soil, sand and vermiculite mixture (3:1:1). The plantlets were covered with transparent plastic to maintain the high humidity and were watered once a day and kept in culture room. The plastic sheets were removed periodically. After 25–30 days, the plantlets were shifted to the soil.
Statistical analysis
The experiments were set up in completely randomized design (CRD) and repeated thrice. The data were analyzed by Analysis of Variance (ANOVA) followed by duncan’s multiple range test. Data analysis was carried out by using SPSS version 13.
Results
Shoot organogenesis and proliferation
In the preliminary experiment affectivity of basal medium and different PGRs were tested for the shoot bud induction in nodal segments (Table 1). The explants failed to respond on PGR-free basal medium; the cut ends, initially, and the whole explant subsequently gets blackened due to phenolic exudation leading to death of the explants within 3 weeks of culture. When the medium was supplemented with 1.0 mg/l of different cytokinins like BAP, TDZ, 2-ip and zeatin, the nodal segments responded to shoot bud formation; however, the frequency of shoot bud induction was very low in case of 2-ip and zeatin (data not shown) and also the explant exhibited the signs of stress like yellowing of colour, wrinkled surface and finally necrosis. TDZ and BAP were observed as suitable cytokinins for inducing many shoot buds per explant, with and without callus respectively. The two auxins viz. IAA and NAA induced callus with different frequency and appearance. This preliminary examination exhibited the effectiveness of BAP and TDZ for direct shoot regeneration and so different concentrations of these two hormones alone as well as in combination with IAA was used for further shoot organogenesis study (Table 2).
Of different concentration of BAP and TDZ (0.5–1.5 mg/l), supplemented individually to the medium, more than 90 % explant responded to shoot bud formation on BAP while response varied from 80 % to 90 % on TDZ. The BAP at 1.0 mg/l, of the three concentrations tested, induced maximum number of shoot buds (3.25) (Fig. 1a) as observed after 21 days of culturing and yielded maximum number of healthy, proliferating shoots (4.85) after 60 days of culturing. On the other hand, TDZ, at all the three concentrations, proved optimal for shoot bud induction, yielding nearly 10–12 shoot buds per explant after 21 days of culture (Fig. 1b). However these shoot buds proliferated poorly resulting in maximum of 4.15 mean number of shoots, after 60 days of culturing, on lowest concentration of TDZ; higher concentration showing a negative effect on shoot proliferation.
Fig. 1.
Bud break from nodal explants as observed on 21st day of culturing on medium containing—a 1.0 mg/l BAP, b 1.0 mg/l TDZ
To study the synergistic effect (if any) of auxins and cytokinins, the optimal concentration of BAP (1.0 mg/l) and TDZ (0.5 mg/l) was tested with different concentrations of IAA (0.5 and 1.0 mg/l) for shoot organogenesis and proliferation from nodal segments (Table 2). Of the various combinations tried, 1.0 mg/l BAP in conjunction with 0.5 mg/l IAA proved to be the best shoot proliferating medium, giving 13.9 mean numbers of shoots after 60 days of culture, and so was selected as the optimal shoot multiplication medium (SM) for the further study (Fig. 2). In fact, addition of 0.5 mg/l IAA to 1.0 mg/l BAP supplemented media has resulted in nearly threefold increase in the shoot proliferation as compared to that on 1.0 mg/l BAP alone, while to TDZ supplemented medium it further lowered the shoot proliferation (Table 2).
Fig. 2.

Shoot proliferation from nodal segments on Shoot multiplication (SM) medium (WPM supplemented with 1.0 mg/l BAP and 0.5 mg/l IAA), as observed on 60th day of culturing
The shoots obtained on TDZ supplemented medium were thick, hyperhydric and stunted in appearance. On the other hand shoots obtained on BAP supplemented medium, at the end of 2 months culture period, exhibited early leaf fall.
