Abstract
Thermal stability of antioxidant defense enzymes was investigated in leaf and inflorescence of heat adaptive weed Chenopodium album. Leaf samples were taken at early and late seedling stage in December (LD, 20 °C/4 °C) and March (LM, 31 °C/14 °C). Young inflorescence (INF) was sampled at flowering in April (40 °C/21 °C). LD, LM and INF crude protein extracts were subjected to elevated temperatures (5 to 100 °C) for 30′. Superoxide dismutase (SOD) was the most heat stable enzyme followed by Ascorbate peroxidase (APX). Two heat stable SOD isozymes were visible on native-PAGE at 100 °C in both leaf and INF. Some heat stable APX isozymes were more abundant in INF than leaf. Thermostability of catalase (CAT) increased with age and increasing ambient temperatures in leaves. CAT activity was observed up to 60 °C in leaves and INF while peroxidase (POX) retained activity up to 100 °C in INF due to one thermostable isozyme. Glutathione reductase (GR), dehydroascorbate reductase (DHAR, EC 1.8.5.1) and monodehydroascorbate reductase (MDHAR) showed activity up to 70 °C in both leaves and INF. DHAR activity was stable up to 60 °C while GR and MDHAR declined sharply after 40 °C. Constitutive heat stable isozymes of SOD and APX in leaves and INF may contribute towards heat tolerance in C. album.
Keywords: Heat tolerance, Chenopodium album, Antioxidant defense, Thermostable SOD, Thermostable APX
Introduction
Heat stress (HS) has considerable effect on growth and development in plants. Transitory or constantly HS causes an array of morpho-anatomical, physiological and biochemical changes in plants, which affect plant growth and development and may lead to a drastic reduction in economic yield (Long and Ort 2010). Plants can survive exposure to temperature above those optimal for growth (basal thermotolerance) or acquire tolerance to otherwise lethal temperatures (heat acclimation) if exposed to moderately high temperature (Iba 2002).
Heat stress leads to accelerated production of toxic metabolic by-products i.e. reactive oxygen species (ROS). ROS, such as 1O2, H2O2, O−2 and HO· are highly reactive molecules capable of causing oxidative damage to proteins, DNA and lipids (Wahid et al. 2007). Antioxidants such as ascorbic acid and glutathione, and ROS scavenging enzymes such as superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11), catalase (CAT, EC 1.11.1.6), peroxidases (POX, EC 1.11.1.7), glutathione reductase (GR, EC 1.6.4.2), dehydroascorbte reductase (DHAR, EC 1.8.5.1) and monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) have been found in almost all cellular compartments, demonstrating the importance of ROS detoxification for cellular survival (Iba 2002). ROS scavenging enzymes are major components of cellular defense during HS induced oxidative stress in plants (Wahid et al. 2007).
Chenopodium album is widely adapted weed plant facing wide array of temperatures (5 to 45 °C) during growth and development in north India and faces very high temperatures (Tmax~45 °C) during reproductive development. C. album is used as a model system to study the biochemical basis of thermotolerance of photosynthetic system and some heat shock proteins from chloroplasts have been isolated and characterized in ecotypes of C. album (Downs et al. 1999). Thermal optimum and lethal temperature limit of photosynthetic enzymes changes seasonally in many thermotolerant plants. Activity and thermal stability of photosynthetic enzymes in Tidestromia oblongifolia increased when plants were facing natural high air temperatures (Berry and Björkman 1980).
We have earlier reported a heat stable chloroplastic Cu/Zn SOD from C. murale which was purified and characterized (Khanna-Chopra and Sabarinath 2004; Sabrinath et al. 2009). There is dearth of information on oxidative stress and antioxidant defense in C. album under HS. Hence, a study was initiated on antioxidant defense enzymes in C. album, with the aim to study the in vitro heat stability of different enzymes in leaves and inflorescence.
Materials and methods
Plant material and sampling
Chenopodium album (Lambs quarter or Bathua) grows as a weed and its growth season extends form December to June. During its growth and development it experiences extremes of temperature ranging from Tmin of 4 °C to Tmax of 45 °C. Naturally growing population of C. album growing in wheat fields of Water Technology Center, Indian Agricultural Research Institute, New Delhi was used for the study. Sampling of young fully emerged leaves was done at early seedling stage i.e. about 25 days after emergence (DAE) in December (LD, Tmax, 20 °C and Tmin, 4 °C) and at late seedling stage, 90 DAE in March (LM, Tmax, 31 °C and Tmin, 14 °C). Sampling of young green inflorescence (INF) was done at flowering stage i.e. about 120 DAE in April (Tmax, 40 °C and Tmin, 21 °C).
Antioxidant enzymes extraction and in vitro heat stress treatment
Fresh leaf or INF tissue (0.2 g) was frozen in liquid nitrogen and stored in deep freezer (−80 °C). Tissue samples were homogenized in liquid nitrogen, suspended in 1.5 mL of 50 mM sodium-phosphate buffer (pH 7.0) containing 2 mM EDTA, 5 mM β-mercaptoethanol. 4 % (w/v) PVP-40 was added at the time of homogenization (Donahue et al. 1997). The enzyme extraction procedure using crude extracts was same for all the enzymes except APX where homogenization buffer contained in addition 5 mM sodium ascorbate. The homogenate was centrifuged at 30,000 × g for 30 min at 4 °C. The supernatant was used for measuring antioxidant enzyme activity. LD, LM and INF protein extracts were incubated in a sealed container at different temperatures i.e. 25, 30, 40, 50, 60, 70, 80, 90 °C and boiling for 30 min in continuous shaking water bath and then cooled by keeping it on ice. After incubation, the sample was centrifuged at 5,000 × g for 5 min, to remove the precipitated protein and the supernatant was used for further analysis. The extract without prior incubation was kept in ice (5 °C) served as control. The enzyme activity at each incubation temperature was expressed relative (%) to control i.e. extract kept in ice (5 °C) without incubation.
