Abstract
The flowers of Bassia latifolia are known to contain 2-acetyl-1-pyrroline (2AP), the compound responsible for pleasant aroma in basmati and other scented rice. Four growth stages of Bassia flowers were identified and 2AP contents were analysed in each stage. It was found that 2AP (3.30 ppm) gets synthesized only in fleshy corolla of mature flowers (fourth stage). The activity of γ-aminobutyraldehyde dehydrogenase (AADH); an enzyme responsible for synthesis of γ-aminobutyricacid (GABA) from γ-aminobutyraldehyde (GABald) was assessed in these four stages. The AADH activity was absent in the fourth stage. It was concluded that ceased activity of AADH in fourth stage flowers leads to the accumulation of γ-aminobutyraldehyde which is cyclised spontaneously to Δ1-pyrroline, the key precursor of 2AP. Δ1-pyrroline further reacts unenzymatically with methylglyoxal to form 2AP.
Keywords: γ-aminobutyraldehyde dehydrogenase, Bassia latifolia, 2 acetyl-1-pyrroline, Biosynthesis, SPME
Introduction
Bassia latifolia Roxb. (syn. Madhuca latifolia L., Madhuca indica Gmel.), commonly known as Mahua tree, is widely distributed throughout India. The tree species has been domesticated by tribal people for its uses as food (flower), feed (leaves and flower), timber (wood) and alcoholic beverage (fermented flowers) and thus have vital socio-economical value (Kapilan and Reddy 2008). Midya and Brahmachary (1996) reported that the fresh flowers of Mahua (B. latifolia Roxb.), that emit fragrance contain 2AP, the compound responsible for pleasant aroma in basmati and other scented rice. Among other natural sources, the leaves of P. amaryllifolius Roxb. and flowers of V. glabra are known to synthesize 2AP at elevated levels (Buttery et al. 1983a; Wongpornchai et al. 2003).
Recently the mechanism of 2AP biosynthesis has become much clear in scented rice (Bradbury et al. 2008; Chen et al. 2008). It was found that the loss of function of enzyme betaine aldehyde dehydrogenase 2 (BADH2) is responsible for the accumulation of γ-aminobutyraldehyde (GABald)/Δ1-pyrroline; the immediate precursor of 2AP. The addition of acetyl group via methylglyoxal; a product of sugar degradation takes place in Δ1-pyrroline to form 2AP (Huang et al. 2007, 2008). The synthesis of 2AP in aromatic rice as well as plants like P. amaryllifolius and V. glabra is constitutive in all aerial parts however, in B. latifolia the synthesis of 2AP takes place only in flowers (fleshy corolla), that too at mature stage (Wongpornchai et al. 2003; Lorieux et al. 1996; Buttery et al. 1983a). To elucidate specific synthesis of 2AP in B. latifolia flowers, several experiments were done and the results of the same are discussed to uncover the mechanism of 2AP biosynthesis in them.
Materials and methods
Plant material and sample preparation
Fresh collection of different growth stages of Bassia flowers and leaves was done from Amboli village of Khed tahsil, Pune district (Maharashtra, India) in early morning hours. Four different growth stages were identified viz. Stage 1. Bud completely closed, Stage 2. Bud closed with the style protruding, Stage 3. Flower partly open with the style protruding and Stage 4. Fully ripe flower with shedding corolla (Fig. 1). The collected plant material was brought to laboratory in polystyrene box having temperature at ∼4 °C. In laboratory the four stages were separated and fleshy corollas of these different flower stages were analyzed for their 2AP contents. The fleshy corolla was cut into small pieces before analysis. Mature flowers were found to emit higher 2AP contents, hence were selected for the optimization of headspace (HS) solid Phase Micro Extraction (SPME) analysis conditions. Leaves were also collected and cut leaf pieces were used for 2AP analysis.
Fig. 1.