Influence of different additives on shoot multiplication
In order to prevent the premature leaf fall and to further enhance the shoot multiplication, different additives viz. adenine sulphate, glutamine and phloroglucinol were supplemented, in different concentration and combinations, to the SM medium (WPM with 1.0 mg/l BAP and 0.5 mg/l IAA, the optimal medium for shoot proliferation as standardized above, Table 2) (Table 3). The inclusion of glutamine to the shoot multiplication medium exhibited no significant effect on shoot multiplication; however, a significant influence on premature leaf fall was observed. Glutamine, at a concentration of 200 mg/l, not only prevented the early leaf fall but also increased the stem girth; making the shoots healthier but no significant effect was observed in shoot length. The higher concentration, 400 mg/l, was found inhibitory as it caused enhanced leaf fall and yellowing of leaf colour.
Retaining the 200 mg/l of glutamine in the shoot multiplication medium, adenine sulphate (ADS) was added in various concentrations (50–200 mg/l) (Table 3). The shoot multiplication was found to increase significantly with increase in concentration of ADS from 50 mg/l to 150 mg/l; maximum number (32) of shoots being obtained on 150 mg/l ADS (Fig. 3). In fact the supplementation of 200 mg/l glutamine in combination with 150 mg/l ADS to the SM medium not only enhanced the shoot multiplication by 2.5 times but also yielded longer and healthier shoots. The higher concentration (200 mg/l) was not found suitable for shoot multiplication.
Fig. 3.

Enhanced shoot multiplication from nodal explant on SM medium modified with 200 mg/l glutamine and 150 mg/l ADS), as observed on 45th day of culturing
The inclusion of phloroglucinol (PG), instead of ADS, at different concentrations (50–150 mg/l) to the SM, having 200 mg/l glutamine, increased the shoot multiplication as well as shoot length as compared to that on SM with 200 mg/l glutamine only (Table 3). The most suitable concentration of PG was 100 mg/l, showing nearly 0.5 times increase in the shoot multiplication. This exhibited the less effective role of PG in shoot multiplication as compared to that of ADS, as reported above (Table 3).
An attempt was also made to explore the effects of phloroglucinol, adenine sulphate and glutamine together in the SM medium. The combination of 200 mg/l glutamine, 150 mg/l ADS and 100 mg/l PG exhibited the synergistic effect towards shoot multiplication (giving 35.6 average numbers of shoots). Also shoots obtained exhibited good vigour as well proper leaf expansion and shoot elongation was also observed.
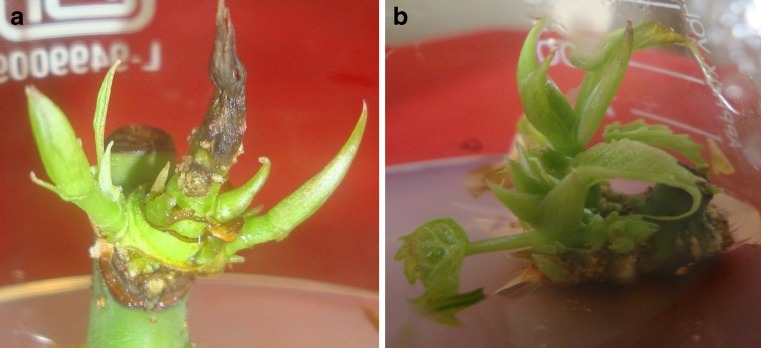
The shoots obtained above were separated into clumps and cultured on fresh medium. Two combinations, one-SM supplemented with 200 mg/l glutamine + 150 mg/l ADS, and another-SM supplemented with 200 mg/l glutamine + 150 mg/l ADS + 100 mg/l PG (as both showed enhanced shoot multiplication, as reported above) (Table 3) were selected for shoot multiplication in subsequent subcultures and were called as maintenance medium-1 (MM-1) and maintenance medium-2 (MM-2), respectively. The number of shoots per clump increased with the original multiplication rate up to 3 subcultures on MM-1 (Fig. 4) and thereafter decrease in multiplication was observed (Fig. 5, bar) along with some occurrence of leaf rolling, leaf tip necrosis as well as yellowing of leaf colour. On the other hand, shoots sub cultured on MM-2, failed to multiply and proliferate properly even after first subculture (Fig. 5, bar) and drastic decrease in shoot length and increase in stress like symptoms were observed after second subculture.
Fig. 4.

