Abstract
The effect of elevated CO2 concentrations (545 and 700 μmol mol−1) on gas exchange and stomatal response of four high Δ9-THC yielding varieties of Cannabis sativa (HPM, K2, MX and W1) was studied to assess their response to the rising atmospheric CO2 concentration. In general, elevated CO2 concentration (700 μmol mol−1) significantly (p < 0.05) stimulated net photosynthesis (PN), water use efficiency (WUE) and internal CO2 concentration (Ci), and suppressed transpiration (E) and stomatal conductance (gs) as compared to the ambient CO2 concentration (390 μmol mol−1) in all the varieties whereas, the effect of 545 μmol mol−1 CO2 concentration was found insignificant (p < 0.05) on these parameters in most of the cases. No significant changes (p < 0.05) in the ratio of internal to the ambient CO2 concentration (Ci/Ca) was observed in these varieties under both the elevated CO2 concentrations (545 and 700 μmol mol−1). An average increase of about 48 %, 45 %, 44 % and 38 % in PN and, about 177 %, 157 %, 191 % and 182 % in WUE was observed due to elevated CO2 (700 μmol mol−1) as compared to ambient CO2 concentration in HPM, K2, MX and W1 varieties, respectively. The higher WUE under elevated CO2 conditions in Cannabis sativa, primarily because of decreased stomatal conductance and subsequently the transpiration rate, may enable this species to survive under expected harsh greenhouse effects including elevated CO2 concentration and drought conditions. The higher PN, WUE and nearly constant Ci/Ca ratio under elevated CO2 concentrations in this species reflect a close coordination between its stomatal and mesophyll functions.
Keywords: Cannabis sativa, Cannabaceae, Elevated CO2, Photosynthesis
Introduction
Increasing atmospheric CO2 concentration and global warming are of major environmental concern around the world. The concentration of CO2 in the atmosphere has increased by more than 30 %, from about 280 μmol mol−1 in the eighteenth century to the present level of 390 μmol mol−1 (Mauna Loa Observatory-MLO, Hawaii), and is projected to reach as high as 700 μmol mol−1 by the end of the twenty first century (Houghton et al. 1996). Thus, the future environment will be characterized by elevated CO2, higher temperature and drier climate in certain parts of the globe. The increase in CO2 concentration may have considerable direct and indirect effects on various life forms. Particular effects are related to the distribution, abundance and productivity of vegetation (Pounds and Puschendorf 2004). Therefore, understanding the effects of the increasing atmospheric CO2 concentration on plants and vegetation has become an important issue. A large number of studies have shown enhancement in growth of the plants subjected to both short and long-term CO2 exposure (Kimball 1983; Cure and Acock 1986) by affecting a number of basic physiological processes, particularly photosynthesis and other gas exchange parameters (Ceulemans et al. 1995). A close correlation between net photosynthesis (PN) and crop yield has been reported since more than 90 % of the dry matter of live plants is derived from photosynthetic CO2 assimilation (Zelitch 1975). However, the magnitude of the enhancement in photosynthesis and growth of the plants appear to be species and genotype/variety specific (Minorsky 2002). Doubling in CO2 concentration has been reported to increase the yield by 30 % or more in many crops (Poorter 1993). On the other hand, inhibition of photosynthesis with increasing CO2 concentration has also been reported in many plant species (Bazzaz and Garbutt 1988; Juurola 2003).
Cannabis sativa L. (Cannabaceae) is a widely distributed plant around the world. It has a long history of medicinal use as far back as the 6th century B.C. Cannabis sativa is the natural source of the cannabinoids, a unique group of terpeno-phenolic compounds that accumulate in the glandular trichomes of the plant. Δ9-Tetrahydrocannabinolic acid (Δ9-THCA) is the major cannabinoid which upon decarboxylation with age or heating gives rise to Δ9-THC, the primary psychoactive agent (Pertwee 2006). The pharmacologic and therapeutic potency of Cannabis preparations and Δ9-THC have been extensively reviewed (Grinspoon and Bakalar 1993; Mattes et al. 1994 and Brenneisen et al. 1996). Despite of its medicinal importance and widespread occurrence, to the best of our knowledge, no information (except a previous note by our group on—one Cannabis variety, Chandra et al. 2008) is available on the consequences of rising atmospheric CO2 concentration on its photosynthesis and growth performance. This study describes the short term effect of elevated CO2 on photosynthetic characteristics and stomatal response in four different high Δ9-THC yielding varieties of Cannabis sativa.
