Abstract
Efficient methods were developed for both in vitro seed germination and micropropagation of an economically important dye yielding multipurpose tree, Bixa orellana L. Mature seeds were inoculated onto Murashige and Skoog (MS) medium supplemented with different concentrations of gibberellic acid (GA3). Highest frequency of germination (93.3 %) was recorded on medium supplemented with 3 μM GA3 against 13.33 % in control. Nodal explants cultured on MS medium fortified with 5 μM isopentanyl adenine (2-iP) produced maximum explants response (93.3 %) and highest number of shoots (35.71). Addition of relatively higher concentration (15 μM) of benzyl adenine (BA) resulted in the production of significantly (P < 0.05) reduced number of shoots (12.66). Sucrose at 87.6 mM was found to be the best carbohydrate source for multiple shoot induction compared to glucose and fructose. Regenerated shoots (3–4 cm) were rooted (95.5 %) on agar gelled MS medium supplemented with 10 μM indole-3-butyric acid (IBA). In vitro developed plantlets with well-developed roots were potted and acclimatized initially in the growth chamber and then moved to a green house with 83.3 % survival. The present protocol avoids the use of auxins in shoot multiplication medium, which will lower the cost, avoid callus formation and thus reduces the possibility of somaclonal variation in the regenerated plants. The method is efficient to produce over 32,000 hardened plants within a 10-month culture period starting from a single nodal explant.
Keywords: Carbohydrate source, Nodal segment, 2-iP, Multiple shoots, Rhizogenesis
Introduction
Annatto (Bixa orellana L.) is a small sized tree cultivated in the tropical and subtropical regions of the world. The plant is chiefly valued for its pigmented seeds which are the source of orange red natural colorant. In commerce, annatto ranks second among the natural colorants with a world consumption of 10,650 tons/year (Satyanarayana et al. 2003). This natural pigment has an ever-increasing demand from food and cosmetic industries, as the natural color is safe for consumption and for skin applications. It is one of the 13 basic pigments derived from natural sources that are currently permitted by the US-FDA and also specified as permitted color in European Union. In India, it is accepted under Prevention of Food Adulteration Act. Hence there is an ever-growing market for annatto (Carvalho 1999; Collins and Hughes 1991). The whole plant is medicinally important and is used in various systems of medicine.
Traditionally the plant is propagated by cuttings and seeds. Vegetative propagation by cuttings has serious drawbacks such as the difficult nature of cuttings to root due to leaching of gummy substances (Sharon and D’Souza 2000). Currently commercial plantations of this species are being raised through seeds (Aparnathi et al. 2003). Commercial cultivation of this crop is not very attractive due to the lack of quality planting materials. Therefore in vitro propagation of annatto could be an alternative to produce large number of superior planting materials. Moreover, development of a reliable in vitro regeneration system is a pre requisite for genetic transformation system and generating polyploids aimed at increasing the levels of carotenoids in the seed coat.
To initiate in vitro cultures, seeds were preferred as starting material (Benson et al. 2000), as they are most capable, fully organized structures to respond in an in vitro environment. In vitro germination of seeds yields large number of aseptic plants that can be inoculated for initiating tissue culture (Mercier and Kerbauy 1997). In vitro protocols using seedling explants have been successfully employed for rapid propagation and manipulation of several woody perennials e.g., Pterocarpus marsupium (Husain et al. 2007).
Previous reports on micropropagation of B. orellana are also based on seedling-derived explants. In vitro response of explants like hypocotyl segments (Neto et al. 2003; Parimalan et al. 2007), nodal segments and shoot tips derived from young seedling (D’Souza and Sharon 2001; Nassar et al. 2001; Parimalan et al. 2008, 2009, 2010; Ramamurthy et al. 1999) and leaf segments from seedling (Almeida et al. 1996) are available. Role of ethylene in in vitro rooting of micropropagated shoots are also available (Neto et al. 2009). In the previous reports however, raising in vitro seedling was a tedious process coupled with a reduced rate of multiplication. An efficient micropropagation protocol is still lacking in B. orellana using nodal explants. The present study sought to develop an effective protocol for micropropagation using low levels of 2-iP or BA without an intervention of auxin in the multiplication medium.
Materials and methods
In vitro seed germination
Due to severe infection, seeds from fully mature dehiscent fruits were avoided, instead 7-week after fruit set, mature, indehiscent fruits collected from a 4-year-old tree growing in the Botanic garden of University of Kerala, Kariavattom was used as seed source.