Enzyme assays and native PAGE activity staining
The supernatant obtained after in-vitro HS treatment at different temperatures was used for antioxidant enzyme assays. Protein content was determined by Lowry et al. (1951) using BSA as standard. Total SOD activity was measured spectrophotometrically based on inhibition in the photochemical reduction of nitroblue tetrazolium (Beauchamp and Fridovich 1971). One unit of SOD is defined as the amount of enzyme that inhibited the nitroblue tetrazolium (NBT) reduction by 50 %. APX activity was determined spectrophotometrically by monitoring the decrease in ascorbate at A290 (ε = 2.8 mM−1 cm−1) as described by Nakano and Asada (1981). CAT activity was measured by following the reduction of H2O2 (ε = 39.4 mM−1 cm−1) at 240 nm according to the method of Aebi (1984). POX activity was determined at 470 nm by its ability to convert guaiacol to tetraguaiacol (ε = 26.6 mM−1 cm−1) according to the method of Chance and Maehly (1955). GR was estimated by monitoring the oxidation of NADPH (ε = 6.22 mM−1 cm−1) at 340 nm according to Schaedle and Bassham (1977). Corrections were made by subtracting values obtained in the absence of either substrate or enzyme extracts. DHAR activity was measured by the method of Asada (1984) by following the reduction of DHA to ascorbate by GSH at pH 6.5. An increase in absorbance due to ascorbate formation was measured at 265 nm and an increase in 0.1 absorbance equal to 7.14 nmol ascorbate formed was used for calculating DHAR activity. MDHAR activity was assayed by following the decrease in absorbance at 340 nm due to oxidation of NADH (ε = 6.22 mM−1 cm−1) (Navari-Izzo et al. 1998). In this assay, MDHA was generated by ascorbate oxidase (EC 1.10.3.3).
Equal amount of soluble protein was separated on polyacrylamide gel electrophoresis (PAGE) under non-denaturing and non-reducing conditions using Protean llxi cell electrophoresis unit (Bio-Rad, Hercules, CA) at 4 °C followed by specific activity staining of the gels. Isozymes of SOD were separated on a 10 % native-PAGE and visualized by following the method of Beauchamp and Fridovich (1971). The different types of SODs were differentiated by the inhibition assay as described by Salin and Lyon (1983). APX isozymes were separated on 10 % native-PAGE gels and were stained as per the method of Mittler and Zilinskas (1993). CAT isozymes were separated on 7 % native-PAGE gels and were visualized by following the method of Woodbury et al. (1971). POX isozymes were separated on 7 % native-PAGE gels and were visualized by staining the gel as described by Seevers et al. (1971). DHAR isozymes were detected by using the method of de Pinto et al. (2000).
Results and discussion
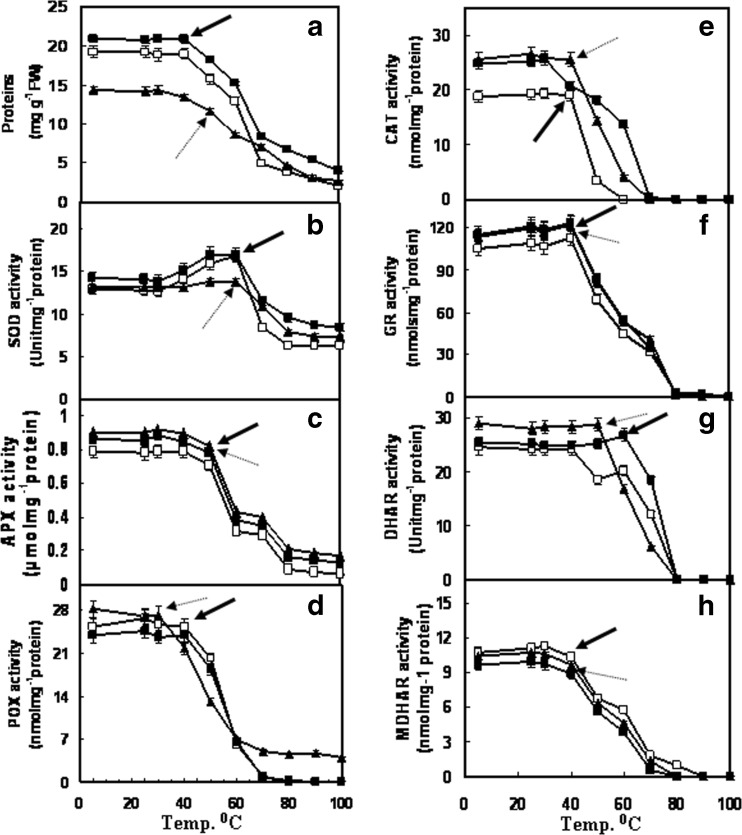
With increasing incubation temperature beyond 40 °C progressive loss in leaf and INF soluble protein content was observed (Fig. 1a). Loss in soluble protein content with increasing temperature has been observed earlier in Chenopodium murale (Khanna-Chopra and Sabarinath 2004). The rate of protein loss was sharper between 50 to 70 °C, being 2.8 %/°C, 2.35 %/°C and 1.6 %/°C in LD, LM and INF respectively. At 100 °C, 10 % proteins were stable in LD while about 20 % proteins were stable in LM and INF. Thermostability of proteins can increase in response to high temperature due to heat acclimation as observed in Tidestromia oblingifolia and Lolium perenne (Berry and Björkman 1980).
Fig. 1.