Materials and methods
Plant material
Plants of four drug-type varieties of C. sativa, namely HPM (High Potency Mexican Variety, seeds originally acquired from Mexico), K2, MX and W1 (all from Switzerland), were grown from seeds in the climate controlled indoor growing facility (16 m length × 6 m width) at the University of Mississippi, USA. Since it is the female plants of this species that are medicinally used (higher concentration of THC and higher biomass), male plants were removed after onset of flowering and only female plants were kept for the experiment. Five female plants from each variety were selected and five cuttings were made from each plant for the photosynthetic study. Throughout the study, all the plants were kept under strict controlled environmental conditions (25 ± 3 °C temperature and 55 ± 5 % RH). Indoor light (18 h photoperiod, ~700 ± 24 μmol m−2s−1 at plant canopy level, measured by LI-COR quantum meter, model LI-189, Lincoln, Nebraska, USA) was provided with seven full spectrum 1000 watt HID (high intensity discharge) lamps in combination with seven 1000 watt high pressure sodium bulbs (Sun Systems, CA), hung above plants and covering 110 square meter area. A hot air suction fan was attached to each light. The bulbs were kept at least three to four feet from the plants to avoid heating caused by the HID bulbs. All the plants were grown in the equal size plastic pots (30 cm diameter × 28 cm height) containing 1:1:1 ratio of top soil, sand and manure and were watered equally and regularly to maintain identical growth condition. Out of the 25 cuttings of each variety, 5 healthy and well established randomly selected female clones from each variety were used for the photosynthetic measurements and comparison.
Gas exchange measurements
To evaluate the effect of different CO2 levels (390, 545 and 700 μmol mol−1) on the photosynthetic and stomatal response of C. sativa, the gas exchange measurements were made on the three upper undamaged, fully expanded and healthy leaves of the five selected plants of each variety with the help of a closed portable photosynthesis system (Model LI-6400; LI-COR, Lincoln, Nebraska, USA). Different levels of CO2 were produced by portable CO2 cylinders compatible with a LI-6400 portable photosynthetic system controlled by a microprocessor. Photosynthetic measurements were recorded at steady state condition with the chamber air temperature maintained constant at 25 °C. Light (1500 μmol m−2s−1) was provided with an artificial light source (Model LI-6400-02; light emitting silicon diode; LI-COR), fixed above the leaf chamber and recorded with the help of quantum sensor kept in the range of 660–675 nm, mounted at the leaf level. The temperature of the cuvette chamber was controlled by the integrated Peltier coolers controlled by the microprocessor. The CO2 concentration supplied to the cuvette of the climatic unit (LI-6400-01, LI-COR Inc., USA) was controlled by mixing pure CO2 with CO2 free air and the CO2 concentration was measured by infrared gas analyzer. Air flow rate (500 μmol s−1) and relative humidity (55 ± 3 %) were kept nearly constant throughout the experiment. Since steady state photosynthesis is reached within 30–45 min (Joshi and Palni 1998; Bag et al. 2000; Joshi 2006 and Chandra et al. 2008), the leaves were kept for about 45–60 min under each set of light conditions before the observations were recorded. Four gas exchange parameters viz., photosynthetic rate (PN), transpirational water loss (E), stomatal conductance (gs) and intercellular CO2 concentration (Ci) were measured simultaneously at steady state under controlled light and temperature conditions. To evaluate the leaf stomatal and mesophyll photosynthetic efficiency, the ratio of intercellular CO2 concentration to ambient CO2 concentration (Ci/Ca) and the ratio of intercellular CO2 concentration to stomatal conductance (Ci/gs) were calculated, respectively. Water use efficiency (WUE) was calculated as the ratio of the rate of photosynthesis and transpiration.
Statistical analysis
Values presented here are the mean of 15 replicates (Five samples per replication with a total of three replications) with ± SE. Two-way ANOVA was performed using SYSTAT-11 (Systat Software Inc. San Jose, CA, USA) statistical software.