The collected fruits were processed by removing bristles like outgrowth by using a fine razor blade. The intact fruits were then treated with diluted (1 % v/v) solution of polysorbitol detergent (Labolene, Mfg. Qualigen’s Fine Chemicals, Mumbai, India) for 30 min, followed by several rinses in water and then submerged in 0.1 % (w/v) carbondazim fungicide (Bavistin, Mfg. BASF, Mumbai, India) for 1 h and washed. Subsequently the fruits were treated with mercuric chloride (0.1 %) solution containing few drops of surfactant (Tween 20) for 10 min followed by several rinses with sterile distilled water in the laminar air flow chamber and finally the fruits were dipped in alcohol and flamed. Seeds were aseptically excised from the fruits and used for inoculation on to germination medium (15 ml) prepared in culture tubes (25 × 150 mm) plugged with non-absorbent cotton. Single seed per culture tube was inoculated.
Effect of GA3 on germination
The seeds were cultured in vitro, on MS medium supplemented with GA3 (0.0, 1.5, 3.0, 4.5 and 6.0 μM) and 3 % sucrose. The pH of the medium was adjusted to 5.8 and solidified with 0.7 % Difco Bactoagar (Qualigen’s, Mumbai, India) before autoclaving at 121 °C and 108 kPa for 20 min. A set of seeds inoculated on hormone free MS medium served as the control.
The number of germinated seeds was recorded from the fifth day onwards at 5 days interval for 45 d. Mean time taken for germination (MTG) was calculated (Hartman and Kester 1983) using the equation 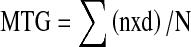 , where n = number of seeds germinated, d = period of incubation in days, N = total number of germinated seeds at the end of the test.
, where n = number of seeds germinated, d = period of incubation in days, N = total number of germinated seeds at the end of the test.
If stated otherwise, all cultures were incubated at 25 ± 2 °C in a culture room with 50-μmolm−2 s−1 irradiance provided by cool white fluorescent tubes (40 W; Philips, India) and were exposed to a photoperiod of 16-h and 55 ± 5 % of relative humidity.
In vitro shoot multiplication
Nodal segments excised from 20-d-old axenic seedlings were used for in vitro shoot multiplication. The nodal segments (1.5–2.0 cm) were excised and cultured on MS medium supplemented with different concentrations (0.5, 1.0, 2.5, 5.0, 10.0 and 15.0 μM) of BA or 2-iP. When most of the axillary shoots attained a length of 3–4 cm, usually within 4 weeks were excised and segmented into 1–1.5 cm size having a single node were subcultured on to MS medium supplemented with 5 μM 2-iP for further multiplication. The multiplication through this way was continued for the six subcultures without adversely affecting rate of multiplication and undesirable callus formation. Also in vitro-raised shoots (>3 cm) from 3rd subculture product onwards was used for rooting experiments.
To study the influence of carbohydrate sources on multiple shoot proliferation, nodal segments were cultured on MS medium supplemented with different carbohydrates viz. sucrose, glucose or fructose at different concentrations.
In vitro rooting
In vitro rooting of shoots were conducted using excised shoots (>3 cm). The MS medium supplemented with auxins viz., IBA and NAA at varying concentrations (2.5, 5.0, 10.0 and 15.0 μM) along with 3 % sucrose and 0.7 % agar was tested. Cultures for rooting were kept at 16-h light conditions.
To identify suitable length of shoots for rooting, shoots were classified into five groups (< 1.0 cm, 1.1–2.0 cm, 2.1–3.0 cm, 3.1–4.0 cm, 4.1–5.0 cm). These shoots were planted on agar gelled MS medium containing 10 μM IBA.
After root initiation (20 d), the plantlets were transferred to half strength MS liquid medium containing 2 % sucrose for root elongation.
Acclimatization
Plantlets with well-developed shoot and roots thus obtained were deflasked. They were washed gently under running tap water and transplanted into plastic pots containing soil: vermiculite (1:1). Potted plants were incubated in a growth chamber at 25 ± 1 °C and 16-h photoperiod with a light intensity of 50-μmolm−2 s−1. Initially, for the first 2 weeks, plants were covered with micro holed transparent polyethylene bags to maintain humidity. They were watered on 5 days intervals with one fourth-strength MS salt solution. When the plants were completely weaned (4 weeks) they were moved to shade house. Three-month-old plants were planted in the field and their performance is being observed.