Soluble protein content in crude extracts from leaves (sampled in December, LD and March, LM) and inflorescence (INF) of C. albuma. Activity of antioxidant enzymes –b superoxide dismutase (SOD), c ascorbate peroxidase (APX), d guaiacol peroxidase (POX), e catalase (CAT), f glutathione reductase (GR), g dehydroascorbate reductase (DHAR) and h monodehydroascorbate reductase (MDHAR) in leaves and inflorescence of C. album. Crude extracts were subjected to different incubation temperatures (5–100 °C) for 30′. LD (white square), LM (black square) and INF (black triangle). Error bars indicate ± SE (n = 3). In some cases error bars are smaller than the symbol. FW indicates fresh weight. Arrows indicate the temperature at which sharp decline in activity was observed
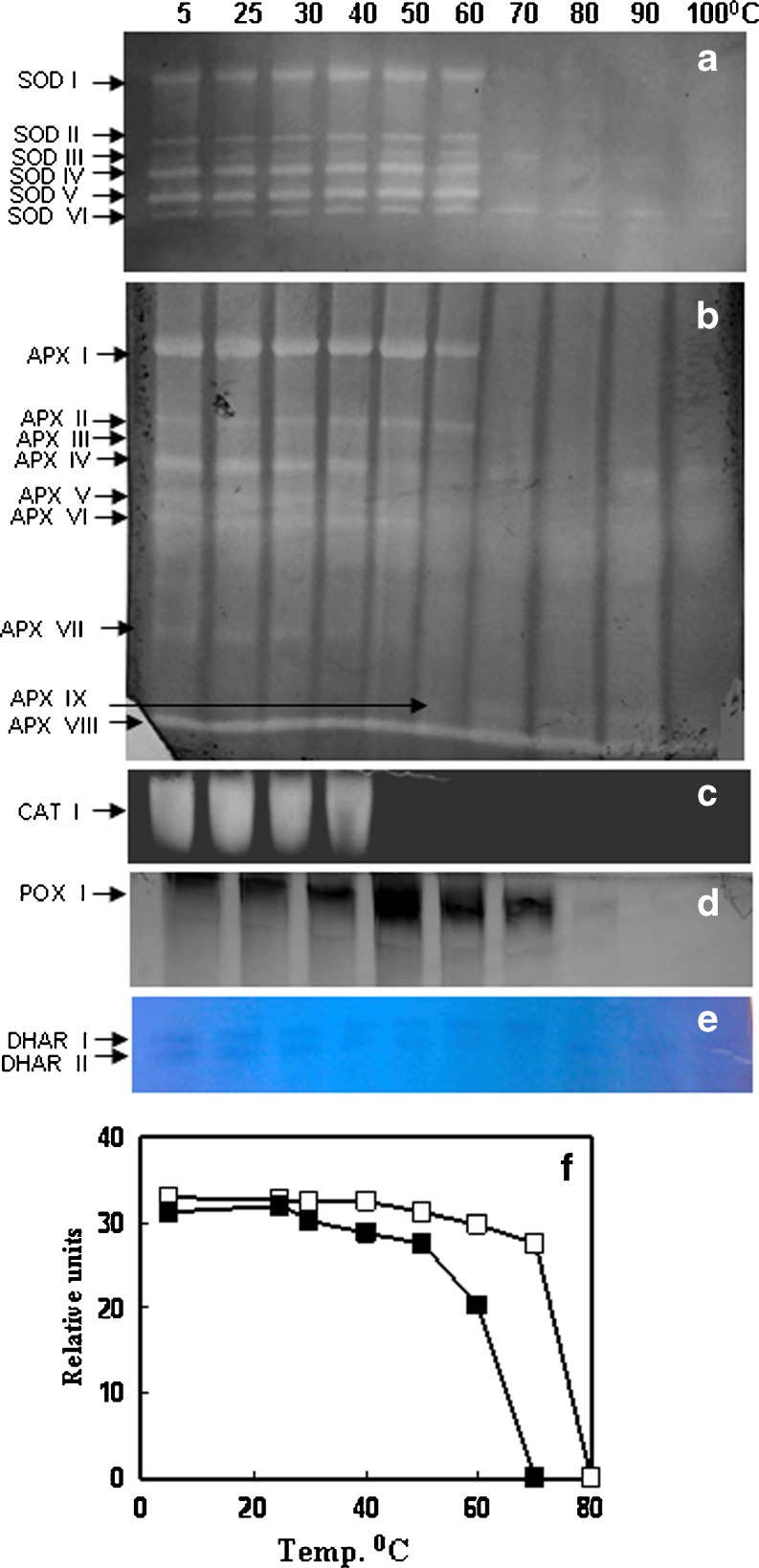
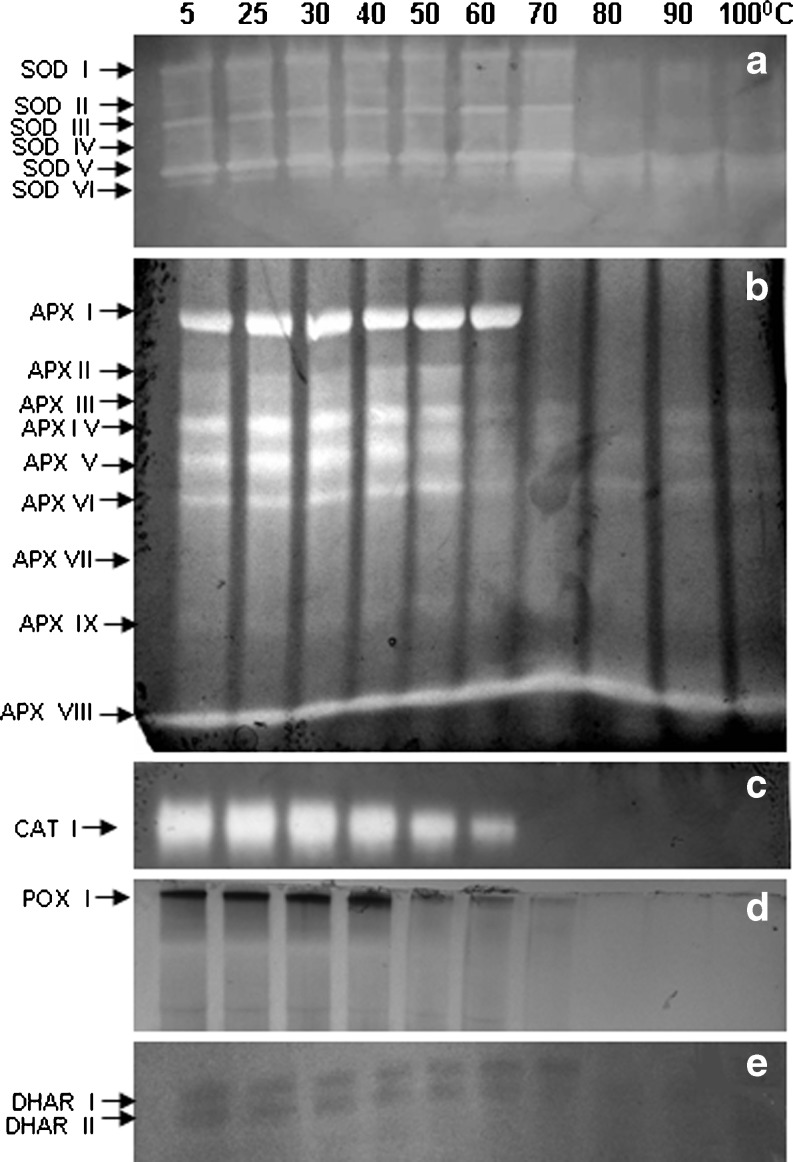
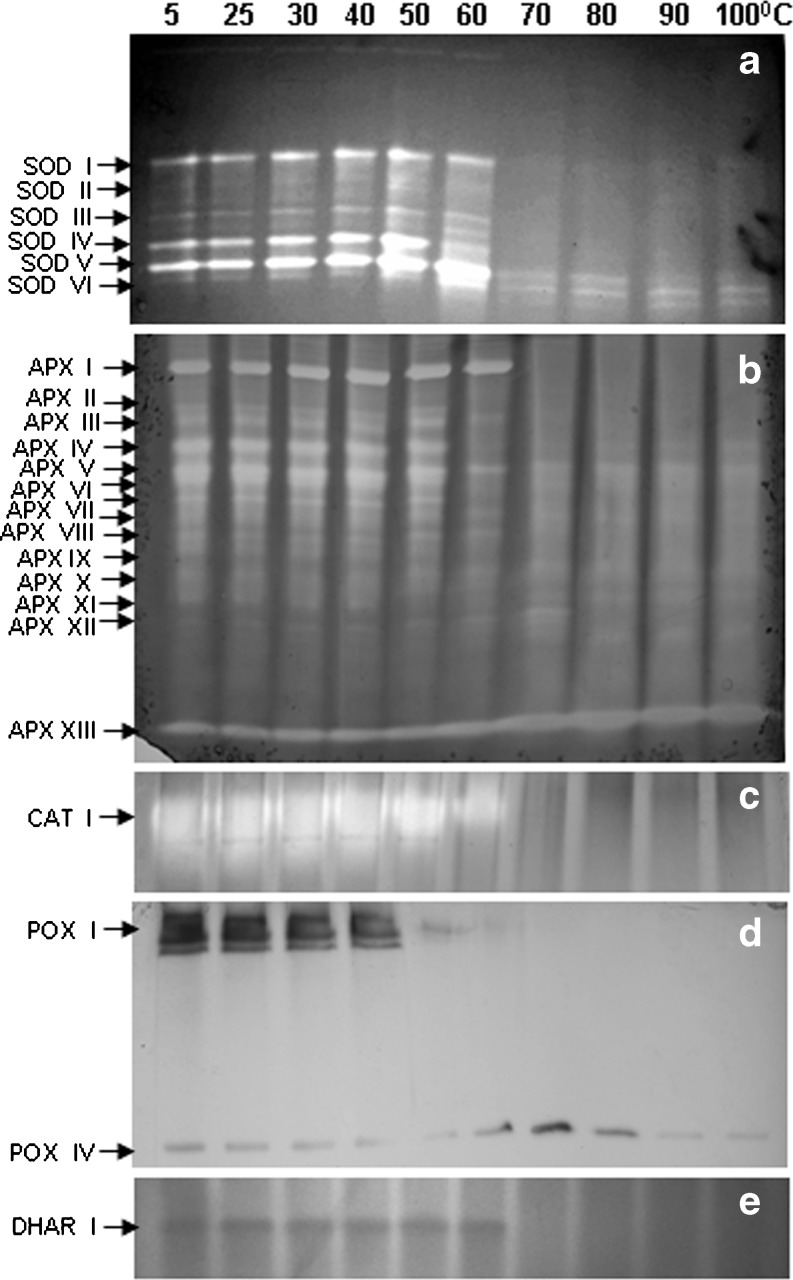
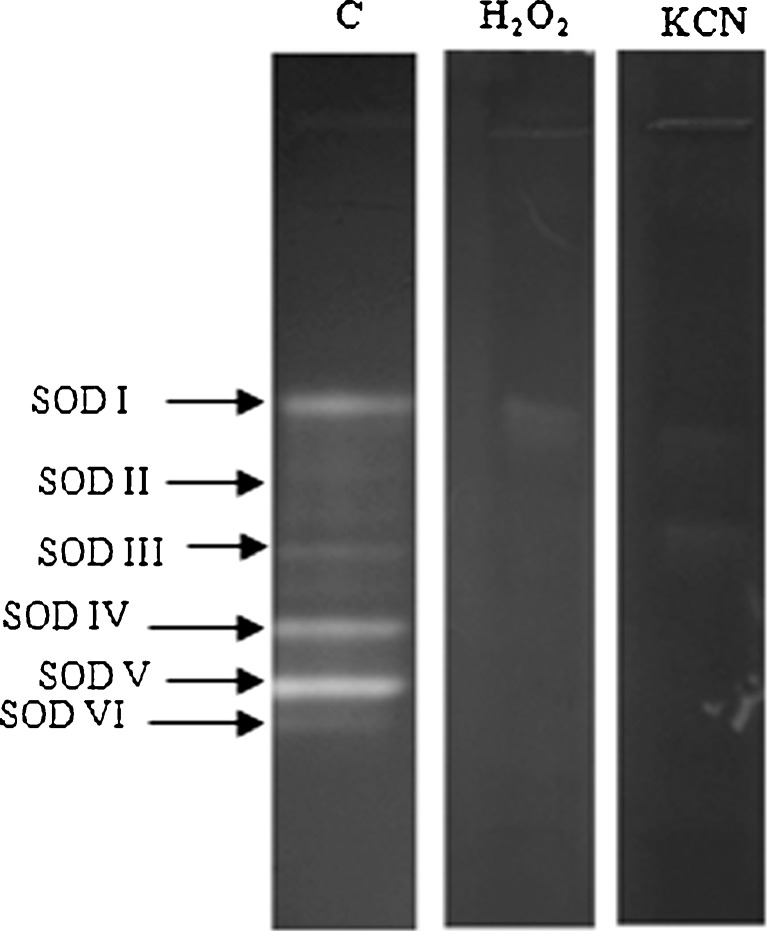
SOD is the first line of defense for oxidative stress and is well recognized for thermostability. SOD was the most heat stable enzyme followed by APX among the antioxidant enzymes studied in C. album (Fig. 1b). In LD, LM and INF 50, 60 and 55 % SOD activity was retained respectively even at 100 °C. SOD activity increased by 30 % at 60 °C. Six SOD bands were observed in LD, LM and INF in C album. All SOD isozymes were stable up to 60 °C (Table 1; Figs. 