Result and discussion
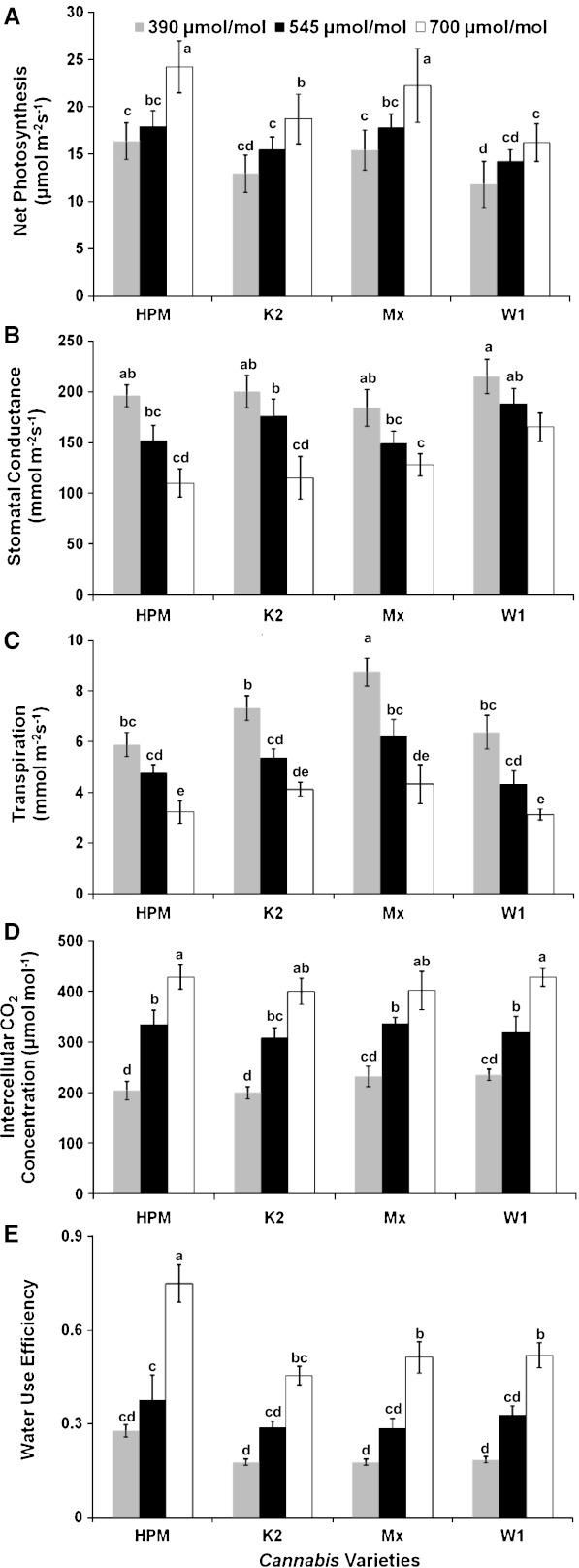
There is a growing concern that human-induced climatic change will affect the eco-physiological processes of plant species, and hence their productivity and distribution (Sanz-Elorza et al. 2003). Further, the responsiveness of the species to recent and past climate changes also suggests that climate change could act as a major cause of extinction of some plant species in the near future (Thomas et al. 1994). The ability of plant species to grow and to survive in a particular environment depends on their photosynthetic capacity (Berry and Downton 1982, Joshi et al. 2007). It has been reported that plants with high rates of photosynthesis and WUE have the potential to grow faster and yield more than the species with low photosynthesis rate and WUE under fluctuating environmental conditions (Jones 1992; Zhang et al. 1996). Elevated atmospheric CO2 concentration in the environment has also been reported to enhance the photosynthesis and growth of many plant species (Kimball 1983, Cure and Acock 1986). It is also generally suspected that the studies on short term exposure of elevated CO2 overestimate the relative enhancements in CO2 assimilation rates in plants as compared to those under the long term exposures (Sage et al. 1989). Nonetheless, short term studies serve a key role in providing first approximation and indication of plants behavior under future environmental conditions (Joshi 2006). Figure 1a shows the effect of ambient and elevated CO2 concentrations on net photosynthesis of different varieties of C. sativa. In all the varieties, a slight but statistically insignificant (p < 0.05) increase in PN was observed when measurements were made at 545 μmol mol−1 CO2 concentration as compared to ambient (390 μmol mol−1) CO2, whereas, a significant increase (p < 0.05) of about 48 %, 45 %, 44 % and 38 % in PN was observed when the measurements were made at 700 μmol mol−1 CO2 concentration as compared to ambient level in HPM, K2, MX and W1 varieties, respectively. The increase in PN due to the short term increase in CO2 concentration is reported to be primarily because of an increase in carboxylation efficiency and secondarily, due to the reduction in photorespiration (Minorsky 2002). However, the magnitude of enhancement was reported to be species and genotype/variety specific. It has also been reported that doubling of ambient CO2 concentration increases PN in the plant species whose photosynthesis is not saturated by the present ambient CO2 level (Joshi 2006). Since photosynthesis of C3 plant species is not saturated at the present ambient CO2 concentration, these plants are thus reported to benefit more than C4 plants (Bowes 1993; Joshi 2006). Average increase of about 33 % in the rate of photosynthesis and productivity of C3 plants has been reported with doubling of atmospheric CO2 concentration (Kimball 1983; Bazzaz and Garbutt 1988; Cure and Acock 1986, Joshi 2006). In the present investigation, under elevated CO2 concentration (700 μmol mol−1) the photosynthetic rate of Cannabis sativa was ~ 44 % higher relative to that under ambient CO2 (390 μmol mol−1).