Flower developmental stages in Bassia latifoliaa Inflorescence representing different stages of flower development, b Detached fleshy corollas, c Growth stages of flower
Chemicals and reagents
Authentic 2AP was a generous gift from Dr. P. Srinivas (Central Food and Technology Research Institute, Mysore, India). Purity of authentic 2AP was confirmed by GC-MS (Shimadzu QP 5050A, Japan). The mass spectrum showed major ions 41, 42, 43(100), 68, 69, 83, 111 which were identical to the ions of 2AP in NIST147 library and also with the ions mentioned in previous literature (Buttery et al. 1983b). Dilutions of 2AP were made using Benzene (Merck, Mumbai, India). 4-aminobutyraldehyde-diethylacetal was from Aldrich, Steinheim, Germany and methylglyoxal solution and betaine aldehyde were from Sigma, Steinheim, Germany. Dithiothreitol (DTT) and nicotinamide adenine dinucleotide (NAD) were from Himedia, Mumbai, India.
Optimization of HS-SPME conditions for quantification of 2AP
One gram cut fleshy corolla (stage 4) was placed in 4 ml screw top vial (15 × 45 mm) with PTFE silicon septa (Chromatography research supplies, USA). The basis of SPME literature suggests that an equilibration time of 10–15 min is generally sufficient for most of the volatile compounds; hence 10 min was selected as an equilibration time. Adsorption temperatures ranging from 40 °C to 80 °C with an interval of 20 °C each and adsorption time ranging from 15 min to 45 min at the interval of 10 min each were varied to standardize the temperature and time for optimization of maximum adsorption of 2AP in the headspace of B. latifolia tissue. The quantity of plant material was varied from 5 mg to 1,000 mg. All the experiments for optimization of HS-SPME conditions from Bassia flowers were performed in triplicate. The carboxen/DVB/PDMS SPME fiber of 1 cm attached to manual holder (Supelco, Bellefonte, PA) was selected for analysis of 2AP based on earlier report (Grimm et al. 2001; Wakte et al. 2010). The fiber was conditioned as per manufacturer’s guidelines and then exposed to headspace of the sample in a 4 ml vial at varied temperature and time regimes as specified above. The SPME fiber was desorbed for 5 min on a GC (Shimadzu 17 A, Japan) with BP-20 capillary column (30 m × 0.32 μm, SGE, Ringwood, Australia) and Flame Ionization Detector (FID). The injector temperature was held at 250 °C. The GC oven temperature was held for 2 min at 50 °C and then ramped to 100 °C at the rate of 7 °C/min with a hold of 1 min and then ramped to 250 °C at the rate of 20 °C/min with a final hold of 2 min.
Quantification of 2AP from different growth stages of flower
Quantification of 2AP was done by using standard 2AP calibration curve following the method reported in our earlier report (Wakte et al. 2010). Standard 2AP solutions in a concentrations series of 25, 50, 75, 100 and 125 ng/l were prepared in benzene and subjected to HS-SPME-GC analysis. Calibration curve was developed with linear correlation coefficient (R2 value) of 0.987. Following the optimized conditions 2AP contents from four different growth stages of Bassia flowers and leaves were quantified in triplicates.
Preparation of crude BADH2 extracts
Two gram of fresh fleshy corolla from each stage of Bassia flowers were homogenized in 10 ml extraction buffer, 50 mM Tris–HCl (pH 7.5), 10 % glycerol, 1 mM EDTA, 0.5 mM dithiothreitol (DTT), 5 % polyvinylpyrrolidone. The homogenate was cleared by centrifugation at 13,000 rpm for 10 min. The supernatant was adjusted to 50 % (NH4)2SO4, transferred to a new tube and centrifuged at 18,000 rpm for 30 min. The supernatant was precipitated with 70 % (NH4)2SO4 and centrifuged at 16,000 rpm for 20 min. The pellet was dissolved in 1 ml of protein buffer containing 10 mM Tris–HCl (pH 7.5), 10 % glycerol, 1 mM EDTA and 0.2 mM DTT. The protein amount was determined by the method of Lowry, Rosebrough, Farr, and Randall with bovine serum albumin as standard (Lowry et al. 1951). The enzyme extract (200 μg/reaction) was used for assessing enzyme activity.
Preparation of 4-aminobutyraldehyde
4-aminobutyraldehyde was prepared according to methods of Trossat et al. (1997) and Srivong et al. (2007). Ten microliter of 4-aminobutyraldehyde diethylacetal was hydrolyzed with 600 μl of 1 M HCl by heating at 70 °C for 1 h. The hydrolyzate was stored in ice and used within 4 h. The 4-aminobutyraldehyde product was analyzed by TLC using silica gel as stationary phase and methanol: acetone: concentrated HC1 (90:10:4, v/v) as mobile phase and detected with 4 % ninhydrin in alcohol. The hydrolyzate was neutralized by KOH just before the assay.