2a, 3a and 4a). Only 2 SOD isozymes, SOD V and SOD VI were visible at 70 °C and above in both leaves and INF (Figs. 2a, 3a and 4a). Both thermostable SODs were Cu/Zn SODs as both showed sensitivity to KCN and H2O2 in both leaves (data not shown) and INF (Fig. 5). Thermostable Cu/Zn SODs have been reported in several plants such as Potentilla astroanguinea, Citrus limon and C. murale etc. (Bafana et al. 2011). Presence of thermostable Cu/Zn SOD in the reproductive tissues of C. album is being reported for the first time. However thermostable MnSOD showing ~20 % activity after incubation at 95 °C (1 h) has been reported earlier in floral tissues of Nicotiana species (Carter and Thornburg 2000). SOD protein is known to have high melting temperature which may be one reason behind its themostability (Schafer and Kardinahl 2003). Overexpression of MnSOD in Brassica napus (Gusta et al. 2009) and thermostable superoxide reductase (SOR) isolated from hyperthermophile Pyrococcus furious in Arabidopsis (Im et al. 2009) resulted in enhanced thermotolerance. Hence SOD plays an important role in thermotolerance.
Table 1.
Thermostability of isozymes of antioxidant enzymes in leaves (sampled in December, LD and March, LM) and inflorescence (INF) crude extracts of C. album. The crude extracts were incubated at different temperatures 5–100 °C for 30′