Shoot multiplication after 3rd sub-culture on MM-1 (SM modified with 200 mg/l glutamine and 150 mg/l ADS)
Shoot elongation and rooting
The shoots, after third and fourth subculture on MM-1, were shifted to basal medium supplemented with a combination of 0.5 mg/l BAP, 0.5 mg/l IAA and 0.5 mg/l GA3 (elongation medium), with (200 mg/l) and without glutamine. Shoots exhibited a significant increase in length (2–3 cm) on both type of elongation medium after 3–4 weeks of culture. However severe leaf fall and yellowing of leaf and stem was observed on medium lacking glutamine. This shows the necessary requirement of this organic nitrogen source for long term maintained cultures of Ficus religiosa.
Rooting of the in vitro regenerated shoots was not observed on PGRs free medium. So the elongated shoots were transferred to semi-solid rooting medium, supplemented with different combinations of IAA, NAA and IBA, along with 200 mg/l glutamine (to avoid the occurrence of leaf fall) (Table 4). Rooting was induced with different frequencies in different medium. More than 85 % shoots rooted well on 2.0 mg/l IBA in combination with different concentration of NAA and IAA. Maximum frequency of root induction (95 %) was observed on the combination of 2.0 mg/l IBA with 0.1 mg/l IAA. The same medium also resulted in significantly higher mean number of roots per shoot and the roots obtained were also sufficiently long on it, so this medium was considered as optimal rooting medium (RM). The liquid form of this RM was further tested for root induction and the significant difference in the rooting frequency was not observed (Fig. 6).
Fig. 6.

In vitro rooting of micro-shoots on semi-solid and liquid medium supplemented with 2.0 mg/l IBA along with 0.1 mg/l IAA
Acclimatization and transplantation
In vitro formed plantlets were transferred to soil, sand and vermiculite mix (3:1:1) and were maintained in culture room under plastic sheets for 25–30 days (Fig. 7a). Periodical removal of plastic sheets, so as to lower down the high atmospheric humidity gradually, was observed as effective way for the acclimatization process as more than 85 % of the plantlets survived through this. After it, the plantlets were transplanted to soil conditions where more than 90 % plantlets showed the successful establishment (Fig. 7b).
Fig. 7.
a Acclimatized plant after 20 days, b Potted plants after 2 months
Discussions
A highly efficient micropropagation protocol has been developed for 45–50 year old Ficus religiosa L. through nodal segments explants, during this study. Jaiswal and Narayan (1985) reported indirect regeneration of plantlets via callus phase in Ficus religiosa. The direct in vitro shoot regeneration of Ficus religiosa has been carried out earlier by Deshpande et al. (1998), where maximum of 6 shoots per node was obtained, and Hassan et al. (2009) who reported the regeneration of 16–24 shoots per culture. However no mention of any problem, common with the tissue culture of higher age woody plant, like leaf fall and yellowing of leaf colour, has been made in any of the above report. In the present study we were able to induce maximum of 32 shoots per nodal segment, with successful control towards early leaf fall and less vigour of shoots.
In this study, 2-ip and zeatin were ineffective for axillary bud sprouting, while TDZ and BAP promoted shoot organogenesis by bud break. TDZ, at all the three levels, was found even more effective than BAP in initial shoot bud induction, however, TDZ induced buds proliferated poorly into shoots after another 30 days culture on same medium and the shoots obtained were stunted and hyperhydric in appearance. TDZ is a substituted phenyl urea (N-phenyl-1,2,3-thidiazol-5-yl urea) that has immense potential as a cytokinin in shoot organogenesis in a large number of plant systems, especially in the woody species (Ahmad and Anis 2007; Liu and Pijut 2008; Mansouri and Preece 2009). On the other hand, many studies also report the negative impact of TDZ on shoot proliferation, and the shoots proliferated being the deformed ones showing various levels of hyper-hydration, whenever it is used above the threshold level and/or for prolonged cultures (Bosela and Michler 2008; Sivanesan and Jeong 2010; Feng et al. 2010). In our study we observed the negative effects of TDZ after 60 days of culture period, probably due to the supra optimal level of it or more exposure time. Although exact mechanism of action of TDZ is not clear, it is believed to be involved in regulation of endogenous levels of various growth regulators.