Assay of BADH2 activity
Two substrates namely betaine aldehyde and 4-aminobutyraldehyde were used to determine BADH2 activity in leaves and four stages of B. latifolia flowers. The BADH2 activity was determined using the reaction mixture (1 ml) containing 50 mM phosphate buffer, pH 8.0, l0 mM DTT, and substrate. The reactions were started by adding 1.5 mM NAD+. Blank was set in the absence of NAD+. Betaine aldehyde (10 μl of 100 mM solution) and neutralized 4-aminobutyraldehyde (10 μl of 37 mM solution) were used as substrates. The reactions were monitored for 10 min with increase in absorbance at 340 nm due to conversion of NAD+ to NADH. Conversion of A340 to NADH concentration was performed by NADH extinction coefficient of 6,220 M−1 cm−1. Enzyme activities were expressed as initial rates for the reduction of NAD+ per mg total protein.
Synthesis and characterization of 2AP
The neutralized solution of 4-aminobutyraldehyde (4 ml of 37 mM solution) was mixed with methylglyoxal (1 ml of 30 % solution) in a 5 ml of phosphate buffer (0.1 M final concentration, pH 7.2). After stirring for 15 min at room temperature (25 °C), the mixture was subjected to HS-SPME-GC/GCMS analysis for characterization and quantification of 2AP in triplicate. One milliliter solution was taken in 4 ml screw top vial and incubated with SPME fibre for 15 min at room temperature. The fibre was desorbed in injector of GC/GC-MS following method and programme described in optimization of HS-SPME conditions for the quantification of 2AP section.
Data analysis
Comparisons between the mean values of 2AP were made using Duncan’s multiple range test (DMRT). The analysis was performed using SPSS software (Version 9, Chicago, IL, USA).
Results and discussion
Optimization of time and temperature for quantification of 2AP
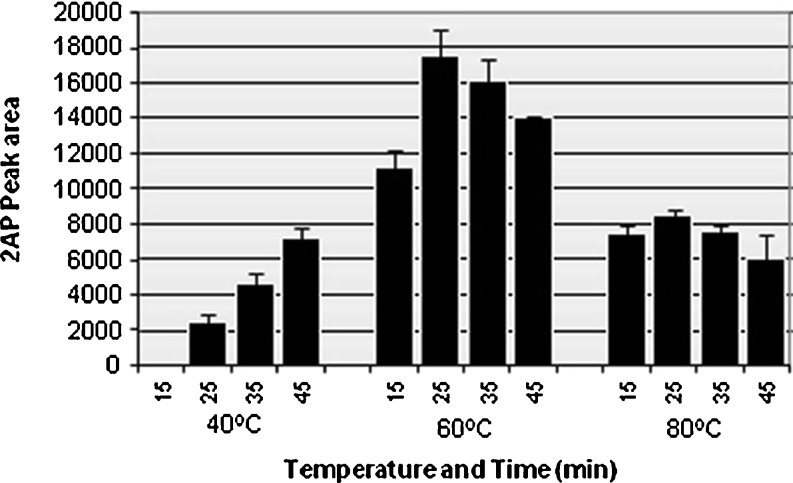
When temperature and time was varied, 2AP peak area was found to vary considerably (Fig. 2). Among the varied temperature and time combinations, 60 °C temperature for 25 min was found to be the most effective combination for maximum release of 2AP in HS of B. latifolia corolla and its effective adsorption by SPME fiber. Grimm et al. (2001) reported 80 °C temperature and 40 min as optimal conditions for maximum adsorption of 2AP from rice samples. Higher temperatures may reduce adsorption time, however it can cause a premature desorption of analytes from the fiber or formation of new compounds (Wakte et al. 2010). Our results agreed with the reduction in 2AP peak area with increase in temperature above 60 °C (Fig. 2). Quantity of the plant material to be used for maximum adsorption of 2AP was also optimized. It was found that 10 mg of cut corolla pieces were most effective in terms of 2AP abundance (Table 1). In our earlier experiments for optimization of protocol for quantification of 2AP from P. amaryllifolius 10 mg powdered leaf material was found optimum (Wakte et al. 2010).