Fig. 1.
Effect of different CO2 concentrations (390, 545 and 700 μmol mol−1) on net photosynthesis (a), stomatal conductance (b), transpirational water loss (c), intercellular CO2 concentration (d) and water use efficiency (e) of Cannabis sativa varieties namely HPM, K2, MX and W1. Values within the same panel followed by the same letter are not significantly different at p < 0.05
Increasing CO2 concentration decreased the stomatal conductance (Fig. 1b) and subsequently, the transpiration rate (Fig. 1c), thereby increasing the water use efficiency (Fig. 1e) of C. sativa. Under elevated CO2 (545 μmol mol−1), about 22 %, 12 %, 19 % and 13 % decrease in gs, 19 %, 27 %, 29 % and 32 % decrease in E and, 36 %, 63 %, 62 % and 78 % increase in WUE was observed in HPM, K2, MX and W1 varieties, respectively, as compared to those under ambient CO2 (390 μmol mol−1); whereas, under 700 μmol mol−1 CO2 concentration, about 44 %, 43 %, 30 % and 23 % decrease in gs, 45 %, 44 %, 50 % and 51 % decrease in E and, 177 %, 157 %, 191 % and 182 % increase in WUE was observed in HPM, K2, MX and W1 respectively, as compared to those under ambient CO2 level. It is important to mention that compared to the report presented earlier by Chandra et al. (2008), in the present study differences were observed in PN and WUE of HPM variety under the ambient CO2 concentration. These differences could be attributed to the growth stages of plants at the time of observations recorded. However, the effect of doubling of CO2 on these parameters was found comparable in both the studies. In the present study, the observed decrease in stomatal conductance and the reduction in the transpirational water loss under elevated CO2 concentration has been previously reported in many plant species (Eamus et al. 1993; Thomas et al. 1994). Elevated CO2 has also been reported to improve the water use efficiency of plants (Morison 1993; Joshi 2006; Chandra et al. 2008). The increase in Ci (Fig. 1d), ratio for Ci and gs (Ci/gs) which reflects the mesophyll efficiency of plants and no change in Ci/Ca ratio (Table 1) in response to the elevated CO2 levels were observed in Cannabis sativa. Constant Ci/Ca values under the different environmental conditions represent a stress free state and a close coordination between stomatal and mesophyll functions regulating CO2 uptake and water loss in plants (Jarvis et al. 1999). A similar result has been reported for many C3 and C4 plant species exposed to elevated CO2 levels (Morison 1993).
Table 1.