The BAP was found as more suitable cytokinin for shoot proliferation in the present study. The edge of BAP over other cytokinins is well documented in many other woody tree species also (Pradhan et al. 1998; Jha et al. 2004; Husain et al. 2008). In our study we found 1.0 mg/l of BAP as the most effective concentration for the shoot bud induction as well as shoot proliferation. Deshpande et al. (1998), however, has reported 5 mg/l BAP as suitable for initial bud break and 1.0 mg/l BAP as for further shoot proliferation of Ficus religiosa, while Hassan et al. (2009) found 0.5 mg/l BAP in combination with 0.1 mg/l IAA as most suitable for shoot multiplication. This difference in observations might be due to the differences in the basal medium, genotype, and physiological state as well as environmental conditions of the mother plant. As compared to BAP alone in the medium, more shoot proliferation (nearly threefold) was observed when it was supplemented with 0.5 mg/l IAA. A similar synergistic effect of cytokinin-auxin combinations towards shoot proliferation has been reported earlier also (Mishra et al. 2008; Negi and Saxena 2010; Hassan et al. 2009).
The shoots obtained on BAP, either alone or in combination with IAA, were thin and exhibited the early leaf fall, during the present investigation. Such reports also exist for some other plant species mentioning the occurrence of early leaf fall (Sanjaya et al. 2005) and low vigour of shoots (Husain et al. 2008) during in vitro shoot multiplication. The early leaf fall could effectively be controlled, throughout this study, by the use of glutamine (200 mg/l). The effectiveness of organic nitrogen source particularly glutamine for multiplication and maintenance of healthy in vitro tissue for long time periods have been reported by many workers (Sanjaya et al. 2005; Green et al. 1990; Ogita et al. 2001; Vasudevan et al. 2004).
Adenine sulphate, supplied in combination with glutamine, enhanced the shoot multiplication by many folds during the present investigation. The promotive role of ADS for shoot multiplication in Ficus religiosa has also been reported by Deshpande et al. (1998) where they found 1.5 mg/l of ADS in combination with 1.5 mg/l of BAP as the most effective level for shoot multiplication (maximum of 3.4 shoots). In our study we tried higher concentrations and observed 150 mg/l of ADS as highly effective for enhancing the shoot multiplication (giving 32 shoots per nodal segment); enhanced vigour of the shoots along with leaf expansion and shoot elongation was also observed. This effect might be the due to the synergistic effect of ADS and glutamine. Similar effects of ADS have been reported in Petrocarpus marsupium also (Husain et al. 2008). Adenine in the form of ADS can stimulate cell growth and shoot multiplication probably by acting as organic nitrogen source and/or acting as pre-cursor for natural cytokinin synthesis. The stimulative role of ADS in shoot multiplication has been emphasized time to time in different woody species like Melia azedarach (Husain and Anis 2004), Bauhinia vahlii (Dhar and Upreti 1999), Petrocarpus marsupium (Husain et al. 2008).
Phloroglucinol, a phenolic compound, is reported to promote the shoot proliferation in many plant species like Vitex negundo L. (Steephen et al. 2010), Minuartia valentine (Ibanez and Amo-marco 1998) while root induction in some others like Prunus avium (Hammatt 1994) and Petrocarpus marsupium (Husain et al. 2008). During the present study, it promoted the shoot multiplication but was found less effective for this as compared to ADS, when used in combination with glutamine. The exact mechanism of PG activity is not clear but probably it affects the endogenous synthesis of auxins and cytokinins.
Gibberellins influence cell division and promote shoot elongation (Davies 2004). Many studies have reported the effective elongation of in vitro shoots by the GA3 in the medium (Anwar et al. 2008). On the other hand, some other studies find transfer of shoots to low PGR (lower to the level on which shoot multiplication achieved) medium or PGR-free medium as a successful way for elongation of shoots (Sivanesan and Jeong 2010). Deshpande et al. (1998) achieved the in vitro shoot elongation of Ficus religiosa on MS basal medium with 2.0 mg/l BAP (lower to the BAP level optimal for shoot multiplication), 0.5 mg/l NAA and 0.3 % (w/v) charcoal. During the present investigation we applied both the above mentioned strategies i.e. addition of gibberellin as well lowering the cytokinin level and successfully achieved the shoot elongation by 2–3 cm. Auxins most frequently incorporated in the medium to induce rooting are IAA, IBA and NAA. Jaiswal and Narayan (1985) reported the root induction along with callus at the base of in vitro shoots of Ficus religiosa at 1.0 mg/l NAA. Deshpande et al. (1998) found 2 mg/l IBA in combination with 0.1 mg/l NAA as the most effective for root induction of in vitro shoots of Ficus religiosa, while Hassan et al. (2009) reported 2.0 mg/l IBA along with 0.1 mg/l NAA as the most suitable for the same. However, during the present investigation we observed the combination of 2.0 mg/l IBA and 0.5 mg/l IAA as the most suitable with respect to high per cent root induction as well as maximum number of roots per shoot. Micropropagated plantlets showed more than 90 % survival in the field after acclimatization in the culture room using plastic sheets cover on pots and grew well.