Fig. 2.
2AP Peak area abundance in accordance to varied time and temperature conditions in Bassia latifolia flowers
Table 1.
Quantity wise 2AP contents in the fleshy corolla of B. latifolia
| Fleshy corolla (mg) | 2AP contents (ppm) ± SE |
|---|---|
| 5 | 3.27 ± 0.15a |
| 10 | 3.57 ± 0.78a |
| 25 | 1.68 ± 0.22b |
| 50 | 0.67 ± 0.08c |
| 100 | 0.37 ± 0.01c |
| 250 | 0.15 ± 0.01c |
| 500 | 0.08 ± 0.01c |
| 1,000 | 0.06 ± 0.01c |
Mean followed by same superscript in a column is not significantly different at P = 0.01 level
Quantification of 2AP in different growth stages of flowers (Fleshy corolla)
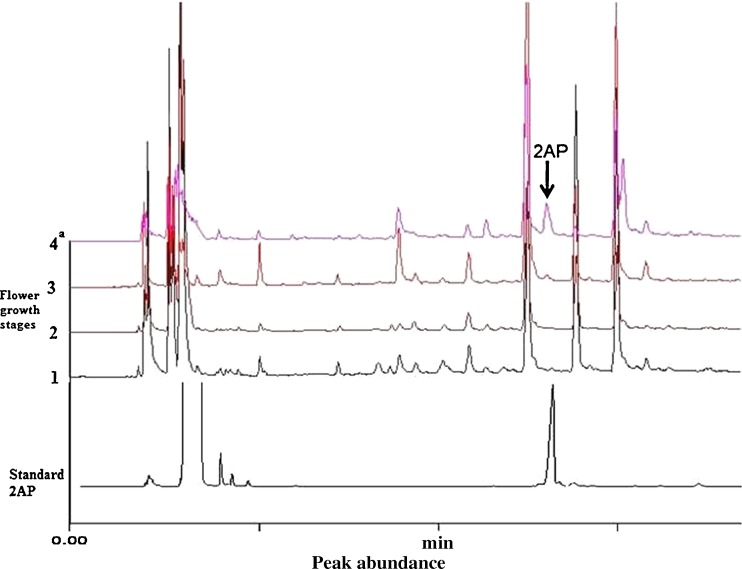
Four different growth stages of B. latifolia were analyzed for 2AP contents using optimized conditions. Stages 1 and 2 were found to be totally devoid of 2AP, whereas in stage 3 marginal concentration of 2AP (0.37 ppm) was recorded. However, stage 4 was found to contain elevated levels of 2AP (3.30 ppm) (Fig. 3). By using similar kind of approach (SPME-GC) we reported 6.85 ppm of 2AP contents in P. amaryllifolius (Wakte et al. 2010). Wongpornchai et al. (2003) quantified concentration of 2AP in fresh flowers of V. glabra (3.36 ppm), P. amaryllifolius (10.26 ppm), and KDML brown rice (3.00 ppm). In comparison to these 2AP natural sources, 2AP in B. latifolia was in order of 3.30 ppm, almost equal to fresh flowers of V. glabra, marginally more than KDML brown rice and about 3 times less than P. amaryllifolius.
Fig. 3.