Effect of different CO2 concentrations on Ci/Ca and Ci/gs ratios in Cannabis sativa varieties. Values within a parameter (Ci/Ca or Ci/gs) followed by the same letter are not significantly different at p < 0.05. Ci—intercellular CO2 concentration, Ca—ambient CO2 concentration, gs—stomatal conductance
| Parameters | CO2 Levels (μmol mol−1) | Cannabis varieties | |||
|---|---|---|---|---|---|
| HPM | K2 | MX | W1 | ||
| Ci/Ca | 390 | 0.58 ± 0.06a | 0.58 ± 0.07 a | 0.57 ± 0.04 a | 0.60 ± 0.08 a |
| 545 | 0.66 ± 0.05 a | 0.59 ± 0.05 a | 0.61 ± 0.06 a | 0.57 ± 0.05 a | |
| 700 | 0.64 ± 0.08 a | 0.56 ± 0.08 a | 0.57 ± 0.05 a | 0.61 ± 0.07 a | |
| Ci/gs | 390 | 1.12 ± 0.14 a | 1.02 ± 0.15 a | 1.22 ± 0.18 a | 1.03 ± 0.16 a |
| 545 | 2.19 ± 0.19 ab | 1.62 ± 0.13 ab | 2.31 ± 0.27 ab | 1.15 ± 0.13 ab | |
| 700 | 3.84 ± 0.22 b | 3.36 ± 0.26 b | 3.15 ± 0.25 b | 2.48 ± 0.21 ab | |
In conclusion, the effects of elevated CO2 concentrations on Cannabis sativa varieties were significant, leading to an increase in PN and WUE of this species. However, the magnitude of the increase was ‘variety-specific’. The decreases in gs and E, and a constant Ci/Ca ratio under the elevated CO2 concentrations are the indicators of survival potential of this species under the climatic changes leading to drought conditions in the future.
Acknowledgements
This work was supported in part by the National Institute on Drug Abuse (NIDA), National Institute of Health (NIH), Department of Health and Human Services, USA, Contract No. N01DA-10-7773.
Abbreviations
- Ca
Ambient CO2 Concentration
- Ci
Intercellular CO2 Concentration
- E
Transpiration
- gs
Stomatal Conductance
- PN
Net Photosynthesis Rate
- WUE
Water Use Efficiency
References
- Bag N, Chandra S, Sharma S, Palni LMS. Micropropagation of Dev-Ringal (Thamnocalamus spathiflorus [(Trin.) Munro]—A temperate Bamboo, and comparison of In vitro and conventionally propagated plants. Plant Sci. 2000;156:125–135. doi: 10.1016/S0168-9452(00)00212-0. [DOI] [PubMed] [Google Scholar]
- Bazzaz FA, Garbutt K. The response of annuals in competitive neighborhoods: effect of elevated CO2. Ecol. 1988;69:937–946. doi: 10.2307/1941249. [DOI] [Google Scholar]
- Berry J, Downton WJS. Environmental regulation of photosynthesis. In: Govindjee, editor. Photosynthesis, Vol. II, Development, carbon metabolism and plant productivity. New York: Academic; 1982. pp. 263–343. [Google Scholar]
- Bowes G. Facing the inevitable: plant and increasing atmospheric CO2. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:309–332. doi: 10.1146/annurev.pp.44.060193.001521. [DOI] [Google Scholar]
- Brenneisen R, Egli A, ElSohly MA, Henn V, Spiess Y. The effect of orally and rectally administered Δ9-tetrahydrocannabinol on spasticity. A pilot study with two patients. Int J Clin Pharmacol Therap. 1996;34:446. [PubMed] [Google Scholar]
- Ceulemans R, Jiang XN, Shao BY. Effect of elevated CO2 on growth, biomass production and nitrogen allocation of two populous clones. J Biogeogr. 1995;22:261–268. doi: 10.2307/2845920. [DOI] [Google Scholar]
- Chandra S, Lata H, Khan IA, ElSohly MA. Photosynthetic response of Cannabis sativa L. to variations in photosynthetic photon flux densities, temperature and CO2 conditions. Physiol Mol Biol Plants. 2008;14:299–306. doi: 10.1007/s12298-008-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure JD, Acock B. Crop response to carbon dioxide doubling: a literature survey. Agric For Meteorol. 1986;38:127–145. doi: 10.1016/0168-1923(86)90054-7. [DOI] [Google Scholar]
- Eamus D, Berryman DA, Duff GA. Assimilation, stomatal conductance, specific leaf area and chlorophyll responses to elevated CO2 of Maranthes corymbosa, a tropical monsoon rain forest species. Aust J Plant Physiol. 1993;20:741–755. doi: 10.1071/PP9930741. [DOI] [Google Scholar]