Experimental design and statistical analysis
All experiments were conducted using a completely randomized block design. Every treatment composed of three replications and each replication block represented by ten culture tubes. Data on various parameters were evaluated by analysis of variance (ANOVA) and mean values were compared with Duncan’s New Multiple Range Test (DNMRT). Data scored in percentages were subjected to arcsine transformation before analysis, and then converted back to percentages for presentation in the tables (Snedecor and Cochran 1962). The shoot number, maximum shoot length and the percentage of explants producing shoots were recorded 4 weeks after inoculation. The mean number and length of roots developed from the regenerated shoots and the percentage of shoots producing roots were also determined after four-week incubation on rooting medium.
Results and discussion
In vitro seed germination
Method adopted for fruit sterilization, excision of seeds from fruits and subsequent culture resulted in the establishment of cent percent aseptic cultures. In the previous works on tissue culture of annatto, sterilization of seeds reported as tedious and lengthy process including one-week agitation in gyratory shaker and warm water treatment to break the dormancy (Parimalan et al. 2007, 2008; 2009; Sharon and D’Souza 2000). The present sterilization and seed culture establishment is therefore rapid and efficient to provide axenic seedlings for further in vitro culture of annatto.
Effect of GA3 on germination
The effect of different concentrations of GA3 on in vitro seed germination of B. orellana was examined (Table 1). The addition of GA3 in MS basal medium significantly (P < 0.05) enhanced in vitro germination. Highest germination (93.3 %) was observed in 3 μM GA3 supplemented medium. In the control, least germination (13.3 %) was observed. The seeds inoculated on to 3 μM GA3 supplemented medium showed emergence of radicle within a week and recorded least MTG (15.16). Concentration of GA3, beyond 3 μM did not show any significant (P < 0.05) difference (Table 1) in MTG. In all the concentrations of GA3 tested, significant (P < 0.05) decrease in the time taken for germination was noted. The seedlings become 4-leaf stage within a period of 20 d (Fig. 1a). In the previous reports on in vitro multiplication of annatto (Parimalan et al. 2009), the seed germination and seedling growth together took 2 months. Thus by using GA3 in the germination medium the time can be considerably reduced. Addition of GA3 (3 μM) in the germination medium did not cause any morphological abnormality to in vitro raised seedlings. In B. orellana, seeds were sensitive to exogenous application of GA3 and found to enhance seed germination (Joseph et al. 2010).
Table 1.
Effect of different concentrations of GA3 supplemented MS medium on in vitro germination of B. orellana seeds
| GA3 Conc. (μM) | % of germinationa | MTGa |
|---|---|---|
| 0.0 | 13.33d | 29.13a |
| 1.5 | 56.66b | 21.33b |
| 3.0 | 93.33a | 15.16c |
| 4.5 | 53.33b | 15.36c |
| 6.0 | 26.66c | 15.60c |
| Main Effect F Df (n-1) 4 | 85.80*** | 110.83** |
aMeans with in a column followed by same letters are not significantly different as determined by DNMRT (P < 0.05).**significant at P < 0.01 level; ***significant at P < 0.001 level
Fig. 1.
In vitro propagation of B. orellana using in vitro raised seedling explant a In vitro seed germination on MS medium supplemented with 3 μM GA3, b Axillary bud elongation and initiation of multiple shoots from nodal segments cultured on MS medium containing 5 μM 2-iP, c Adventitious shoot bud proliferation on MS medium with 5 μM 2-iP, d Shoot multiplication and subsequent elongation of shoots in MS + 5 μM 2-iP e 4-week-old well rooted in vitro raised plantlet before potting, f Acclimatized plants growing in green house (2-months after potting)
In vitro shoot multiplication
Nodal segments from 20 d-old in vitro seedlings were used for shoot multiplication. Enlargement of shoot buds at the nodal region was observed in 7 d-old cultures. Development of adventitious shoot buds was noticed in 20 d after culture from the lower cut end of nodal explants. Nodal segments cultured on various hormonal concentrations showed significant (P < 0.001) response. Cultures growing in 2-iP (5 μM) supplemented medium showed emergence of axillary shoots (Fig. 1b) and development of adventitious shoot buds (Fig. 1c). Axillary shoot development could not be induced on growth regulator free medium. ANOVA revealed significant (P < 0.001) interaction between cytokinin type and concentration in evoking both explants response and shoot bud development (Table 2). 2-iP (5 μM) supplemented MS medium thus standardized as the best medium for multiple shoot induction (35.7 shoots per explant) from nodal segments of seedlings with 93.3 % response. Shoots developed in this hormonal concentration sustained an active growth and therefore elongated in to 5.96 cm size on fourth week of culture (Fig. 1d). Subculturing of nodal segments excised from 5 μM 2-iP supplemented medium was continued for six multiplication cycles without any decline in the rate of multiplication and morphological abnormality of nascent shoots.