HS-SPME/GC-FID chromatogram of 2AP from four stages of Bassia latifolia flowers. Note the 2AP peak abundance in 4th stage. (1–4 represents flower stages). Superscript ‘a’ at stage 4 represents that 2AP values at stage 4 are statistically different from other stages at P = 0.01 level
Substrate specificity and activity of BADH2
The accumulation of 2AP in scented rice is due to absence of BADH2 activity (Bradbury et al. 2005). BADH2 usually oxidizes betaine aldehyde to osmoprotectant glycine betaine (Ishitani et al. 1993). However recent studies have shown that BADH2 is capable of metabolizing a range of substrates including omega-aminoaldehydes, often more efficiently than betaine aldehyde (Sebela et al. 2000; Livingstone et al. 2003). When expressed in E. coli, BADH2 showed greater affinity for GABald than for betaine aldehyde and is capable of oxidizing GABald to GABA (Bradbury et al. 2008; Chen et al. 2008). In present study, both betaine aldehyde and GABald were used as substrate for assessing the activity of BADH2. It was found that BADH2 shows a greater affinity towards GABald and with no activity towards betaine aldehyde. BADH2 activity was detected in first three growth stages of flowers for substrate GABald. The result revealed that, BADH2 participates in the pathway of GABA synthesis by oxidizing GABald and had no role to play in the synthesis of glycine betaine from betaine aldehyde. Some researchers reported that aminoaldehyde dehydrogenase (AADH) has high homology with BADH2 but no affinity to betaine aldehyde, leading to suggestion that BADH2 and AADH are the same enzyme (Trossat et al. 1997; Sebela et al. 2000; Livingstone et al. 2003). Authors agree with these reports because the enzyme is basically involved in conversion of GABald to GABA. Hence, it is more appropriate to refer the enzyme as AADH. Here onwards BADH2 will be designated as AADH. In leaves AADH showed very less activity as compared to enzyme activity detected in first three flower stages (Table 2). The enzyme activity increased as flower matured (till stage 3) and ceased in stage 4 (fleshy corolla). The cessation of AADH activity can be correlated with the detachment of fleshy corolla from the mother plant. The leaves recorded AADH activity (8.0 nmoles min−1 mg protein−1) which was considerably less in comparison to activity recorded in flower growth stages 1–3 (Avg. 52 nmoles min−1 mg protein−1). These results indicate that flower has a higher activity than that of leaf. This might be due to availability of elevated substrate concentration or higher transcript levels of AADH in fleshy corolla of flower. A detailed study in this regard is needed. The increase in the activity in stage 3 also indicates availability of higher substrate concentration of GABald. The activity of AADH ceases in stage 4 flower (mature fleshy corolla) leading to accumulation of GABald which is already synthesized at elevated levels as revealed by the higher activity of AADH in flowers of third stage. GABald is nearly entirely transformed into Δ1-pyrroline when AADH is inhibited or absent. Ceased activity of AADH at stage 4 flower leads to accumulation of elevated level of Δ1-pyrroline which leads to higher levels of 2AP synthesis in B. latifolia flowers (fleshy corolla). Availability of free Δ1-pyrroline has been observed to be the rate controlling factor in synthesis of 2AP. Consumption of GABald by converting it into GABA inhibits 2AP synthesis, whereas the accumulation of GABald/Δ1-pyrroline results in increased 2AP synthesis (Chen et al. 2008). However, when AADH is available Δ1-pyrroline is nearly entirely transformed into GABald and is converted into GABA. In biological systems AADH is capable of utilizing only GABald and any kind of suppression, inhibition or absence of AADH leads to its cyclization into Δ1-pyrroline and formation of its polymers (Fogel et al. 1979). Δ1-pyrroline cannot be used as a substrate by aldehyde-metabolizing enzymes in biological systems (Fogel et al. 1979). Thus levels of Δ1-pyrroline are treated as a direct reflection of diamine oxidase activity which is involved in conversion of Putrescine to GABald (Fogel et al. 1979). Total conversion of GABald to Δ1-pyrroline in such experiments is obtained by adding inhibitors for aldehyde metabolizing enzymes.
Table 2.