In conclusion, the present investigation describes a highly efficient regeneration system for Ficus religiosa. The protocol outlined above is a great modification over the earlier reported protocols and has the potential for large scale rapid propagation of this medicinally important tree. This regeneration system would facilitate its use in future tree improvement programme using genetic transformation studies as well as in understanding the molecular mechanism of secondary metabolites synthesis.
Acknowledgements
The award of junior research fellowship to Anita Rani Gill under the ‘Rajiv Gandhi National Fellowship Scheme’ by the ‘University Grants Commission’ New Delhi, India is acknowledged. We also acknowledge the financial assistance provided by Ch. Devi Lal University, Sirsa, Haryana, India, for all the laboratory requirements.
Abbreviations
- WPM
Woody Plant Medium
- BAP
Benzylaminopurine
- TDZ
Thidiazuron
- 2-ip
2-isopentenylphosphate
- NAA
Naphathalene Acetic Acid
- IAA
Indole Acetic Acid
- IBA
Indole Butyric Acid
- ADS
Adenine Sulphate
- PG
Phloroglucinol
- SM
Shoot Multiplication Medium
- MM
Maintenance Medium
- RM
Rooting Medium
References
- Ahmad N, Anis M. Rapid clonal multiplication of a woody tree, Vitex negundo L. through axillary shoots proliferation. Agrofor Syst. 2007;71:195–200. doi: 10.1007/s10457-007-9078-1. [DOI] [Google Scholar]
- Ananda RJ, Kunjani J. Indigenous knowledge and uses of medicinal plants by local communities of the kali Gandaki Watershed Area, Nepal. J Ethnopharmacol. 2000;73:175–183. doi: 10.1016/S0378-8741(00)00301-9. [DOI] [PubMed] [Google Scholar]
- Anwar F, Sharmila P, Saradhi PP. An optimal protocol for in vitro regeneration, efficient rooting and stable transplantation of chickpea. Physiol Mol Biol Plants. 2008;14(4):329–335. doi: 10.1007/s12298-008-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey LH, Bailey EZ. Hortus third—a concise dictionary of plants cultivated in the US and Canada. New York: Macmillan Pub. Co. Inc.; 1976. [Google Scholar]
- Beruto M, Lanteri L, Portogallo C. Micropropagation of tree peony (Paeonia suffruticosa) Plant Cell Tissue Org Cult. 2004;79:249–255. doi: 10.1007/s11240-004-0666-8. [DOI] [Google Scholar]
- Bosela MJ, Michler CH. Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinins types. In Vitro Cell Dev Biol Plant. 2008;44:316–329. doi: 10.1007/s11627-008-9114-5. [DOI] [Google Scholar]
- Chandrasekar SB, Bhanumathy M, Pawar AT, Somasundaram T. Phytopharmocology of Ficus religiosa. Phcog Rev. 2010;4:195–199. doi: 10.4103/0973-7847.70918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damanpreet S, Rajesh KG. Anticonvulsant effect of Ficus religiosa: role of serotonergic pathways. J Ethnopharmacol. 2009;123:330–334. doi: 10.1016/j.jep.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Davies PJ. Plant hormones: biosynthesis, signal transduction, action. Dordrecht: Kluwer; 2004. [Google Scholar]
- Deshpande SR, Josekutty PC, Prathapasenam G. Plant regeneration from axillary buds of a mature tree of Ficus religiosa L. Plant Cell Rep. 1998;17(6–7):571–573. doi: 10.1007/s002990050444. [DOI] [PubMed] [Google Scholar]
- Dhar U, Upreti J. In vitro regeneration of a mature leguminous liana (Bauhinia vahlii) (Wight and Arnott) Plant Cell Rep. 1999;18:664–669. doi: 10.1007/s002990050639. [DOI] [Google Scholar]