Table 2.
Effect of BA and 2-iP on shoot multiplication using nodal explants of B. orellana
| Cytokinin | Conc. (μM) | Explant responsea (%) | No. of shoots/explanta | Mean shoot lengtha (cm) |
|---|---|---|---|---|
| Control | 0.0 | 0.00g | 0.00i | 0.00i |
| 2-iP | 0.5 | 4.5f | 1.43hi | 1.50cde |
| 1.0 | 43.2de | 4.58fg | 2.25bc | |
| 2.5 | 63.5bc | 20.11c | 5.83a | |
| 5.0 | 93.3a | 35.71a | 5.96a | |
| 10.0 | 73.5bc | 3.53gh | 1.47cde | |
| 15.0 | 33.3e | 0.94i | 1.33cde | |
| BA | 0.5 | 1.2fg | 1.72hi | 1.05de |
| 1.0 | 33.3e | 5.99ef | 2.65b | |
| 2.5 | 43.3de | 7.75e | 1.96bcd | |
| 5.0 | 56.7cd | 10.66d | 1.42cde | |
| 10.0 | 76.8b | 23.26b | 0.92e | |
| 15.0 | 63.5c | 12.66d | 0.70ef | |
| Treatment Df (n-1) | 12 | 37.43*** | 220.60*** | 37.22*** |
| Auxin type(T) Df (n-1) | 1 | 5.20 | 2.69 | 80.391*** |
| Auxin conc.(C) Df (n-1) | 5 | 50.60*** | 206.374*** | 34.780*** |
| T X C Df (n-1) | 11 | 26.79*** | 204.923*** | 33.074*** |
aMeans with in a column followed by same letters are not significantly different as determined by DNMRT (P < 0.05). ***significant at P < 0.001 level
The type and concentration of carbohydrate significantly influenced shoot proliferation (Table 3). Explants cultured on medium containing 87.6 mM sucrose as carbohydrate produced the maximum response (93.3 %). The number of shoots (35.71) and shoot length (5.96) was also found to be the highest at this concentration of sucrose. Callusing was observed at higher concentrations (131.2 mM) of sucrose and lower concentrations of glucose (43.3 and 87.6 mM). No callus formation was observed in all the three concentrations of fructose. The lowest culture response (43.3 %) was observed in 43.3 mM fructose supplemented medium.
Table 3.
Effect of different carbohydrate sources and its concentrations on multiple shoot induction using nodal explants of B. orellana cultured in 5 μM 2-iP supplemented MS medium
| Carbon source | Conc. (mM) | % response | No. of shoots | Shoot length | Callus development |
|---|---|---|---|---|---|
| Glucose | 43.3 | 73.5cd | 10.76e | 2.14d | + |
| 87.6 | 67.7cde | 8.16f | 2.56c | ++ | |
| 131.2 | 60.0de | 4.30i | 3.50b | − | |
| Fructose | 43.3 | 43.3f | 5.23hi | 2.66c | − |
| 87.6 | 53.3ef | 6.20g | 1.63e | − | |
| 131.2 | 70.4cd | 12.30d | 1.10f | − | |
| Sucrose | 43.3 | 76.8bc | 14.66c | 2.96c | − |
| 87.6 | 93.3a | 35.71a | 5.96a | − | |
| 131.2 | 87.0b | 19.40b | 3.56b | + | |
| Treatment Df (n-1) | 8 | 9.55*** | 537.59*** | 106.78*** | |
| Carbon source (S) | 2 | 27.04*** | 1329.05*** | 230.34*** | |
| Df (n-1) | |||||
| Carbon conc.(C) | 2 | 2.24 | 187.11*** | 29.77*** | |
| Df (n-1) | |||||
| S X C. Df (n-1) | 8 | 9.55*** | 537.59*** | 106.78*** |
aMeans with in a column followed by same letters are not significantly different as determined by DNMRT (P < 0.05). ***significant at P < 0.001 level
In the previous reports on B. orellana (Table 6), the number of shoots obtained was only 3–5 per explant (D’Souza and Sharon 2001) in B5 medium supplemented with very high concentration of triacontanol along with 2-iP. However, in the present study 2-iP was found to be the best cytokinin for nodal explants to produce 35.7 shoots. In contrast to present findings that superiority of 2-iP over BA, Ramamurthy et al. (1999) has reported effectiveness of BA in multiple shoot induction from nodal explants. Earlier reports on organogenesis in B. orellana (Table 6) indicate rate of multiplication limited to around 10 shoots per explant (Parimalan et al.2008, 2009). In the previous report (Parimalan et al. 2008) both auxin, (phenyl acetic acid, 14.7 μM) and cytokinin (BA 31.1 