Leaf and flower stages wise AADH enzyme activity and 2AP contents in B. latifolia
| Plant material form | Vmax nmoles min−1 mg protein−1 | 2AP contents ppm ± S.E. |
|---|---|---|
| Leaf | 8.0 | ND |
| Flower stage 1 | 20.0 | ND |
| Flower stage 2 | 36.0 | ND |
| Flower stage 3 | 100.0 | 0.37 ± 0.07a |
| Flower stage 4 | ND | 3.30 ± 0.08b |
Mean followed by same superscript in a column is not significantly different at P = 0.01 level
Synthesis and characterization of 2AP
The neutralized solution of 4-aminobutyraldehyde mixed with Methylglyoxal in phosphate buffer lead to formation of 2AP. HS-SPME-GC/GCMS analysis of solution mixture revealed that 4-aminobutyraldehyde (which is in equilibrium with Δ1-pyrroline) is converted to 2AP. The mass spectrum of 2AP synthesized showed major ions 41, 42, 43(100), 68, 69, 83, 111 which were identical to the ions of 2AP in NIST147 library and also with the ions mentioned in previous literature (Buttery et al. 1983b). This also confirms non-enzymatic formation of 2AP as also suggested by other researchers (Chen et al. 2008). Methylglyoxal was identified as the carbon source for 2AP (Huang et al. 2007, 2008). They synthesised 2AP non-enzymatically at room temperature simply by stirring mixture of P5C (Δ1-pyrroline-5-carboxylic acid) and Methylglyoxal for 30 min. Methylglyoxal is also known to react with and modify other molecules to form new compounds non-enzymatically (Yadav et al. 2005). Schieberle (1989) reported that the thermal reactions between proline, 1-pyrroline, and several metabolites of the glycolytic pathway were shown to be responsible for 2AP formation in wheat bread crust. They also reported significant amount of 2AP in 1-pyrroline/Methylglyoxal model system under boiling condition (Schieberle 1990). This leads to suggestion that formation of 2AP in final step (reaction between Δ1-pyrroline/Methylglyoxal) is non-enzymatic.
Mechanism of 2AP biosynthesis in flowers (fleshy corolla) of B. latifolia
Romanczyk et al. (1995) demonstrated increase in 2AP contents in presence of high levels of proline, ornithine and glutamate in Bacillus cereus cultures. They conducted several labelling experiments and established that proline and glutamate are the two nitrogen sources that contribute in synthesis of 2AP. They also found high amounts of 2AP in carbon sources like amylose and glucose. Later on proline was identified and confirmed as the precursor molecule of 2AP with a tracer experiments using 15N-proline radiolabeled isotope (Yoshihashi et al. 2002). An increase in concentration of 2AP was obtained when proline, ornithine, and glutamate were added in solution, with proline addition yielded more than 3-fold rise in 2AP when compared to that of the control. Trace experiments with 15N-proline, 15N-glycine, and proline-1-13C indicated that the nitrogen source of 2AP was proline, whereas the carbon source of the acetyl group was not its carboxyl group (Yoshihashi et al. 2002). Costello and Henschke (2002) suggested role of ornithine as nitrogen source of 2AP via Putrescine pathway. Intermediate 1-pyrroline could then be subjected to acylation of C-2 position of acyl-CoA derivatives, thus leading to synthesis of 2AP.
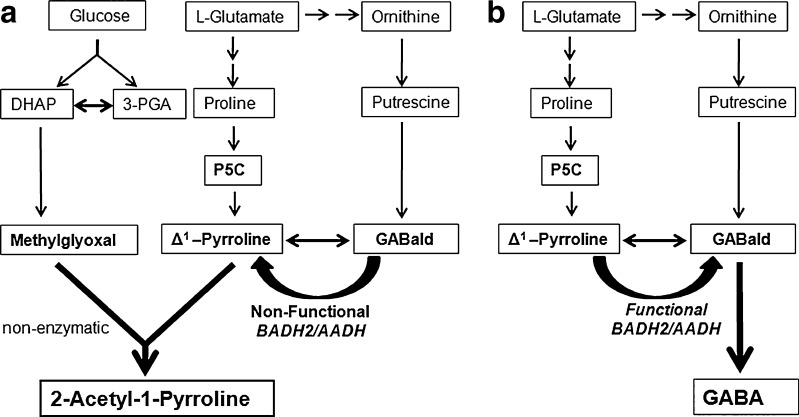
The accumulation of 2AP in rice is due to absence of BADH2 activity leading to an increased level of its substrate, GABald/Δ1-pyrroline, the immediate precursor of 2AP (Bradbury et al. 2005). Consumption of GABald/Δ1-pyrroline by converting it into GABA inhibits 2AP synthesis, whereas the accumulation of GABald/Δ1-pyrroline results in increased 2AP synthesis (Chen et al. 2008). Stewart in experiments with [14C] Proline, recovered 4.9 % radioactivity in GABA, which suggests that considerable amount of GABald/Δ1-pyrroline, is formed, during proline oxidation (Stewart 1977). Thus, availability of GABald/Δ1-pyrroline formed by degradation of polyamines and proline is controlling factor for 2AP synthesis. Fleshy corolla of B. latifolia has very high sugar contents which act as a precursor for methylglyoxal, the other immediate substrate for synthesis of 2AP (Swain et al. 2007). Formation of methylglyoxal is correlated to the excess glucose metabolized through Embedn-Meyerhof pathway (Phillips and Thornalley 1993). Hence it is hypothesized that fleshy corolla (stage 4) of B. latifolia have elevated levels of Methylglyoxal and Δ1-pyrroline which react with each other non-enzymatically due to their unstable and highly reactive nature, leading to formation of 2AP (Fig. 4). Thus, the flowers of B. latifolia produce 2AP only after the corolla starts detaching from the other parts of flower (AADH is inactivated) and finally detached corolla falls on ground releasing fragrance similar to aromatic rice varieties.