- Feng JC, Yu XM, Shang XL, Li JD, Wu YX. Factors influencing efficiency of shoot regeneration in Ziziphus jujube Mill. ‘Huizao’. Plant Cell Tissue Org Cult. 2010;101:111–117. doi: 10.1007/s11240-009-9663-2. [DOI] [Google Scholar]
- Green B, Tabone T, Felker P. A comparison of amide and ureide nitrogen sources in tissue culture of tree legume Prosopis alba clone B2 V50. Plant Cell Tissue Org Cult. 1990;21:83–86. doi: 10.1007/BF00034497. [DOI] [Google Scholar]
- Hammatt N. Promotion of phloroglucinol of adventitious root formation in micrpropagated shoots of adult wild cherry (Prunus avium L.) Plant Growth Reg. 1994;14:127–132. doi: 10.1007/BF00025213. [DOI] [Google Scholar]
- Hassan AKMS, Afroz F, Jahan MAA, Khatun R. In vitro regeneration through apical and axillary shoot proliferation of Ficus religiosa L.—a multipurpose woody medicinal plant. Plant Tissue Cult Biotechnol. 2009;19(1):71–78. [Google Scholar]
- Huetteman CA, Preece JE. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Org Cult. 1993;33:105–119. doi: 10.1007/BF01983223. [DOI] [Google Scholar]
- Husain MK, Anis M. In vitro proliferation of shoots of Melia azedarach L. from mature trees. In: D’Souza L, Anuradha M, Nivas S, Hegde S, Rajendera K, editors. Biotechnology for a better future. Mangalore: SAC; 2004. pp. 294–301. [Google Scholar]
- Husain MK, Anis M, Shahzad A (2008) In vitro propagation of a multipurpose leguminous tree (Pterocarpus marsupium Roxb.) using nodal explants. Acta Physiol Plant. doi:10.1007/s11738-007-0130-6
- Ibanez MR, Amo-marco JB. Promotion by phoroglucinol of micropropagation of Minuartia valentine—an endangered, endemic Spanish plant. Plant Growth Reg. 1998;26:49–56. doi: 10.1023/A:1006050122173. [DOI] [Google Scholar]
- Jaiswal VS, Narayan P. Regeneration of plantlets from the callus of stem segments of adult plants of Ficus Religiosa L. Plant Cell Rep. 1985;4:256–258. doi: 10.1007/BF00269371. [DOI] [PubMed] [Google Scholar]
- Jha AK, Prakash S, Jain N, Nanda K, Gupta SC. Micropropagation of Sesbania rostrata from the cotyledonary node. Biol Plant. 2004;48:289–292. doi: 10.1023/B:BIOP.0000033458.88441.67. [DOI] [Google Scholar]
- Kirana H, Agrawal SS, Srinivasan BP. Aqueous extract of Ficus religiosa Linn: reduces oxidative stress in experimentally induced type 2 diabetic rats. Indian J Exp Biol. 2009;47:822–826. [PubMed] [Google Scholar]
- Liu X, Pijut M. Plant regeneration from in vitro leaves of mature black cherry (Prunus serotina) Plant Cell Tissue Org Cult. 2008;94:113–123. doi: 10.1007/s11240-008-9393-x. [DOI] [Google Scholar]
- Lloyd GB, McCown BH. Commercially-feasible micropropagation of mountain laurel (Kalmia latifolia) by use of shoot-tip culture. Proc Intl Plant Prop Soc. 1980;30:421–437. [Google Scholar]
- Mallurvar VR, Pathak AK. Studies on immunomodulatory activity of Ficus religiosa. Indian J Pharm Educ Res. 2008;42(4):343–347. [Google Scholar]
- Mansouri K, Preece JE. The influence of plant growth regulators on explant performance, bud break, and shoot growth from large stem segments of Acer saccharinum L. Plant Cell Tissue Org Cult. 2009;99:313–318. doi: 10.1007/s11240-009-9606-y. [DOI] [Google Scholar]