μM) were used. Excessive concentrations of auxins and/or cytokinins in the shoot multiplication medium, however, can result in the development of morphologically variant plants (George 1996), callus development and subsequent shoot regeneration. Shoots regenerated through callus can often variant from its source material. The appearance of somaclonal variation (Larkin and Scowcroft 1981) in the in vitro regenerated plants can be greatly reduced by using lower levels of 2-iP (Debnath and McRae 2001; Marcotrigiano and McGlew 1991). In another report using nodal shoot tip explants (Parimalan et al. 2010), 12–13 elongated shoots were produced by the incorporation of putrescine in the medium, still then callus formation was noticed. In the present work use of auxin in shoot multiplication medium was avoided. This omission reduces the cost as well as chance of somaclonal variation in the regenerated plants.
Table 6.
Summary of tissue culture work on Bixa orellana
| Reference | Explant and mode of regeneration | Treatment/Medium | Result | Remarks |
|---|---|---|---|---|
| Ramamurthy et al. 1999 | Node (Indirect) | B5 + BA 1.5 mgl−1 + IBA 1 mgl−1 for shoots and rooting in IBA 1 mgl−1 | 4–6 shoots along with callus | Low frequency of multiplication, requires 5–6 months to get plantlets with 30–60 % response |
| Nassar et al. 2001 | Coty. node, node (Direct) | WPM + 1 mgl−1 lBA/Kin + 0.2 mgl−1 NAA + 100 mg Tyrosine + 40 mgl−1 AS for shoot and rooting on WPM/1/2 MS + 3 mgl−1 NAA | Few shoots | low frequency of multiplication |
| D’Souza and Sharon 2001 | Shoot apex, node (Direct) | B5 + 2iP 4.9 μM + TRIA for shoot and rooting in 0.05 μM/2.7 μM NAA | 9 shoots per shoot apex and 5 shoots per node explants | low frequency of multiplication, high conc. of TRIA |
| Khan et al. 2002 | Seed (Indirect) | MS + 10 μM BA + 5 μM NAA for shoot and 1/2MS + 5 μM IBA for rooting | 6–7shoots per callus | Indirect regeneration, low frequency of multiplication |
| Neto et al. 2003 | Hypocotyl (HY), rooted HY (Direct) | MS + 4.56 μM Zeatin for shoot and rooting in 5.0 μM IBA | 3–4 shoots | Mechanical scarification of seeds may cause contamination and damage to embryo |
| Parimalan et al. 2007 | HY, rooted HY, coty. leaf, nodal shoot tips (Direct) | MS + TDZ + CW/BA + NAA for shoots. and IBA 3 mgl−1 for rooting | rooted hypocotyls give 22 shoots per explant | Seed dormancy breaking and sterilization tedious, chances for contamination of seeds, shoot length is only 10–16 mm |
| Parimalan et al. 2008 | Nodal shoot tip explants (Direct) | MS + 31.1 μM BA + 14.7 μM PAA for shoot and 4.9 μM IBA for rooting | 6–13 primary shoots | Seed dormancy breaking and sterilization tedious process, shoot length is less than 1.5 cm, callusing,high conc. of hormones |
| Parimalan et al. 2009 | Nodal shoot tip (Direct) | MS + 8.87 μM BA + 0.05 μM IAA + 11.2 μM TRIA for shoot and rooting in 4.9 μM IBA | 6–18 primary shoots | Seed dormancy breaking and sterilization tedious process, shoot ength is less than 1.5 cm, callusing |
| Parimalan et al. 2010 | Nodal shoot tip (Direct) | MS + 6.6 μM BA + 4.9 IBA + Putrescine 800 μM for shoot and rooting in 4.9 μM IBA | 12–13 shoots with maximum shoot length of 7.3 cm | Seed dormancy breaking and sterilization tedious process, callusing, |
| Present study | Node (Direct) | MS + 5 μM 2-iP for shoot and rooting in 10 μM IBA | 35.71 shoots per explant | Sterilization protocol simple, avoids contamination chances and seed germination percentage significantly high with the use of GA3 in germination medium. High frequency regeneration without auxin in multiplication medium, avoid callusing, chances for variation and reduce cost of production. Time taken for rooting is less. |