| Sampling | Enzyme | Number of isoforms | Temp. up to which activity was observed (°C) | Number of isoforms at 100 °C |
|---|---|---|---|---|
| LD | SOD | 6 | 100 | 2 |
| APX | 9 | 100 | 3 | |
| CAT | 1 | 40 | 0 | |
| POX | 2 | 60 | 0 | |
| GR | – | 70 | – | |
| DHAR | 2 | 70 | 0 | |
| MDHAR | – | 70 | – | |
| LM | SOD | 6 | 100 | 2 |
| APX | 9 | 100 | 4 | |
| CAT | 1 | 60 | 0 | |
| POX | 2 | 60 | 0 | |
| GR | – | 70 | – | |
| DHAR | 2 | 70 | 0 | |
| MDHAR | – | 70 | – | |
| INF | SOD | 6 | 100 | 2 |
| APX | 13 | 100 | 6 | |
| CAT | 1 | 60 | 0 | |
| POX | 4 | 100 | 1 | |
| GR | – | 70 | – | |
| DHAR | 1 | 70 | 0 | |
| MDHAR | – | 70 | – |
Fig. 2.
Isozyme pattern of antioxidant enzymes a SOD, b APX, c CAT, d POX and e DHAR from leaves (sampled in December. LD) of C. album. Lanes indicate different incubation temperatures (5–100 °C, 30′). (F) Relative activity of DHAR isozymes as determined by scanning the gels using gel documentation system, area expressed as relative units. Equal amount of protein, 50 μg for SOD, APX, POX and DHAR and 15 μg for CAT activity was loaded in each lane
Fig. 3.
Isozyme pattern of antioxidant enzymes a SOD, b APX, c CAT, d POX and e DHAR leaves (sampled in March, LM) of C. album. Lanes indicate different incubation temperatures (5–100 °C, 30′). Equal amount of protein, 50 μg for SOD, APX, POX and DHAR and 15 μg for CAT activity was loaded in each lane
Fig. 4.