Fig. 4.
Proposed pathway for 2AP biosynthesis in mature Bassia latifolia flowers (a) against in other parts (b)
Conclusion
It is concluded that ceased activity of AADH in the mature (fourth stage) flowers lead to the accumulation of γ-aminobutyraldehyde which is cyclised spontaneously to Δ1-pyrroline. Δ1-pyrroline further reacts with methylglyoxal to form 2AP. Thus the biosynthesis of 2AP in B. latifolia flowers takes place in unenzymatic way.
Acknowledgements
Authors are thankful to Dr. P. Srinivas (Central Food and Technology Research Institute, Mysore, India) for generous gift of authentic 2AP.
References
- Bradbury LMT, Fitzgerald TL, Henry RJ, Jin Q, Waters DLE. The gene for fragrance in rice. Plant Biotech J. 2005;3:363–370. doi: 10.1111/j.1467-7652.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- Bradbury LM, Gillies SA, Brushett DJ, Waters DLE, Henry RJ. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol Bio. 2008;68:439–449. doi: 10.1007/s11103-008-9381-x. [DOI] [PubMed] [Google Scholar]
- Buttery RG, Juliano BO, Ling LC (1983a) Identification of rice aroma compound 2-acetyl-1-pyrroline in Pandan leaves. Chemistry and Industry (London), p 478
- Buttery RG, Ling LC, Juliano BO, Turnbaugh JG. Cooked rice aroma and 2-acetyl-1-pyrroline. J Agric Food Chem. 1983;31:823–826. doi: 10.1021/jf00118a036. [DOI] [Google Scholar]
- Chen S, Yang Y, Shi W, Ji Q, He F, Zhang Z, Cheng Z, Liu X, Xu M. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;20:1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello PJ, Henschke PA. Mousy off-flavor of wine: Precursors and biosynthesis of the causative N-heterocycles 2-ethyltetrahydropyridine, 2-acetyl tetrahydropyridine and 2-acetyl-1-pyrroline by Lactobacillus hilgardii DSM 20176. J Agric Food Chem. 2002;50:7079–7087. doi: 10.1021/jf020341r. [DOI] [PubMed] [Google Scholar]
- Fogel WA, Bieganski T, Maslinski C. Effects of inhibitors of aldehyde metabolizing enzymes on putrescine metabolism in guinea pig liver homogenates. Inflamm Res. 1979;9(1):42–44. doi: 10.1007/BF02024104. [DOI] [PubMed] [Google Scholar]
- Grimm CC, Bergman C, Delgado JT, Bryant R. Screening for 2-acetyl-1-pyrroline in the headspace of rice using SPME/GC-MS. J Agric Food Chem. 2001;49:245–249. doi: 10.1021/jf0008902. [DOI] [PubMed] [Google Scholar]
- Huang TC, Huang YW, Hung HJ, Ho CT, Wu ML. Δ1-Pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp. natto expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J Agric Food Chem. 2007;55:5097–5102. doi: 10.1021/jf0700576. [DOI] [PubMed] [Google Scholar]
- Huang TC, Teng CS, Chang JL, Chuang HS, Ho CT, Wu ML. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J Agric Food Chem. 2008;56:7399–7404. doi: 10.1021/jf8011739. [DOI] [PubMed] [Google Scholar]
- Ishitani M, Arakawa K, Mizuno K, Kishitani S, Takabe T. Betaine aldehyde dehydrogenase in the gramineae: levels in leaves both betaine-accumulating and nonaccumulating cereal plants. Plant Cell Physiol. 1993;34:493–495. [Google Scholar]
- Kapilan N, Reddy R. Evaluation of Methyl esters of Mahua oil (Madhuca Indica) as diesel fuel. J Am Oil Chem Soc. 2008;85(2):185–188. doi: 10.1007/s11746-007-1179-5. [DOI] [Google Scholar]
- Livingstone JR, Maruo T, Yoshida I, Tarui Y, Hirooka K, Yamamoto Y, Tsutui N, Hirasawa E. Purification and properties of betaine aldehyde dehydrogenase from Avena sativa. J Plant Res. 2003;116:133–140. doi: 10.1007/s10265-003-0077-7. [DOI] [PubMed] [Google Scholar]