*HY hypocotyl, AS Adenine sulphate, CW Coconut water
In vitro rooting
Shoots were rooted after 10–15 d of culture on rooting medium. In vitro rooting was accompanied with elongation of shoots. Rooting response of micro shoots cultured in different auxin (NAA or IBA) supplemented medium varied significantly (Table 4). The maximum rooting response (95.5 %) and number of roots per shoot (20.23) was recorded in medium supplemented with 10 μM IBA. Choice of auxin and concentration had significant (P < 0.001) effect on rooting. Increasing concentration of auxins caused development of callus at the basal portion of the micro shoots. The number of roots increased with increasing concentration from 2.5 to 10 μM and decreases thereafter. With respect to rooting percentage and number of roots, there was significant (P < 0.001) interaction between auxin type and concentration. D’Souza and Sharon (2001) found that the average root length of annatto was affected by increased concentrations of NAA (0.05–4.03 μM) in the rooting medium.
Table 4.
Effect of auxins on rooting of B. orellana micro shoots in vitro
| Auxin | Conc. (μM) | % of response | Root number | Root length(cm) |
|---|---|---|---|---|
| Control | 0.0 | 0.0f | 0.00h | 0.00e |
| IBA | 2.5 | 50.0c | 11.15d | 3.13a |
| 5.0 | 73.5b | 16.86b | 2.61b | |
| 10.0 | 95.5a | 20.23a | 1.70c | |
| 15.0 | 33.3d | 12.83c | 0.63d | |
| NAA | 2.5 | 53.4c | 5.31f | 3.06a |
| 5.0 | 60.1bc | 6.92e | 1.90c | |
| 10.0 | 46.6cd | 2.90g | 1.78c | |
| 15.0 | 16.4e | 2.46g | 0.77d | |
| TreatmentDf(n-1) | 8 | 59.10*** | 227.535*** | 106.015*** |
| Auxin type (T)Df(n-1) | 1 | 37.61** | 991.74*** | 3.22 |
| Auxin conc.(C)Df(n-1) | 3 | 40.11 *** | 40.81*** | 156.66*** |
| T X C Df(n-1) | 7 | 28.21 *** | 179.57*** | 70.15*** |
aMeans with in a column followed by same letters are not significantly different as determined by DNMRT (P < 0.05). **significant at P < 0.01 level; ***significant at P < 0.001 level
Rooting response of different sized shoots varied significantly (P < 0.05). Shoots length ranging 3.1–4.0 cm showed 95.5 % rooting. Lowest rooting response (4.5 %) was observed in shoots of <1.0 cm (Table 5). The efficient rooting of regenerated shoots and the survival of plantlets in the soil are the important steps in the successful micropropagation. In this study both IBA and NAA produced roots. Addition of IBA is found to be more effective to induce roots in B. orellana. The superior effect of IBA has been previously documented in other tree species (Siril and Dhar 1997; Tiwari et al. 2002). De Klerk et al. (1997) reported the strong inhibition of root growth with NAA (4 mM) in apple rootstock Jork 9, whereas IBA produced longer roots (11 mM) at the optimal concentrations. The superior effect of IBA on the root development in these plant species including B. orellana might be due to preferential uptake, transport and stability over NAA and subsequent gene activation (Ludwig-Muller 2000). Shoots planted in hormone free medium (control) failed to produce roots. Time taken for rooting in the present protocol is less compared to previous report (Parimalan et al. 2008) with the same type of growth hormone (Table 6). This attributes to the effect of explants type or due to the genotypic difference. Due to the lack of rooting in hormone free medium and dramatic increase of rooting due to exogenous auxin supplementation, annatto, in accordance to Marks and Simpson (2000) can be considered as a difficult-to-root category of woody plants.
Table 5.