Isozyme pattern of antioxidant enzymes a SOD, b APX, c CAT, d POX and e DHAR from inflorescence of C. album. Lanes indicate different incubation temperatures (5–100 °C, 30′). Equal amount of protein, 50 μg for SOD, APX, POX and DHAR and 15 μg for CAT activity was loaded in each lane
Fig. 5.
Native PAGE stained for SOD activity in INF, inhibitor studies with 5 mM H2O2 or 3 mM KCN. Control (c) indicates gel stained without inhibitor. 50 μg protein was loaded in each lane
APX is the most important H2O2 scavenging enzyme in plant cells followed by CAT and POX. APX and POX were more heat stable in INF than LD and LM at 100 °C in C. album (Fig. 1c, d). APX and POX showed sharp decline in activity at 50 °C and 40 °C respectively in both leaf and INF. Thermostability of CAT enhanced with age and ambient temperature in leaves as CAT activity declined sharply at 40 °C in LD and INF but was stable upto 60 °C in LM (Fig. 1e). At 100 °C LD, LM and INF retained 10, 15 % and 20 % APX activity respectively (Fig. 1c). Diversity in APX isozymes profile was observed with INF showing more isozymes than leaves. At 100 °C, three, four and six APX isozymes showed activity in LD and LM and INF respectively (Table 1; Figs. 2b, 3b and 4b). Some (probably low molecular weight) APX isozymes (APX VIII in leaves and APX XIII in INF) which are unique in C. album showed increase in activity with increasing incubation temperatures in both leaf and INF (Figs. 2b, 3b and 4b). To the best of our knowledge, this is the first report on plant APX isozymes showing activity even after boiling treatment. Thermostability of APX has been reported in Vinca (60 °C) (Anderson and Padhye 2004) and rice (80 °C for 45′) (Sharma and Dubey 2004). APX is known to be regulated by HS transcription factors. Heat shock transcription factor3 (HSF3) overexpressing Arabidopsis plants showed the appearance of a unique HS induced thermostable isozyme of APX which was absent in wild type plants (Panchuk et al. 2002). Thermotolerance was enhanced in transgenics overexpressing APX in Arabidopsis (Shi et al. 2001) while a mutant deficit in cytosolic APX1 exhibited HS sensitivity (Koussevitzky et al. 2008). APX was upregulated by high temperatures in tomato microspores showing its importance in preventing the reproductive parts from oxidative damage in heat tolerant cultivar (Frank et al. 2009).
CAT activity generally declines under HS (Dat et al. 1998). CAT activity was lost completely at 60 °C in LD and 70 °C in both LM and INF in C. album (Fig. 1e). A single CAT isozyme showed activity only up to 40 °C in LD and 60 °C in both LM and INF respectively (Figs. 2c, 3c and 4c). In Vinca leaves complete loss of CAT activity was observed at 60 °C (15′) (Anderson and Padhye 2004). However some CAT isozymes in mouse liver showed activity upto 68 °C (Sun 1997). Optimum temperature and stability of enzymes can change seasonally as in case of mung bean nitrate reductase (NR). NR optimum temperature was 30 °C in rainy season while during summers it was 50 °C (Khanna-Chopra l983). POX activity was retained (14 %) upto 100 °C in INF while leaves retained ~25 % POX activity only upto 60 °C in LD and LM respectively (Fig. 1d). Two POX isozymes were seen in leaves which were lost at 70 °C (Figs. 2d and 3d). Inflorescence showed 4 POX isozymes of which POX IV was visible even at 100 °C (Fig. 4d). This isozyme was not observed in leaves. Thermostability of plant peroxidases is well documented, having heat inactivation temperature as high as 90.5 °C in soybean (McEldoon and Dordick 1996) and 100 °C (10′) in olives (Tzika et al. 2009). POXs also have regeneration capacity after heat inactivation (100 °C, 1.5′) if incubated at 25 °C (20 h) subsequently (Schwimmer 1944). The role of POXs is complicated by their involvement in diverse physiological functions. An increase in POXs has been reported in various stresses and has been linked with protection from oxidative damage, lignification and cross linking of cell wall (Brisson et al. 1994).
GR, DHAR and MDHAR are the enzymes responsible for maintenance of redox (AsA and GSH) pool of the cell. GR, DHAR and MDHAR showed activity upto 70 °C in both leaves and INF in C. album (Fig. 1f, g, h). DHAR activity was stable up to 60 °C while GR and MDHAR declined significantly at 40 °C. DHAR plays an important role in regeneration of reduced ascorbate. Two DHAR isozymes were visible in leaves (Figs. 2e and 3e) while only one DHAR band was visible in INF gel up to 60 °C (Fig. 4e). Lack of thermostable DHAR activity resulted in chronic photooxidative damage and yellowing in Ficus microcarpa (Yamasaki et al. 1999). AsA synthesis declines during HS (Song et al. 2005) which may be due to down regulation of AsA biosynthesis genes (Ioannidi et al. 2009). Heat stable AsA regeneration system therefore may help in maintaining the reduced AsA pool under heat stress. At 70 °C LD, LM and INF retained ~33 % GR activity (Fig. 1f) and 17 %, 6 % and 13 % MDHAR activity respectively (Fig. 1h). HS generally results in enhanced levels of GSH and high GSH/GSSG ratio in plants (Srivalli and Khanna-Chopra 2008). Maintaining high GSH/GSSG ratio during HS is correlated with thermotolerance in many plants like wheat and rice (Song et al. 2005; Szalai et al. 2009). A well coordinated balance between these enzymes may be one of the heat adaptive character in C. album as maintenance of AsA-GSH redox pool is highly important for tolerance and survival under HS. AsA-GSH pool becomes more important when enzymatic antioxidant defense is not sufficient. Double mutants of cytosolic APX1 and APX2 in rice showed higher accumulation of ROS, oxidative damage and enhanced AsA-DHA ratio during abiotic stresses. This increase in total antioxidant capacity observed during abiotic stresses shows the importance of compensatory mechanisms for ROS scavenging in plants (Rosa et al. 2010).