- Lorieux M, Petrov M, Huang N, Guiderdoni E, Ghesquiere A. Aroma in rice: genetic analysis of a quantitative trait. Theor Appl Genet. 1996;93:1145–1151. doi: 10.1007/BF00230138. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-Phenol reagents. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Midya S, Brahmachary R. The aroma of Bassia flower. Curr Sci. 1996;71:430. [Google Scholar]
- Phillips SA, Thornalley PJ. The formation of Methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem. 1993;212:101–105. doi: 10.1111/j.1432-1033.1993.tb17638.x. [DOI] [PubMed] [Google Scholar]
- Romanczyk LJ, Jr, McClelland CA, Post LS, Aitken WM. Formation of 2-acetyl-I-pyrroline by several Bacillus cereus strains isolated from cocoa fermentation boxes. J Agric Food Chem. 1995;43:469–475. doi: 10.1021/jf00050a040. [DOI] [Google Scholar]
- Schieberle P (1989) Formation of 2-acetyl-1-pyrroline and other important flavor compounds in wheat bread crust. In Thermal generation of aromas pp. 268–275, ACS Symposium series 409. American Chemical Society, Washington
- Schieberle P. The role of free amino acids present in yeast as precursors the odorants 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine in wheat bread crust. Z Lebensm Unters Forsch. 1990;191:206–209. doi: 10.1007/BF01197621. [DOI] [Google Scholar]
- Sebela M, Brauner F, Radova A, Jacobsen S, Havlis J, Galuszka P, Pec P. Characterisation of a homogeneous plant aminoaldehyde dehydrogenase. Biochim Biophys Acta. 2000;1480:329–341. doi: 10.1016/S0167-4838(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Srivong P, Wangsomnuk P, Pongdontri P. Characterization of a fragrant gene and enzymatic activity of betaine aldehyde dehydrogenase in aromatic and nonaromatic Thai rice cultivars. KKU Sci J. 2007;36(4):290–301. [Google Scholar]
- Stewart CR. Inhibition of proline oxidation by water stress. Plant Physiol. 1977;59:930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain MR, Kar S, Sahoo AK, Ray RC. Ethanol fermentation of mahula (Madhuca latifolia L) flowers using free and immobilized yeast Saccharomyces cerevisiae. Microbiol Res. 2007;162:93–98. doi: 10.1016/j.micres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Trossat C, Rathinasabapathi B, Hanson AD. Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and omega-aminoaldehydes. Plant Physiol. 1997;113:1457–1461. doi: 10.1104/pp.113.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakte KV, Thengane RJ, Jawali N, Nadaf AB. Optimization of HS-SPME conditions for quantification of 2-acetyl-1-pyrroline and study of other volatiles in Pandanus amaryllifolius Roxb. Food Chem. 2010;121:595–600. doi: 10.1016/j.foodchem.2009.12.056. [DOI] [Google Scholar]
- Wongpornchai S, Sriseadka T, Choonvisase S. Identification and quantitation of the rice aroma compound, 2-acetyl-1-pyrroline, in bread flowers (Vallaris glabra Ktze) J Agric Food Chem. 2003;51:457–462. doi: 10.1021/jf025856x. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK. Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett. 2005;579:6265–71. doi: 10.1016/j.febslet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Yoshihashi T, Huong NTT, Inatomi H. Precursors of 2-acetyl-1-pyrroline, a potent flavour compound of an aromatic rice variety. J Agric Food Chem. 2002;50:2001–2004. doi: 10.1021/jf011268s. [DOI] [PubMed] [Google Scholar]