- Grinspoon L, Bakalar JB. Marijuana, the forbidden medicine. New Haven: Yale University Press; 1993. [Google Scholar]
- Houghton JT, Meira-Filho LG, Calander BA, Harris N, Kattenburg A, Maskll K. IPCC Climatic Change Assessment 1995. The science of climatic change. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Jarvis AJ, Mansfield TA, Devis WJ. Stomatal behavior, photosynthesis and transpiration under rising CO2. Plant Cell Environ. 1999;22:639–648. doi: 10.1046/j.1365-3040.1999.00407.x. [DOI] [Google Scholar]
- Jones HG. Plants and microclimate: quantitative approach to environmental plant physiology. Vegetatio. 1992;104:193–209. [Google Scholar]
- Joshi SC. Photosynthetic response of Thysanolaena maxima (Roxb.) Kuntze, A multipurpose and monotype plant, to different levels of CO2. Physiol Mol Biol Plants. 2006;12:241–245. [Google Scholar]
- Joshi SC, Palni LMS. Clonal variation in temperature response of photosynthesis in tea. Plant Sci. 1998;13:225–232. doi: 10.1016/S0168-9452(98)00015-6. [DOI] [Google Scholar]
- Joshi SC, Chandra S, Palni LMS. Differences in photosynthetic characteristics and accumulation of osmoprotectants in sapling of evergreen plants grown inside and outside a greenhouse during the winter season. Photosynthetica. 2007;45:594–600. doi: 10.1007/s11099-007-0102-5. [DOI] [Google Scholar]
- Juurola E. Biochemical acclimation pattern of Betula pandula and Pinus sylvestris seedlings to elevated CO2 concentration. Tree Physiol. 2003;23:85–95. doi: 10.1093/treephys/23.2.85. [DOI] [PubMed] [Google Scholar]
- Kimball BA. Carbon dioxide and agricultural yield: an assemblage and analysis of 430 prior observations. Agron J. 1983;75:779–788. doi: 10.2134/agronj1983.00021962007500050014x. [DOI] [Google Scholar]
- Mattes RD, Egelman K, Shaw LM, ElSohly MA. Cannabinoids appetite stimulation. Pharmacol Biochem Behav. 1994;49:187–195. doi: 10.1016/0091-3057(94)90475-8. [DOI] [PubMed] [Google Scholar]
- Mauna Loa Observatory–MLO, Hawaii -Trends in atmospheric carbon dioxide, http://www.esrl.noaa.gov/gmd/ccgg/trends
- Minorsky PV. Global warming-effect on plants. Plant Physiol. 2002;129:1421–1422. doi: 10.1104/pp.900042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL (1993) Response of plants to CO2 under water limited conditions. Vegetatio 104/105:193–209
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol Chemother. 2006;147:163–171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H. Inter-specific variation in the growth response of plant to an elevated CO2 concentration. Vegetatio. 1993;104:77–97. doi: 10.1007/BF00048146. [DOI] [Google Scholar]
- Pounds JA, Puschendorf R. Clouded futures. Nature. 2004;427:107–109. doi: 10.1038/427107a. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiol. 1989;89:590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Elorza M, Dana ED, Gonzalez A, Sobrino E. Changes in the high-mountain vegetation of the central Iberian peninsula and a probable sign of global warming. Ann Bot. 2003;92:273–280. doi: 10.1093/aob/mcg130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RB, Lewis JD, Strain BR. Effect of leaf nutrient status on photosynthetic capacity in loblolly pine (Pinus taeda L.) seedling grown in elevated CO2. Tree Physiol. 1994;14:947–960. doi: 10.1093/treephys/14.7-8-9.947. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Improving the efficiency of photosynthesis. Science. 1975;188:626–633. doi: 10.1126/science.188.4188.626. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Marshall JD, Fins L. Correlated population differences in dry matter accumulation, allocation and water use efficiency in three sympatric conifer species. Forensic Sci. 1996;42:242–249. [Google Scholar]