The present study shows that the high temperature tolerant C. album has constitutive heat stable isozymes of SOD and APX in leaf and inflorescence. C. album also shows acquired thermotolerance as themostable protein fraction and heat stability of SOD, APX, CAT and POX increased with age when plants were facing high ambient temperatures (Table 1; Fig. 1). The in vitro heat stable isozymes of antioxidant defense enzymes may play an important role in heat acclimation process in C. album which needs to be confirmed under in vivo conditions. Both basal and acquired thermotolerance in terms of antioxidant defense may play a major role in heat adaptation as ROS scavenging enzymes play an important role in preventing oxidative damage under HS in tobacco, tomato and alfalfa etc. (Iba 2002). HS often results in protein degradation in plants and hence maintaining stable and functional proteins is essential for proper metabolic functions under extremes of temperatures. Heat stability of proteins is dependent on other processes such as chaperones activity and HSPs (Sumesh et al. 2008). HSPs play a crucial role in protecting plants against heat stress by reestablishing normal protein confirmation and thus cellular homeostasis (Wang et al. 2004). Heat stability of different enzymes in plants acclimated to high temperatures (Berry and Björkman 1980) and thermophiles (Brock 1967) have long been known to be higher and is essential for their survival and growth under high temperatures. Thermal optimum and heat stability of different enzymes in plants can change seasonally with change in ambient temperatures (Berry and Björkman 1980; Khanna-Chopra l983).
Survival and succession of plants is highly dependent on their reproductive success. The onset of reproductive phases, its duration and the quality and quantity of reproductive products is influenced by HS. HS during reproductive development can result in chronic oxidative damage and enhanced expression of defense genes such as APX, HSPs etc. (Zinn et al. 2010). Inefficient antioxidant defense system in developing spikes resulted in sterility due to oxidative stress in rice (Selote and Khanna-Chopra 2004) and loss of both male and female gametophyte functions in many plants such as Brassica napus, tomato, wheat etc. (Zinn et al. 2010). The presence of more heat stable isozymes of antioxidant defense enzymes SOD, APX and POX in inflorescence of C. album may contribute to its reproductive stability and survival at high temperatures.
Acknowledgement
This research was supported by the financial grants of ICAR National Fellow Scheme awarded to Dr. (Mrs.) R. K. Chopra.
Abbreviations
- AsA
Ascorbate
- APX
Ascorbate peroxidase
- CAT
Catalase
- DHAR
Dehydroascorbate reductase
- GSH
Reduced glutathione
- GR
Glutathione reductase
- GSSG
Oxidized glutathione
- MDHAR
Monodehydroascorbate reductase
- POX
Guaiacol peroxidase
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Padhye SR. Protein aggregation, redical scavenging capacity, and stability of hydrogen peroxide defense systems in heat-stresses vinca and sweet pea leaves. J Am Soc Hort Sci. 2004;129:54–59. [Google Scholar]
- Asada K. Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol. 1984;105:422–429. doi: 10.1016/S0076-6879(84)05059-X. [DOI] [Google Scholar]
- Bafana A, Dutt S, Kumar S, Ahuja PS. Superoxide dismutase: an industrial perspective. Crit Rev Biotechnol. 2011;31:65–76. doi: 10.3109/07388551.2010.490937. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Berry J, Björkman O. Photosynthetic response and adaptation to temperatures in higher plants. Ann Rev Plant Physiol. 1980;31:491–453. doi: 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TD. Life at high temperatures. Science. 1967;158:1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Carter C, Thornburg RW. Tobacco Nectarin I. Purification and characterization as germ-like manganese superoxide dismutase implicated in the defense of floral reproductive tissues. J Biol Chem. 2000;275:26726–36733. doi: 10.1074/jbc.M006461200. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998;116:1351–1357. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L. Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco bright yellow 2. Plant Physiol Biochem. 2000;38:541–550. doi: 10.1016/S0981-9428(00)00773-7. [DOI] [Google Scholar]
- Donahue JL, Okpodu CM, Cramer CL, Grabau EA, Alscher RG. Responses of anti-oxidants to paraquat in pea leaves, relationships to resistance. Plant Physiol. 1997;113:249–257. doi: 10.1104/pp.113.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CA, Rayen SL, Heckathorn SA. The chloroplast small heat-shock protein: evidence for a general role in protecting Photosystem-II anainst oxidative stress and photoinhibition. J Plant Physiol. 1999;155:477–487. [Google Scholar]
- Frank G, Pressman E, Ophir R, Altman L, Shaked R, Freedman M, Shen S, Firon M. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones and sugars in the HS response. J Exp Bot. 2009;60:3891–3908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta LV, Benning NT, Wu G, Luo X, Liu X, Gusta ML, McHughen A. Superoxide dismutase: an all-purpose gene for agri-biotechnology. Mol Breed. 2009;24:103–115. doi: 10.1007/s11032-009-9274-y. [DOI] [Google Scholar]
- Iba K. Acclimation responses to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Im YJ, Ji M, Lee A, Killens R, Grunden AM, Boss WF. Expression of Pyrococcus furiosus superoxide reductase in Arabidopsis enhances heat tolerance. Plant Physiol. 2009;151:893–904. doi: 10.1104/pp.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidi E, Kalamaki MS, Engineer C, Peteraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot. 2009;60:663–678. doi: 10.1093/jxb/ern322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Chopra R. Effects of temperature on the in vivo assay of Nitrate reducatse in some C3 and C4 species. Ann Bot. l983;5l:3l–34. [Google Scholar]