Effect of shoot size on in vitro rooting response of B. orellana shoots
| Shoot size (cm) | % of response | Root number | Root length |
|---|---|---|---|
| <1.0 | 4.5d | 0.66e | 3.66a |
| 1.1–2.0 | 46.6c | 10.53d | 2.83ab |
| 2.1–3.0 | 83.6ab | 16.33b | 1.76ab |
| 3.1–4.0 | 95.5a | 20.23a | 1.70ab |
| 4.1–5.0 | 63.5bc | 5.80c | 0.53c |
| Main Effect F Df (n-1) 4 | 34.66*** | 101.41*** | 2.07 |
aMeans with in a column followed by same letters are not significantly different as determined by DNMRT (P < 0.05). ***significant at P < 0.001 level
Acclimatization
Four-week-old rooted plantlets (Fig. 1e) were transferred to plastic pots containing soil: vermiculite mixture (1:1) were survived (83.3 %). Potted plants showed emergence of new leaves within 3-weeks of planting. In vitro raised plants showed uniform morphological features (Fig. 1f) and active growth in ambient conditions. Three-month-old, well-acclimatized plants were planted in the field.
The above study on in vitro multiplication demonstrates a possible step towards efficient in vitro propagation using MS medium containing 5 μM 2-iP for multiple shoot induction and 10 μM IBA for rooting. Direct shoot proliferation from nodal segments seems to be suitable for the rapid propagation of annatto. Shoot multiplication can be enhanced with repeated subculture under a mass propagation or production strategy. These results will be helpful to future research on in vitro propagation of adult trees. Using present method it is estimated that a single nodal segment can produce over 32,000 hardened plants within a 10-month culture period. This simple and rapid propagation protocol is reproducible and can be used as a supporting system in the improvement program of B. orellana.
Acknowledgements
We thank Dr. Ashalatha S Nair, Professor and Head, Department of Botany for providing the facilities and Kerala State Council for Science, Technology and Environment (KSCSTE) Govt. of Kerala, Thiruvananthapuram, India, for the financial support (No.028/SRSLS/2007/CSTE).
References
- Almeida JL, Almeida FCG, Nunes RDEP, Almeida FAG. Bud initiation in leaf explants of annatto seedlings in different cytokinins. Cinencia Rural. 1996;26:45–49. [Google Scholar]
- Aparnathi K, Lata R, Sharma R. Annatto (Bixa orellana L.): its cultivation, preparation and usage. Int J Trop Agric. 2003;8:80–88. [Google Scholar]
- Benson E, Danahar JE, Pimbley IM, Anderson CT, Wake JE, Daley S, Adams LK. In vitro propagation of Primula scotica: a rare Scottish plant. Biodivers Conserv. 2000;9:711–726. doi: 10.1023/A:1008941726419. [DOI] [Google Scholar]
- Carvalho PR. Technological advances and perspectives. Arch Latinoam Nutr. 1999;49:71–73. [PubMed] [Google Scholar]
- Collins P, Hughes S. Report in prospective in natural food symposium. England: Oversea food Ltd.; 1991. [Google Scholar]
- D’Souza MC, Sharon M. In vitro clonal propagation of annatto (Bixa orellana L.) In Vitro Cell Dev Biol-Plant. 2001;37:168–172. doi: 10.1007/s11627-001-0029-7. [DOI] [Google Scholar]
- De Klerk G, Brugge J, Marinova S. Effectiveness of indole-acetic acid, indole butyric acid and naphthalene acetic acid during adventitious root formation in vitro in Malus Jork 9. Plant Cell Tiss Organ Cult. 1997;49:39–44. doi: 10.1023/A:1005850222973. [DOI] [Google Scholar]
- Debnath SC, McRae KB. An efficient in vitro shoot propagation of cranberry (Vaccinium macrocarpon AIT.) by axillary bud proliferation. In Vitro Cell Dev Biol-Plant. 2001;37:243–249. doi: 10.1007/s11627-001-0043-9. [DOI] [Google Scholar]
- George EF. Plant propagation by tissue culture, Part 2: in practice. London: Exegetics Ltd; 1996. p. 799. [Google Scholar]
- Hartman HT, Kester KE. Plant propagation and practices. New Delhi: Prentice Hall, India Pvt. Ltd; 1983. [Google Scholar]
- Husain MK, Anis M, Shahzad A. In vitro propagation of Indian kino (Pterocarpus marsupim Roxb.) using Thidiazuron. In Vitro Cell Dev Biol-Plant. 2007;43:59–64. [Google Scholar]