- Mishra Y, Patel PK, Yadav S, Shirin F, Ansari SA. A micropropagation system for cloning of Bambusa tulda Roxb. Sci Hortic. 2008;115(3):315–318. doi: 10.1016/j.scienta.2007.10.002. [DOI] [Google Scholar]
- Nasir E, Ali SI (1974) (Verbenaceae by Jafri, M.H.) Flora of west Pakistan, Herbarium, Department of Botany, University of Karachi 77:23–27
- Negi D, Saxena S (2010) In vitro propagation of Bambusa nutans Wall. Ex Munro through axillary shoot proliferation. Plant Biotechnol Rep. doi:10.1007/s11816-010-0154-z
- Ogita S, Sasamoto H, Yeung EC, Thorpe TA. The effects of glutamine on the maintenance of embryogenic cultures of Cryptomeria japonica. In Vitro Cell Dev Biol Plant. 2001;37:268–273. doi: 10.1007/s11627-001-0048-4. [DOI] [Google Scholar]
- Panit R, Phadke A, Jagtap A. Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;128:462–466. doi: 10.1016/j.jep.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Pradhan C, Kar S, Pattnaik S, Chand PK. Propagation of Dalbergia sissoo Roxb. through in vitro shoot proliferation from cotyledonary nodes. Plant Cell Rep. 1998;18:122–126. doi: 10.1007/s002990050543. [DOI] [Google Scholar]
- Prasad PV, Subhakthe PK, Narayana A, Rao MM. Medico historical study of “asvattha” (sacred fig tree) Bull Indian Inst Hist Med Hyderabad. 2006;36:1–20. [PubMed] [Google Scholar]
- Sanjaya T, Rathore S, Ravishanker Rai V. Micropropagation of Pseudoxytenanthera stocksii Munro. Vitro Cell Dev Biol. 2005;41(3):333–337. doi: 10.1079/IVP2004625. [DOI] [Google Scholar]
- Shah NC. Herbal folk medicines in northern India. J Ethnopharmacol. 1982;6:293–301. doi: 10.1016/0378-8741(82)90052-6. [DOI] [PubMed] [Google Scholar]
- Singh AK, Raghubanshi AS, Singh JS. Medical ethnobotany of the tribals of sonaghati of sonbhadra district, Uttar Pradesh, India. J Ethnopharmacol. 2002;81:31–41. doi: 10.1016/S0378-8741(02)00028-4. [DOI] [PubMed] [Google Scholar]
- Sivanesan I, Jeong BR (2010) Micropropagation of Cotoneaster wilsoni Nakai-a rare endemic ornamental plant. Plant Cell Tissue Org Cult. doi:10.1007/s11240-010-9841-2
- Steephen M, Nagarajan S, Ganesh D. Phoroglucinol and silver nitrate enhances axillary shoot proliferation in nodal explants of Vitex negundo L.—an aromatic medicinal plant. Iran J Biotechnol. 2010;8(2):82–89. [Google Scholar]
- Swami KD, Bisht NP. Constituents of Ficus religiosa and Ficus infectoria and their biological activity. J Indian Chem Soc. 1996;73:631. [Google Scholar]
- Thimmappaiah SRA, Sadhana PH. In vitro propagation of cashew from young trees. Vitro Cell Dev Boil Plant. 2002;38:152–156. doi: 10.1079/IVP2001263. [DOI] [Google Scholar]
- Van Stedan J, Zazimalova E, George EF. Cytokinins, their analogues and antagonist. In: George EF, Hall M, Delkleck GJ, editors. Plant Propagation by tissue culture. The background. Plant growth regulators II, vol 1. The Netherlands: Springer; 2008. pp. 205–226. [Google Scholar]
- Vasudevan A, Selveraj N, Ganapathi A, Kasthurirengan ARV, Manickavasagam M. Glutamine: a suitable nitrogen source for enhanced shoot multiplication in Cucumis sativus L. Biol Plant. 2004;48(1):125–128. doi: 10.1023/B:BIOP.0000024288.82679.50. [DOI] [Google Scholar]
- Vinutha B, Prashanth D, Salma K. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2007;109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]