- Khanna-Chopra R, Sabarinath S. Heat stable chloroplastic Cu/Zn SOD in Chenopodium murale. Biochem Biophys Res Commun. 2004;320:1187–1192. doi: 10.1016/j.bbrc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armiji L, Sha W, Cortes D, Shulaev V, Mittler R. Ascorbate peroxidase 1 is plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem. 2008;283:34197–34203. doi: 10.1074/jbc.M806337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ort DR. More than taking heat: crops and global climate change. Curr Opin Plant Biol. 2010;13:241–248. doi: 10.1016/j.pbi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall HJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McEldoon JP, Dordick JS. Unusual thermal stability of soybean peroxidase. Biotech Prog. 1996;12:555–558. doi: 10.1021/bp960010x. [DOI] [Google Scholar]
- Mittler R, Zilinskas BA. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate dependent reduction of nitroblue tetrazolium. Anal Biochem. 1993;212:540–546. doi: 10.1006/abio.1993.1366. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Navari-Izzo F, Quartacci MF, Pinzino C, Vecchia FD, Sgherri CLM. Thylakoid bound and stromal anti-oxidative enzymes in wheat treated with excess copper. Physiol Plant. 1998;104:630–638. doi: 10.1034/j.1399-3054.1998.1040416.x. [DOI] [Google Scholar]
- Panchuk II, Volkov RA, Schŏffl F. HS and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002;129:838–853. doi: 10.1104/pp.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JAG, Ferreira-Silva SL, Abreu-Neto J, Margis R, Margis-Pinheiro M. Cytosolic APX knockdown indicates an ambiguous redox response in rice. Phytochemistry. 2010;71:548–558. doi: 10.1016/j.phytochem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Sabrinath S, Khanna S, Khanna-Chopra R. Purification and characterization of thermostable Cu/Zn superoxide dismutase from Chenopodium murale. Physiol Mol Biol Plants. 2009;15:199–209. doi: 10.1007/s12298-009-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin ML, Lyon DS. Iron-containing superoxide dismutase in eukaryotes: localization in chloroplasts in water lily, Nuphar luteum. In: Cohen G, Greenwald RA, editors. Oxy radicals and their scavenger systems. Amsterdam: Elesvier; 1983. pp. 344–347. [Google Scholar]
- Schaedle M, Bassham A. Chloroplast glutathione reductase. Plant Physiol. 1977;53:1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer G, Kardinahl S. Iron superoxide dismutases: structure and function of an archaic enzyme. Biochem Society Trans. 2003;31:1130–1134. doi: 10.1042/BST0311130. [DOI] [PubMed] [Google Scholar]
- Schwimmer S. Regeneration of heat inactivated peroxidase. J Biol Chem. 1944;154:487–495. [Google Scholar]
- Seevers PM, Daly JM, Catedral FF. The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol. 1971;48:353–360. doi: 10.1104/pp.48.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote DS, Khanna-Chopra R. Drought induced spikelet sterility is associated with inefficient antioxidant defense in rice panicle. Physiol Plant. 2004;121:462–471. doi: 10.1111/j.1399-3054.2004.00341.x. [DOI] [Google Scholar]
- Sharma P, Dubey RS. Ascorbate peroxidase from rice seedlings: properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci. 2004;167:541–550. doi: 10.1016/j.plantsci.2004.04.028. [DOI] [Google Scholar]
- Shi WM, Muramoto Y, Ueda A, Takabe T. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene. 2001;273:23–27. doi: 10.1016/S0378-1119(01)00566-2. [DOI] [PubMed] [Google Scholar]
- Song XS, Hu WH, Mao WH, Ogweno O, Zhou YH, Yu JQ. Response of ascorbate peroxidase isozymes and ascorbate regeneration system to abiotic stresses in Cucumis sativus L. Plant Physiol Biochem. 2005;43:1082–1088. doi: 10.1016/j.plaphy.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Srivalli S, Khanna-Chopra R. Role of glutathione in abiotic stress tolerance. In: Khan NA, Singh S, Umar S, editors. Sulfur assimilation and abiotic stress in plants. Berlin: Springer-Verlag; 2008. pp. 207–225. [Google Scholar]
- Sumesh KV, Sharma-Natu P, Ghildiyal MC. Starch synthase activity and heat shock protein in relation to thermal tolerance of developing wheat grains. Biol Plant. 2008;52:749–753. doi: 10.1007/s10535-008-0145-x. [DOI] [Google Scholar]
- Sun Y. Multiplicity of antioxidant enzyme catalase in mouse liver cells. Free Radical Res. 1997;26:343–350. doi: 10.3109/10715769709097814. [DOI] [PubMed] [Google Scholar]
- Szalai G, Kellős T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatore molecule in plants under abiotic stress conditions. Plant Growth Reg. 2009;28:66–80. doi: 10.1007/s00344-008-9075-2. [DOI] [Google Scholar]
- Tzika ED, Sortiroudis TG, Papadimitriou V, Xenakis A. Partial purification and characterization of peroxidase from olives (Olea eeuropaea cv. Koroneiki) Eur Food Res Technol. 2009;228:487–495. doi: 10.1007/s00217-008-0956-1. [DOI] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Expt Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat shock proteins and molecular chaperones in abiotic stress response. Treads Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Woodbury W, Spencer AK, Stahmann MA. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971;44:301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Takahashi S, Heshiki R. The tropical fig Ficus microcarpa L. f. cv. Golden leaves lack heat-stable dehydoascorbate reductase activity. Plant Cell Physiol. 1999;40:640–646. [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot. 2010;61:1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]