- Joseph N, Siril EA, Nair GM. Imbibition duration, seed treatment, seed mass and population influence germination of annatto (Bixa orellana L.) seeds. Seed Technol. 2010;32:37–45. [Google Scholar]
- Khan PSSV, Prakash E, Rao KR. Callus induction and plantlet regeneration in Bixa orellana L., an annatto-yielding tree. In Vitro Cell Dev Biol-Plant. 2002;38:186–190. doi: 10.1079/IVP2001284. [DOI] [Google Scholar]
- Larkin PJ, Scowcroft WR. Somaclonal variation- a novel source of variability from cell cultures for plant improvement. Theor Appl Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- Ludwig-Muller J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000;32:219–230. doi: 10.1023/A:1010746806891. [DOI] [Google Scholar]
- Marcotrigiano M, McGlew SP. A two-stage micropropagation system for cranberries. J Am Soc Hort Sci. 1991;116:911–916. [Google Scholar]
- Marks TM, Simpson SE. Interaction of explants type and indole-3-butyric acid during rooting in vitro in a range of difficult and easy-to-root woody plants. Plant Cell Tiss Organ Cult. 2000;62:65–74. doi: 10.1023/A:1006443124007. [DOI] [Google Scholar]
- Mercier H, Kerbauy GB. Micropropagation of ornamental bromeliads (Bromeliaceae) Biotechnol Agric For. 1997;40:43–57. [Google Scholar]
- Nassar AH, Khalifa SF, Hammouda FM, Shams KA. In vitro propagation of Bixa orellana L. (annatto) an economically important plant newly introduced to Egypt. Egyptian J Bot. 2001;41:241–253. [Google Scholar]
- Neto VBP, de Botelho MN, Aguiar R, Silva EAM, Otoni WC. Somatic embryogenesis from immature zygotic embryos of annatto (Bixa orellana L.) In Vitro Cell Dev Biol-Plant. 2003;39:629–634. doi: 10.1079/IVP2003465. [DOI] [Google Scholar]
- Neto VPB, Reis LB, Finger FL, Barros RS, Carvalho CR, Otoni WC. Involvement of ethylene in the rooting of seedling shoot cultures of Bixa orellana L. In Vitro Cell Dev Biol-Plant. 2009;45:693–700. doi: 10.1007/s11627-009-9236-4. [DOI] [Google Scholar]
- Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA. Organogenesis from cotyledon and hypocotyls derived explants of japhara (Bixa orellana L.) Act Bot Croat. 2007;66:153–160. [Google Scholar]
- Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA. Mass multiplication of Bixa orellana L. through tissue culture for commercial propagation. Ind Crops and Prod. 2008;28:122–127. doi: 10.1016/j.indcrop.2008.01.012. [DOI] [Google Scholar]
- Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA. Micropropagation of Bixa orellana using phytohormones and triacontanol. Biol Plant. 2009;53:347–350. doi: 10.1007/s10535-009-0064-5. [DOI] [Google Scholar]
- Parimalan R, Giridhar P, Ravishankar GA. Enhanced shoot organogenesis in Bixa orellana L. in the presence of putrescine and silver nitrate. Plant Cell Tiss Organ Cult. 2010;105:285–290. doi: 10.1007/s11240-010-9865-7. [DOI] [Google Scholar]
- Ramamurthy N, Savithramma N, Usha R, Swamy PM. Multiple shoot induction and regeneration of japhara (Bixa orellana L.) through axillary bud derived callus cultures. J Plant Biol. 1999;26:231–235. [Google Scholar]
- Satyanarayana A, Rao PG, Rao DG. Chemistry, processing and toxicology of annatto (Bixa orellana L.) J Food Sci Technol. 2003;40:131–141. [Google Scholar]
- Sharon M, D’Souza MC. In vitro clonal propagation of annatto (Bixa orellana L.) Curr Sci. 2000;78:1532–1535. [Google Scholar]
- Siril EA, Dhar U. Micropropagation of mature Chinese tallow tree (Sapium sebiferum Roxb.) Plant Cell Rep. 1997;16:637–640. doi: 10.1007/BF01275506. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 4. Iowa: The Iowa State University Press; 1962. [Google Scholar]
- Tiwari SK, Tiwari KP, Siril EA. An improved micropropagation protocol for teak. Plant Cell Tiss Organ Cult. 2002;71:1–6. doi: 10.1023/A:1016570000846. [DOI] [Google Scholar]



